Abstract
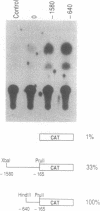
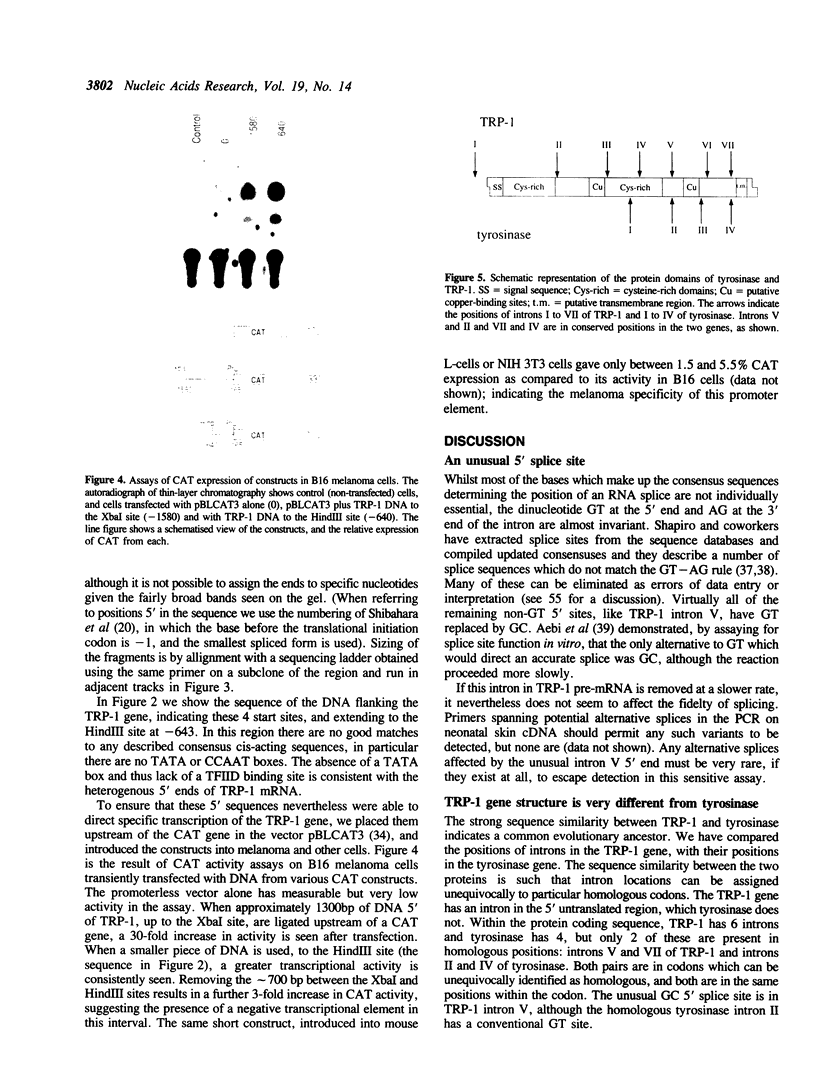
We have determined the exon structure of the mouse tyrosinase-related protein-1 (TRP-1) gene. The gene is only 15kb in length, but contains seven introns, in contrast to the tyrosinase gene which is almost 100kb long with only four introns. Only two introns are located in homologous positions in both genes. Intron I of TRP-1 has three alternative 5' splice sites clustered within 21bp, which all splice to the same 3' site. Intron V has a very unusual 5' splice site, which has the dinucleotide GC rather than the conventional GT. We show that as little as 370bp of 5'-flanking DNA is sufficient to direct cell-specific expression of the chloramphenicol acetyl transferase gene. The flanking DNA of TRP-1, unlike tyrosinase, does not contain a TATA box or a CCAAT box. Both mouse genes, however, share an 11bp sequence, also found in human tyrosinase, which we suggest may be a melanocyte-specific promoter element.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi M., Hornig H., Weissmann C. 5' cleavage site in eukaryotic pre-mRNA splicing is determined by the overall 5' splice region, not by the conserved 5' GU. Cell. 1987 Jul 17;50(2):237–246. doi: 10.1016/0092-8674(87)90219-4. [DOI] [PubMed] [Google Scholar]
- Ali S., Clark A. J. Characterization of the gene encoding ovine beta-lactoglobulin. Similarity to the genes for retinol binding protein and other secretory proteins. J Mol Biol. 1988 Feb 5;199(3):415–426. doi: 10.1016/0022-2836(88)90614-6. [DOI] [PubMed] [Google Scholar]
- Barber J. I., Townsend D., Olds D. P., King R. A. Dopachrome oxidoreductase: a new enzyme in the pigment pathway. J Invest Dermatol. 1984 Aug;83(2):145–149. doi: 10.1111/1523-1747.ep12263381. [DOI] [PubMed] [Google Scholar]
- Beermann F., Ruppert S., Hummler E., Bosch F. X., Müller G., Rüther U., Schütz G. Rescue of the albino phenotype by introduction of a functional tyrosinase gene into mice. EMBO J. 1990 Sep;9(9):2819–2826. doi: 10.1002/j.1460-2075.1990.tb07470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalier-Smith T. Intron phylogeny: a new hypothesis. Trends Genet. 1991 May;7(5):145–148. [PubMed] [Google Scholar]
- Chen C. J., Clark D., Ueda K., Pastan I., Gottesman M. M., Roninson I. B. Genomic organization of the human multidrug resistance (MDR1) gene and origin of P-glycoproteins. J Biol Chem. 1990 Jan 5;265(1):506–514. [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibb N. J., Newman A. J. Evidence that introns arose at proto-splice sites. EMBO J. 1989 Jul;8(7):2015–2021. doi: 10.1002/j.1460-2075.1989.tb03609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didier D. K., Schiffenbauer J., Woulfe S. L., Zacheis M., Schwartz B. D. Characterization of the cDNA encoding a protein binding to the major histocompatibility complex class II Y box. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7322–7326. doi: 10.1073/pnas.85.19.7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Giebel L. B., Strunk K. M., King R. A., Hanifin J. M., Spritz R. A. A frequent tyrosinase gene mutation in classic, tyrosinase-negative (type IA) oculocutaneous albinism. Proc Natl Acad Sci U S A. 1990 May;87(9):3255–3258. doi: 10.1073/pnas.87.9.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebel L. B., Strunk K. M., Spritz R. A. Organization and nucleotide sequences of the human tyrosinase gene and a truncated tyrosinase-related segment. Genomics. 1991 Mar;9(3):435–445. doi: 10.1016/0888-7543(91)90409-8. [DOI] [PubMed] [Google Scholar]
