Abstract
Background
The effects of dexmedetomidine on the propofol-sparing effect and intraoperative hemodynamics during remifentanil-based propofol-supplemented anesthesia have not been well investigated.
Methods
Twenty patients undergoing breast surgery were randomly allocated to receive dexmedetomidine (group DEX) or placebo (group C). In the DEX group, dexmedetomidine was loaded (1 µg/kg) before anesthesia induction and was infused (0.6 µg/kg/h) during surgery. Anesthesia was induced with a target-controlled infusion (TCI) of propofol (effect site concentration, Ce; 3 µg/ml) and remifentanil (plasma concentration, Cp, 10 ng/ml). The Ce of TCI-propofol was adjusted to a bispectral index of 45-55, and Cp of TCI-remifentanil was fixed at 10 ng/ml in both groups. Mean arterial blood pressure (MAP) and heart rate (HR) were recorded at baseline (T-control), after the loading of study drugs (T-loading), 3 min after anesthesia induction (T-induction), tracheal intubation (T-trachea), incision (T-incision), 30 min after incision (T-incision30), and at tracheal extubation (T-extubation). MAP% and HR% (MAP and HR vs. T-control) were determined and the propofol infusion rate was calculated.
Results
The propofol infusion rate was significantly lower in the DEX group than in group C (63.9 ± 16.2 vs. 96.4 ± 10.0 µg/kg/min, respectively; P < 0.001). The changes in MAP% at T-induction, T-trachea and T-incision in group DEX (-10.0 ± 3.9%, -9.4 ± 4.6% and -11.2 ± 6.3%, respectively) were significantly less than those in group C (-27.6 ± 13.9%, -21.7 ± 17.1%, and -25.1 ± 14.1%; P < 0.05, respectively).
Conclusions
Dexmedetomidine reduced the propofol requirement for remifentanil-based anesthesia while producing more stable intraoperative hemodynamics.
Keywords: Dexmedetomidine, Propofol, Remifentanil
Introduction
Dexmedetomidine (DEX), a highly selective α2-adrenoreceptor agonist, is used for sedation management in various clinical settings and shows an anesthetic-sparing effect [1-5]. DEX reduces the propofol requirement in remifentanil-based anesthesia for faster postoperative recovery and more stable intraoperative hemodynamics [6-8], but the possible propofol-sparing effect during remifentanil-based anesthesia has not been well investigated.
DEX has complex vasodilative and vasoconstrictive hemodynamic effects specific to its activation of pre- and post-synaptic α2-receptors. These effects are dose-dependent and biphasic: vasodilation at lower dosages, vasoconstriction at higher dosages and an initial short-term increase in blood pressure (BP) followed by a longer lasting reduction in BP and heart rate (HR). Several investigations have identified the cardiovascular effects of DEX in various clinical settings [1,9-11], however its effect on intraoperative hemodynamics during a propofol-supplemented remifentanil-based anesthesia regimen, which produces a strong vasodilatory effect, has not been investigated.
We conducted this study to determine whether DEX affects the requirement for propofol and to describe the intraoperative hemodynamics during remifentanil-based propofol-supplemented anesthesia for breast surgery.
Materials and Methods
Study population
After obtaining approval from the institutional review board and written informed consent from all patients, only American Society of Anesthesiologists physical status I female patients undergoing elective breast surgery were prospectively investigated.
Patient exclusion criteria were: 1) patient age < 18 or > 80 years, 2) preoperative hypotension (Mean arterial blood pressure < 60 mmHg), 3) preoperative bradycardia (Heart rate < 45 beats/min), and 4) preoperative dysrrhythmia.
Using the sealed envelope method, 20 patients were randomly allocated into a DEX group (n = 10) or normal saline group (placebo, group C, n = 10) before anesthesia induction.
Study drugs (DEX or normal saline) in 50 ml syringes were prepared by a pharmacist and anesthesiologists were blinded to the syringe contents.
Anesthetic regimens and study drug administration
Invasive arterial BP monitoring in the radial artery contralateral to the surgical site and routine non-invasive patient monitoring, including pulse oximetry, electrocardiography and bispectral index (BIS) were established upon the patient's arrival in the operating room. In group DEX, DEX (1 µg/kg) was loaded intravenously for 10 min before anesthesia induction and was continuously infused at 0.6 µg/kg/h until the end of surgery. The same volume of normal saline was administered in the same manner to group C.
