Abstract
Background
The present study evaluated the efficacy of biphasic human insulin 30 (BHI 30) in type 2 diabetes patients who had failed in therapy with two or more oral antidiabetes drugs (OADs).
Methods
This open-label, nonrandomized, 4-month, multicenter, clinical observational study was conducted in Shanghai, China. A total of 660 insulin-naive type 2 diabetes patients with poor glycemic control (glycosylated hemoglobin [HbA1c] ≥7.5%), despite treatment with two or more OADs for more than 6 months, were recruited and received BHI 30 monotherapy or BHI 30 plus OAD(s) (metformin only, α-glucosidase inhibitor only, or both).
Results
Among the 660 subjects, 644 completed the 4-month study. At the end of the study, the median level of HbA1c decreased by 2.0% (from 9.1% to 7.0%) in the BHI 30 monotherapy group and also 2.0% (from 9.5% to 7.3%) in the BHI 30 plus OAD group. More patients achieved the HbA1c <7.0% target in the BHI 30 monotherapy group than in the BHI 30 plus OAD(s) group (47.9% vs. 35.3%, P=0.002). Compared with the expenses of the prior treatment strategy, the median daily cost decreased by 39.8% (4.5 yuan, Chinese RMB) at the end point in the BHI 30 monotherapy group but increased by 20.0% (2.2 yuan) in the BHI 30 plus OAD(s) group (P<0.0001). Moreover, patients in the BHI 30 plus OAD(s) group had fewer minor hypoglycemic episodes than in the BHI 30 monotherapy group (mean of 1.06 vs. 2.77 per patient per year, P<0.0001).
Conclusions
Short-term BHI 30 therapy can improve glycemic control in insulin-naive type 2 diabetes patients after failure of two or more OADs. With higher baseline glucose level, the BHI 30 plus OAD(s) group had lower pharmacoeconomic efficacy than the BHI 30 monotherapy group despite having fewer hypoglycemia events.
Introduction
The maintenance of a near-normal glucose level remains a cornerstone for the management of type 2 diabetes.1 However, as the disease progresses, islet β-cell deterioration with subsequent decrease in endogenous insulin production makes glycemic control gradually more difficult, regardless of the initial hypoglycemic reagents used. Therefore, exogenous insulin treatment is eventually required to maintain a satisfactory glucose level.2
According to the International Diabetes Federation, insulin therapy should be initiated when oral antidiabetes drug (OAD) therapy can no longer maintain glycosylated hemoglobin (HbA1c) at ≤7.5%.2 The European Diabetes Policy Group recommends that for the majority of type 2 diabetes patients, premixed insulin should be applied upon initiating insulin therapy.3 Premixed insulin accounts for more than 80% of insulin used in both type 1 and type 2 diabetes worldwide.4
One of the most popular types of premixed insulin is biphasic human insulin 30 (BHI 30), consisting 30% soluble human insulin and 70% neutral protamine Hagedorn (NPH) insulin. The 30% soluble portion is meant to mimic the physiological pattern of postprandial insulin secretion, wheres NPH supplies the basal insulin. Injection of BHI 30 half an hour before breakfast and dinner provides stable glycemic control in type 2 diabetes patients.5–7
Despite its side effects (e.g., hypoglycemia and body weight gain8), premixed human insulin is still considered a reasonable regimen for type 2 diabetes.9 In China, BHI 30 has been widely applied for decades. In the present study, we investigated the efficacy and comparative financial costs of BHI 30 as a monotherapy versus in combination with OAD(s) in insulin-naive type 2 diabetes patients when OADs had failed.
Subjects and Methods
Patients and research design
This open-label, nonrandomized, multicenter, 4-month observational study was conducted from September 2007 to April 2008. Twenty-two medical organizations in Shanghai, China, participated, including eleven first-class hospitals, eight second-class hospitals, and three third-class hospitals.
A total of 660 insulin-naive type 2 diabetes patients, ranging from 30 to 75 years of age, were enrolled in the study with the following inclusion criteria: (1) diabetes lasting more than 1 year, (2) HbA1c ≥7.5% after over 6 months of treatment with two or more OADs, and (3) at least one of the OADs was an insulin secretagogue. Patients were excluded if they had type 1 or secondary diabetes or contraindications (such as clinical signs of heart, kidney, or liver failure) for the drugs used in this study. This study was approved by the local ethics committee, and all patients provided written informed consent before the study recruitment.
