Abstract
The cytokine thymic stromal lymphopoietin (TSLP) functions as a regulator of bone marrow B-cell development and a key initiator of allergic inflammation. In the current study, we show that mature B cells, derived from transgenic mice with systemically elevated levels of TSLP (K5-TSLP mice), exhibit markedly enhanced mitogenic responses in vitro and that this enhanced responsiveness leads to polyclonal B-cell activation and development of autoimmune hemolytic anemia in vivo. In contrast, B cells derived from K5-TSLP mice lacking CD4+ T cells failed to show polyclonal activation. Furthermore, neither mature B-cell activation nor hemolytic anemia occurred in IL-4-deficient K5-TSLP mice. Consistent with these findings, activation of mature B cells occurred independently of B-cell intrinsic TSLP signals. Taken together, our results demonstrate that systemic alterations in TSLP, through induction of IL-4 from CD4+ T cells and other cell types, functions as an important factor in peripheral B-cell homeostasis and promotion of humoral autoimmunity.
Keywords: autoantibody, cytokine, hemolytic anemia, Th2
Introduction
Autoimmune diseases can be caused by autoreactive T cells and/or B cells which are normally deleted during their development and/or cannot respond to self-antigens due to anergy (1–3). Under some conditions, self-reactive B cells can escape deletion and/or anergy and be activated to produce autoantibodies that contribute to autoimmune diseases, including systemic lupus erythematosus, hemolytic anemia and glomerulonephritis (4–6). While recent studies have begun to clarify how autoreactive B cells can survive and become activated, the underlying mechanisms responsible for loss of peripheral B-cell tolerance in vivo remain relatively poorly defined.
Thymic stromal lymphopoietin (TSLP) is a cytokine originally identified as a growth and differentiation factor of T cells and B cells (7). TSLP can replace the activity of IL-7 in supporting B-cell development in vitro (8), and administration of TSLP into neonatal mice can induce an expansion of immature B cells in the bone marrow (9). This function is similar to IL-7 and, consistent with this idea, TSLP overexpression can partially restore the B-lineage compartment in IL-7-deficient mice (10). This functional similarity is explained by the observation that the functional TSLP receptor is composed of a heterodimer complex that includes both the IL-7 receptor α chain (IL-7Rα) and the TSLP-specific TSLP receptor (TSLPR) (11, 12). TSLPR engagement also leads to Stat5 activation (13–15). Recently, it was revealed that Jak1 and Jak2 are important for the TSLP-mediated activation of Stat5 in primary CD4+ T cells (16).
Another important feature of TSLP is its ability to act as an initiator of allergic inflammation in both human and mouse (17–19). Elevated TSLP levels are found in affected skin and lung from patients of atopic dermatitis or allergic asthma, respectively (20,21). Consistent with this, mice overexpressing TSLP or administered with recombinant TSLP exhibit severe Th2-polarlized inflammation in the sites (22–25). TSLP also regulates intestinal immunity and inflammation (26), contact hypersensitivity response (27) and is important in helminth infections (26, 28). TSLP stimulation can induce maturation of dendritic cells (DCs) along with induction of OX40L expression (29). TSLP-primed DCs activate naive CD4+ T cells and induce Th2 polarization in an OX40L- to OX40-dependent manner (29, 30). TSLP-stimulated DCs can also produce Th2-attracting chemokines such as CCL17/TARC and CCL22/MDC and contribute to generation of Th2-biased conditions (14, 20, 22). TSLP also stimulates naive CD4+ T cells directly to induce IL-4 production and Th2 polarization (31, 32).
Skin-specific, tetracycline-inducible TSLP transgenic mice (K5-TSLP mice) display an increase of systemic TSLP after doxycycline (dox) treatment and develop abnormalities in B-cell development and maturation (33). Immature bone marrow B cells, especially B cells after the late pro B-cell stage (also known as Hardy Fraction C (34)), are dramatically increased, and there was a concomitant increase in immature phenotype B cells in the periphery. These mice also display a marked expansion of splenic mature B cells, while marginal zone and marginal zone precursor B cells are dramatically reduced. In addition, B-1 cells are markedly increased, via a direct effect on bone marrow B-1 progenitors as well as CD5− B-1b cells within the peritoneal cavity (35). Finally, K5-TSLP mice, as well as TSLP transgenic mice in which the transgene is driven by Lck proximal promoter (Lck-TSLP mice), develop cryoglobulinemic glomerulonephritis (33,36).
In this study, we show that systemic expression of TSLP leads to a polyclonal activation of mature B cells in the spleen, which is mediated by IL-4 produced by activated CD4+ T cells. Using B-cell transfer experiments, we show that TSLPR expression on B cells is not required for this TSLP-mediated B-cell activation, indicating that this is not a direct effect of TSLP on B cells. In addition, we show that K5-TSLP mice develop severe autoimmune hemolytic anemia. Interestingly, Tcrb-deficient or Il4-deficient K5-TSLP mice failed to develop similar B-cell activation or anemia. Together, these findings indicate that TSLP can play a potent role in immune homeostasis in vivo by regulating Th1–Th2 balance and subsequent mature B-cell activation.
Methods
Mice
K5-TSLP mice, which are double transgenic mice of K5-rtTA transgenic and tetO-TSLP transgenic mice, were generated as described previously (23). K5-TSLP mice were backcrossed at least seven times with BALB/c mice purchased from Taconic Farms (Hudson, NY, USA) or The Jackson Laboratory (Bar Harbor, ME, USA). TCRβ KO (Tcrb−/−) mice (Tcrbtm1Mom), IL-4 KO (Il4−/−) mice (Il4tm2Nnt) and Rag1 KO (Rag1−/−) mice (Rag1tm1Mom) were purchased from The Jackson Laboratory and crossed with K5-TSLP mice. TSLPR KO (Tslpr−/−) mice (Crlf2tm1Jni) were generated as described previously (37) and backcrossed at least 11 times with BALB/c mice. Nude (BALB/c-nu/nu) mice were purchased from CLEA Japan (Tokyo, Japan). Mice were treated with dox (Sigma) at a concentration of 1 mg ml−1 in the drinking water to induce rtTA transactivation of the TSLP transgene. Transgene-negative or single-transgenic littermates were used as controls. All mice were maintained under specific pathogen-free conditions in the animal facility of Benaroya Research Institute at Virginia Mason. All mouse works were conducted under conditions approved by the Benaroya Research Institute Animal Care and Use Committee.
Flow cytometry
RBC-lysed single-cell suspensions were prepared from the bone marrow and spleen. After Fc blocking with the culture supernatant of 2.4G2 hybridoma, cells are incubated in staining buffer (PBS containing 3% FCS) with antibody cocktails on ice for 20 min in the dark. For intracellular cytokine staining, splenocyte were stimulated with 50 ng ml−1 phorbol myristate acetate, 500 ng ml−1 ionomycin and 1 μl ml−1 GolgiPlug (BD Biosciences) for 4 h and stained for surface antigens. Cells were fixed in 1.25× PBS containing 1% PFA, permeabilized with 1× Permeabilization Buffer (eBioscience) and stained for cytokines in 1× Permeabilization Buffer. Following antibodies were used FITC-conjugated anti-CD24 (clone CT-HSA, CALTAG or clone M1/69; BioLegend), PE-conjugated anti-CD43 (clone S7; BD Biosciences), PE-Cy7-conjugated anti-B220/CD45R (clone RA3-6B2; eBioscience), biotin-conjugated anti-BP-1 (clone 6C3; eBioscience), FITC-conjugated anti-CD21/CD35 (clone 7G6; BD Bioscience), biotin-conjugated anti-CD23 (clone B3B4; eBioscience), allophycocyanin (APC)-conjugated anti-MHC class II (clone M5/114.15.2; eBioscience), biotin-conjugated anti-CD4 (clone RM4-4; BD Biosciences), FITC-conjugated anti-CD8α (clone 53-6.7; eBioscience), PE-conjugated anti-IL-7Rα (clone A7R34; eBioscience), biotin-conjugated anti-IL-4Rα (clone mIL4R-M1; BD Biosciences), FITC-conjugated anti-IFN-γ (clone XMG1.2; BioLegend), PE-conjugated anti-IL-4 (clone 11B11; eBioscience), FITC-conjugated anti-IL-10 (clone JES5-16E3; BD Biosciences) and PE-conjugated anti-IL-5 (clone TRFK5; BioLegend). Anti-TSLP receptor (clone 22H9) (15) was purified from hybridoma supernatant and labeled with Alexa Fluor 647 using Monoclonal Antibody Labeling Kit (Molecular Probes) according to the manufacturer’s instruction. Biotinylated cells were visualized by incubation with PE- or APC-conjugated streptavidin (eBioscience or BioLegend). After washing twice with staining buffer, cells were suspended in the staining buffer and data were acquired with FACSCalibur (BD Biosciences) or FACSCanto II (BD Biosciences) and analyzed with FlowJo software (Treestar).