- Hearing V. J., Jiménez M. Analysis of mammalian pigmentation at the molecular level. Pigment Cell Res. 1989 Mar-Apr;2(2):75–85. doi: 10.1111/j.1600-0749.1989.tb00166.x. [DOI] [PubMed] [Google Scholar]
- Huber M., Hintermann G., Lerch K. Primary structure of tyrosinase from Streptomyces glaucescens. Biochemistry. 1985 Oct 22;24(22):6038–6044. doi: 10.1021/bi00343a003. [DOI] [PubMed] [Google Scholar]
- Jackson I. J. A cDNA encoding tyrosinase-related protein maps to the brown locus in mouse. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4392–4396. doi: 10.1073/pnas.85.12.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
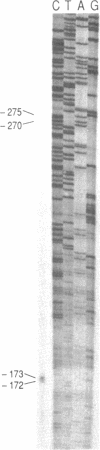
- Jackson I. J. A reappraisal of non-consensus mRNA splice sites. Nucleic Acids Res. 1991 Jul 25;19(14):3795–3798. doi: 10.1093/nar/19.14.3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson I. J., Bennett D. C. Identification of the albino mutation of mouse tyrosinase by analysis of an in vitro revertant. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7010–7014. doi: 10.1073/pnas.87.18.7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi H., Hara S., Ishiguro S., Tamai M., Watanabe M. Detection of point mutation in the tyrosinase gene of a Japanese albino patient by a direct sequencing of amplified DNA. Hum Genet. 1990 Jun;85(1):123–124. doi: 10.1007/BF00276337. [DOI] [PubMed] [Google Scholar]
- Kikuchi H., Miura H., Yamamoto H., Takeuchi T., Dei T., Watanabe M. Characteristic sequences in the upstream region of the human tyrosinase gene. Biochim Biophys Acta. 1989 Dec 22;1009(3):283–286. doi: 10.1016/0167-4781(89)90115-2. [DOI] [PubMed] [Google Scholar]
- Kirchgessner T. G., Chuat J. C., Heinzmann C., Etienne J., Guilhot S., Svenson K., Ameis D., Pilon C., d'Auriol L., Andalibi A. Organization of the human lipoprotein lipase gene and evolution of the lipase gene family. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9647–9651. doi: 10.1073/pnas.86.24.9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klüppel M., Beermann F., Ruppert S., Schmid E., Hummler E., Schütz G. The mouse tyrosinase promoter is sufficient for expression in melanocytes and in the pigmented epithelium of the retina. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3777–3781. doi: 10.1073/pnas.88.9.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon B. S., Halaban R., Chintamaneni C. Molecular basis of mouse Himalayan mutation. Biochem Biophys Res Commun. 1989 May 30;161(1):252–260. doi: 10.1016/0006-291x(89)91588-x. [DOI] [PubMed] [Google Scholar]
- Kwon B. S., Haq A. K., Pomerantz S. H., Halaban R. Isolation and sequence of a cDNA clone for human tyrosinase that maps at the mouse c-albino locus. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7473–7477. doi: 10.1073/pnas.84.21.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon B. S., Haq A. K., Wakulchik M., Kestler D., Barton D. E., Francke U., Lamoreux M. L., Whitney J. B., 3rd, Halaban R. Isolation, chromosomal mapping, and expression of the mouse tyrosinase gene. J Invest Dermatol. 1989 Nov;93(5):589–594. doi: 10.1111/1523-1747.ep12319693. [DOI] [PubMed] [Google Scholar]
