Abstract
The study evaluated the damage caused by Haemonchus contortus in terms of blood loss, faecal clearance of plasma protein and elevated serum enzyme activity in Sahabadi sheep. Apparently healthy Sahabadi sheep (n = 15) were selected randomly based on phenotypic characteristics and divided into two groups; infected (n = 8) and uninfected control (n = 6) and one sheep was used as donor animal. Each animal of infected group were orally infected with 700 third stage larvae (L3) of H. contortus/kg body weight. Blood from all the fourteen animals were collected at weekly intervals starting from day one to 42 day post infection. Parameters studied were haemoglobin concentration (Hb), packed cell volume (PCV), total erythrocyte count (TEC), total serum protein, serum albumin, serum globulin, alkaline phosphatase, Alanine amino transferase and Aspartate amino transferase. Statistical analysis showed that significant decreases in Hb, PCV, TEC and serum protein concentration and significant increases in serum enzymes level in infected sheep compared to uninfected control. The present study concluded that experimental H. contortus infection caused disturbances to the haemopoietic system resulting anaemia and severe damage to abomasal mucosa resulting lower serum protein and higher enzyme activities.
Keywords: Haemonchus, Haematology, Serum protein, Serum enzymes, Sheep
Introduction
Sahabadi sheep is one of the important breed of sheep in India and it plays an essential role in the economy of small, marginal farmers and landless labourers in rural India. The rearing practices adopted by the rural people in India make them highly prone to gastrointestinal nematodosis which causes severe economic losses owing to reduced weight gain (Jas and Ghosh 2009). Amongst GI helminths of sheep Haemonchus contortus is the predominant species. Main pathological lesion caused by H. contortus infection is anaemia. Both adult and fourth stage larvae suck blood and in addition, migration of adult and larvae cause haemorrhages into the abomasum (Soulsby 1982). The average blood loss due to H. contortus infection is 0.03 ml/parasite/day (Urquhart 1996). Inhabiting the abomasum of sheep and goats it causes blood loss resulting in reduced feed intake, decreased body weight and wool growth (Hayat et al. 1996). Although a number of studies were already been done regarding haematological and enzymatic assays in sheep and goats infected with H. contortus (Costa et al. 1986; Ahmad and Ansari 1989; Siddiqua et al. 1989; Kuttler and Marble 1966; Raisinghani et al. 1971). However, different technique, standards, breed of animals involved, species-strain and stage of the parasites used by various workers did not show a regular pattern. The present study was conducted to determine extent of damage caused by H. contortus in terms blood loss, faecal clearance of plasma protein and elevated levels of serum enzyme activity in Sahabadi sheep.
Materials and methods
Experimental animals
A total of 15 Sahabadi sheep in the age group of 4–12 months were randomly selected from villages located in Bankura district of West Bengal. These animals were then maintained in intensive system of management for 3 months before they were used in the study. Pre-existing gastrointestinal parasites were eliminated by treatment with Fenbendazole (Panacur®, Intervet, @ 5 mg/kg body weight). One sheep was used as donor animal and the rest were divided into two groups viz. infected (n = 8) and uninfected control group (n = 6).
Experimental infection
Infective third stage larvae (L3) of H. contortus were obtained through culture of eggs isolated from the adult female worms (Soulsby 1982). Infective larvae were orally administered in donor sheep (@ 700 larvae/kg body weight), after overnight withdrawal of feed. After the patency of the infection, faecal sample of the donor sheep was cultured and infective larvae were harvested (Anon 1971). Culture infective larvae were used for artificial infection to 8 Sahabadi sheep and the remaining 6 sheep as uninfected control.
Quantitative examination of faecal samples
Faecal samples of infected sheep were qualitatively examined daily by salt floatation technique (Soulsby 1982) from second week post infection to determine the prepatent period of the infection. Quantitative examination of faecal samples was done by Modified McMaster’s technique (Soulsby 1982) at 2 days interval from 21 days post infection (DPI) onwards till the end of the experiment.