[3H] thymidine uptake proliferation assay
Splenic B cells were purified using a MACS system (Miltenyi Biotec, Bergisch Gladbach, Germany) following incubation with biotin-conjugated anti-CD43 (BD Biosciences) and streptavidin-coupled microbeads (Miltenyi Biotec) according to the manufacturer’s instruction. B cells (2 × 105) were cultivated in 0.2 ml of RPMI1640 medium supplemented with 10% FCS, 50 μM 2-mercaptoethanol, 100 U/ml penicillin and 100 μg ml−1 streptomycin in a 96-well plate. Cells were stimulated with various concentrations of F(ab’)2 goat anti-mouse IgM (Jackson ImmunoResearch Laboratories, West Grove, PA, USA), LPS (Sigma) or anti-CD40 (clone HM40-3; BD Biosciences). Cells were pulse-labeled with [3H]-thymidine (1 μCi per well) during the last 8 h of a 24-h culture period. Cells were harvested onto glass fiber filters and counted in a liquid scintillation counter (Wallac).
In vitro and in vivo 5-bromo-2-deoxyuridine incorporation assay
For in vitro assay, purified B cells were cultured with anti-IgM, LPS or anti-CD40 in the presence of 40 μM 5-bromo-2-deoxyuridine (BrdU; Sigma). After 24-h incubation, cells were washed and fixed overnight in ethanol at −20°C. Cells were then incubated in 2 N HCl for 20 min to denature the DNA and neutralized with 0.1 M sodium borate pH 8.5 for 2 min. After washing with PBS twice, cells were incubated with FITC-conjugated anti-BrdU antibody (clone 3D4; BD Biosciences) for 30 min. Finally, 100 mg ml−1 RNase (Sigma) and 50 mg ml−1 propidium iodide (Molecular Probes) were added and the cells were analyzed by flow cytometry. For in vivo assay, K5-TSLP or normal littermate control (NLC) mice were inoculated with 0.8 mg of BrdU in PBS every 12 h for the last 3 days of 3-week dox treatment. Spleen cells were stained for surface expression of B220, CD21/CD35 and CD23 as described above and then fixed using IC Fixation Buffer (eBioscience) and permeabilized with Permeabilization Buffer (eBioscience). Subsequently, cells were washed with Permeabilization Buffer, incubated with DNase I, washed and then stained with Alexa Fluor 647-conjugated anti-BrdU (clone PRB-1; Molecular Probes) for 20 min in the dark. Stained cells were analyzed by flow cytometry.
Depletion of CD4+ T cells by anti-CD4 antibodies
Purified anti-CD4 antibodies (clone GK1.5) were purchased from University of California San Francisco. K5-TSLP or NLC mice were intraperitoneally treated with 200 μg per mouse of anti-CD4 antibodies or rat IgG (Sigma) every 5 days since 5 days before starting dox treatment. Blood was collected from mouse leg at days 0, 7 and 14 of dox treatment and analyzed by flow cytometry to confirm the depletion of CD4+ cells.
Reconstitution of K5-TSLP/Rag1 KO mice or nude mice
CD4+ T cells were purified from spleen and lymph nodes of wild-type (WT) or TSLPR KO mice using MACS system following magnetic labeling with CD4+ T Cell Isolation Kit according to the manufacturer’s instruction. B cells were isolated from spleens of WT or TSLPR KO mice as described above. WT or TSLPR KO B cells (8.8 × 106) with or without 6.5 × 106 of CD4+ T cells were intravenously (i.v.) injected into K5-TSLP/Rag1 KO mice from their tail vein. Dox was added in the dinking water at the next day of i.v. injection, and mice were sacrificed and analyzed after 3-week dox treatment. For nude mice, 3.5 × 106 of CD4+ T cells were injected i.v., and the next day, mice were injected i.v. with 50 μg of TSLP cDNA cloned into pLIVE plasmid vector (Mirus Bio, Madison, WI, USA) in TransIT-EE Hydrodynamic Delivery Solution (Mirus Bio). Treated nude mice were analyzed after 4 weeks.
Enzyme-linked immunosorbent assay
Serum cytokine concentrations were determined with mouse IL-4 ELISA MAX kit (BioLegend) or mouse TSLP ELISA MAX kit (BioLegend) according to the manufacturer’s instructions.
Determination of hematocrit
Blood was collected into centrifuge tubes containing heparin from the heart after euthanasia and 70 μl of blood was transferred to capillary tubes (Fisher Scientific, Pittsburgh, PA, USA). The tubes were sealed by Tube Sealing Compound (Fisher Scientific) and spun in a centrifuge at 1730 × g for 5 min; the hematocrit was determined as the relative height of the RBC column expressed in percent.
Flow cytometric detection of anti-RBC antibodies
Blood collected during hematocrit measurement was diluted 1:10 with staining buffer and 0.5 μl of diluted blood was used for staining. RBCs were washed with staining buffer and incubated in the staining buffer containing FITC-conjugated anti-mouse IgM (clone eB121-15F9; eBioscience), FITC-conjugated goat F(ab’)2 anti-mouse IgG (CALTAG) or control antibodies for 20 min on ice in the dark. After washing twice with staining buffer, RBCs were analyzed using flow cytometry as described above.
Statistical analysis
P values were determined by two-tailed unpaired Student’s t-test. P values of <0.05 were considered significant.
Results
Mature B cells from K5-TSLP mice exhibit an activated cell phenotype
We have previously shown an increase of immature and mature B cells, and loss of marginal zone B cells, in spleens from K5-TSLP transgenic mice (33). In order to further define the potential of effects of TSLP in vivo, we focused our attention on mature B cells derived from K5-TSLP mice following dox treatment. Follicular (FO) B cells from dox-treated K5-TSLP mice showed lower CD21/CD35 and higher CD23 expression compared with their counterparts from NLC mice (Fig. 1A). Furthermore, K5-TSLP-derived mature B cells exhibited features of activation, including increased forward scatter (FSC) and an increase in MHC class II cell surface expression (Fig. 1A). Next, to assess the activation status of B cells from the K5-TSLP mice, we examined their proliferation potential in vitro using [3H] thymidine incorporation assay. Following polyclonal stimulation in vitro, primary B cells from K5-TSLP mice exhibited significantly higher levels of [3H] thymidine incorporation than NLC B cells as early as 24-h post-stimulation (Fig. 1B).
Fig. 1.
FO B cells in spleen of K5-TSLP mice showed activated phenotype. (A) Alteration of cell surface phenotype of K5-TSLP FO B cells. After 3 weeks of 1 mg ml−1 dox treatment, single cell was isolated from normal littermate control mice (NLC) or K5-TSLP mice spleens and analyzed with flow cytometry. Cells were gated on FO B cells (B220+CD21/CD35lowCD23+). Data (left) represent one of more than seven independent experiments; right, summary of mean fluorescence intensity relative to NLC. *P < 0.005. (B) Proliferative response of spleen B cells from K5-TSLP or NLC mice. B cells purified from spleens were cultured with indicated stimulations for 24 h. Cells were pulse-labeled with [3H]-thymidine during the last 8 h. Error bars, SD of three replicates. Data represent one of three independent experiments. (C) In vitro BrdU incorporation assay of splenic B cells from K5-TSLP or NLC mice. B cells purified from spleens were cultured with 40 μM of BrdU and indicated stimulations for 24 h. Cells were washed, fixed with ethanol, stained with anti-BrdU antibodies and propidium iodide and analyzed. Numbers near gated areas indicate percent of the population. Data represent one of two independent experiments. (D) In vivo BrdU incorporation in immature (B220+CD21/CD35−CD23−) and FO B cells in NLC and K5-TSLP mice. Mice were given i.p. inoculations of 0.8 mg of BrdU in PBS at 12-h interval during the last 3 days of 3-week dox treatment. Splenocytes were stained with antibodies for surface antigens, fixed and permeabilized. Following DNase treatment, cells were stained with anti-BrdU antibodies and analyzed. Data represent one of two independent experiments.