The glycemic level and other clinical characteristics of each patient were assessed at baseline. Subjects were then allocated by the clinicians to receive either BHI 30 monotherapy or BHI 30 plus OAD(s) for 4 months. As a nonrandomized clinical observational study, whether the patients needed additional OADs were decided by the physicians participating in the study. Based on their experiences and common practice, the prescription was individualized according to each subject's glucose level and concomitant diseases at baseline. The three BHI 30 plus OAD(s) treatment groups included BHI 30 plus metformin (MET), BHI 30 plus α-glucosidase inhibitor (AGI), and BHI 30 plus MET and AGI. The daily BHI 30 dose of 20 units was distributed before breakfast and dinner. Each physician made his or her own decision on the distribution of the 20-unit insulin administration according to the fasting and postprandial glucose level.
All patients were required to test their fasting and postprandial capillary blood glucose levels at least twice a week at home or in the community health service centers. During the initial 4 weeks, the patients visited their clinicians once a week, and insulin dosage was titrated according to their blood glucose records. All the individuals were asked to visit the clinicians every 2 weeks from week 5. In addition, both the fasting and 2-h postprandial plasma glucose (FPG and 2hPG, respectively) values were tested at baseline and the end point.
Outcome measurements
The primary outcome was the improvement of HbA1c. The HbA1c targets were set below 6.5% and 7.0%, according to the criteria recommended by the American Diabetes Association10 and International Diabetes Federation.11 Secondary outcomes included FPG and 2hPG, body weight change, daily insulin dose, and daily drug costs. The plasma glucose concentration was measured using a glucose oxidase method, and HbA1c was measured by high-performance liquid chromatography at the clinical laboratory of each participating center. All measurements met the Shanghai Center for Clinical Laboratory criteria.
Safety measurements
The safety of BHI 30 treatment was evaluated by the frequency of hypoglycemic events. A minor hypoglycemic episode was defined as an occurrence where the patient could be self-treated with a blood glucose value <3.1 mmol/L, with or without sympathetic activation symptoms. A major hypoglycemic event was defined as neurological symptoms consistent with hypoglycemia requiring assistance and either a plasma glucose value <3.1 mmol/L or reversal of symptoms after food intake, intravenous glucose, or glucagon.12
Cost assessment
The prevailing retail prices were adopted to evaluate the daily costs of hypoglycemic drugs at the time when the study was conducted. The change in daily hypoglycemic drug costs equals daily drug costs at the end of the study minus that of the prior treatment. The costs of diabetes complications were not included.
Statistical analysis
All continuous variables were tested for parametric distribution by Kolmogorov–Smimov normality test. Normally distributed variables were expressed as mean±SD values. One-way analysis of variance was used to test the significance of difference in means for multiple groups, paired-samples t test for individuals before–after comparison, and independent-samples t test for two groups. Skewed variables were expressed as median (interquartile range). The Mann–Whitney U test was used for two groups, and the Wilcoxon test was used for each individual's before–after comparison. The χ2 test was used in the analysis of stratified categorical data. An analysis of univariate general linear model was conducted to test the mean changes of outcome variables among the different treatment groups adjusted by the corresponding variables as the covariates. According to the range of HbA1c level from baseline to the end point, we divided the HbA1c level into multiple ranks (from the lowest to the highest) with intervals of 1%. Then the frequencies of the patients with different ranks of HbA1c were sorted out in a rows (baseline and end point)×columns (ranks) table. The χ2 test was used to analyze the difference of frequency distribution from baseline to the end of the study. A P value of <0.05 (two-tailed) was considered statistically significant. All the data were analyzed using Statistical Package for the Social Sciences software, version 16.0 (SPSS, Chicago, IL).
Results
Of 660 participants enrolled, 644 (97.6%) completed the 4-month follow-up study. Among the 16 patients who withdrew, 6 were dropped for noncompliance, and 10 were lost to follow-up.
Baseline characteristics of participants
The participants' characteristics by treatment group are shown in Table 1. The median (range) ages and diabetes durations for all subjects were 61.9 (52.9, 71.6) years and 7.0 (3.9, 10.8) years, respectively. All patients were assigned to a treatment group: 409 in the BHI 30 monotherapy group and 235 in the BHI 30 plus OAD(s) groups. Of the latter 235 who received both BHI 30 and OAD(s), 160 received one OAD only (92 with MET and 68 with AGI), and 75 were administered both MET and AGI.
Table 1.
