Abstract
Erythropoietin is a cytokine which specifically regulates the proliferation and differentiation of erythroid progenitor cells. The expression of erythropoietin receptor on the cell membrane of the progenitor cells is a critical event during the erythroid differentiation process. In order to clarify the tissue-specific and differentiation stage-specific expression of the erythropoietin receptor gene, its transcriptional regulation was examined by transient expression assay, gel mobility shift assay and DNase I footprinting. The results clearly showed that GATA-1 transactivates the gene expression through a single GATA motif located around -200 bp upstream from the ATG codon in a dose dependent manner. Furthermore, Northern blot analysis revealed that erythropoietin receptor-mediated signals strongly enhanced GATA-1 gene expression in accordance with the appearance of hemoglobin-positive cells. Taken together with other observations, these results suggested the following scheme of erythroid differentiation: 1)GATA-1 is expressed in the early stage of blood cell development; 2) GATA-1 transactivates the erythropoietin receptor gene; 3) erythropoietin binds its receptor and the receptor-mediated signals enhance GATA-1 gene expression in erythroid progenitor cells; and 4) GATA-1 finally transactivates hemoglobin synthesis-related genes and globin genes in relatively matured erythroid cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brady H. J., Sowden J. C., Edwards M., Lowe N., Butterworth P. H. Multiple GF-1 binding sites flank the erythroid specific transcription unit of the human carbonic anhydrase I gene. FEBS Lett. 1989 Nov 6;257(2):451–456. doi: 10.1016/0014-5793(89)81594-7. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- D'Andrea A. D., Lodish H. F., Wong G. G. Expression cloning of the murine erythropoietin receptor. Cell. 1989 Apr 21;57(2):277–285. doi: 10.1016/0092-8674(89)90965-3. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T., Felsenfeld G. The erythroid-specific transcription factor Eryf1: a new finger protein. Cell. 1989 Sep 8;58(5):877–885. doi: 10.1016/0092-8674(89)90940-9. [DOI] [PubMed] [Google Scholar]
- Frampton J., Walker M., Plumb M., Harrison P. R. Synergy between the NF-E1 erythroid-specific transcription factor and the CACCC factor in the erythroid-specific promoter of the human porphobilinogen deaminase gene. Mol Cell Biol. 1990 Jul;10(7):3838–3842. doi: 10.1128/mcb.10.7.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser J. K., Lin F. K., Berridge M. V. Expression of high affinity receptors for erythropoietin on human bone marrow cells and on the human erythroleukemic cell line, HEL. Exp Hematol. 1988 Nov;16(10):836–842. [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramochi S., Ikawa Y., Todokoro K. Characterization of murine erythropoietin receptor genes. J Mol Biol. 1990 Dec 5;216(3):567–575. doi: 10.1016/0022-2836(90)90384-x. [DOI] [PubMed] [Google Scholar]
- Kuramochi S., Sugimoto Y., Ikawa Y., Todokoro K. Transmembrane signaling during erythropoietin- and dimethylsulfoxide-induced erythroid cell differentiation. Eur J Biochem. 1990 Oct 5;193(1):163–168. doi: 10.1111/j.1432-1033.1990.tb19318.x. [DOI] [PubMed] [Google Scholar]
- Martin D. I., Tsai S. F., Orkin S. H. Increased gamma-globin expression in a nondeletion HPFH mediated by an erythroid-specific DNA-binding factor. Nature. 1989 Mar 30;338(6214):435–438. doi: 10.1038/338435a0. [DOI] [PubMed] [Google Scholar]
- Martin D. I., Zon L. I., Mutter G., Orkin S. H. Expression of an erythroid transcription factor in megakaryocytic and mast cell lineages. Nature. 1990 Mar 29;344(6265):444–447. doi: 10.1038/344444a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mignotte V., Eleouet J. F., Raich N., Romeo P. H. Cis- and trans-acting elements involved in the regulation of the erythroid promoter of the human porphobilinogen deaminase gene. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6548–6552. doi: 10.1073/pnas.86.17.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin S. H. Globin gene regulation and switching: circa 1990. Cell. 1990 Nov 16;63(4):665–672. doi: 10.1016/0092-8674(90)90133-y. [DOI] [PubMed] [Google Scholar]
