Abstract
Onchocerciasis is a disease of public health and socio-economic importance in Ethiopia. The aim of this study was to assess parasitological and clinico-epidemiological features of onchocerciasis in the Anfilo District, West Wellega, prior to implementation of Community Directed Treatment with Ivermectin (CDTI) to generate epidemiological and parasitological data for use in control program of the disease and subsequent evaluation of CDTI. A cross-sectional study was conducted in Anfilo District of West Wellega zone during a period of 1 month: from mid-August to mid-September 2006. Data on socio-demographic characteristics were collected using a standardized questionnaire prepared for this purpose. All persons were examined clinically for skin signs and symptoms of onchocerciasis. Two skin snips, one from each side of the gluteal fold were taken using blood lancet and sterilized razor blade and examined for microfilaria. All data were categorized, coded, entered in a data base and analyzed using SPSS version 15.0. for windows. A total of 1114 individuals ≥15 years were examined for microfilariae (mf) of Onchocerca volvulus and onchocercal skin disease (OSD). The prevalence of onchocercal (mf) carrier was 74.8% (833/1114). In both genders, the prevalence of onchocerciasis showed direct correlations with the age of individuals (R2 = 0.79, P < 0.05). The infection rate varied with the occupation of the study subjects, with preponderance among farmers. Among the subjects with onchocerciasis, the mf density ranged from 1.0 to 711.0 per mg of skin snip with a mean density (SD) and median values of 32.1 (61.5) and 10.4 respectively. The overall community microfilariae load (CMFL), the most sensitive parasitological indicator of onchocerciasis was 19.6. The pervasiveness of OSD among the study subjects was 26.4%. OSD was more frequent in males (32.4%) than their female counterparts (20.8%, P < 0.05). The overall prevalence of onchocercal nodule carrier, the symptom opted for determining the community-wide prevalence of onchocerciasis was 12.1%. Leopard skin, the proxy of longstanding infection of onchocerciasis in the community, was also relatively high (19.1%). The abundance of mf in skin would definitely lead to high transmission potential in the Anfilo District. The situation in the Anfilo District should call for continued CDTI, owing to success of similar recommendations for such programmes in other parts of the country and elsewhere.
Keywords: Onchocerciasis, Microfilariae carriers, Onchocercal skin disease, Neglected disease, Ethiopia
Introduction
Human onchocerciasis is caused by the filarial parasitic nematode Onchocerca volvulus. Adult worms live in subcutaneous nodules and form deeper worm bundles, where fertilized females can produce, during an average of 10 years, millions of microfilariae (mf) responsible for the morbidity associated with the infection (Basáñez et al. 2006). Microfilariae enter a female blackfly when it is having blood meal from an infected person. A small percentage of these reach the insect’s thoracic muscles where after several moults, they become third-stage infective larvae. They then migrate to the insect’s salivary glands and are ready to be transferred to humans during the next blood meal. Several simuliid species have been incriminated in the transmission of O. volvulus (Crosskey 1990). In Africa, the Simulium damnosum sensu lato (s.l.) species complex is responsible for more than 95% of onchocerciasis (Crosskey 1990). The species breeds in fast flowing rivers and streams and hence transmission is most intense and the disease is more severe in communities located in river valleys (WHO 2010).
Recent results obtained from Rapid Epidemiological Mapping of Onchocerciasis (REMO) estimated that there are about 37 million people carry O. volvulus with 90 million at risk of the disease in Africa (APOC 2005). Of those infected with onchocerciasis, approximately 270,000 are blind and 500,000 have severe visual impairment. More than 99% of all cases of onchocerciasis and onchocerciasis related blindness are found in Africa (Hoerauf et al. 2003; Vision 2010). In most of endemic countries, the disease has an enormous economic and social impact, preventing people from working, receiving education or taking care of children. Studies conducted in Ethiopia, Nigeria and Sudan have shown that onchocerciasis is responsible for poor school performance, higher dropout rate among children due to itching, lack of sleep and other associated consequences. More so, low productivity, low income and higher health related costs are found among adults with onchocerciasis (Vision 2010).
In Ethiopia, onchocerciasis has been known since 1939 as a result of investigation by Italians in southwestern Ethiopia. In the last seven decades, the disease has been spreading to previously non-endemic regions of the country partly due to agricultural development projects and resettlement of millions of people from the highlands into endemic areas (Zein 1990). The REMO carried out in Ethiopia involving six regions of the country indicated, all the six regions were endemic for onchocerciasis, and four out of the six had areas that were meso- or hyperendemic to onchocerciasis. Particularly the disease is widespread in western Ethiopia extending from the Takazi valley in the northwest to the Omo valley in the southwest in varying levels of endemicity (De Sole and Walton 1976; Zein 1986; Gundersen et al. 1988; Tatichef et al. 1987; Terranova et al. 2007). Currently more than 10 million Ethiopians are at risk of onchocerciasis and three million are infected. Simulium damnosum complex and Simulium woodi ethiopiense are implicated disease vectors in Ethiopia (Zein 1990).