Patient Characteristics at Enrollment
| Total (n=644) | BHI 30 monotherapy (n=409) | BHI 30 plus one OAD (n =160) | BHI 30 plus two OADs (n=75) | P value | |
|---|---|---|---|---|---|
| Gender, male (%) | 336 (52.2) | 203 (49.6) | 99 (61.9)a | 34 (45.3) | 0.014 |
| Age (years) | 61.9 (52.9, 71.6) | 61.9 (52.7, 71.4) | 61.0 (53.0, 71.3) | 63.8 (53.4, 73.5) | 0.332 |
| Duration of diabetes (years) | 7.0 (3.9, 10.8) | 6.7 (3.7, 10.2) | 7.7 (4.6, 11.5) | 8.0 (4.3, 11.4) | 0.029 |
| BMI (kg/m2) | 24.3 (22.4, 26.7) | 24.5 (22.5, 26.8) | 24.1 (22.1, 26.3) | 24.6 (22.8, 26.6) | 0.455 |
| Weight (kg) | 65.8 (60.0, 74.0) | 65.0 (60.0, 74.0) | 65.0 (60.0, 73.0) | 67.0 (60.0,74.3) | 0.557 |
| HbA1c (%) | 9.2 (8.2, 10.6) | 9.1 (8.1, 10.3) | 9.2 (8.3, 10.7) | 9.8 (8.5, 11.0)b | 0.007 |
| FPG (mmol/L) | 9.4 (8.0, 11.6) | 9.0 (7.9, 11.1) | 10.0 (8.5, 12.7)c | 9.8 (8.7, 12.1)d | 0.001 |
| 2hPG (mmol/L) | 14.7 (12.0, 18.0) | 14.3 (11.5, 18.0) | 15.1 (12.4, 18.0) | 15.3 (12.8, 17.9) | 0.149 |
| HbA1c ≥9.0% (%) | 356 (55.3) | 213 (52.1) | 93 (58.1) | 50 (66.7) | 0.046 |
Data are presented as median (interquartile range) for non-normally distributed variables and n (%) for categorical variables, unless specified otherwise.
P value for difference comparison among the three groups: aP <0.05 (the BHI 30 plus one OAD group vs. the BHI 30 monotherapy group); bP<0.01 (the BHI 30 plus two OADs group vs. the BHI 30 monotherapy group); cP<0.01 (the BHI 30 plus one OAD group vs. the BHI 30 monotherapy group); dP<0.05 (the BHI 30 plus two OADs group vs. the BHI 30 monotherapy group).
BHI, biphasic human insulin 30; BMI, body mass index; FPG, fasting plasma glucose; HbA1c, glycosylated hemoglobin; 2hPG, 2-h postprandial plasma glucose; OAD, oral antidiabetes drug.
The percentages of men in the three treatment arms of BHI 30 monotherapy, BHI 30 plus one OAD, and BHI 30 plus two OADs, were 49.6%, 61.9%, and 45.3%, respectively (P=0.014 for among the three treatment groups). At the beginning of the study, median HbA1c levels in the three groups of BHI 30 monotherapy, BHI 30 plus one OAD, and BHI 30 plus two OADs were 9.1%, 9.2%, and 9.8% (P=0.007), respectively, and the respective FPG levels were 9.0 mmol/L, 10.0 mmol/L, and 9.8 mmol/L (P=0.001). Compared with the treatment arms that included OAD(s), the BHI 30 monotherapy arm had the lowest HbA1c (P=0.011) and FPG (P=0.003) levels. Accordingly, the percentage of subjects who had HbA1c ≥9.0% was also the lowest in the BHI 30 monotherapy arm compared with the BHI 30 plus one OAD and the BHI 30 plus two OADs groups (52.1%, 58.1%, and 66.7%, respectively; P=0.046). There were no significant differences in body weight or body mass index among the three treatment arms.