To extend these studies, BrdU incorporation was used to determine the number of cells entering the cell cycle 24 h after stimulation. In the absence of stimulation, very few cells from either NLC or K5-TSLP mice entered the cell cycle, suggesting that B cells in the K5-TSLP mice were not actively proliferating in vivo prior to isolation (Fig. 1C). Anti-IgM and LPS stimulation, however, induced much higher levels of BrdU+ cells, in cells derived from K5-TSLP versus NLC animals (Fig. 1C). We also performed an in vivo BrdU labeling assay to determine whether elevated systemic TSLP levels resulted in enhanced cycling of B cells in vivo. NLC and K5-TSLP mice were treated with dox for 3 weeks, injected with BrdU at 12-h interval during the last 3 days of dox treatment and analyzed. There was no significant difference in BrdU incorporation seen in either immature B cells (B220+CD21/CD35−CD23−) or mature FO B cells from K5-TSLP and NLC mice (Fig. 1D). These results suggest that B cells from dox-treated K5-TSLP mice are in a pre-activated state, thereby allowing them to respond more rapidly than their NLC counterparts to mitogenic signals.
Peripheral B cells in K5-TSLP mice do not express a functional TSLPR complex
To begin to determine whether the increased systemic levels of TSLP directly altered mature B-cell activation, we first examined peripheral B cells for expression of the components of the TSLPR complex. The functional receptor for TSLP consists of IL-7 receptor α chain (IL-7Rα) and TSLP-specific TSLPR (11, 12). TSLPR was detected at similar levels on all splenic B-cell subsets [including transitional 1 (T1) B cells (B220+CD21/CD35−CD23−), transitional 2 (T2) B cells (B220+CD21/CD35highCD23+), FO B cells (B220+CD21/CD35lowCD23+) and marginal zone (MZ) B cells (B220+CD21/CD35highCD23−)], derived from either K5-TSLP or NLC mice with similar levels of expression (Supplementary Figure 1A, top, is available at International Immunology Online). On the other hand, IL-7Rα expression was not detected on any of these B-cell subsets in either group (Supplementary Figure 1A, bottom, is available at International Immunology Online). These results were confirmed using quantitative reverse transcription–PCR to assess Tslpr and Il7r transcripts in mature B cells from NLC and K5-TSLP mice. Consistent with the results of flow cytometry, Tslpr mRNA was detected in mature B cells from K5-TSLP and NLC mice, but Il7r transcripts were not found (Supplementary Figure 1B is available at International Immunology Online).
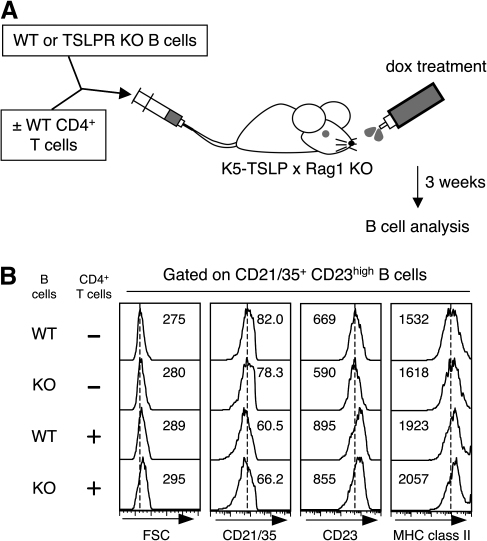
The above results implied that B cells do not have a functional TSLPR, and thus, are unlikely to be capable of responding directly to TSLP stimulation and that the activation of mature B cells present in the K5-TSLP mice was also likely to be independent of a direct affect of TSLP on B cells. To formally test this idea, mature splenic B cells were isolated from WT or TSLPR-deficient (Tslpr−/−) mice (37) and transferred into K5-TSLP/Rag1−/− mice, followed by 3 weeks of dietary dox treatment (Fig. 2A). The phenotypes of the B cells in mice receiving Tslpr−/− B cells were indistinguishable from mice that received WT B cells (Fig. 2B). These data are consistent with the effects of TSLP on B-cell activation being indirect.
Fig. 2.
CD4+ T cells, but not TSLPR on B cells, are required for B-cell activation in reconstituted K5-TSLP/Rag1−/− mice. (A) Schematic representation of transfer experiment using K5-TSLP crossed with Rag1 KO mice. B cells purified from WT or TSLPR KO (Tslpr−/−) mice were i.v. transferred with or without CD4+ T cells isolated from WT mice. Dox treatment (1 mg ml−1) was started the next day of transfer, and mice were analyzed after 3 weeks of dox treatment. (B) Analysis of FO B cells from spleen of K5-TSLP/Rag1−/− mice transferred with WT or Tslpr−/− B cells with or without WT CD4+ T cells and treated for 3 weeks with 1 mg ml−1 of dox in the drinking water. Splenocytes were stained for B220, CD21/35, CD23 and MHC class II and analyzed with flow cytometry. Cells were gated on FO B cells and FSC and expression of CD21/CD35, CD23 and MHC class II were shown. Dotted line indicates the peak of each histogram of B cells from K5-TSLP/Rag1−/− mice transferred with WT B cells without CD4+ T cells. Numbers indicate mean of FSC or fluorescent intensity in each histogram. Results represent one of two independent experiments.
CD4+ T cells are required for polyclonal B-cell activation in K5-TSLP mice
The data presented above showed that B cells do not need to directly respond to TSLP to become activated in the K5-TSLP mice. To begin to examine what TSLP-responsive cell populations are required, we focused on T cells, which are also absent in Rag1-deficient animals. WT CD4+ T cells were co-transferred with B cells from either WT or Tslpr−/− mice into K5-TSLP/Rag1−/− recipient mice. B-cell activation was subsequently assessed after 3 weeks of dietary dox treatment. As shown in Fig. 2(B), B cells from both groups of mice displayed signs of B-cell activation, slightly higher FSC, CD23, MHC class II and lower CD21/35, equivalent to that present in intact K5-TSLP animals. Thus, CD4+ T cells are likely required for the effect of TSLP overexpression on mature B-cell activation.
To extend these studies, we took two approaches, one genetic and one deletional, to deplete CD4+ T cells from K5-TSLP mice and determine the effect on B-cell activation. First, K5-TSLP mice were bred with mice with TCRβ-deficient (Tcrb−/−) mice and given dietary dox for 3 weeks. B-cell development and activation were subsequently assessed. Consistent with our previous findings (33), the late pro B cells (Hardy Fraction C) were dramatically increased in the bone marrow in both K5-TSLP/Tcrb+/− and K5-TSLP/Tcrb−/− mice, showing that the response of bone marrow B-cell progenitors to TSLP is T cell independent (Supplementary Fig. 2A is available at International Immunology Online). However, FO B cells in the spleen from K5-TSLP/Tcrb−/− mice lacked the activated phenotype present in TCRβ-sufficient K5-TSLP mice and more closely resembled the phenotype of FO B cells derived from NLC/Tcrb+/− or NLC/Tcrb−/− mice (Supplementary Fig. 2B is available at International Immunology Online). These results suggested that polyclonal activation of B cells induced in K5-TSLP mice depends on αβ T cells, and that T cells themselves, or some factor produced by T cells, might play an important role in this phenotype.