After the intravenous administration of 0.075 mg of palonosetron, a target-controlled infusion (TCI) of propofol with an effect-site concentration (Ce) of 3 µg/ml and a TCI of remifentanil with a plasma concentration (Cp) of 10 ng/ml were started to induce anesthesia. Bolus rocuronium (0.6 mg/kg) was administered to facilitate tracheal intubation in both groups. After tracheal intubation, volume-controlled ventilation with an air/O2 mixture (FiO2, 0.3-0.4) was followed with a tidal volume of 7 ml/ideal body weight and a respiratory rate to maintain end-tidal CO2 (EtCO2) at 35-40 mmHg with an I : E ratio of 1 : 2. The Cp of TCI-remifentanil was fixed at 10 ng/ml, and the Ce of TCI-propofol was reduced to the minimum dosage needed to maintain a BIS of 45-55. Additional rocuronium (0.1 mg/kg) was administered under the guidance of peripheral neuromuscular monitoring in both groups.
Intravenous phenylnephrine (50-100 µg) was administered if MAP dropped to < 60 mmHg. A HR of < 45 beats/min was treated with intravenous administration of 0.2 mg of glycopyrrolate or 0.5 mg of atropine. Patients who were administered atropine were excluded from the study.
During suturing of the subcutaneous tissue at the operation site, 1.0 µg/kg of fentanyl and 30 mg of ketorolac were administered intravenously and the anesthetics were stopped. Tracheal extubation was performed after confirming sufficient recovery (TOF ratio > 95%; BIS > 80, ability to open the eyes, ability to obey anesthesiologist's verbal commands and ability to maintain a regular breathing pattern) and patients were then transferred to a post-anesthesia care unit.
Data measurements
Operation time, extubation time (the time from stopping the administration of anesthetic agents to tracheal extubation) and the total volume of intraoperative intravascular fluids administered were recorded. The total doses of remifentanil, propofol and DEX were recorded, and their mean infusion rates were calculated.
To stabilize the patients' vital signs, monitoring was performed 5 min after arrival in operation theater. MAP and HR were then measured after the 5 min (T-control), after the loading of the study drugs (T-loading), 3 min after the start of TCI-propofol and remifentanil (T-induction), at tracheal intubation (T-trachea), at surgical incision (T-incision), at 30 min after surgical incision (T-incision30) and at tracheal extubation (T-extubation). The lowest values of MAP and HR for a 3 min observation were recorded as baseline values at T-control and the MAP and HR values with the greatest deviation from baseline during 3 min observations at T-trachea, T-incision, T-incision30 and T-extubation were recorded.
The percentile data (%) of these values versus T-control values and the changes in MAP% and HR% at each time point from those at T-control (ΔMAP% and ΔHR%) were determined.
Statistical analysis
A sample size of 10 in each group was determined to be appropriate for identifying a 15% difference with a power of 0.8 and an α value of 0.05, for a mean propofol infusion rate of 96.6 ± 10.6 µg/kg/min, which was determined in nine volunteer patients in a preliminary study.
Inter-group differences in the data collected at each measured time point were determined using a t-test (student t-test) and intra-group differences in MAP and HR in each group were determined by a Friedman test. Sigmastat ver.3.1 (Systat Software Inc, San Jose, CA, USA) was used for all statistical analyses and P < 0.05 was considered to indicate statistical significance.
Results
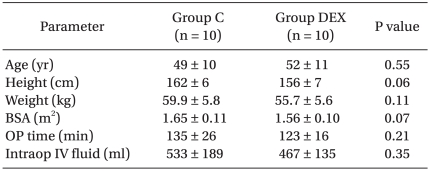
The patient demographic profiles, operation time and volume of intravascular fluids administered were not significantly different between the two groups (Table 1).
Table 1.
Demographic Data and Perioperative Parameters
The values are expressed as means ± SD. P values determined by a t-test. C: remifentanil-propofol, DEX: remifentanil-propofol-dexmedetomidine, BSA: body surface area, OP time: operation duration, Intraop: intraoperative, IV: intravenous.
No patient in either group required bolus phenylephrine, ephedrine, atropine or glycopyrrolate.