Glycemic control and daily insulin dose
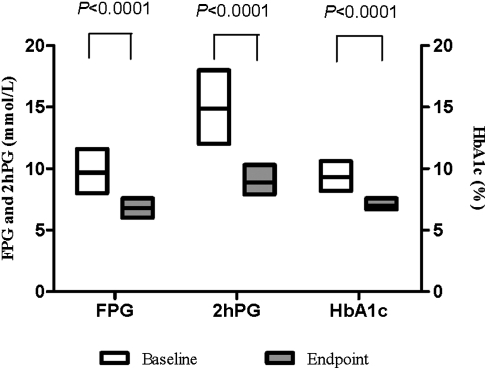
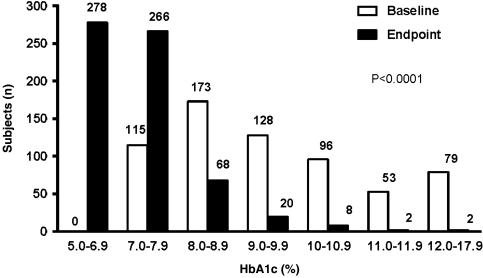
After the 4-month therapy, the overall median HbA1c, FPG, and 2hPG decreased from 9.2% to 7.0%, 9.4 mmol/L to 6.8 mmol/L, and 14.7 mmol/L to 8.9 mmol/L, respectively (all P<0.0001; Fig. 1). The frequency distribution of the HbA1c levels significantly improved at the end of the study after BHI 30 therapy, with or without OAD(s) (P<0.0001; Fig. 2). HbA1c levels significantly decreased from 9.1% to 7.0% in the BHI 30 monotherapy group and from 9.5% to 7.3% in the BHI 30 plus OAD(s) group. FPG levels significantly decreased from 9.0 mmol/L to 6.7 mmol/L in the BHI 30 monotherapy group and from 10.0 mmol/L to 7.1 mmol/L in the BHI 30 plus OAD(s) group. Significant improvements in 2hPG were also observed in both of these treatment arms: 14.3 mmol/L to 8.6 mmol/L in the BHI 30 monotherapy group and 15.3 mmol/L to 9.6 mmol/L in the BHI 30 plus OAD(s) group (Table 2).
FIG. 1.
Overall improvements of glycosylated hemoglobin (HbA1c), fasting plasma glucose (FPG), and 2-h postprandial plasma glucose (2hPG) from baseline to end point were all significant using Wilcoxon's test (paired samples). All P<0.0001. Data are median (interquartile range) values.
FIG. 2.
The distribution of the frequencies of the different glycosylated hemoglobin (HbA1c) levels of all the patients was compared using a nonparametric χ2 test and found to be different from baseline to end point (P<0.0001).
Table 2.
Changes in Glucose Parameters, Body Weight, and Daily Cost from Baseline to the End Point with Two Different Treatments
| Baseline | End point | Change | P valuea | |
|---|---|---|---|---|
| BHI 30-monotherapy (n=409) | ||||
| HbA1c (%) | 9.1 (8.1, 10.3) | 7.0 (6.6, 7.4) | −2.0 (−3.3, −1.2) | <0.0001 |
| FPG (mmol/L) | 9.0 (7.9, 11.1) | 6.7 (5.9, 7.4) | −2.5 (−4.3, −1.2) | <0.0001 |
| 2hPG (mmol/L) | 14.3 (11.5, 18.0) | 8.6 (7.9, 10.1) | −5.2 (−8.5, −2.6) | <0.0001 |
| HbA1c [n (%)] | ||||
| ≥9.0% | 213 (52.1) | 12 (2.9) | NA | <0.0001 |
| <7.0% | NA | 196 (47.9) | NA | NA |
| <6.5% | NA | 70 (17.1) | NA | NA |
| Weight (kg) | 65.0 (60.0, 74.0) | 66.0 (60.0, 74.0) | 0.2 (−0.5, 1.0) | 0.004 |
| Daily cost of therapy (RMB) | 11.3 (7.1, 15.9) | 6.3 (5.0, 8.0) | −4.5 (−9.4, 0.1) | <0.0001 |
| Daily BHI 30 dose (IU/day) | NA | 30.0 (24.0, 38.0) | NA | NA |
| BHI 30 plus OAD(s) (n=235) | ||||
| HbA1c (%) | 9.5 (8.4, 10.5) | 7.3 (6.8, 7.9) | −2.0 (−3.4, −1.3) | <0.0001 |
| FPG (mmol/L) | 10.0 (8.6, 12.5) | 7.1 (6.3, 8.1) | −2.7 (−4.8, −1.4) | <0.0001 |
| 2hPG (mmol/L) | 15.3 (12.5, 18.0) | 9.6 (8.2, 10.8) | −5.2 (−8.0, −2.8) | <0.0001 |
| HbA1c [n (%)] | ||||
| ≥9.0% | 143 (60.9) | 19 (8.1) | NA | 0.002 |
| <7.0% | NA | 83 (35.3) | NA | NA |
| <6.5% | NA | 28 (11.9) | NA | NA |
| Weight (kg) | 66.0 (60.0, 73.0) | 66.0 (60.0, 73.0) | 0.0 (−1.0, 1.0) | 0.485 |
| Daily cost of therapy (RMB) | 11.0 (7.2, 14.9) | 12.2 (8.5, 15.8) | 2.2 (−4.2, 7.3) | 0.002 |
| Daily BHI 30 dose (IU/day) | NA | 30.0 (24.0, 38.0) | NA | NA |
Data are presented as median (interquartile range) for non-normally distributed variables and n (%) for categorical variables, unless specified otherwise.