In Ethiopia, the main symptom of the disease is dermal manifestations that are characterized by intense itching and thickening of the skin, hanging groin and depigmentation of the skin, less appreciated but definitely more widespread form of onchocerciasis (Murdoch et al. 1993). While the disastrous effects of ocular damage by river blindness have always been high priority as a public health problem, skin disease has received scant attention. Nonetheless, large multi-country studies have recently shown that onchocercal skin disease (OSD) entails a grave burden and that its symptoms have significant personal and psycho-social effects, not only on the affected individuals but also on their families and communities (Murdoch et al. 2002). In Ethiopia, eye lesions and blindness due to the disease appear to be absent or uncommon (WHO 2010).
Since the initiation of African program for onchocerciasis control (APOC), the main focus of onchocerciasis control is community-directed treatment with Ivermectin (CDTI). Ethiopia is a member State of APOC and it launched the first CDTI in Kaffa-Sheka project in the year 2000. Similar implementations were effectuated by the national onchocerciasis task force in some other parts of the country. The success gained by onchocerciasis control program is utterly pleasing, however for materialization of the ambitious goal to eliminate the disease, accurate data on the disease before the commencement of CDTI and after CDTI are instrumental in the implementation and subsequent monitoring of such strategies. This study was therefore conducted to get data on onchocerciasis before the implementation of CDTI in the District. In particular it aimed at determining the prevalence of onchocerciasis, intensity of infection, sex-age related distribution of the infection and to shed light on the magnitude of OSD in the area. The obtained data will also be valuable for the subsequent evaluation and monitoring of the CDTI recently implemented in the District.
Materials and methods
Study area
The study was conducted in the Anfilo District which is situated 750 km west of Addis Ababa. The Anfilo District comprises 46 kebeles with a total population of about 75,000 (CSA 2007). The kebele is the lowest administrative unit in Ethiopia. Over 90% of the district’s population is comprised of farmers which depend mostly on rain-fed agriculture. Coffee is an important cash crop. Over 50 square kilometers are planted with this crop. There are several fast flowing rivers and streams in the District. Eda and Ega rivers are amongst the perennial rivers in the study area (Fig. 1).
Fig. 1.
Map of the study area
Study design and sample size
A cross-sectional study was conducted in Anfilo District of West Wellega zone during a period of one month: from mid-August to mid-September 2006. A multistage sampling technique was used. Firstly, four kebeles (namely: Dollea, Shebel, Waba, and Yelli) were selected by random sampling. Secondly, sensitization and advocacy was made by community health workers. After adequate community mobilization and sensitization, a total of 1114 persons were recruited for study. All volunteers aged ≥ 15 years, who gave a written informed consent, were included in the study. Children under 15 were intentionally excluded for it is well known that young children, who stay most of the time within the village, are relatively little exposed to the bites of S. damnosum s.l., which is only found biting at low densities in the clearings around the houses (Remme et al. 1986).
Clinical examination of the study subjects
Each study participant underwent through a clinical examination. The clinical examination was conducted by trained nurses in a separate room to maintain the privacy of study participants. All persons were examined clinically for skin signs and symptoms of onchocerciasis. Nodules were palpated from head to the ankles. Individuals were also asked if they had any palpable nodules on their bodies. Additional signs and symptoms recorded were pruritus, leopard skin, darkened skin (chronic onchodermatitis), skin lesions, lymphadenopathy and hanging groin.
Data and specimen collection
Data on socio-demographic characteristics like, age, gender, marital status, occupation, length of time spent in the study area and previous treatments received for onchocerciasis were collected using a standardized questionnaire prepared for this purpose. Two skin snips, one from each side of the gluteal fold were taken using blood lancet and sterilized razor blade. Skin snips were placed in eppendorf tubes containing 100 μl of physiological saline and incubated at room temperature for 24 h to assure complete emergence of mf from the skin biopsies. After 24 h, 100 μl of 4% formaldehyde was added to preserve the morphological features of mf and conserve skin snips. All samples were transported to Medical Faculty of Addis Ababa University, Microbiology laboratory. The fluid in each tube was thoroughly mixed and pipetted onto a glass slide for microscopic examination and counting of mf. Each skin biopsy, after blotting to remove excess moisture was weighed using analytical balance and the number of mf from each biopsy expressed as mf per milligram (mf/mg) of the skin snip. The geometric mean of the mf from the two skin biopsies from each patient was calculated and used as a measure of intensity of infection. Every 20th skin snip samples among positives were stained with Giemsa staining solution to identify the mf of O. volvulus from mf of Dipetalonema streptocerca.