- Pevny L., Simon M. C., Robertson E., Klein W. H., Tsai S. F., D'Agati V., Orkin S. H., Costantini F. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature. 1991 Jan 17;349(6306):257–260. doi: 10.1038/349257a0. [DOI] [PubMed] [Google Scholar]
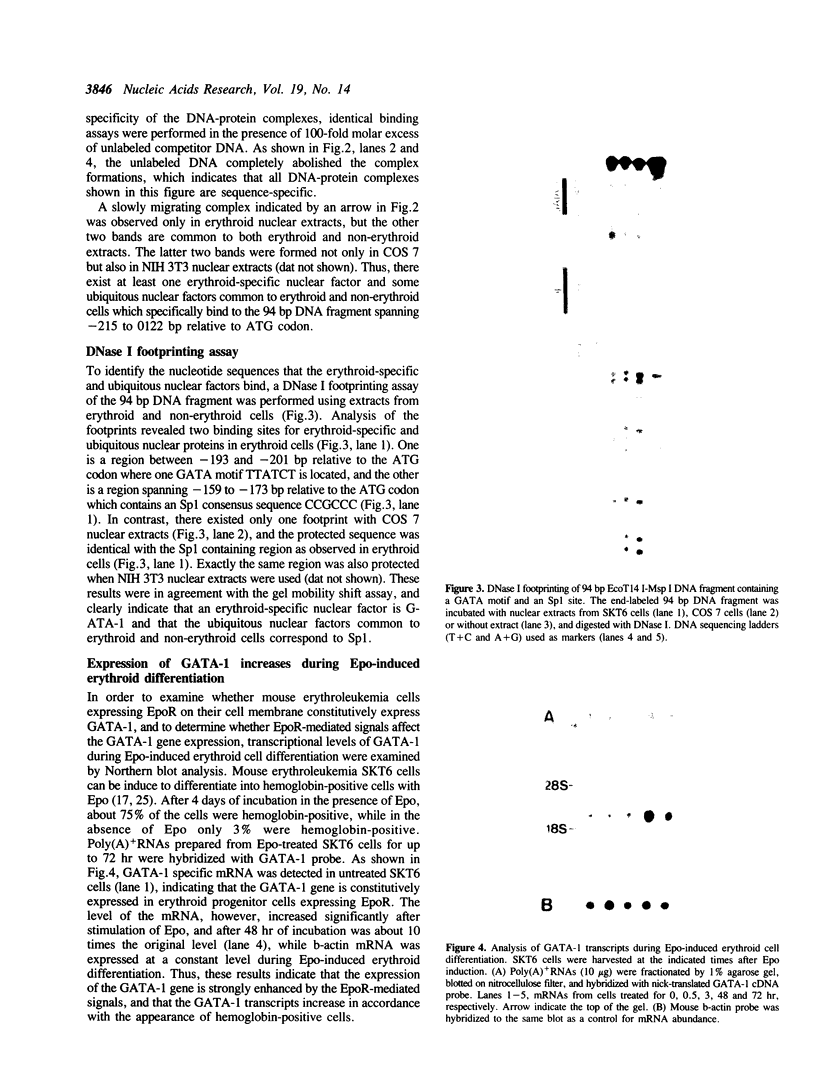
- Romeo P. H., Prandini M. H., Joulin V., Mignotte V., Prenant M., Vainchenker W., Marguerie G., Uzan G. Megakaryocytic and erythrocytic lineages share specific transcription factors. Nature. 1990 Mar 29;344(6265):447–449. doi: 10.1038/344447a0. [DOI] [PubMed] [Google Scholar]
- Takebe Y., Seiki M., Fujisawa J., Hoy P., Yokota K., Arai K., Yoshida M., Arai N. SR alpha promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol Cell Biol. 1988 Jan;8(1):466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todokoro K., Kanazawa S., Amanuma H., Ikawa Y. Specific binding of erythropoietin to its receptor on responsive mouse erythroleukemia cells. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4126–4130. doi: 10.1073/pnas.84.12.4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todokoro K., Watson R. J., Higo H., Amanuma H., Kuramochi S., Yanagisawa H., Ikawa Y. Down-regulation of c-myb gene expression is a prerequisite for erythropoietin-induced erythroid differentiation. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8900–8904. doi: 10.1073/pnas.85.23.8900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor C. D., Evans T., Felsenfeld G., Boguski M. S. Structure and evolution of a human erythroid transcription factor. Nature. 1990 Jan 4;343(6253):92–96. doi: 10.1038/343092a0. [DOI] [PubMed] [Google Scholar]
- Tsai S. F., Martin D. I., Zon L. I., D'Andrea A. D., Wong G. G., Orkin S. H. Cloning of cDNA for the major DNA-binding protein of the erythroid lineage through expression in mammalian cells. Nature. 1989 Jun 8;339(6224):446–451. doi: 10.1038/339446a0. [DOI] [PubMed] [Google Scholar]
- Wall L., deBoer E., Grosveld F. The human beta-globin gene 3' enhancer contains multiple binding sites for an erythroid-specific protein. Genes Dev. 1988 Sep;2(9):1089–1100. doi: 10.1101/gad.2.9.1089. [DOI] [PubMed] [Google Scholar]
- Wilson D. B., Dorfman D. M., Orkin S. H. A nonerythroid GATA-binding protein is required for function of the human preproendothelin-1 promoter in endothelial cells. Mol Cell Biol. 1990 Sep;10(9):4854–4862. doi: 10.1128/mcb.10.9.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Ko L. J., Leonard M. W., Beug H., Orkin S. H., Engel J. D. Activity and tissue-specific expression of the transcription factor NF-E1 multigene family. Genes Dev. 1990 Oct;4(10):1650–1662. doi: 10.1101/gad.4.10.1650. [DOI] [PubMed] [Google Scholar]
- Youssoufian H., Zon L. I., Orkin S. H., D'Andrea A. D., Lodish H. F. Structure and transcription of the mouse erythropoietin receptor gene. Mol Cell Biol. 1990 Jul;10(7):3675–3682. doi: 10.1128/mcb.10.7.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zon L. I., Tsai S. F., Burgess S., Matsudaira P., Bruns G. A., Orkin S. H. The major human erythroid DNA-binding protein (GF-1): primary sequence and localization of the gene to the X chromosome. Proc Natl Acad Sci U S A. 1990 Jan;87(2):668–672. doi: 10.1073/pnas.87.2.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deBoer E., Antoniou M., Mignotte V., Wall L., Grosveld F. The human beta-globin promoter; nuclear protein factors and erythroid specific induction of transcription. EMBO J. 1988 Dec 20;7(13):4203–4212. doi: 10.1002/j.1460-2075.1988.tb03317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]