P value for the difference between baseline and end point tested by Wilcoxon signed ranks test.
BHI, biphasic human insulin 30; FPG, fasting plasma glucose; HbA1c, glycosylated hemoglobin; 2hPG, 2-h postprandial plasma glucose; NA, not applicable; OAD, oral antidiabetes drug.
The overall percentage of patients with HbA1c <9.0% at baseline who had reached the HbA1c <7.0% target was greater than those with HbA1c ≥9.0% (56.6 vs. 31.2%, P<0.0001). The probability that patients that would reach the HbA1c <7.0% target was 1.7 times higher in those with HbA1c <9.0% at baseline compared with those with HbA1c ≥9.0% (odds ratio, 2.70; 95% confidence interval, 1.96–3.72; P<0.0001).
There was no significant difference in the median daily BHI 30 dosage (30.0 IU vs. 30.0 IU, P=0.304) between the BHI 30 monotherapy group and the BHI plus OAD(s) group at the end of the study.
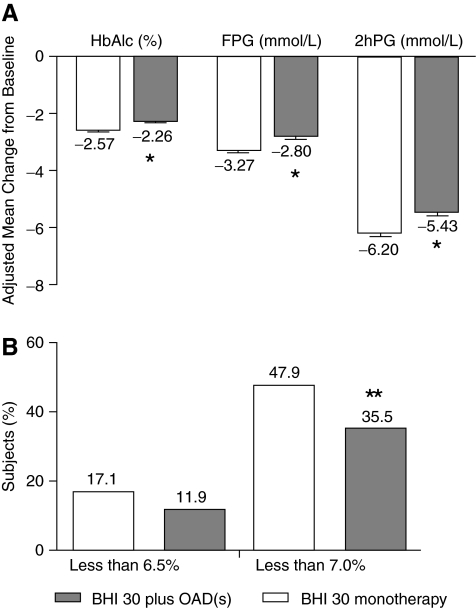
Comparison in glucose improvements between the BHI 30 monotherapy and BHI 30 plus OAD(s) groups was shown in Figure 3. After adjustment by the baseline level of HbA1c, FPG, 2hPG, age, sex, diabetes duration, and body mass index, from the beginning to the end of the study, the changes in HbA1c (mean±SE, −2.57±0.04% vs. −2.26±0.06%), FPG (mean±SE, −3.27±0.06 vs. −2.80±0.08 mmol/L), and 2hPG (mean±SE, −6.20±0.10 vs. −5.43±0.13 mmol/L) were significantly larger in the BHI monotherapy group than those in the group administered BHI plus OAD(s) (all P<0.0001). More patients achieved the HbA1c <7.0% target level in the BHI 30 monotherapy group than in the BHI 30 plus OAD(s) group (47.9% vs. 35.3%, P=0.002), despite the similar total daily BHI 30 dosage at the end of the study. However, there was no difference in the proportion of subjects who achieved HbA1c <6.5% between the two groups (17.1% vs. 11.9%, P=0.077).
FIG. 3.
(A) Comparison of mean changes in glycemic indices from baseline to end point between the two treatment groups. After adjustment for age, sex, baseline glycosylated hemoglobin (HbA1c), fasting plasma glucose (FPG), 2-h postprandial plasma glucose (2hPG), and body mass index, mean changes of HbA1c, FPG, and 2hPG were significantly different in the biphasic human insulin 30 (BHI 30) monotherapy group compared with the BHI 30 plus oral antidiabetes drug(s) [OAD(s)] group. Data are mean±SE values. (B) At the end of the study, there was a greater percentage of subjects achieving the HbA1c target of<7.0% in the BHI 30 monotherapy group than in the BHI plus OAD(s) group. There was no significant difference in the percentage of subjects who achieved HbA1c of <6.5%. *P<0.0001, **P<0.01.