Statistical analysis
Demographic data, clinical examination and laboratory results were categorized, coded, entered in a data base and analyzed using SPSS version 15.0. for windows. Frequencies and proportions were calculated for the descriptive data analysis involving nodule carrier rate (NCR), mf carrier rate (MFCR) and OSD. Associations between participant’s characteristics and onchocercal related features were analyzed using the Pearson chi-square and correlation test. Student t test was also used to compare the means of microfilarial load and to compare onhocerciasis related features between different study groups. Differences and associations were deemed significant when P value less than 0.05 is obtained. The community microfilariae load (CMFL), the geometric mean number of microfilariae per skin snip among adults aged 20 years or above, was calculated using a log (x + 1) transformation.
Ethical consideration
This study was conducted after obtaining ethical clearance from Ethical review committee of Faculty of Medicine, Addis Ababa University, Ethiopia. The objective of the study was explained to the participants or their guardians in Afaan Oromo (local language). Skin snip samples were taken after informed written consent was obtained. All positive study subjects were treated with standard dose of Ivermectin.
Results
Socio-demographic characteristics of study subjects
The study included a total of 1114 subjects from Anfilo District, western Ethiopia. The ages of the study participants ranged from 15 to 100 years with a mean (SD) and median ages of 36 (16.6) and 32 years respectively. The majority (51.9%) of the study subjects were females, 62.4% were married, 50.1% were farmers and 54.7% were protestant Christians. The socio-demographic characteristics of the study subjects is given in Table 1.
Table 1.
Socio-demographic characteristics of study subjects in Anfilo district, western Ethiopia; august–september 2006
| Characteristics | Females n (%) |
Male n (%) |
Total n (%) |
|---|---|---|---|
| Age | |||
| 15–24 | 188 (16.9) | 127 (11.4) | 315 (28.3) |
| 25–34 | 132 (11.8) | 126 (11.3) | 258 (23.2) |
| 35–44 | 112 (10.1) | 82 (7.4) | 194 (17.4) |
| 45–54 | 83 (7.4) | 84 (7.5) | 167 (15.0) |
| ≥55 | 63 (5.7) | 117 (10.5) | 180 (16.2) |
| Marital status | |||
| Married | 371 (33.3) | 324 (29.1) | 695 (62.4) |
| Single | 140 (12.6) | 183 (16.4) | 323 (29.0) |
| Divorced | 25 (2.2) | 11 (1.0) | 36 (3.2) |
| Widowed | 42 (3.8) | 18 (1.6) | 60 (5.4) |
| Occupation | |||
| Farmer | 133 (11.9) | 425 (38.2) | 558 (50.1) |
| Housewife | 336 (30.2) | – | 336 (30.2) |
| Student | 101 (9.1) | 92 (8.3) | 193 (17.4) |
| Civil servant | 4 (0.4) | 7 (0.6) | 11 (1.0) |
| Daily labourer | 4 (0.4) | 12 (1.1) | 16 (1.5) |
| Religion | |||
| Orthodox Christian | 224 (20.1) | 227 (20.4) | 451 (40.5) |
| Protestant Christian | 330 (29.6) | 280 (25.1) | 610 (54.7) |
| Muslim | 24 (2.2) | 29 (2.6) | 53 (4.8) |
Parasitological data
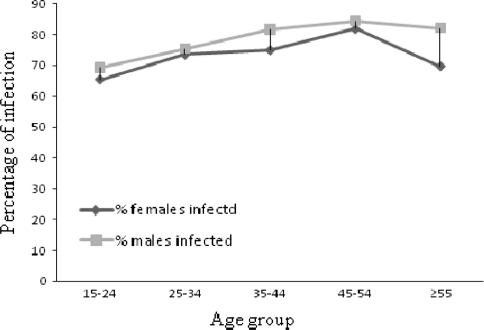
Onchocerciasis was prevalent in all of the Kebeles included in the study. The overall onchocercal MFCR was 74.8% (n = 1114). The pervasiveness of onchocercal mf carrier was higher among male study subjects (77.8%) than their female counterparts (71.9%) with statistically significant difference (χ2 = 5.0, P < 0.05). Among the subjects with onchocerciasis, the mf density ranged from 1.0 to 711.0 per mg of skin snip; and with a mean density (SD) and median values of 32.1 (61.5) and 10.4 respectively. The higher magnitude of MFCR was among the age group 45–54 years in both genders. The percentage of mf carriers in both genders and age group is shown in Fig. 2. In both genders, the prevalence of onchocercal infection showed direct relationship with the age of persons (R2 = 0.79, P < 0.05). However in the elderly study subjects (≥55 years), a slight decline in the prevalence of mf carrier was observed (Fig. 2). The infection rate varied with the occupation of the individuals. The highest prevalence of infection (82.1%) was detected among farmers while the least infection rate (62.7%) was observed among students (Pearson χ2 = 26.6, P < 0.05). The MFCR vs. occupation of the participants is shown in Table 2.