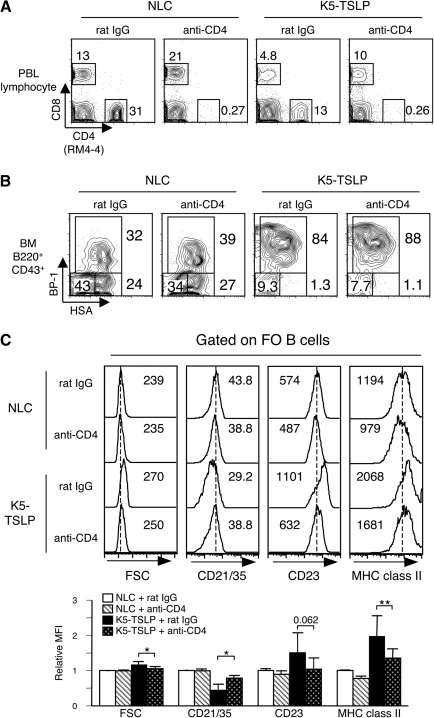
As an alternative approach to assess the role of CD4+ T cells and to exclude a possibility of a developmental effect on B cells in Tcrb−/− background, we used acute elimination of CD4+ T cells from K5-TSLP mice before and during dox treatment. K5-TSLP or NLC mice were treated with 200 μg of purified anti-CD4 antibody (clone GK1.5) or control antibody (rat IgG) per mouse every 5 days, beginning 5 days before starting dietary dox treatment. NLC and K5-TSLP mice injected with anti-CD4 antibody had no detectable CD4+ T cells in their peripheral blood after 3 weeks of dox treatment (Fig. 3A). As was the case in the K5-TSLP/Tcrb−/− mice, fraction C late pro B cells in the bone marrow of anti-CD4-treated K5-TSLP mice increased to the same extent as control antibody-treated K5-TSLP mice (Fig. 3B). In their spleen, however, FO B cells in anti-CD4-treated K5-TSLP mice had a phenotype similar to that seen in NLC mice, including higher levels of CD21/CD35, lower levels of MHC class II and reduced FSC compared with B cells in control antibody-treated K5-TSLP mice (Fig. 3C). These combined results demonstrate that CD4+ T cells are required for the polyclonal activation of mature B cells in K5-TSLP mice.
Fig. 3.
Depletion of CD4+ T cells inhibits polyclonal activation of spleen B cells in K5-TSLP mice. (A) Peripheral blood lymphocytes from NLC or K5-TSLP mice injected with anti-CD4 (clone GK1.5) antibodies or control rat IgG. All mice were treated with 1 mg ml−1 dox for 3 weeks. Cells were stained with anti-CD4 (clone RM4-4, different clone from the antibody for depletion) and anti-CD8 and analyzed with flow cytometry. Numbers near boxed area indicate percent of gated subset. (B) B-cell subsets in bone marrow from NLC or K5-TSLP mice injected with anti-CD4 or control rat IgG. All mice were treated with 1 mg ml−1 dox for 3 weeks. Cells were gated on B220+CD43+ live lymphocyte, and immature B-cell subsets were identified by staining for HSA/CD24 and BP-1. Numbers near boxed area indicate percent of gated subset. (C) Analysis of FO B cells from spleen of NLC or K5-TSLP mice injected with anti-CD4 or control rat IgG and treated for 3 weeks with 1 mg ml−1 of dox in the drinking water. Splenocytes were stained for B220, CD21/35, CD23 and MHC class II and analyzed with flow cytometry. Cells were gated on FO B cells and FSC and expression of CD21/CD35, CD23 and MHC class II were shown (top). Dotted line indicates the peak of each histogram of B cells from NLC mice injected with control rat IgG. Numbers indicate mean of FSC or fluorescent intensity in each histogram. Data represent one of four independent experiments. The bar graph (bottom) shows summary of mean fluorescence intensity relative to NLC injected with rat IgG. *P < 0.005; **P < 0.05.
TSLP receptor on CD4+ T cells is required for Th2 differentiation and polyclonal B-cell activation induced by TSLP overexpression in vivo
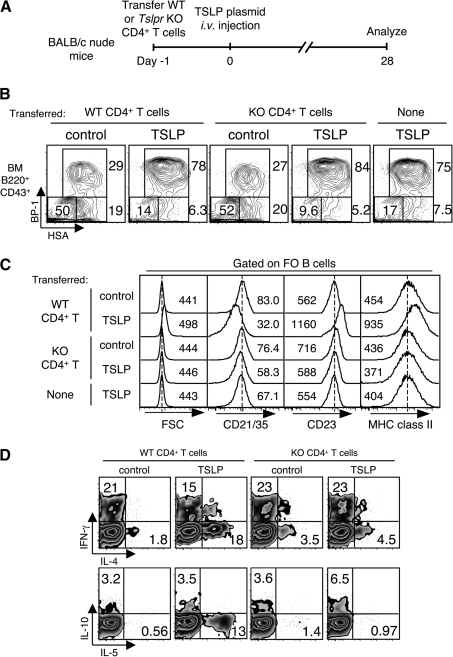
We and others have shown that TSLP can activate not only DCs but also CD4+ T cells to induce Th2 differentiation (31, 32). To examine whether TSLPR on CD4+ T cells is required for polyclonal B-cell activation, we transferred purified WT or Tslpr−/− CD4+ T cells into nude mice and induced TSLP expression by hydrodynamic injection of TSLP plasmid DNA (38, 39) (Fig. 4A). Four weeks after plasmid injection, we detected 866 (±219) pg ml−1 of TSLP in the serum of TSLP plasmid-injected nude mice, similar to K5-TSLP mice that have received dox for 2–3 weeks (33). The late pro B cells were expanded in bone marrow in both CD4+ T cell-transferred and non-transferred nude mice that received TSLP plasmid, as seen in dox-treated K5-TSLP mice (Fig. 4B). Splenic FO B cells from WT CD4+ T cell-transferred and TSLP plasmid-injected nude mice displayed higher FSC, CD23, MHC class II and lower CD21/35, equivalent to that seen in dox-treated K5-TSLP animals. However, FO B cells from nude mice that received Tslpr−/− CD4+ T cells and TSLP plasmid showed normal phenotype, similar to that seen for B cells from non-transferred or control plasmid-injected mice (Fig. 4C). Furthermore, there were many fewer IL-4- and IL-5-producing Th2-type cells isolated from the spleens of nude mice that received Tslpr−/− CD4+ T cells and TSLP plasmid (Fig. 4D). These results suggest that TSLP signaling in CD4+ T cells is essential for the production of Th2-type cytokines and polyclonal activation of B cells after TSLP overexpression in vivo.
Fig. 4.
Polyclonal B-cell activation in K5-TSLP spleen depends on TSLPR on CD4+ T cells. (A) Schematic representation of transfer experiment using BALB/c nude mice. CD4+ cells purified from WT or TSLPR KO (KO) mice were transferred. The next day, plasmid DNA encoding TSLP was i.v. injected, and mice were analyzed after 4 weeks. (B) Immature B-cell subsets in bone marrow from CD4+ T cell-transferred and DNA-injected nude mice 4 weeks after DNA injection. Cells were gated on B220+CD43+ live lymphocyte, and immature B-cell subsets were identified by staining for HSA/CD24 and BP-1. Numbers near boxed area indicate percent of gated subset. (C) Analysis of FO B cells from spleen of CD4+ T cell-transferred and DNA-injected nude mice 4 weeks after DNA injection. Splenocytes were stained for B220, CD21/35, CD23 and MHC class II and analyzed with flow cytometry. Cells were gated on FO B cells (B220+CD21/CD35lowCD23+) and FSC and expression of CD21/CD35, CD23 and MHC class II were shown. Dotted line indicates the peak of each histogram of B cells from WT CD4+ T cell-transferred and control DNA-injected nude mice. Numbers indicate mean of FSC or fluorescent intensity in each histogram. Both results represent one of two independent experiments. (D) Intracellular staining of splenic CD4+ T cells from transferred nude mice. Splenocytes were stimulated with phorbol myristate acetate and ionomycin for 4 h, fixed, permeabilized, stained for cytokines and analyzed with flow cytometry. Cells were gated on CD4+ cells. Data represent one of two independent experiments.