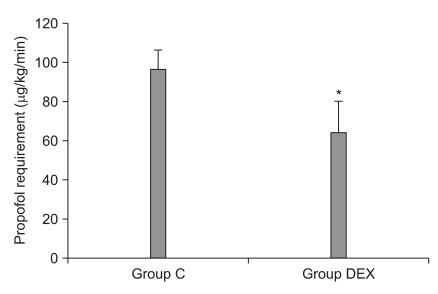
The mean infusion rate of propofol in group DEX was significant lower than that in group C (63.9 ± 16.2 vs. 96.4 ± 10.0 µg/kg/min, respectively; P < 0.001) (Fig. 1). The mean remifentanil infusion rate did not significantly differ between the two groups (0.367 ± 0.045 µg/kg/min in group C vs. 0.379 ± 0.051 µg/kg/min in the group DEX, P = 0.57).
Fig. 1.
Inter-group comparison of supplemental propofol requirements. C: remifentanil-propofol, DEX: remifentanil-propofol-dexmedetomidine. *Indicates P < 0.05 vs. group C.
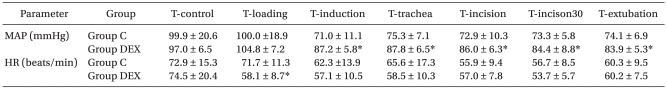
MAP and HR at T-control were not significantly different between the groups (Table 2). The MAPs at T-induction, T-incision, T-incison30 and T-extubation were significantly lower than that at T-control in group DEX (p < 0.05: Table 3). In group C, the MAPs at T-induction, T-incision, T-incision30 were significantly lower than that at T-control (Table 3). The MAPs at T-induction, T-trachea, T-incision, T-incison30 and T-extubation in group DEX were significantly higher than those in group C (P < 0.001, P < 0.001, P = 0.001, P = 0.004, and P = 0.002, respectively: Table 2).
Table 2.
Inter-group Comparisons in Mean Arterial Pressure and Heart Rate
The values are expressed as means ± SD. P values determined by a t-test. Group C: remifentanil-propofol, Group DEX: remifentanil-propofol-dexmedetomidine, MAP: mean arterial blood pressure, HR: heart rate, T-control: at the arrival to operation theater, T-loading: at after the loading of study drugs, T-induction: at 3 min after the start of anesthesia induction, T-trachea: at tracheal intubation, T-incision: at surgical incision, T-incision30: at 30 min after surgical incision, T-extubation: at tracheal extubation. *Indicates P < 0.05 vs. Group C at each measured time point.
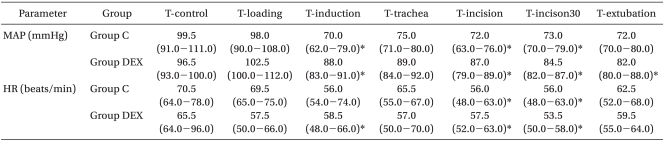
Table 3.
Intra-group Comparisons in Mean Arterial Pressure and Heart Rate
The values are expressed as median and range (25-75%) and significance was determined by a Friedman test. Group C: remifentanil-propofol, Group DEX: remifentanil-propofol-dexmedetomidine, MAP: mean arterial blood pressure, HR: heart rate, T-control: at the arrival to operation theater, T-loading: at after the loading of study drugs, T-induction: at 3 min after the start of anesthesia induction, T-trachea: at tracheal intubation, T-incision: at surgical incision, T-incision30: at 30 min after surgical incision, T-extubation: at tracheal extubation. *Indicates P < 0.05 vs. T-control in the same group.
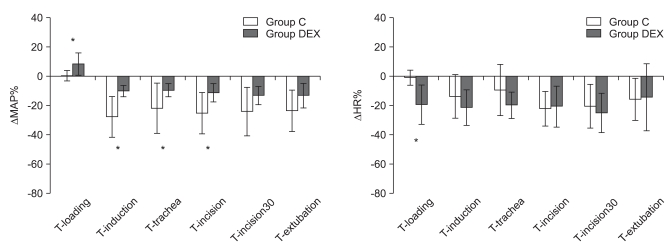
The ΔMAP% values at T-induction, T-trachea, and T-incision were significantly less in group DEX (-10.0 ± 3.9%, -9.4 ± 4.6% and -11.2 ± 6.3%, respectively) than in group C (-27.6 ± 13.9%, -21.7 ± 17.1% and -25.1 ± 14.1%; P = 0.001, P = 0.042, and P = 0.011, respectively). The ΔMAP% at T-loading was significantly greater in group DEX (8.3 ± 7.5%) than in group C (0.5 ± 3.6%; P = 0.001: Fig. 2).