Estimation of haemato-biochemical parameters
Blood samples were collected from all the experimental sheep at weekly interval from 0 to 42 DPI. The haemoglobin concentration (Hb), packed cell volume (PCV), total erythrocyte count (TEC) were estimated by cyanomethaemoglobin, Wintrobe’s haematocrit and haemocytometer method, respectively (Jain 1993). Total serum protein (TSP) and serum albumin (SA) were estimated by Biuret method (Reinhold 1953) and the method described by Dumas et al. (1971), respectively. The concentration of serum globulin (SG) was estimated by subtracting the value of SA from that of TSP. Serum alkaline phosphatase (ALP) activity (IU/l) was estimated in UV–Vis-spectrophotometer (ELICO, India) by Kind and King’s method (Varley 1975). Serum Alanine amino transferase (ALT) and Aspartate amino transferase (AST) activity (IU/l) was estimated in UV–Vis-spectrophotometer (ELICO, India) by 2,4-dinitro phenylhydramine (2,4-DNPH) method (Reitman and Frankel 1957).
Statistical analysis
All the parameters for each group on different post infection days were compared (Analyze-Compare Means) for obtaining the mean value along with standard error (S.E). Variation among groups and between post infection days was observed using one-way-analysis of variance (ANOVA).
Results
Faecal egg count (EPG)
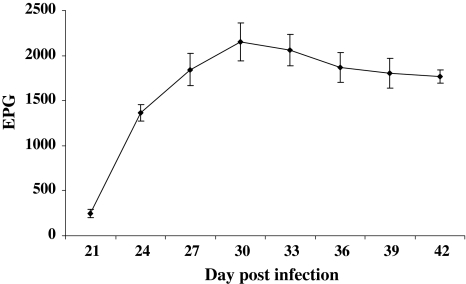
Parasite egg in faecal samples of infected sheep was first detected after 16 DPI and subsequently all the infected sheep were found to be positive on 18 DPI. After the infection attained patency, mean eggs per gram (EPG) of faeces was found to be (245 ± 45.69) on 21 DPI. Infected sheep was recorded non-significant increase of EPG level (Fig. 1) till the end of the experiment (42 DPI).
Fig. 1.
Mean faecal egg count (EPG) in H. contortus infection in Sahabadi sheep
Haematological profiles
Haemonchus contortus infection resulted in gradual decline in Hb concentration during the post infection period, but the level of decline was found to be statistically significant (P < 0.01) after 21 DPI as compared to pre-infection value (Table 1). Although PCV was also gradually declining but the level of decline was not statistically significant. However the PCV values in the infected group was significantly (P < 0.05) decreased on 21 and 28 DPI compared to the control group (Table 1). TEC decrease significantly (P < 0.01) in infected animal from 21 DPI (Table 1).
Table 1.
Changes in haematological profile due to Haemonchus contortus infection in Sahabadi sheep
| Day post infection | Mean (±SE) of Hb (gm/dl) value | Mean (±SE) of PCV (%) value | Mean (±SE) of TEC (×106/cm) value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Infected (n = 6) | Control (n = 6) | P value | Infected (n = 6) | Control (n = 6) | P value | Infected (n = 6) | Control (n = 6) | P value | |
| 0 | 9.10a ± 0.19 | 9.20 ± 0.34 | 0.803 | 30.2 ± 1.16 | 32.4 ± 1.21 | 0.225 | 20.42a ± 0.36 | 19.62 ± 0.96 | 0.432 |
| 7 | 9.00a ± 0.22 | 9.30 ± 0.41 | 0.536 | 30.0 ± 1.38 | 33.0 ± 1.30 | 0.153 | 19.06ab ± 0.56 | 20.15 ± 0.63 | 0.703 |
| 14 | 8.50a ± 0.44 | 9.05 ± 0.15 | 0.277 | 27.8 ± 2.22 | 33.0 ± 1.87 | 0.111 | 18.66ab ± 0.77 | 20.06 ± 0.36 | 0.595 |
| 21 | 7.00by ± 0.27 | 8.80x ± 0.19 | 0.001 | 26.0y ± 1.79 | 31.8x ± 1.28 | 0.030 | 11.13cy ± 0.87 | 19.27x ± 0.44 | 0.000 |
| 28 | 7.20by ± 0.1 | 8.80x ± 0.26 | 0.001 | 25.6y ± 1.36 | 31.2x ± 1.59 | 0.028 | 10.56cy ± 0.72 | 19.10x ± 1.08 | 0.000 |
| 35 | 7.40by ± 0.18 | 8.50x ± 0.20 | 0.004 | 27.6 ± 0.81 | 32.0 ± 2.30 | 0.109 | 17.50b ± 0.89 | 19.90 ± 0.60 | 0.276 |
| 42 | 7.50by ± 0.22 | 8.80x ± 0.12 | 0.001 | 28.0 ± 0.81 | 32.0 ± 2.30 | 0.109 | 17.89b ± 0.87 | 19.37 ± 0.35 | 0.134 |
Values bearing superscripts x, y in a row and a, b, c,…in a column differs significantly (P < 0.05)
Serum protein profile
Total serum protein concentration of infected sheep was significantly (P < 0.05) reduced as compared to pre-infection value as well as from uninfected control from 28 DPI (Table 2). SA level in infected sheep was found to be significantly (P < 0.01) decrease from 7 DPI till 42 DPI. No significant variation was observed in SG level during the post infection period in infected animal. No significant difference was also found in SG value between infected and uninfected control group.