IL-4 plays a central role in polyclonal B-cell activation in K5-TSLP mice
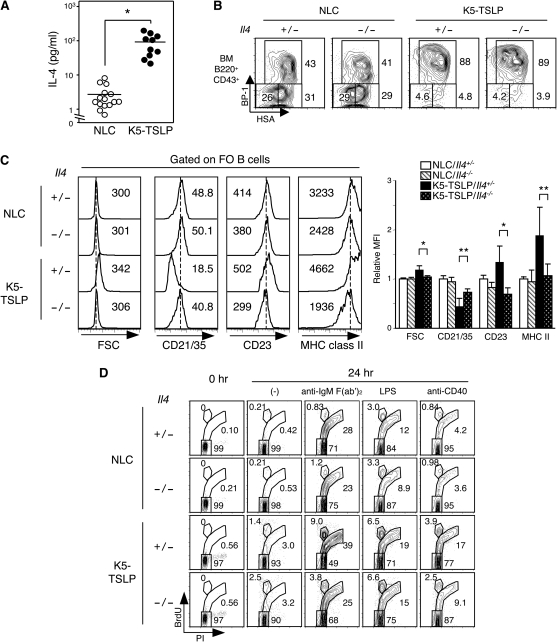
Based upon previous studies, we reasoned that IL-4 was a prominent candidate factor produced by CD4+ T cells for the promotion of polyclonal B-cell activation in dox-treated K5-TSLP mice. IL-4 is produced by various cell types including Th2 cells, mast cells, basophils and eosinophils (39–43). The majority of CD4+ T cells in K5-TSLP mice has a Th2-type phenotype and produce large amounts of IL-4 (23). In addition, IL-4 has been shown to stimulate B cells and to rescue B cells from apoptotic cell death, and constitutive expression of IL-4 in vivo leads to polyclonal B-cell activation and autoimmune disease in mice (41, 44). Indeed, serum IL-4 levels in K5-TSLP mice were significantly increased after dox treatment (Fig. 5A), and it was decreased on Tcrb−/− background or by depletion of CD4+ T cells (Supplementary Fig. 3A and B is available at International Immunology Online). To test whether IL-4 was important for polyclonal activation in K5-TSLP mice, we crossed K5-TSLP mice with IL-4 gene-deficient (Il4−/−) mice and analyzed these animals following dox treatment. K5-TSLP/Il4+/− mice displayed comparable level of serum IL-4 to K5-TSLP/Il4+/+ mice (Supplementary Fig. 3C is available at International Immunology Online). The late pro B cells (Hardy Fraction C) were increased in bone marrow of K5-TSLP/Il4−/− mice, as well as K5-TSLP/Il4+/− mice, consistent with this phenotype being IL-4-independent (Fig. 5B). However, splenic FO B cells from K5-TSLP/Il4−/− mice displayed a nearly normal cell surface phenotype. Cell size and surface expression of CD21/CD35, CD23 and MHC class II in these mice more closely resembled those in B cells from NLC rather than in K5-TSLP/Il4+/− mice (Fig. 5C). FO B cells from K5-TSLP/Il4−/− mice also displayed in vitro proliferative responses that were significantly reduced compared with B cells derived from K5-TSLP/Il4+/− mice (Fig. 5D). We also found that IL-4 receptor α chain (IL-4Rα) on mature B cells was up-regulated in K5-TSLP mice after dox treatment (Supplementary Fig. 4 is available at International Immunology Online). These results suggest that IL-4, most likely produced by Th2-type CD4+ T cells, plays a prominent role in the polyclonal B-cell activation induced after systemic TSLP overexpression in K5-TSLP mice.
Fig. 5.
IL-4 is required for polyclonal B-cell activation in K5-TSLP mice. (A) IL-4 concentration in serum samples from NLC or K5-TSLP mice received 1 mg ml−1 of dox in the drinking water for 3 weeks. Samples are diluted and analyzed by ELISA for IL-4. *P < 0.02. (B) B-cell subsets in bone marrow from NLC/Il4+/−, NLC/Il4−/−, K5-TSLP/Il4+/− and K5-TSLP/Il4−/− mice treated for 3 weeks with 1 mg ml−1 of dox. Cells were gated on B220+CD43+ live lymphocyte, and immature B-cell subsets were identified by staining for HSA/CD24 and BP-1. Numbers near boxed area indicate percent of gated subset. (C) Flow cytometry analysis of FO B cells from spleen of NLC/Il4+/−, NLC/Il4−/−, K5-TSLP/Il4+/− and K5-TSLP/Il4−/− mice treated for 3 weeks with 1 mg ml−1 of dox. Splenocytes were stained for B220, CD21/35, CD23 and MHC class II and analyzed. Cells were gated on FO B cells and FSC and expression of CD21/CD35, CD23 and MHC class II were shown (left). Dotted line indicates the peak of each histogram of NLC/Il4+/− B cells. Numbers indicate mean of FSC or fluorescent intensity in each histogram. Data represent one of seven independent experiments. The bar graph (right) shows summary of mean fluorescence intensity relative to NLC/Il4+/−. *P < 0.005; **P < 0.01. (D) In vitro BrdU incorporation assay of splenic B cells from NLC/Il4+/−, NLC/Il4−/−, K5-TSLP/Il4+/− and K5-TSLP/Il4−/− mice treated for 3 weeks with 1 mg ml−1 of dox. B cells purified from spleens were cultured with 40 μM of BrdU and indicated stimulations for 24 h. Cells were washed, fixed with ethanol, stained with anti-BrdU antibodies and propidium iodide and analyzed. Numbers near gated areas indicate percent of the population. Data represent one of two independent experiments.