Cost-effectiveness
Compared with the total daily costs of more than two OADs administered before the study, the overall daily hypoglycemic drug cost per person was reduced by 2.8 yuan (from 11.9 yuan to 9.0 yuan, P<0.0001) at the end of the study. In the BHI 30 monotherapy group, the median costs per person per day decreased by 39.8% (from 11.3 to 6.3 yuan, P<0.0001), but they increased by 20.0% (from 11.0 to 12.2 yuan, P=0.002) in the BHI 30 plus OAD(s) group simultaneously. After adjustment by age, diabetes duration, baseline HbA1c, and weight at the end point, the daily cost to reduce per 1% HbA1c was less in the BHI 30 monotherapy group than in the BHI 30 plus OAD(s) group (mean±SE, 4.0±0.6 yuan vs. 9.8±0.9 yuan, P<0.0001.)
Safety
One hundred eighteen of the 409 patients (28.8%) receiving BHI 30 monotherapy and 78 of the 235 patients (23.5%) receiving BHI 30 plus OAD(s) experienced at least one hypoglycemic event (P=0.717). During the 16-week study, the rate of minor hypoglycemic events per patient per year was 2.77 in the BHI 30 monotherapy group and 1.06 in the BHI 30 plus OAD(s) group (P<0.0001). The rate of hypoglycemic events with symptoms only (without confirmed blood glucose reading) was 0.78 per patient per year in both treatment groups. Major events occurred only twice in the BHI 30 monotherapy group.
Body weight changes
At the end of the study, the body weight increased by 0.2 kg in the BHI 30 monotherapy group (P=0.004) but did not change in the BHI 30 plus OAD(s) group (P=0.485) (Table 2). The percentage of the patients losing weight was higher in the BHI 30 plus OAD(s) group than in the BHI 30 monotherapy group (32.8% vs. 25.2%, P=0.039).
Discussion
This 4-month study showed that a twice-daily injection of BHI 30 either with or without OAD(s) was effective in achieving optimal glycemic control in those type 2 diabetes patients who had previously failed in oral antidiabetes therapy. Using changes from the baseline as a reference, the glycemic control was worse in those patients administered both BHI 30 and at least one OAD than in those receiving only BHI 30. Our results also showed that achieving the target glycemic level was more difficult in those subjects with poorer glycemic control at baseline.
At the end of our study, the median HbA1c of the BHI 30 monotherapy group was reduced by 2.0% (from 9.1% to 7.0%), and about 47.9% of subjects reached HbA1c <7.0% with median daily insulin dosage of 30.0 IU per person. Although our results for the reduction of HbA1c and the percentage of the patients who achieved HbA1c level ≤7% looked better than the results reported by Janka et al.,13 there were several differences between these two studies. The baseline weight was heavier (85.1 kg) in the previous study than in ours (66.9 kg). Therefore, at the end of the study, the daily insulin dose was more in the study of Janka et al.13 (64.5 IU/day) than in ours (31.7 IU/day). Because the level of baseline HbA1c in the previous study (8.8%) was lower than that in ours (9.5%), at the end of the study the reduction in HbA1c was less in the study of Janka et al.13 (−1.31%) than in ours (−2.0%), and the percentage of the patients who reached the target of HbA1c <7.0% was lower in the previous study (39%) than in ours (47.9%). All these differences were explainable according to the characteristics of the patients in the two studies. Despite all the difference between the two studies, we can still draw the conclusion that the HbA1c can be improved after initiating biphasic human insulin therapy. Our data also showed that about 35.3% of subjects reached the glycemic target of HbA1c <7.0% in the BHI 30 plus OAD(s) group, with a 2.0% reduction (from 9.5% to 7.3%) of median HbA1c (median daily insulin dosage=30.0 IU per person). This observation approached the results reported by Schwartz et al.,14 who found the HbA1c reduction was 1.96% and that 32% of subjects achieved the same target after treatment with BHI 30 (62.98 IU per day per person) plus MET for 24 weeks.
Body weight gain is one of the major side effects of insulin therapy. We observed only a slight body weight gain in those subjects treated with BHI 30 monotherapy (change in body weight=0.2 kg, P=0.004). In the group receiving both BHI 30 and OAD(s), body weights did not significantly change. The percentage of patients losing weight was higher in the BHI 30 plus OAD(s) group than in the BHI 30 monotherapy group (32.8% vs. 25.2%, P=0.039). In clinical practice, MET is one of the most widely used regimens in both obese15 and nonobese16 type 2 diabetes patients as a monotherapy or in combination with insulin, because of its ability to ameliorate insulin resistance16 and the potential effect of weight loss. However, some studies have documented that MET induced weight loss only when its daily dosage reached 2,000–3,000 mg.16,17 In our study, the body weight change in those patients receiving a mean MET dosage of 1,260±408 mg/day did not reach statistical significance. Besides the pharmacodynamics of MET, the synergistic effects of delayed gastric emptying and appetite inhibition caused by combined AGI and MET may also have offset the weight gain of insulin.