Fig. 2.
Percentage of mf carriers by gender and age groups, Anfilo district, western Ethiopia; august–september 2006
Table 2.
Prevalence of MFCR vs. occupation of subjects, Anfilo district, western Ethiopia; august–september 2006
| Occupation | Total examined n (%) |
Mf carrier n (%) |
|---|---|---|
| Farmer | 558 (50.1) | 458 (82.1) |
| Housewife | 336 (30.2) | 235 (69.9) |
| Student | 193 (17.4) | 121 (62.7) |
| Civil servant | 11 (1.0) | 7 (63.6) |
| Daily laborer | 16 (1.5) | 12 (75.0) |
| Total | 1114 (100) | 833 (74.8) |
Of the studied Kebeles, the highest prevalence of MFCR (87.6%) was observed among study subjects from Yelli Kebele while the lowest prevalence (62.6%) was found among study participants from Dollea Kebele. The highest infected males (91.5%) were from Yelli Kebele and the least infected males were from Dollea Kebele. Similar trend of infection rate was observed among female study participants. A significant difference in the prevalence of infection was observed among the Kebeles (P < 0.05). The highest density mf carrier was observed among individuals living in Shebel Kebele.
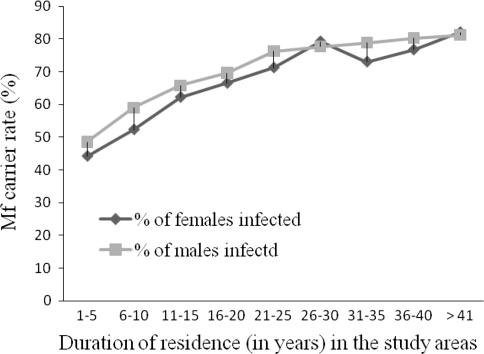
The prevalence of onchocerciasis infection increased with the duration of stay in the area. Accordingly a linear relationship (R2 = 0.87, P < 0.05) between the period lived in the area and prevalence of onchocerciasis was observed (Fig. 3). The highest prevalence of MFCR was observed among persons who lived in the area for longer period of time. Irrespective of the length of stay, both males and females had high prevalence of infection in all kebeles (Fig. 3).
Fig. 3.
Prevalence of mf carriers among study subjects along with the duration lived in the Anfilo district, Western Ethiopia; august–september 2006
The overall CMFL, the geometric mean number of microfilariae per skin snip among adults aged ≥20 years in the community including those with negative counts was 19.6. The CMFL was significantly higher (25.6) among males than in females (15.2) (P < 0.05). The highest CMFL (42.2) was documented among study subjects from Yelli Kebele of the study area and the lowest CMFL (8.4) was in study subjects from Dollea Kebele of the study site (P < 0.05).
Influence of previous treatments on the magnitude of onchocerciasis
Accessing treatment for infectious disease from different source is a common practice in endemic countries. Likewise some of our study participants individually sought treatment for filariasis from different source such as private pharmacies and drug peddlers. Of the 1114 study subjects, 445 (39.9%) reported that they had received treatment for filariasis at least once (Table 3). Most of the persons 52.6% (234/445) who sought treatment, did so only once. Few among them (11) reported that they sought treatment for about five times.
Table 3.
Frequency of treatment with anti-filarials as reported by the study participants of Anfilo district, western Ethiopia; august–september 2006
| Frequency of treatment | Frequency n (%) |
Mf carrier rate n (%) |
|---|---|---|
| 1X | 234 (52.6) | 174 (74.4) |
| 2X | 124 (27.9) | 86 (69.4) |
| 3X | 57 (12.8) | 40 (70.2) |
| 4X | 19 (4.3) | 9 (47.4) |
| 5X | 11 (2.5) | 4 (57.8) |
| Total | 445 (100) | 313 (70.3) |
*Percentages were calculated for subjects who sought treatment for filariasis before collection of data for this study
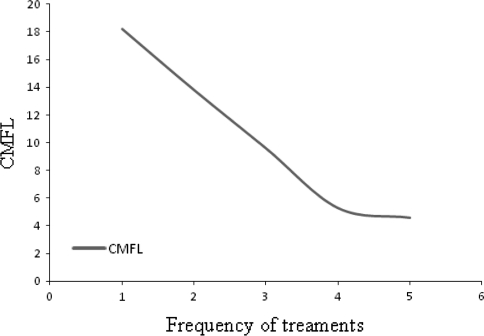
The mf carrier rate was significantly higher among the study subjects who had never been treated with anti-filarials (77.7%) than persons who previously sought treatment (70.3%, P < 0.05). Similar trend of difference has also been observed with CMFL (Fig. 4). Among previously treated study subjects, the highest CMFL was observed among group that sought treatment only once while the lowest CMFL was detected among the group that was treated five times for filariasis (Fig. 4). There was also a strong positive correlation between the frequency of treatment sought and CMFL (R2 = 0.96, P < 0.05).