K5-TSLP mice develop autoimmune hemolytic anemia that depends on IL-4
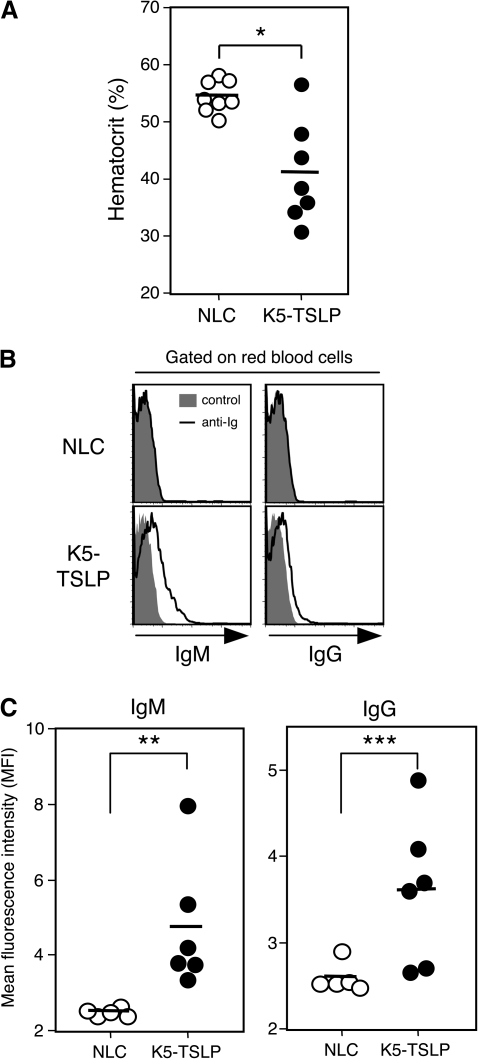
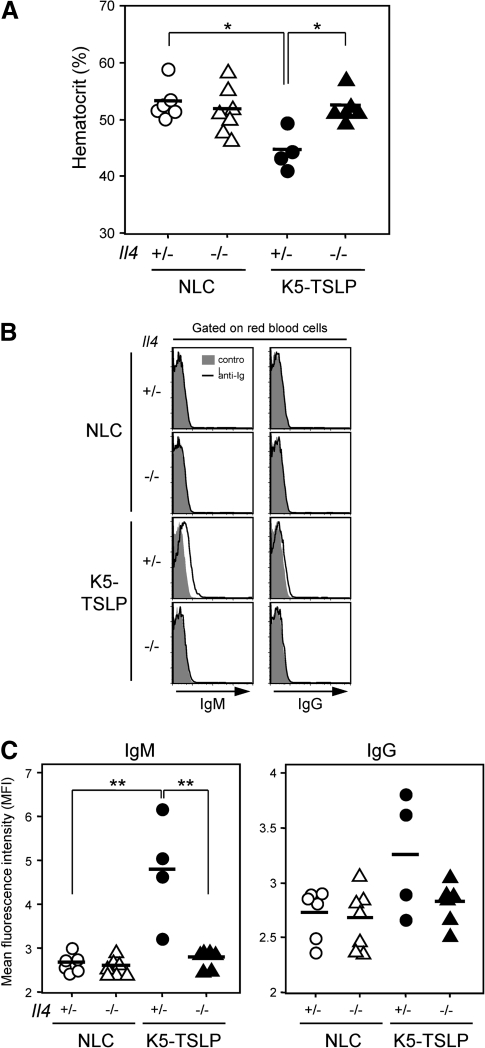
K5-TSLP mice display autoimmune features, including cryoglobulinemia and deposition of immune complexes in kidneys, after dox treatment (33). In addition to these previous findings, we noted that K5-TSLP mice develop significant anemia, exemplified by a drop in hematocrit following dox treatment (Fig. 6A). The fall in hematocrit directly correlated with the presence of anti-RBC-specific autoantibodies, consistent with onset of hemolytic anemia (Fig. 6B and C). Consistent with our data, previous studies using IL-4 transgenic mice, with expression driven via an MHC class I promoter, have reported the development of anemia, glomerulonephritis with complement and immune complex deposition and increased production of autoantibodies (44). Notably, the decrease in hematocrit after dox treatment was not seen in K5-TSLP mice crossed with Tcrb-deficient mice or Il4-deficient mice (Fig. 7A and Supplementary Figure 5A is available at International Immunology Online), and RBC-bound autoantibodies were also absent in K5-TSLP/Tcrb−/− and K5-TSLP/Il4−/− mice although the difference in anti-RBC IgG between K5-TSLP/Il4+/− and K5-TSLP/Il4−/− mice was not significant (Fig. 7B and C and Supplementary Figure 5B is available at International Immunology Online). Together, these results suggest that Th2-produced IL-4 is essential to drive the B-cell autoimmunity in K5-TSLP mice following the induction of systemic TSLP expression.
Fig. 6.
K5-TSLP mice developed autoimmune hemolytic anemia. (A) Hematocrit in the blood of NLC mice and K5-TSLP mice that were treated with 1 mg ml−1 of dox for 3 weeks. Averages were indicated by horizontal bars. (B) Detection of anti-RBC antibodies by flow cytometry. RBCs form Dox-treated NLC or K5-TSLP mice were stained with anti-mouse IgM and anti-mouse IgG and analyzed. Data represent one of four independent experiments. (C) Mean fluorescence intensities of NLC or K5-TSLP RBCs stained with anti-mouse IgM or IgG. Averages were indicated by horizontal bars. *P < 0.01; **P < 0.005; ***P < 0.05.
Fig. 7.
Autoimmune hemolytic anemia in K5-TSLP mice depends on IL-4. (A) Hematocrit in the blood of NLC/Il4+/−, NLC/Il4−/−, K5-TSLP/Il4+/− and K5-TSLP/Il4−/− mice after 1 mg ml−1, 3-week dox treatment. Averages were indicated by horizontal bars. (B) Detection of anti-RBC antibodies by flow cytometry. RBCs form dox-treated NLC/Il4+/−, NLC/Il4−/−, K5-TSLP/Il4+/− and K5-TSLP/Il4−/− mice were stained with anti-mouse IgM and anti-mouse IgG and analyzed. Data represent one of three independent experiments. (C) Mean fluorescence intensities of RBCs from NLC/Il4+/−, NLC/Il4−/−, K5-TSLP/Il4+/− and K5-TSLP/Il4−/− mice and stained with anti-mouse IgM or IgG. Averages were indicated by horizontal bars. *P < 0.005; **P < 0.05.
Discussion
We have previously shown that elevated systemic levels of TSLP lead to variety of abnormalities in B-cell development and maturation (33). That report focused on the bone marrow B lineage targets for TSLP, and identified late pro-B cells (Hardy fraction C) and B-1 progenitors as the primary target cell populations. Using the same model of increased systemic TSLP (K5-TSLP mice), we extended these findings, focusing on the effect of TSLP on mature splenic B cells in K5-TSLP mice. We show that mature FO B cells (B220+CD21/CD35lowCD23+) in the K5-TSLP mice display features of polyclonal activation, including down-regulation of CD21/CD35 expression, elevated CD23 and MHC class II expression, increased cell size and increased mitogenic responsiveness. Furthermore, we show that these phenotypic changes are not a direct effect of TSLP on mature B cells but, instead, require TSLPR-bearing CD4+ T cells and IL-4. Finally, we show that an additional manifestation of systemically elevated TSLP is development of autoimmune hemolytic anemia, a process that also requires CD4+ T cells and IL-4.
It has been reported that TSLP activates both DCs and CD4+ T cells and can induce Th2 differentiation (20, 22, 31, 32). We have shown that TSLPR expression by CD4+ T cells is essential for Th2 cytokine production and polyclonal B-cell activation after systemic overexpression of TSLP, and our results are very similar to those of other inflammation models (45–47). These results indicate that CD4+ T cells are important target of TSLP in vivo.
IL-4 is a multi-functional cytokine produced by various cell types, including CD4+ T cells, eosinophils, mast cells and basophils. Interestingly, both mast cells and eosinophils are attracted to sites of TSLP overexpression (22,23), suggesting the possibility that eosinophils and mast cells are a source of IL-4 during systemic TSLP overexpression. However, mature B cells from K5-TSLP/Il4−/− spleens displayed similar phenotypes as those from K5-TSLP/Tcrb−/− or CD4+ T cell-depleted K5-TSLP mice. These results indicate that CD4+ T cells are the primary source of IL-4 that induces the polyclonal B-cell activation in K5-TSLP mice. Consistent with this model, dox-treated K5-TSLP mice have a marked increase in IL-4 producing CD4+ T cells in both spleen and lymph nodes (23) as well as high circulating levels of IL-4 that was dependent on CD4+ T cells. However, CD4+ T cell-deficient (Tcrb−/− or CD4+ T cell-depleted) K5-TSLP mice still showed higher serum IL-4 than NLC mice. These results suggest that CD4+ T cells are not only source of IL-4 in these mice and that TSLP can stimulate other cells to produce IL-4. One possible candidate is basophils, which produce IL-4 and have been shown to be TSLP responsive (39, 43). Moreover, IL-4Rα expression on mature B cells was increased in K5-TSLP after dox treatment, likely through a positive feedback from IL-4 signaling (48). IL-4 plays an important role in B-cell physiology by enhancing proliferation, maintaining cell survival, changing expression of surface molecules and inducing class switch recombination of Ig (41). Thus, elevated IL-4 levels in K5-TSLP mice may enhance B-cell response to autoantigens that crosslink B-cell receptor (BCR) and thereby also promote humoral autoimmunity. However, the slightly higher mitogenic response and lower CD21/CD35 expression observed with B cells derived from K5-TSLP/Il4−/− versus NLC mice suggests that an additional factor(s) may play a partially redundant role in mature B-cell activation in vivo.
Using an in vivo BrdU incorporation assays, we demonstrated that FO B cells in the K5-TSLP mice do not proliferate spontaneously in vivo. In contrast, our combined data are most consistent with the interpretation that TSLP and IL-4 signals promote development of mature B cells that are poised for rapid and robust responses to mitogenic stimulation in vitro. This idea is consistent with previous data showing that IL-4 does not directly promote B-cell cycle entry but rather acts as a co-stimulator in combination with other mitogens (41). In addition, and also consistent with the data presented here, in vivo treatment of mice with IL-4 does not lead to increased proliferation of splenic mature B cells (49). Finally, previous work has shown that IL-7Rα expression is induced following antigen or anti-CD40 signaling in mature B cells (50,51). Mature B cells from K5-TSLP mice do not express IL-7Rα further supporting the idea that this population is not spontaneously activated through BCR or CD40 in vivo. Taken as a whole, our finding suggest that the marked increase in mature B cells in K5-TSLP mice reflects the combined effect of increased bone marrow production of immature B cells in concert with prolonged IL-4-mediated B-lineage survival within the periphery.