Table 2.
Changes in serum protein profile due to Haemonchus contortus infection in Sahabadi sheep
| Day post infection | Mean (±SE) of TSP (gm/dl) value | Mean (±SE) of SA (gm/dl) value | Mean (±SE) of SG (gm/dl) value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Infected (n = 6) | Control (n = 6) | P value | Infected (n = 6) | Control (n = 6) | P value | Infected (n = 6) | Control (n = 6) | P value | |
| 0 | 7.86a ± 0.23 | 8.04 ± 0.38 | 0.697 | 4.03a ± 0.07 | 4.24 ± 0.12 | 0.160 | 3.83 ± 0.29 | 3.80 ± 0.28 | 0.950 |
| 7 | 7.25abc ± 0.25 | 8.17 ± 0.33 | 0.055 | 3.45by ± 0.06 | 4.21x ± 0.15 | 0.001 | 3.80 ± 0.20 | 3.97 ± 0.17 | 0.544 |
| 14 | 7.72ab ± 0.26 | 8.11 ± 0.25 | 0.31 | 3.59by ± 0.12 | 4.25x ± 0.13 | 0.006 | 4.13 ± 0.20 | 3.86 ± 0.18 | 0.326 |
| 21 | 7.40abc ± 0.35 | 8.06 ± 0.23 | 0.153 | 3.00cy ± 0.19 | 3.97x ± 0.09 | 0.002 | 4.40 ± 0.27 | 4.08 ± 0.15 | 0.346 |
| 28 | 6.93bcdy ± 0.21 | 7.89x ± 0.31 | 0.032 | 2.64cy ± 0.14 | 4.12x ± 0.06 | 0.000 | 4.29 ± 0.33 | 3.78 ± 0.26 | 0.248 |
| 35 | 6.81cdy ± 0.20 | 7.86x ± 0.30 | 0.019 | 2.67cy ± 0.10 | 3.94x ± 0.16 | 0.000 | 4.13 ± 0.2 | 3.92 ± 0.15 | 0.41 |
| 42 | 6.30dy ± 0.41 | 7.79x ± 0.24 | 0.014 | 2.60cy ± 0.17 | 3.99x ± 0.04 | 0.000 | 3.70 ± 0.36 | 3.80 ± 0.22 | 0.824 |
Values bearing superscripts x, y in a row and a, b, c,…in a column differs significantly (P < 0.05)
Serum enzymes activity
Serum ALP activity was significantly (P < 0.01) increased in infected animal from 14 DPI till the end of the experiment. The ALP level of infected sheep elevated significantly (P < 0.05) as compared to control group from 21 DPI till 42 DPI (Table 3). Serum ALT activity of infected animal was increased significantly (P < 0.01) in infected sheep (Table 3) from 21 DPI till the end of the experiment. Serum AST activity was consistently elevated due to H. contortus infection throughout the post infection period and this increase was significant (P < 0.05) in Sahabadi sheep (Table 3) on 35 and 42 DPI compared to uninfected control sheep.