Fig. 4.
Correlation of numbers of previous treatment with CMFL in Anfilo district, western Ethiopia; august–september 2006
Clinical examinations results
All of the study participants were underwent clinical examination to check for the common symptoms of onchocerciasis. Pruritus was the most frequently encountered (64.3%). The frequency of the symptoms of onchocerciasis is shown in Table 4. Among the commonly encountered symptoms of onchocerciasis, hanging groin is the least prevalent (5.2%). Overall, the commonly diagnosed symptoms of onchocerciasis are appeared to be more prevalent in male subjects than their female counterparts (P < 0.05).
Table 4.
Frequency of the symptoms of onchocerciasis among the study subjects, Anfilo district, western Ethiopia; august–september 2006
| Clinical symptoms | Frequency | χ2, P-value | ||
|---|---|---|---|---|
| Females n (%) |
Males n (%) |
Total n (%) |
||
| Pruritus | 351 (60.7) | 365 (68.1) | 716 (64.3) | 6.6, 0.01 |
| Onchocercomata | 47 (8.1) | 88 (16.4) | 135 (12.1) | 18.1, <0.01 |
| Leopard skin | 73 (12.6) | 140 (26.1) | 213 (19.1) | 32.7, <0.01 |
| Skin lesions | 62 (10.7) | 64 (11.9) | 126 (11.3) | 0.4, >0.05 |
| Hanging groin | – | 28 (5.2) | 28 (5.2) | NA |
| Lymphadenopathy | 80 (13.8) | 103 (19.2) | 183 (16.4) | 11.9, <0.05 |
NA not applicable
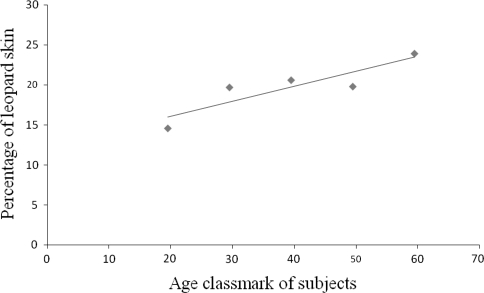
The overall prevalence of onchocercal nodule carrier, the symptom usually opted for determining the community-wide prevalence of onchocerciasis was 12.1% (Table 4). The incidence of onchocercomata was more frequent in male subjects than their female counterparts (Table 4). Leopard skin, the sign of the longstanding infection in the community was also relatively common among the study subjects (19.1%). The preponderance of leopard skin was correlated with the age of persons (R2 = 0.79, P < 0.05) (Fig. 5).
Fig. 5.
Correlation between leopard skin with age of individuals in Anfilo district, western Ethiopia; august–september 2006
Onchocercal skin disease
Onchocercal skin disease was deemed present when one of the following clinical spectra of onchocerciasis was coupled with pruritus: onchocercal nodule, leopard skin, lizard skin, lymphadenopathy, skin lesion and hanging groin. The overall prevalence of OSD among the 1114 study subjects was found to be 26.4% (Table 5); being more prevalent in males (32.4%) than in females (20.8%, P < 0.05). Among study participants of the four Kebele’s, the highest prevalence of OSD (36.9%) was observed in study subjects from Yelli Kebele. The prevalence in other villages was: 33.6% in Waba, 23.8% in Dollea and 22.9% in Shebel.
Table 5.
Distribution of OSD by age-gender of study participants in Anfilo district, western Ethiopia; august–september 2006
| Age group | # examined | Prevalence of OSD | ||
|---|---|---|---|---|
| Males n (%) |
Females n (%) |
Total n (%) |
||
| 15–24 | 315 | 27 (21.3) | 39 (20.7) | 66 (20.9) |
| 25–34 | 258 | 45 (35.7) | 26 (19.7) | 71 (27.5) |
| 35–44 | 194 | 22 (26.8) | 30 (26.7) | 52 (28.6) |
| 45–54 | 167 | 35 (41.7) | 15 (17.9) | 50 (29.9) |
| >55 | 180 | 44 (37.6) | 10 (8.5) | 54 (30.0) |
| Total | 1114 | 173 (32.4) | 120 (20.8) | 293 (26.3) |
The highest prevalence of OSD was detected in elderly persons (≥55) while the least prevalence was observed among younger persons (15–24 years, P < 0.05) of the study participants (Table 5).
Discussions
There are remarkable pervasiveness and distribution of onchocerciasis in Ethiopia due to the differences in ecological features of the localities. In order to identify the endemic areas and communities at higher risk and to lay down priorities towards the formulation of intervention strategies, it was essential to carry out epidemiological and parasitological studies in different parts of the country. This study was therefore conducted to determine the prevalence, sex-age related intensity of onchocerciasis and to shed light on the burden of OSD in rural areas of Western Ethiopia.