- Kwon B. S., Wakulchik M., Haq A. K., Halaban R., Kestler D. Sequence analysis of mouse tyrosinase cDNA and the effect of melanotropin on its gene expression. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1301–1309. doi: 10.1016/s0006-291x(88)81370-6. [DOI] [PubMed] [Google Scholar]
- Körner A. M., Pawelek J. Dopachrome conversion: a possible control point in melanin biosynthesis. J Invest Dermatol. 1980 Aug;75(2):192–195. doi: 10.1111/1523-1747.ep12522650. [DOI] [PubMed] [Google Scholar]
- Körner A., Pawelek J. Mammalian tyrosinase catalyzes three reactions in the biosynthesis of melanin. Science. 1982 Sep 17;217(4565):1163–1165. doi: 10.1126/science.6810464. [DOI] [PubMed] [Google Scholar]
- Lerch K., Longoni C., Jordi E. Primary structure of tyrosinase from Neurospora crassa. I. Purification and amino acid sequence of the cyanogen bromide fragments. J Biol Chem. 1982 Jun 10;257(11):6408–6413. [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M., Pawelek J. M., Lamoreux M. L. New regulatory factors for melanogenesis: developmental changes in neonatal mice of various genotypes. Dev Biol. 1983 Nov;100(1):120–126. doi: 10.1016/0012-1606(83)90202-6. [DOI] [PubMed] [Google Scholar]
- Müller G., Ruppert S., Schmid E., Schütz G. Functional analysis of alternatively spliced tyrosinase gene transcripts. EMBO J. 1988 Sep;7(9):2723–2730. doi: 10.1002/j.1460-2075.1988.tb03126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinchik E. M., Bangham J. W., Hunsicker P. R., Cacheiro N. L., Kwon B. S., Jackson I. J., Russell L. B. Genetic and molecular analysis of chlorambucil-induced germ-line mutations in the mouse. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1416–1420. doi: 10.1073/pnas.87.4.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J. H. How were introns inserted into nuclear genes? Trends Genet. 1989 Jul;5(7):213–216. doi: 10.1016/0168-9525(89)90084-x. [DOI] [PubMed] [Google Scholar]
- Rogers J. H. The role of introns in evolution. FEBS Lett. 1990 Aug 1;268(2):339–343. doi: 10.1016/0014-5793(90)81282-s. [DOI] [PubMed] [Google Scholar]
- Rogers J. Exon shuffling and intron insertion in serine protease genes. Nature. 1985 Jun 6;315(6019):458–459. doi: 10.1038/315458a0. [DOI] [PubMed] [Google Scholar]
- Ruppert S., Müller G., Kwon B., Schütz G. Multiple transcripts of the mouse tyrosinase gene are generated by alternative splicing. EMBO J. 1988 Sep;7(9):2715–2722. doi: 10.1002/j.1460-2075.1988.tb03125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Senapathy P., Shapiro M. B., Harris N. L. Splice junctions, branch point sites, and exons: sequence statistics, identification, and applications to genome project. Methods Enzymol. 1990;183:252–278. doi: 10.1016/0076-6879(90)83018-5. [DOI] [PubMed] [Google Scholar]