Table 3.
Changes in serum enzymes activity (IU/l) due to Haemonchus contortus infection in Sahabadi sheep
| Day post infection | Mean (±SE) of serum ALP (IU/l) activity | Mean (±SE) of serum ALT (IU/l) activity | Mean (±SE) of serum AST (IU/l) activity | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Infected (n = 6) | Control (n = 6) | P value | Infected (n = 6) | Control (n = 6) | P value | Infected (n = 6) | Control (n = 6) | P value | |
| 0 | 163.1d ± 12.19 | 169.82 ± 21.18 | 0.79 | 34.55bc ± 1.09 | 32.49 ± 1.86 | 0.369 | 124.49b ± 30.15 | 118.91 ± 9.63 | 0.864 |
| 7 | 161.93d ± 16.69 | 147.01 ± 17.74 | 0.557 | 33.31c ± 1.00 | 31.18 ± 1.89 | 0.350 | 147.37ab ± 31.63 | 122.69 ± 9.83 | 0.891 |
| 14 | 195.76c ± 7.49 | 159.68 ± 18.24 | 0.105 | 35.08bc ± 0.34 | 31.8 ± 1.93 | 0.132 | 167.09ab ± 29.07 | 128.41 ± 7.88 | 0.455 |
| 21 | 221.36bcx ± 11.53 | 157.62y ± 8.63 | 0.002 | 37.8abx ± 1.87 | 29.62y ± 0.48 | 0.003 | 150.08ab ± 27.64 | 124.22 ± 6.81 | 0.842 |
| 28 | 246.10abx ± 8.15 | 168.93y ± 8.74 | 0.000 | 39.59ax ± 0.81 | 29.52y ± 0.47 | 0.000 | 175.22ab ± 27.15 | 130.24 ± 5.20 | 0.267 |
| 35 | 253.9abx ± 11.58 | 179.28y ± 9.16 | 0.001 | 40.2ax ± 0.83 | 29.52y ± 0.64 | 0.000 | 195.07ax ± 26.51 | 123.44 ± 18.70 | 0.013 |
| 42 | 256.34ax ± 3.74 | 173.12y ± 21.46 | 0.005 | 39.78ax ± 1.07 | 28.56y ± 0.77 | 0.000 | 206.32ax ± 31.58 | 128.68 ± 18.79 | 0.009 |
Values bearing superscripts x, y in a row and a, b, c,…in a column differs significantly (P < 0.05)
Discussion
Prepatent period of the infection in sheep is 2–3 weeks (Urquhart 1996), which is precisely 15 days as stated by Soulsby (1982). In the present study, the prepatent period of the infection in Sahabadi sheep was found to be within the established range. High faecal egg count in infected sheep was recorded during the entire study period indicating higher worm burden in the abomasums and thereby producing significant effect on haematological and serum biochemical parameters.
Haemonchus contortus infection is known to cause significant changes of haematological parameters like Hb, PCV and TEC and which may result in anaemia in infected animal (Siham et al. 1997; Sharma et al. 2000). The finding of the present study revealed that there was significant decrease in Hb, PCV and TEC value with resultant anaemia in Sahabadi sheep infected with H. contortus. It is estimated that an adult H. contortus can suck 0.03 ml of blood/day (Urquhart 1996), in addition to causing leakage of blood from the site of attachment. The blood letting activities of H. contortus, in addition to reducing the Hb concentration, cause a decrease in TEC and PCV (Soulsby 1976). The PCV and TEC value of infected sheep reduced significantly only on 21 and 28 DPI compared to control group but Hb concentration remained lower (P < 0.01) from 21 DPI till the end of the study. In the early stage (21 and 28 DPI) decrease in PCV and TEC was due to a time lag between the loss of blood and activation of the erythropoietic system of the host to compensate for this blood loss (Dargie and Allonby 1975). On 35 and 42 DPI there was no significant difference in PCV and TEC value between infected and control sheep. The TEC value of infected sheep increased significantly on 35 and 42 DPI compared to 21 and 28 DPI and this increase might be due to increased erythropoietic activity to compensate the blood loss. Significantly lower Hb concentration in infected sheep in stead of non-significant PCV and TEC compared to controls from 35 to 42 DPI indicates that hypochromic normocytic type of anaemia developed due to experimental haemonchosis in Sahabadi sheep.