K5-TSLP mice developed autoimmune hemolytic anemia, manifested by a dramatic decrease in hematocrit and production of RBC-specific autoantibodies. The lack of this phenotype in dox-treated K5-TSLP/Tcrb−/− and K5-TSLP/Il4−/− mice demonstrates that development of autoimmunity, similar to other mature B-cell phenotypes found in the K5-TSLP mice, is dependent on αβ T cells and IL-4. Interestingly, IL-4 transgenic mice also developed severe anemia and exhibit a high mortality rate (44), consistent with a critical role for IL-4 in the development of this autoimmune disease. Finally, in addition to its effects on B-cell activation noted above, IL-4 is also a potent IgG class switch factor (41) and is also capable of rescuing autoreactive B cells from anergy and promoting cell survival in vivo (52, 53) rather than rescuing autoreactive B cells from clonal deletion in vivo (44). Thus, development of autoimmunity in K5-TSLP mice likely reflects a multistep process that progressively impacts B-cell tolerance. We have previously reported that K5-TSLP mice developed cryoglobulinemia and glomerulonephritis (33). However, these phenotypes were not consistently seen in the K5-TSLP mice used in this study (data not shown). One possible explanation for this difference is the genetic background of the mice used. The mice in this study were BALB/c, while those used in the previous report were a mixed background that included BALB/c, C57BL/6 and 129. In addition, another TSLP transgenic strain, Lck-TSLP mice, was C57BL/6 background and developed glomerulonephritis (36), and studies using other strains have reported that only mice with a C57BL/6 background develop glomerulonephritis (54–57). Further analysis is needed to clarify the details.
Altogether, our current and previous data demonstrate that elevated systemic levels of TSLP can induce developmental changes in B cells, including both immature B-cell progenitors in the bone marrow and mature B cells in the spleen. The impact of TSLP on bone marrow B-cell differentiation appears to be direct, while its effects on mature B cells are largely indirect, requiring Th2-polarized CD4+ T cells and IL-4. Interestingly, elevated TSLP levels can lead to the production of RBC-specific autoantibodies and development of autoimmune hemolytic anemia. Induction of full-blown autoimmunity also required CD4+ T cells and IL-4, indicating that the pathology caused by systemic TSLP overexpression is dependent on both Th2 cells and activated B cells. Taken as a whole, these data suggest that TSLP can play a pivotal role in immune homeostasis through the coordinate regulation of Th1–Th2 balance and mature B-cell activation and support the need for future studies designed to assess whether local inflammatory signals that promote TSLP expression may modulate humoral autoimmunity in humans.
Supplementary data
Supplementary data are available at International Immunology Online.
Funding
National Institute of Health grant (AI068731 and AR056113 to S.F.Z.; AI044259 to S.F.Z. and D.J.R); Japan Society for the Promotion of Science grant-in-aid for Young Scientists (B) (21790488 to M.I.). M.I. was a recipient of a Research Fellowship from The Uehara Memorial Foundation.
Supplementary Material
Acknowledgments
The authors would like to thank Theingi Aye for excellent technical assistance and members of the Ziegler laboratory for helpful discussions. We also thank Matt Warren for his administrative support.
References
- 1.Lohr J, Knoechel B, Nagabhushanam V, Abbas AK. T-cell tolerance and autoimmunity to systemic and tissue-restricted self-antigens. Immunol. Rev. 2005;204:116. doi: 10.1111/j.0105-2896.2005.00241.x. [DOI] [PubMed] [Google Scholar]
- 2.Rioux JD, Abbas AK. Paths to understanding the genetic basis of autoimmune disease. Nature. 2005;435:584. doi: 10.1038/nature03723. [DOI] [PubMed] [Google Scholar]
- 3.Melchers F, Rolink AR. B cell tolerance—how to make it and how to break it. Curr. Top. Microbiol. Immunol. 2006;305:1. doi: 10.1007/3-540-29714-6_1. [DOI] [PubMed] [Google Scholar]
- 4.Levesque MC, St Clair EW. B cell-directed therapies for autoimmune disease and correlates of disease response and relapse. J. Allergy Clin. Immunol. 2008;121:13. doi: 10.1016/j.jaci.2007.11.030. [DOI] [PubMed] [Google Scholar]
- 5.Merlini G, Stone MJ. Dangerous small B-cell clones. Blood. 2006;108:2520. doi: 10.1182/blood-2006-03-001164. [DOI] [PubMed] [Google Scholar]
- 6.Clatworthy MR, Smith KG. B cells in glomerulonephritis: focus on lupus nephritis. Semin. Immunopathol. 2007;29:337. doi: 10.1007/s00281-007-0092-1. [DOI] [PubMed] [Google Scholar]
- 7.Friend SL, Hosier S, Nelson A, Foxworthe D, Williams DE, Farr A. A thymic stromal cell line supports in vitro development of surface IgM+ B cells and produces a novel growth factor affecting B and T lineage cells. Exp. Hematol. 1994;22:321. [PubMed] [Google Scholar]
- 8.Ray RJ, Furlonger C, Williams DE, Paige CJ. Characterization of thymic stromal-derived lymphopoietin (TSLP) in murine B cell development in vitro. Eur. J. Immunol. 1996;26:10. doi: 10.1002/eji.1830260103. [DOI] [PubMed] [Google Scholar]
- 9.Sims JE, Williams DE, Morrissey PJ, et al. Molecular cloning and biological characterization of a novel murine lymphoid growth factor. J. Exp. Med. 2000;192:671. doi: 10.1084/jem.192.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chappaz S, Flueck L, Farr AG, Rolink AG, Finke D. Increased TSLP availability restores T- and B-cell compartments in adult IL-7 deficient mice. Blood. 2007;110:3862. doi: 10.1182/blood-2007-02-074245. [DOI] [PubMed] [Google Scholar]
- 11.Pandey A, Ozaki K, Baumann H, et al. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat. Immunol. 2000;1:59. doi: 10.1038/76923. [DOI] [PubMed] [Google Scholar]
- 12.Park LS, Martin U, Garka K, et al. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: formation of a functional heteromeric complex requires interleukin 7 receptor. J. Exp. Med. 2000;192:659. doi: 10.1084/jem.192.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isaksen DE, Baumann H, Trobridge PA, Farr AG, Levin SD, Ziegler SF. Requirement for stat5 in thymic stromal lymphopoietin-mediated signal transduction. J. Immunol. 1999;163:5971. [PubMed] [Google Scholar]
- 14.Reche PA, Soumelis V, Gorman DM, et al. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J. Immunol. 2001;167:336. doi: 10.4049/jimmunol.167.1.336. [DOI] [PubMed] [Google Scholar]
- 15.Isaksen DE, Baumann H, Zhou B, et al. Uncoupling of proliferation and Stat5 activation in thymic stromal lymphopoietin-mediated signal transduction. J. Immunol. 2002;168:3288. doi: 10.4049/jimmunol.168.7.3288. [DOI] [PubMed] [Google Scholar]
- 16.Rochman Y, Kashyap M, Robinson GW, et al. Thymic stromal lymphopoietin-mediated STAT5 phosphorylation via kinases JAK1 and JAK2 reveals a key difference from IL-7-induced signaling. Proc. Natl Acad. Sci. USA. 2010;107:19455. doi: 10.1073/pnas.1008271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ziegler SF, Liu YJ. Thymic stromal lymphopoietin in normal and pathogenic T cell development and function. Nat. Immunol. 2006;7:709. doi: 10.1038/ni1360. [DOI] [PubMed] [Google Scholar]