- Shapiro M. B., Senapathy P. RNA splice junctions of different classes of eukaryotes: sequence statistics and functional implications in gene expression. Nucleic Acids Res. 1987 Sep 11;15(17):7155–7174. doi: 10.1093/nar/15.17.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibahara S., Okinaga S., Tomita Y., Takeda A., Yamamoto H., Sato M., Takeuchi T. A point mutation in the tyrosinase gene of BALB/c albino mouse causing the cysteine----serine substitution at position 85. Eur J Biochem. 1990 Apr 30;189(2):455–461. doi: 10.1111/j.1432-1033.1990.tb15510.x. [DOI] [PubMed] [Google Scholar]
- Shibahara S., Tomita Y., Sakakura T., Nager C., Chaudhuri B., Müller R. Cloning and expression of cDNA encoding mouse tyrosinase. Nucleic Acids Res. 1986 Mar 25;14(6):2413–2427. doi: 10.1093/nar/14.6.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spritz R. A., Strunk K. M., Giebel L. B., King R. A. Detection of mutations in the tyrosinase gene in a patient with type IA oculocutaneous albinism. N Engl J Med. 1990 Jun 14;322(24):1724–1728. doi: 10.1056/NEJM199006143222407. [DOI] [PubMed] [Google Scholar]
- Tafuri S. R., Wolffe A. P. Xenopus Y-box transcription factors: molecular cloning, functional analysis and developmental regulation. Proc Natl Acad Sci U S A. 1990 Nov;87(22):9028–9032. doi: 10.1073/pnas.87.22.9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda A., Tomita Y., Matsunaga J., Tagami H., Shibahara S. Molecular basis of tyrosinase-negative oculocutaneous albinism. A single base mutation in the tyrosinase gene causing arginine to glutamine substitution at position 59. J Biol Chem. 1990 Oct 15;265(29):17792–17797. [PubMed] [Google Scholar]
- Tanaka S., Yamamoto H., Takeuchi S., Takeuchi T. Melanization in albino mice transformed by introducing cloned mouse tyrosinase gene. Development. 1990 Feb;108(2):223–227. doi: 10.1242/dev.108.2.223. [DOI] [PubMed] [Google Scholar]
- Tomita Y., Takeda A., Okinaga S., Tagami H., Shibahara S. Human oculocutaneous albinism caused by single base insertion in the tyrosinase gene. Biochem Biophys Res Commun. 1989 Nov 15;164(3):990–996. doi: 10.1016/0006-291x(89)91767-1. [DOI] [PubMed] [Google Scholar]
- Traut T. W. Do exons code for structural or functional units in proteins? Proc Natl Acad Sci U S A. 1988 May;85(9):2944–2948. doi: 10.1073/pnas.85.9.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winship P. R. An improved method for directly sequencing PCR amplified material using dimethyl sulphoxide. Nucleic Acids Res. 1989 Feb 11;17(3):1266–1266. doi: 10.1093/nar/17.3.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H., Takeuchi S., Kudo T., Sato C., Takeuchi T. Melanin production in cultured albino melanocytes transfected with mouse tyrosinase cDNA. Jpn J Genet. 1989 Apr;64(2):121–135. doi: 10.1266/jjg.64.121. [DOI] [PubMed] [Google Scholar]
- Yokoyama T., Silversides D. W., Waymire K. G., Kwon B. S., Takeuchi T., Overbeek P. A. Conserved cysteine to serine mutation in tyrosinase is responsible for the classical albino mutation in laboratory mice. Nucleic Acids Res. 1990 Dec 25;18(24):7293–7298. doi: 10.1093/nar/18.24.7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zdarsky E., Favor J., Jackson I. J. The molecular basis of brown, an old mouse mutation, and of an induced revertant to wild type. Genetics. 1990 Oct;126(2):443–449. doi: 10.1093/genetics/126.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]