In Ethiopia, several studies were previously undertaken in an attempt to determine the prevalence of onchocerciasis in different regions of the country. Consequently, various estimates of prevalence of infection ranging from low to high rates were reported (Adugna et al. 1996; Jira 1993; Taye et al. 2000; Hailu et al. 2002). The prevalence of onchocerciasis in Anfilo District during our survey was 74.8%. Adopting the endemic rates classification as defined by World Health Organization, these communities can be regarded as hyperendemic for onchocerciasis (WHO 1992). The prevalence observed in our study area was higher than the reports from some parts of the country (Adugna et al. 1996; Taye et al. 2000) even though other reports have previously documented a high prevalence among different ethnic groups of western Ethiopia. For instance, Jira (1993) reported a high prevalence of onchocerciasis (81.0%) among Nilotic people living in Blue Nile Valley of Western Ethiopia.
In the current study, the prevalence of onchocerciasis was higher in male study subjects than their female counterparts (P < 0.05), possibly associated with occupational/behavioural risk factors attributable to male gender. In the Anfilo District, male farmers are more engaged in outdoor activities mainly for farming in areas which are in proximity to the breeding sites of blackflies; while females are more engaged in indoor activities and hence less exposed to the outdoor biting blackflies. Similar trend of prevalence rate of onchocerciasis was reported form Assosa region and other parts of the country (Tatichef et al. 1987; Adugna et al. 1996; Yeneneh et al. 1989). A higher prevalence of river blindness among the male segment of endemic populations has also been documented in other endemic African countries (Okonkwo et al. 1991; Bocharie and Davies 1990).
There was a significant relationship between type of occupation and prevalence of onchocerciasis, farmers being the most affected (Table 2). Farming was the major occupation of the study community. Farmers in the Anfilo District are usually dressed in short sleeves and shorts during farming activity which expose them to bites of blackflies. The higher prevalence rate of onchocerciasis among farmers has also been reported from other endemic countries where farming was the major occupation of the study communities (Akinboye et al. 2010).
In contrast to reports from West African countries, we found no association between the mf and palpable nodule carrier rate. While microscopic examination of skin biopsies yielded 74.8% prevalence rate, the NCR was only 12.1%. The World Health Organization protocol for determining community-wide prevalence is based on the prevalence of nodules in men multiplied by 1.6 to give the community prevalence of onchocerciasis in an endemic area. Application of this formulation yields a prevalence of 26.6% (16.4 × 1.6) cf. 74.8% MFCR. This disparity between the NCR and MFCR is much wider than previously reported in other countries (Remme et al. 1986; Mace et al. 1997; Ngoumou et al. 1994), and may be associated with the variability in parasite strains’ virulence, differences of vectors in their biting proclivities, altered host factors associated with genetic susceptibility or host immunity, and history of co-infection by other parasites (McCarthy et al. 1994). A study from southwest Ethiopia showed even the complete absence of onchocercal nodules among study subjects whilst microscopic examination of the skin snips demonstrated a prevalence of 17.1% (Taye et al. 2000). Additionally Adugna et al. (1996) has reported disproportionally low rate of palpable NCR (1.7%) and microfilaridermia prevalence of 15.3% (Adugna et al. 1996). We are of the view that the World Health Organization protocol for determining community-wide prevalence based on palpable NCR is not suited to the clinico-epidemiological settings of onchoceriasis in Ethiopia.
Onchocercal skin disease is highly prevalent in Anfilo District, and affects more frequently males than females. A large multi-country study has shown that OSD entails a grave burden and that its symptoms have significant personal and psycho-social effects, not only on the affected individuals but also on their families and communities (Murdoch et al. 2002). Of the clinical spectra of onchocerciasis, pruritus was the most frequently observed symptom among the study subjects, which is often very serious and the main cause of sleeplessness, fatigue and weakness. Troublesome itches also leads to scratching, often with stones, twigs or knives and results in bleeding wounds, sores and pain in the different parts of the body. Onchocercal skin depigmentation was also relatively high among the study participants, which may signify the longstanding of infection with O. volvulus in the area. The disease is seriously affecting the quality of life of people living in endemic areas with a consequent impact on overall human development.
The CMFL in Anfilo District was high and comparable to magnitudes reported in Jimma Zone—southwest Ethiopia with CMFL of 17.2 and Asossa zone—western Ethiopia with CMFL of 19.9 (Adugna et al. 1996; Legesse et al. 2010). Relatively lower level of CMFL (13.6) has previously been reported from nearby region of south-western Ethiopia (Taye et al. 2000). The higher magnitude of CMFL observed in our study area might be due to the fact that CDTI was not in place at the time of the survey, notwithstanding the fact that self-treatment was commonplace. CMFL among self-treated individuals in the study population was indeed much lower.