Drug cost is an important factor in treatment decision. When a patient is treated with two or more classes of OADs, the side effects, complex drug titration, and drug costs should be considered. In our study, the overall median daily costs of drugs decreased by 2.7 yuan (from 11.2 yuan to 7.6 yuan, P<0.0001, data not shown). Of all 644 patients, 63.5% receiving BHI 30 alone saved 45% in daily costs compared with the prior treatment, but the remaining 36.5% of patients in the BHI 30 plus OAD(s) group spent an extra 16% in daily costs. The results of the 4T Study showed that the insulin requirements increased during the first 3 years of insulin introduction, and therefore daily cost may increase.18 In the present study, although the daily cost of insulin in the BHI 30 monotherapy group decreased during the short follow-up period, the antidiabetes medicine expense may increase with the increment of insulin dosage in the future.
The unpredictable nature of hypoglycemia and its consequences remain the most undesirable side effect of the treatment with insulin.19 The fear of hypoglycemia remains a serious problem for both patients and physicians when initiating insulin therapy. It has been reported by Janka et al.13 that the probabilities of overall and symptomatic hypoglycemic events induced by BHI 30 were 9.87 and 5.73 occurrences per patient per year, respectively, and the rate of severe hypoglycemic events was 0.05 per patient per year with the mean daily dose of 64.5 IU of insulin. In our study, the frequency of the hypoglycemic events was lower than in the study of Janka et al.13 because of the lower daily insulin dosage. Although the percentage of the patients with hypoglycemic events between the two groups was not different, the rate of minor hypoglycemic episodes (2.77 vs. 1.06 per person per year, P<0.0001) was higher in the BHI 30 monotherapy group than in the BHI 30 plus OAD(s) group. The higher percentage of patients attaining the HbA1c <7.0% target may be the major reason of this result. The duration of insulin treatment is a key predictor of hypoglycemia in insulin-treated type 2 diabetes as reported by Donnelly et al.19 (odds ratio, 1.06, 95% confidence interval, 1.01–1.11; P=0.014). Therefore, a long-term investigation is required to assess the risk of hypoglycemia events.
Because the present study was a short-term follow-up clinical study, the effects of long-term glycemic control, chronic complication prevention, and quality of life in those administered BHI 30 should be further evaluated. In summary, a twice-daily regimen of BHI 30, either with or without OAD(s), appeared to be an effective therapy for insulin-naive subjects with type 2 diabetes for whom two or more OADs had failed. With higher baseline glucose level, the BHI 30 plus OAD(s) group had lower cost-effectiveness than the BHI 30 monotherapy group despite the lower frequency of hypoglycemia.
Acknowledgments
The authors are very grateful to researchers from multiple hospitals in Shanghai, who helped in this study. This work was funded by The National 973 Program (grant 2011CB504001), the major program of Shanghai Municipality for Basic Research (grant 08dj1400601), and the Key Project of Science and Technology of Shanghai (grant 09DZ1950202).
Author Disclosure Statement
All authors have nothing to declare. No competing financial interests exist.
References
- 1.MacIsaac R. Cheung A. Jerums G. Type 2 diabetes—controlling hyperglycaemia with early insulin use. Aust Fam Physician. 2010;39:565–569. [PubMed] [Google Scholar]
- 2.Brussels: International Diabetes Federation; 2005. Force ICGT: Global Guideline for Type 2 Diabetes. [Google Scholar]
- 3.A desktop guide to Type 2 diabetes mellitus. European Diabetes Policy Group 1999. Diabet Med. 1999;16:716–730. [PubMed] [Google Scholar]
- 4.Hauner H. Köster I. von Ferber L. Prevalence of diabetes mellitus in Germany 1998–2001. Secondary data analysis of a health insurance sample of the AOK in Hesse/KV in Hesse. Dtsch Med Wochenschr. 2003;128:2632–2637. doi: 10.1055/s-2003-812396. [DOI] [PubMed] [Google Scholar]