- 18.Liu YJ, Soumelis V, Watanabe N, et al. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu. Rev. Immunol. 2007;25:193. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- 19.Ziegler SF. The role of thymic stromal lymphopoietin (TSLP) in allergic disorders. Curr. Opin. Immunol. 2010;22:795. doi: 10.1016/j.coi.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soumelis V, Reche PA, Kanzler H, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 2002;3:673. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 21.Ying S, O'Connor B, Ratoff J, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J. Immunol. 2005;174:8183. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- 22.Zhou B, Comeau MR, De Smedt T, et al. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat. Immunol. 2005;6:1047. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 23.Yoo J, Omori M, Gyarmati D, et al. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J. Exp. Med. 2005;202:541. doi: 10.1084/jem.20041503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jessup HK, Brewer AW, Omori M, et al. Intradermal administration of thymic stromal lymphopoietin induces a T cell- and eosinophil-dependent systemic Th2 inflammatory response. J. Immunol. 2008;181:4311. doi: 10.4049/jimmunol.181.6.4311. [DOI] [PubMed] [Google Scholar]
- 25.Headley MB, Zhou B, Shih WX, Aye T, Comeau MR, Ziegler SF. TSLP conditions the lung immune environment for the generation of pathogenic innate and antigen-specific adaptive immune responses. J. Immunol. 2009;182:1641. doi: 10.4049/jimmunol.182.3.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor BC, Zaph C, Troy AE, et al. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J. Exp. Med. 2009;206:655. doi: 10.1084/jem.20081499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larson RP, Zimmerli SC, Comeau MR, et al. Dibutyl phthalate-induced thymic stromal lymphopoietin is required for Th2 contact hypersensitivity responses. J. Immunol. 2010;184:2974. doi: 10.4049/jimmunol.0803478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramalingam TR, Pesce JT, Mentink-Kane MM, et al. Regulation of helminth-induced Th2 responses by thymic stromal lymphopoietin. J. Immunol. 2009;182:6452. doi: 10.4049/jimmunol.0900181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ito T, Wang YH, Duramad O, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J. Exp. Med. 2005;202:1213. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seshasayee D, Lee WP, Zhou M, et al. In vivo blockade of OX40 ligand inhibits thymic stromal lymphopoietin driven atopic inflammation. J. Clin. Invest. 2007;117:3868. doi: 10.1172/JCI33559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Omori M, Ziegler S. Induction of IL-4 expression in CD4(+) T cells by thymic stromal lymphopoietin. J. Immunol. 2007;178:1396. doi: 10.4049/jimmunol.178.3.1396. [DOI] [PubMed] [Google Scholar]
- 32.Rochman I, Watanabe N, Arima K, Liu YJ, Leonard WJ. Cutting edge: direct action of thymic stromal lymphopoietin on activated human CD4+ T cells. J. Immunol. 2007;178:6720. doi: 10.4049/jimmunol.178.11.6720. [DOI] [PubMed] [Google Scholar]
- 33.Astrakhan A, Omori M, Nguyen T, et al. Local increase in thymic stromal lymphopoietin induces systemic alterations in B cell development. Nat. Immunol. 2007;8:522. doi: 10.1038/ni1452. [DOI] [PubMed] [Google Scholar]
- 34.Hardy RR, Hayakawa K. B cell development pathways. Annu. Rev. Immunol. 2001;19:595. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- 35.Montecino-Rodriguez E, Leathers H, Dorshkind K. Identification of a B-1 B cell-specified progenitor. Nat. Immunol. 2006;7:293. doi: 10.1038/ni1301. [DOI] [PubMed] [Google Scholar]
- 36.Taneda S, Segerer S, Hudkins KL, et al. Cryoglobulinemic glomerulonephritis in thymic stromal lymphopoietin transgenic mice. Am. J. Pathol. 2001;159:2355. doi: 10.1016/S0002-9440(10)63085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carpino N, Thierfelder WE, Chang MS, et al. Absence of an essential role for thymic stromal lymphopoietin receptor in murine B-cell development. Mol. Cell. Biol. 2004;24:2584. doi: 10.1128/MCB.24.6.2584-2592.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sebestyen MG, Budker VG, Budker T, et al. Mechanism of plasmid delivery by hydrodynamic tail vein injection. I. Hepatocyte uptake of various molecules. J. Gene Med. 2006;8:852. doi: 10.1002/jgm.921. [DOI] [PubMed] [Google Scholar]
- 39.Siracusa MC, Saenz SA, Hill DA, et al. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature. 2011;477:229. doi: 10.1038/nature10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 1986;136:2348. [PubMed] [Google Scholar]
- 41.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu. Rev. Immunol. 1999;17:701. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 42.Voehringer D, Shinkai K, Locksley RM. Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity. 2004;20:267. doi: 10.1016/s1074-7613(04)00026-3. [DOI] [PubMed] [Google Scholar]
- 43.Perrigoue JG, Saenz SA, Siracusa MC, et al. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat. Immunol. 2009;10:697. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Erb KJ, Ruger B, von Brevern M, Ryffel B, Schimpl A, Rivett K. Constitutive expression of interleukin (IL)-4 in vivo causes autoimmune-type disorders in mice. J. Exp. Med. 1997;185:329. doi: 10.1084/jem.185.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Al-Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. J. Exp. Med. 2005;202:829. doi: 10.1084/jem.20050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He R, Oyoshi MK, Garibyan L, Kumar L, Ziegler SF, Geha RS. TSLP acts on infiltrating effector T cells to drive allergic skin inflammation. Proc. Natl Acad. Sci. USA. 2008;105:11875. doi: 10.1073/pnas.0801532105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blazquez AB, Mayer L, Berin MC. Thymic stromal lymphopoietin is required for gastrointestinal allergy but not oral tolerance. Gastroenterology. 2010;139:1301. doi: 10.1053/j.gastro.2010.06.055. [DOI] [PubMed] [Google Scholar]
- 48.Ohara J, Paul WE. Up-regulation of interleukin 4/B-cell stimulatory factor 1 receptor expression. Proc. Natl Acad. Sci. USA. 1988;85:8221. doi: 10.1073/pnas.85.21.8221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mori M, Morris SC, Orekhova T, Marinaro M, Giannini E, Finkelman FD. IL-4 promotes the migration of circulating B cells to the spleen and increases splenic B cell survival. J. Immunol. 2000;164:5704. doi: 10.4049/jimmunol.164.11.5704. [DOI] [PubMed] [Google Scholar]
- 50.Wang YH, Diamond B. B cell receptor revision diminishes the autoreactive B cell response after antigen activation in mice. J. Clin. Invest. 2008;118:2896. doi: 10.1172/JCI35618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hikida M, Nakayama Y, Yamashita Y, Kumazawa Y, Nishikawa SI, Ohmori H. Expression of recombination activating genes in germinal center B cells: involvement of interleukin 7 (IL-7) and the IL-7 receptor. J. Exp. Med. 1998;188:365. doi: 10.1084/jem.188.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Foote LC, Evans JW, Cifuni JM, et al. Interleukin-4 produces a breakdown of tolerance in vivo with autoantibody formation and tissue damage. Autoimmunity. 2004;37:569. doi: 10.1080/08916930400020602. [DOI] [PubMed] [Google Scholar]
- 53.Morris SC, Dragula NL, Finkelman FD. IL-4 promotes Stat6-dependent survival of autoreactive B cells in vivo without inducing autoantibody production. J. Immunol. 2002;169:1696. doi: 10.4049/jimmunol.169.4.1696. [DOI] [PubMed] [Google Scholar]
- 54.Strasser A, Whittingham S, Vaux DL, et al. Enforced BCL2 expression in B-lymphoid cells prolongs antibody responses and elicits autoimmune disease. Proc. Natl Acad. Sci. USA. 1991;88:8661. doi: 10.1073/pnas.88.19.8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Botto M, Dell'Agnola C, Bygrave AE, et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat. Genet. 1998;19:56. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 56.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 57.Bolland S, Ravetch JV. Spontaneous autoimmune disease in Fc(gamma)RIIB-deficient mice results from strain-specific epistasis. Immunity. 2000;13:277. doi: 10.1016/s1074-7613(00)00027-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.