Though onchcerciasis is not making headlines, it is quietly stealing the vitality and hope of people living in the Anfilo District, and adjoining localities. The prevalence of skin MFCR and CMFL in Anfilo District is among the highest in Ethiopia and other African regions. The presence of large number of mf in skin will definitely lead to high transmission potential. Owing to the success of CDTI in some locations of western Ethiopia (Kebede et al. 1993; Legesse et al. 2010) and other endemic African countries (Opara and Fagbemi 2008; Soungalo et al. 1997), the CDTI recently started in the District needs to be re-enforced and continued sustainably and vigorously until onchocerciasis and the associated skin disease will no more become a public health problem. There is also a need for evaluation and monitoring of the CDTI at a reasonable interval period of time.
Acknowledgments
We very much thank the communities in Anfilo District who showed deep interest and willingly participated in the study. The authors would like to acknowledge School of Graduate Studies of Addis Ababa University for facilitating this work by providing financial assistance. We are also grateful to Mr. Shuma Negassa and all staffs of Anfilo Health center for their support in the course of this study. The Technical assistance of Mr. Tesfaye Getachew is highly appreciated.
Contributor Information
Geme Urge Dori, Email: gemeurge@yahoo.com.
Tariku Belay, Email: yibroo03@yahoo.com.
Habtamu Belete, Email: Habtamubel@yahoo.com.
K. N. Panicker, Email: knpanicker@aims.amrita.edu
Asrat Hailu, Email: hailu_a2004@yahoo.com.
References
- Adugna N, Woldegiorgis M, Tilahun D, Kebede A, Hadis M. Assessment of the infection rate of onchocerciasis in the resettled and indigenous communities of Asossa, Western Ethiopia. Ethiop J Health Dev. 1996;10(2):89–95. [Google Scholar]
- Final communique of the 11th session of the Joint Action Forum (JAF) of APOC, Paris, France, 6–9 December 2005. Ouagadougou (Burkina Faso): APOC; 2005. [Google Scholar]
- Akinboye DO, Okwong E, Ajiteru N, Fawole O, Agbolade OM, Ayinde OO, Amosu AM, Atulomah NOS, Oduola O, Owodunni BM, Rebecc SN, Falade M. Onchocerciasis among inhabitants of Ibarapa local government community of Oyo state, Nigeria. Biomed Res. 2010;21(2):174–178. [Google Scholar]
- Basáñez M-G, Pion SDS, Churcher TS, Breitling LP, Little MP, et al. River blindness: a success story under threat? PLoS Med. 2006;3(9):e371. doi: 10.1371/journal.pmed.0030371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocharie MJ, Davies JB. The transmission of onchocerciasis at a forest village in Sierra Leone 11. Man-fly contact, human activity and exposure to transmission. Ann Trop Med Parasitol. 1990;84(6):599–605. doi: 10.1080/00034983.1990.11812515. [DOI] [PubMed] [Google Scholar]
- Crosskey RW. The natural history of blackflies. Chichester: Wiley; 1990. [Google Scholar]
- CSA (2007) Summary and Statistical Report of the 2007 Population and Housing Census Results. Federal Democratic Republic of Ethiopia Population Census commission. http://www.csa.gov.et/pdf/Cen2007_firstdraft.pdf. Accessed 8 Sept 2010
- Sole G, Walton JC. Onchocerciasis in Gemu Gofa: an anthropological and ecological survey. Ethiop Med J. 1976;14:37. [PubMed] [Google Scholar]
- Gundersen SG, Schmitt-Lechner A, Bjorvatn B. Onchocerciasis in the Blue Nile of Western Ethiopia. Trans Roy Soc Trop Med Hyg. 1988;82:122. doi: 10.1016/0035-9203(88)90284-2. [DOI] [PubMed] [Google Scholar]
- Hailu A, Balcha F, Birrie H, Berhe N, Aga A, Mengistu M, Bezuneh A, Ali A, Geber-Micheal T, Gemetchu T. Prevalence of Onchocercal skin disease and infection among workers of Coffee plantation farms in Teppi, south western Ethiopia. Ethiop Med J. 2002;2002(40):259–269. [PubMed] [Google Scholar]
- Hoerauf A, Büttner DW, Adjei O, Pearlman E. Onchocerciasis. BMJ. 2003;326:207–210. doi: 10.1136/bmj.326.7382.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jira C. Prevalence of onchocerciasis in Blue Nile valley of Western Ethiopia. Indian J Public Health. 1993;37(4):135–137. [PubMed] [Google Scholar]