- 5.Guler S. Sharma SK. Almustafa M. Kim CH. Azar S. Danciulescu R. Shestakova M. Khutsoane D. Bech OM. Improved glycaemic control with biphasic insulin aspart 30 in type 2 diabetes patients failing oral antidiabetic drugs: PRESENT study results. Arch Drug Inf. 2009;2:23–33. doi: 10.1111/j.1753-5174.2008.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hermansen K. Colombo M. Storgaard H. Østergaard A. Kølendorf K. Madsbad S. Improved postprandial glycemic control with biphasic insulin aspart relative to biphasic insulin lispro and biphasic human insulin in patients with type 2 diabetes. Diabetes Care. 2002;25:883–888. doi: 10.2337/diacare.25.5.883. [DOI] [PubMed] [Google Scholar]
- 7.Boehm BO. Home PD. Behrend C. Kamp NM. Lindholm A. Premixed insulin aspart 30 vs. premixed human insulin 30/70 twice daily: a randomized trial in Type 1 and Type 2 diabetic patients. Diabet Med. 2002;19:393–399. doi: 10.1046/j.1464-5491.2002.00733.x. [DOI] [PubMed] [Google Scholar]
- 8.Garber AJ. Ligthelm R. Christiansen JS. Liebl A. Premixed insulin treatment for type 2 diabetes: analogue or human? Diabetes Obes Metab. 2007;9:630–639. doi: 10.1111/j.1463-1326.2006.00654.x. [DOI] [PubMed] [Google Scholar]
- 9.Riddle MC. Combined therapy with insulin plus oral agents: is there any advantage? An argument in favor. Diabetes Care. 2008;31(Suppl 2):S125–S130. doi: 10.2337/dc08-s231. [DOI] [PubMed] [Google Scholar]
- 10.American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2005;28(Suppl 1):S4–S36. [PubMed] [Google Scholar]
- 11.IDF Clinical Guidelines Task Force: Global guideline for type 2 diabetes: recommendations for standard, comprehensive, and minimal care. Diabet Med. 2006;23:579–593. doi: 10.1111/j.1464-5491.2006.01918.x. [DOI] [PubMed] [Google Scholar]
- 12.Raskin P. Allen E. Hollander P. Lewin A. Gabbay RA. Hu P. Bode B. Garber A. Initiating insulin therapy in type 2 diabetes: a comparison of biphasic and basal insulin analogs. Diabetes Care. 2005;28:260–265. doi: 10.2337/diacare.28.2.260. [DOI] [PubMed] [Google Scholar]
- 13.Janka HU. Plewe G. Riddle MC. Kliebe-Frisch C. Schweitzer MA. Yki-Jarvinen H. Comparison of basal insulin added to oral agents versus twice-daily premixed insulin as initial insulin therapy for type 2 diabetes. Diabetes Care. 2005;28:254–259. doi: 10.2337/diacare.28.2.254. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz S. Sievers R. Strange P. Lyness WH. Hollander P. Insulin 70/30 mix plus metformin versus triple oral therapy in the treatment of type 2 diabetes after failure of two oral drugs: efficacy, safety, and cost analysis. Diabetes Care. 2003;26:2238–2243. doi: 10.2337/diacare.26.8.2238. [DOI] [PubMed] [Google Scholar]
- 15.Wright A. Burden AC. Paisey RB. Cull CA. Holman RR. Sulfonylurea inadequacy: efficacy of addition of insulin over 6 years in patients with type 2 diabetes in the U.K. Prospective Diabetes Study (UKPDS 57) Diabetes Care. 2002;25:330–336. doi: 10.2337/diacare.25.2.330. [DOI] [PubMed] [Google Scholar]
- 16.Lund SS. Tarnow L. Frandsen M. Nielsen BB. Hansen BV. Pedersen O. Parving HH. Vaag AA. Combining insulin with metformin or an insulin secretagogue in non-obese patients with type 2 diabetes: 12 month, randomised, double blind trial. BMJ. 2009;339:b4324. doi: 10.1136/bmj.b4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siraj ES. Is there a role for metformin or acarbose as a weight-loss agent in the absence of diabetes? Cleve Clin J Med. 2003;70:702–704. doi: 10.3949/ccjm.70.8.702. [DOI] [PubMed] [Google Scholar]
- 18.Holman RR. Farmer AJ. Davies MJ. Levy JC. Darbyshire JL. Keenan JF. Paul SK. Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med. 2009;361:1736–1747. doi: 10.1056/NEJMoa0905479. [DOI] [PubMed] [Google Scholar]
- 19.Donnelly LA. Morris AD. Frier BM. Ellis JD. Donnan PT. Durrant R. Band MM. Reekie G. Leese GP. Frequency and predictors of hypoglycaemia in Type 1 and insulin-treated Type 2 diabetes: a population-based study. Diabet Med. 2005;22:749–755. doi: 10.1111/j.1464-5491.2005.01501.x. [DOI] [PubMed] [Google Scholar]