In infected sheep there was significant decline in TSP with consequent decrease in SA and without any significant changes in globulin. Hypoproteinemia with decreased levels of TSP and SA is an important consequence of haemonchosis, which is responsible for protein loosing enteropathy (Soulsby 1982). Infected animals loose large quantities of serum proteins into the gut and it was reported that mean daily faecal clearance of plasma of Haemonchus infected animal was 210–340 ml/day (Dargie 1975). Hypoalbuminemia, which was found in present study, might be due to selective loss of albumin which is smaller in size and osmotic sensitivity to fluid movement (Tanwar and Mishra 2001). In consequence, the fractional catabolic rate of albumin was markedly increased resulting in hypoalbuminemia. In infected animals SG concentration increases mainly due to increased synthesis of gamma globulin which is an important component of humoral immune response (Tarazona et al. 1982) but in the present study globulin concentration did not increase significantly compared to control animal.
Significant increases in the activity of serum enzymes in H. contortus infected sheep over the controls in the present study were similar to Ahmad and Ansari (1989) who reported a significant rise in serum ALP in sheep and goat haemonchosis in natural and experimental condition. Rise in enzymatic level in haemonchosis observed here, might be attributed to the damage to abomasal mucosa by these parasites similar to that described by Charleston (1965) who reported deep invasion of muscular layer of abomasal mucosa in sheep by H. contortus larvae. The hypothesis is further supported by the findings of Hodson et al. (1962) and Posen (1967) who reported gastrointestinal diseases, such as peptic ulcer and ulcerative colitis, to be responsible for enzyme release from affected mucosa. Variations in serum ALT and AST was significant from 21 DPI between infected and control groups coinciding with the most active period of H. contortus life cycle, i.e. 4th stage larvae approaching adulthood and with their well equipped buccal cavity causing traumatic damage to the lining of abomasal mucosa (Al-Zubaidy et al. 1987). Serum enzymes and total proteins concentration are likely to change in various clinical disease conditions. They are treated as very good tools for diagnosis, prognosis and evaluation of treatment therapy. The elevation of serum ALP, ALT and AST level indicated some disruptive activities in organs of their origin or of altered membrane permeability. Their level could also rise due to lack of excretion or decrease due to impaired synthesis.
Conclusion
Observations, in the present study, on haemato-biochemical changes caused by experimental haemonchosis in sheep carry importance as they may indicate the extent of damage to the haemopoietic system and abomasal mucosa and thereby help in better understanding of the pathogenesis of anaemia especially in the absence of other possible factors which may influence these changes.
Acknowledgments
The authors thankfully acknowledge the financial assistance of the Indian Council of Agricultural Research, New Delhi in conducting this study under the research project entitled “All India Network Programme on Gastrointestinal Parasitism.”