Fig. 2.
Inter-group comparisons of the changes in mean arterial blood pressure and heart rate compared with control values (ΔMAP% and ΔHR%). C: remifentanil-propofol, DEX: remifentanil-propofol-dexmedetomidine, MAP: mean arterial blood pressure, HR: heart rate, T-control: at the arrival to operation theater, T-loading: at after the loading of study drugs, T-induction: at 3 min after the start of anesthesia induction, T-trachea: at tracheal intubation, T-incision: at surgical incision, T-incision30: at 30 min after surgical incision, T-extubation: at tracheal extubation. *Indicates P < 0.05 vs. group C.
The HRs at T-induction, T-incision and T-incison30 were significantly lower than that at T-control in group DEX (P < 0.05), and the HRs at T-incision and T-incision30 were significantly lower than that at T-control in group C (P < 0.05: Table 3). HR at T-loading in group DEX was significantly lower than that in group C (P = 0.008: Table 2).
The ΔHR at T-loading in group DEX (-19.2 ± 13.5%) was significantly greater than that in group C (-0.8 ± 5.2%; P < 0.05: Fig. 2).
The extubation time did not differ between the groups (11 ± 4 min in group C vs. 9 ± 3 min in group DEX, P = 0.41).
Discussion
We evaluated the effect of DEX on the requirement for supplemental propofol and described the intraoperative hemodynamic changes during remifentanil-based anesthesia. DEX reduced the amount of adjuvant propofol needed to maintain a similar BIS score by approximately 30% and provided more stable hemodynamics without compromising postoperative recovery during remifentanil-based anesthesia. These results are consistent with previous investigations showing a 30-50% reduction in the propofol requirement with concomitant use of DEX in adolescent patients and healthy volunteers [2,3]. The sedative effect of DEX is mediated through the locus ceruleus in the brain stem, where DEX decreases sympathetic outflow and increases parasympathetic outflow [4,12-14]. The different mechanisms for producing a sedative effect among DEX, propofol and remifentanil suggest a possible synergism upon combined administration with respect to their sedative effects.
Previous investigations commented on a possible delay in recovery from propofol anesthesia with the concomitant use of DEX, probably due to its quite long duration of action [3,5,11,15]. However, no compromises in prolongation of extubation time or recovery profiles were observed in the present study when employing remifentanil-based propofol-supplemented anesthesia. The reason for this result might be associated with Ce of propofol in the group DEX: the Ce of propofol at the end of surgery in group DEX (1.0-1.5 µg/ml) was relatively lower than that in group C (2.0-2.5 µg/ml) and it was already lower than the usual Ce of propofol for awakening when used alone (~1.5 µg/ml) [16]. Therefore, although DEX might induce delayed recovery or awakening in the DEX group, it may be attenuated by the low Ce of propofol at the end of surgery.
The propofol-sparing effect of DEX may be beneficial for reducing the propofol dosage and avoiding adverse effects such as myocardial depression, metabolic acidosis, impaired platelet aggregation and extended recovery caused by prolonged and large-dose administration of propofol [17-24].