- Kebede A, Taticheff S, Bulto T, Workneh W, Tilahun D. Effect of Ivermectin on microfilarial load in patients with O. volvulus in Bebeka. Ethiop Med J. 1993;31:127–135. [PubMed] [Google Scholar]
- Legesse M, Balcha F, Erko B. Status of onchocerciasis in Teppi area, Southwestern Ethiopia, after four years of annual community-directed treatment with ivermectin. Ethiop J Health Dev. 2010;24(1):51–56. [Google Scholar]
- Mace JM, Boussinesq M, Ngoumou P, Enyegue OJ, Godin C. Country-wide rapid epidemiological mapping of onchocerciasis (REMO) in Cameroon. Ann Trop Med Parasitol. 1997;91(4):379–391. doi: 10.1080/00034989761003. [DOI] [PubMed] [Google Scholar]
- McCarthy JS, Ottesen EA, Nutman TB. Onchocerciasis in endemic and nonendemic populations: differences in clinical presentation and immunologic findings. J Infect Dis. 1994;170:736–741. doi: 10.1093/infdis/170.3.736. [DOI] [PubMed] [Google Scholar]
- Murdoch ME, Hay RJ, Mackenzie CD, Williams JF, Ghalib HW, Cousens S, Abiose, et al. A clinical classification and grading system of the cutaneous changes in onchocerciasis. Br J Dermatol. 1993;1993(129):260–269. doi: 10.1111/j.1365-2133.1993.tb11844.x. [DOI] [PubMed] [Google Scholar]
- Murdoch M, Asuzu M, Haga M, et al. Onchocerciasis The clinical and epidemiological burden of skin disease in Africa. Ann Trop Med Parasitol. 2002;96:283–296. doi: 10.1179/000349802125000826. [DOI] [PubMed] [Google Scholar]
- Ngoumou P, Walsh JF, Mace JM. A rapid mapping technique for the prevalence and distribution of onchocerciasis: a Cameroon case study. Ann Trop Med Parasitol. 1994;88:463–474. doi: 10.1080/00034983.1994.11812893. [DOI] [PubMed] [Google Scholar]
- Okonkwo P, Akpa A, Ihekwaba A, Nwagbo D, Umeh K, Adibua S, Ezike V, Ogbuokin S. Studies on Onchocerciasis in the Forest-Savanna Mosaic Area of Nigeria 1 investigations in Gabragu, Oji River. Ann Trop Med Parasitol. 1991;85:617–623. doi: 10.1080/00034983.1991.11812617. [DOI] [PubMed] [Google Scholar]
- Opara KN, Fagbemi BO. Population dynamics of Onchocerca volvulus microfilariae in human host after six years of drug control. J Vector Borne Dis. 2008;45:29–37. [PubMed] [Google Scholar]
- Remme J, Ba O, Dadzie KY, Karam M. A force-of-infection model for onchocerciasis and its applications in the epidemiological evaluation of the Onchocerciasis Control Programme in the Volta River basin area. Bull World Health Organ. 1986;64(5):667–681. [PMC free article] [PubMed] [Google Scholar]
- Soungalo T, Soumana D, Moussa S, Francois R. Ivermectin in onchocerciasis control in the forest zone of Cote d’Ivoire. Acta Trop. 1997;68:297–300. doi: 10.1016/S0001-706X(97)00103-4. [DOI] [PubMed] [Google Scholar]
- Tatichef S, Abebe M, Workineh W, Hana NG. Onchocerciasis: a prevalence study in Bebeka, Ethiopia. Trop Med Parasit. 1987;38:279. [PubMed] [Google Scholar]
- Taye A, Gebre-Michael T, Taticheff S. Onchocerciasis in Gilgel Ghibe river valley, Southwest Ethiopia. East Afr Med J. 2000;77(2):116–120. doi: 10.4314/eamj.v77i2.46411. [DOI] [PubMed] [Google Scholar]
- Terranova M, Padovese V, Klaus S, Morrone A. Onchocerciasis in Tigray. Int J Dermatol. 2007;46(Suppl 2):39–41. doi: 10.1111/j.1365-4632.2007.03472.x. [DOI] [PubMed] [Google Scholar]
- Vision (2010) (20209 http://www.vision2020.org/main.cfm?type=WIBONCHOC. Accessed 23 Nov 2010
- WHO (1992) Methods of community diagnosis of onchocerciasis to guide ivermectin drug control in Africa. Report of an informal consultation held in Ouagadougou, 19–21 November, 1991 TDR/JDE/ONCH/922. Geneva
- WHO (2010) Onchocerciasis control programme in Ethiopia. http://www.who.int/countries/eth/areas/cds/onchocerciasis/en/. Accessed 12 Oct 2010
- Yeneneh H, Mengistu F, Ayele TA. Multidiciplinary study of onchocerciasis in Bure area, Ethiopia. Ethiop Med J. 1989;27:121. [PubMed] [Google Scholar]
- Zein AZ. The epidemiology of onchocerciasis in northwestern Ethiopia. Trop Geogr Med. 1986;38:33. [PubMed] [Google Scholar]
- Zein AZ. An appraisal of the epidemiologic situation of onchocerciasis in Ethiopia. Parassitologia. 1990;32(2):237–244. [PubMed] [Google Scholar]