References
- Ahmad M, Ansari JA. Effect of haemonchosis on haematology and non-specific phosphomonoesterase activities in sheep and goats. Helminthologia. 1989;26(4):295–302. [Google Scholar]
- Al-Zubaidy AJ, Altaif KI, Al-Qaisy HHK, Makkawi TA. Gross pathology and histopathology of haemonchosis in sheep and goats in Iraq. Vet Parasitol. 1987;23:286–288. doi: 10.1016/0304-4017(87)90010-0. [DOI] [PubMed] [Google Scholar]
- Manual of veterinary parasitological laboratory techniques, technical bulletin No. 18, her Majesty’s Stationery Office. London: Ministry of Agriculture, Fisheries and Food; 1971. [Google Scholar]
- Charleston WAG. Pathogenesis of experimental haemonchosis in sheep with special reference to the development of resistance. J Comp Pathol. 1965;75:55–67. doi: 10.1016/0021-9975(65)90048-4. [DOI] [Google Scholar]
- Costa CAF, Vieita-L da S, Pank KP. Erythrocytes and eosinophil count in sheared lambs before and after anthelmintic treatment. Pesquisaro Agropecuaria Brasileria. 1986;21(2):193–201. [Google Scholar]
- Dargie JD. Application of radioisotope techniques to the study of red cell and plasma protein metabolism in helminth diseases of sheep. Symp Br Soc Parasit. 1975;13:1–26. [Google Scholar]
- Dargie JD, Allonby EW. Pathophysiology of single and challenge infections of Haemonchus contortus in merino sheep: studies on red cell kinetics and “self cure” phenomenon. Int J Parasitol. 1975;5:147–157. doi: 10.1016/0020-7519(75)90021-1. [DOI] [PubMed] [Google Scholar]
- Dumas BT, Arends RL, Pinto PVC. Determination of serum albumin using BCG. In standard methods. Clin Chem. 1971;7:175–189. [Google Scholar]
- Hayat CS, Hussain SM, Iqbal Z, Hayat B, Akhtar M. Effect of parasitic nematodes on haematology and productivity of sheep. Pak Vet J. 1996;16(2):81–83. [Google Scholar]
- Hodson AW, Latner AL, Raine L. Isoenzymes of alkaline phosphatase. Clin Chim Acta. 1962;7:255. doi: 10.1016/0009-8981(62)90018-9. [DOI] [PubMed] [Google Scholar]
- Jain NC. Essentials of veterinary haematology. Philadelphia: Lea and Febiger; 1993. [Google Scholar]
- Jas R, Ghosh JD. Economic impact of gastrointestinal nematodosis in sheep: enhanced meat production by anthelmintic treatment. Indian J Anim Sci. 2009;79(8):3–5. [Google Scholar]
- Kuttler KL, Marble DW. Serum protein changes in lambs with naturally acquired Haemonchus contortus infection. Am J Vet Res. 1966;21:445–448. [PubMed] [Google Scholar]
- Posen S. Alkaline phosphatase. Ann Intern Med. 1967;67:183. doi: 10.7326/0003-4819-67-1-183. [DOI] [PubMed] [Google Scholar]
- Raisinghani PM, Ghosal AK, Singh BB. Studies on serum proteins in lambs experimentally infected with Haemonchus contortus. Ind Vet J. 1971;48:1112–1115. [Google Scholar]
- Reinhold JG (1953) In: Reiner M (ed) Standard methods of clinical chemistry. Academic Press, New York
- Reitman S, Frankel S. Colorimetric determination of GOT and GPT activity. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- Sharma DK, Chauhan PPS, Agrawal RD. Haematological changes in experimental haemonchosis in Barbari goats. J Anim Sci. 2000;70(4):353–355. [Google Scholar]
- Siddiqua A, Mannan MA, Hussain MA. Some biochemical studies in the blood of goats naturally infected with intestinal parasites. Ind Vet J. 1989;66:502–504. [Google Scholar]
- Siham ES, Karrar AE, El Amin SMM. Clinical picture and chemotherapy of experimental Haemonchus contortus and coccidial infection in goats. Sudan J Vet Sci Anim Husbandry. 1997;36(1–2):158–166. [Google Scholar]
- Soulsby EJL. Pathophysiology of parasitic infection. New York: Academic Press; 1976. [Google Scholar]
- Soulsby EJL. Helminths, arthropods and protozoa of domesticated animals. 7. Tindall: The English Language Book Society and Bailliere; 1982. [Google Scholar]
- Tanwar RK, Mishra S. Clinico-haemato-biochemical studies on intestinal helminthiasis in poultry. Vet Practitioner. 2001;2(2):137–140. [Google Scholar]
- Tarazona JM, Sanz-Pastor A, Babin-M-del M, Dominguez T, Parra I, Pastor-A Sanz, Del-Mar-Babin M, Pastor-A Sanz. Caprine Trichostrongylidosis II clinical studies of field infections, Anales-del-Instituto-Nacional-de-Investigaciones Agrarias; Ganadera, Spain. Indian J Parasitol. 1982;14:111–124. [Google Scholar]
- Urquhart GM. Veterinary parasitology. Cambridge: Blackwell Science; 1996. [Google Scholar]
- Varley H. Textbook of clinical practical biochemistry, Vol 1. 5. Delhi: CBS Publishers and Distributors; 1975. [Google Scholar]