DEX shows complex hemodynamic effects, as it produces not only vasodilation by activating pre-synaptic α2-receptors on sympathetic and post-synaptic α2-receptors of the central nervous system (sympatholysis), but also vasoconstriction through post-synaptic α2-receptors on vascular smooth muscle cells [25-28]. Furthermore, the overall effect of DEX on MAP and HR is biphasic and dose-dependent [14,25-27], characterized by an initial short-term increase in BP followed by a longer lasting reduction in BP and HR. Lower DEX dosages (plasma concentrations, 0.7-1.2 ng/ml) reduce norepinephrine release, resulting in an attenuation of vascular and sympathetic tone and an inhibition of sympathetic neurotransmission by activating α2A receptors [9,10,12,29]. Higher DEX dosages (i.e. plasma concentrations, > 1.9 ng/ml) produce α2B receptor-mediated vasoconstriction [9,12,14]. Despite this variability, most previous investigations have shown the cardiovascular depressive effects of DEX, which increases the incidence of hypotension and bradycardia [1,10,11]. We had assumed that DEX would show a more intense depressive effect and would increase the need for vasoactive medication during remifentanil-based anesthesia. However, we observed a significant increase in MAP immediately after loading DEX (1 µg/kg) and a more constant MAP was observed during anesthesia induction, intubation and surgical incision. A significant reduction in MAP was observed in the control group. Some reasons include: first, this result was probably attributable to the dominant hemodynamic effect of DEX through postsynaptic α2B-mediated vasoconstriction at higher dosages [28] compared with the vasodilatory effect of remifentanil at the dosages used in the present study. Second, the reduced propofol dosage owing to adjuvant DEX might have contributed to less of a propofol-induced vasodilatory effect in group DEX. This dose-dependent vasoconstrictive effect and resulting aggravation of pre-existing systemic or pulmonary hypertension should be considered.
In conclusion, DEX (1 µg/kg loading dose and infusion at 0.6 µg/kg/h) reduced the requirement for adjuvant propofol during remifentanil-based anesthesia without compromising the recovery profile, as indicated by extubation time. DEX also provided more stable intraoperative hemodynamics during remifentanil-based anesthesia.
Acknowledgements
This research was supported by Konkuk University School of Medicine.
References
- 1.Aantaa R, Kanto J, Scheinin M, Kallio A, Scheinin H. Dexmedetomidine, an alpha 2-adrenoceptor agonist, reduces anesthetic requirements for patients undergoing minor gynecologic surgery. Anesthesiology. 1990;73:230–235. doi: 10.1097/00000542-199008000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Ngwenyama NE, Anderson J, Hoernschemeyer DG, Tobias JD. Effects of dexmedetomidine on propofol and remifentanil infusion rates during total intravenous anesthesia for spine surgery in adolescents. Paediatr Anaesth. 2008;18:1190–1195. doi: 10.1111/j.1460-9592.2008.02787.x. [DOI] [PubMed] [Google Scholar]
- 3.Dutta S, Karol MD, Cohen T, Jones RM, Mant T. Effect of dexmedetomidine on propofol requirements in healthy subjects. J Pharm Sci. 2001;90:172–181. doi: 10.1002/1520-6017(200102)90:2<172::aid-jps8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 4.Khan ZP, Munday IT, Jones RM, Thornton C, Mant TG, Amin D. Effects of dexmedetomidine on isoflurane requirements in healthy volunteers. 1: Pharmacodynamic and pharmacokinetic interactions. Br J Anaesth. 1999;83:372–380. doi: 10.1093/bja/83.3.372. [DOI] [PubMed] [Google Scholar]
- 5.Bulow NM, Barbosa NV, Rocha JB. Opioid consumption in total intravenous anesthesia is reduced with dexmedetomidine: a comparative study with remifentanil in gynecologic videolaparoscopic surgery. J Clin Anesth. 2007;19:280–285. doi: 10.1016/j.jclinane.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Vuyk J, Mertens MJ, Olofsen E, Burm AG, Bovill JG. Propofol anesthesia and rational opioid selection: determination of optimal EC50-EC95 propofol-opioid concentrations that assure adequate anesthesia and a rapid return of consciousness. Anesthesiology. 1997;87:1549–1562. doi: 10.1097/00000542-199712000-00033. [DOI] [PubMed] [Google Scholar]
- 7.Glass PS, Hardman D, Kamiyama Y, Quill TJ, Marton G, Donn KH, et al. Preliminary pharmacokinetics and pharmacodynamics of an ultra-short-acting opioid: remifentanil (GI87084B) Anesth Analg. 1993;77:1031–1040. doi: 10.1213/00000539-199311000-00028. [DOI] [PubMed] [Google Scholar]
- 8.Westmoreland CL, Hoke JF, Sebel PS, Hug CC, Jr, Muir KT. Pharmacokinetics of remifentanil (GI87084B) and its major metabolite (GI90291) in patients undergoing elective inpatient surgery. Anesthesiology. 1993;79:893–903. doi: 10.1097/00000542-199311000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93:382–394. doi: 10.1097/00000542-200008000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Bloor BC, Ward DS, Belleville JP, Maze M. Effects of intravenous dexmedetomidine in humans: II. hemodynamic changes. Anesthesiology. 1992;77:1134–1142. doi: 10.1097/00000542-199212000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Tufanogullari B, White PF, Peixoto MP, Kianpour D, Lacour T, Griffin J, et al. Dexmedetomidine infusion during laparoscopic bariatric surgery: the effect on recovery outcome variables. Anesth Analg. 2008;106:1741–1748. doi: 10.1213/ane.0b013e318172c47c. [DOI] [PubMed] [Google Scholar]
- 12.Kamibayashi T, Maze M. Clinical uses of alpha2-adrenergic agonists. Anesthesiology. 2000;93:1345–1349. doi: 10.1097/00000542-200011000-00030. [DOI] [PubMed] [Google Scholar]
- 13.Virtanen R, Savola JM, Saano V, Nyman L. Characterization of the selectivity, specificity and potency of medetomidine as an [alpha]2-adrenoceptor agonist. Eur J Pharmacol. 1988;150:9–14. doi: 10.1016/0014-2999(88)90744-3. [DOI] [PubMed] [Google Scholar]
- 14.Arcangeli A, D'Alò C, Gaspari R. Dexmedetomidine use in general anaesthesia. Curr Drug Targets. 2009;10:687–695. doi: 10.2174/138945009788982423. [DOI] [PubMed] [Google Scholar]
- 15.Ohtani N, Kida K, Shoji K, Yasui Y, Masaki E. Recovery profiles from dexmedetomidine as a general anesthetic adjuvant in patients undergoing lower abdominal surgery. Anesth Analg. 2008;107:1871–1874. [Google Scholar]
- 16.Iwakiri H, Nagata O, Matsukawa T, Ozaki M, Sessler DI. Effect-site concentration of propofol for recovery of consciousness is virtually independent of fentanyl effect-site concentration. Anesth Analg. 2003;96:1651–1655. doi: 10.1213/01.ANE.0000062772.28479.2B. [DOI] [PubMed] [Google Scholar]
- 17.Kam PC, Cardone D. Propofol infusion syndrome. Anaesthesia. 2007;62:690–701. doi: 10.1111/j.1365-2044.2007.05055.x. [DOI] [PubMed] [Google Scholar]
- 18.Fourcade O, Simon MF, Litt L, Samii K, Chap H. Propofol inhibits human platelet aggregation induced by proinflammatory lipid mediators. Anesth Analg. 2004;99:393–398. doi: 10.1213/01.ANE.0000123491.08697.CA. [DOI] [PubMed] [Google Scholar]
- 19.Aoki H, Mizobe T, Nozuchi S, Hiramatsu N. In vivo and in vitro studies of the inhibitory effect of propofol on human platelet aggregation. Anesthesiology. 1998;88:362–370. doi: 10.1097/00000542-199802000-00015. [DOI] [PubMed] [Google Scholar]
- 20.De La Cruz JP, Carmona JA, Paez MV, Blanco E, Sanchez De La Cuesta F. Propofol inhibits in vitro platelet aggregation in human whole blood. Anesth Analg. 1997;84:919–921. doi: 10.1097/00000539-199704000-00040. [DOI] [PubMed] [Google Scholar]
- 21.Burow BK, Johnson ME, Packer DL. Metabolic acidosis associated with propofol in the absence of other causative factors. Anesthesiology. 2004;101:239–241. doi: 10.1097/00000542-200407000-00035. [DOI] [PubMed] [Google Scholar]
- 22.Liolios A, Guerit JM, Scholtes JL, Raftopoulos C, Hantson P. Propofol infusion syndrome associated with short-term large-dose infusion during surgical anesthesia in an adult. Anesth Analg. 2005;100:1804–1806. doi: 10.1213/01.ANE.0000153017.93666.BF. [DOI] [PubMed] [Google Scholar]
- 23.Merz TM, Regli B, Rothen HU, Felleiter P. Propofol infusion syndrome--a fatal case at a low infusion rate. Anesth Analg. 2006;103:1050. doi: 10.1213/01.ane.0000239080.82501.c7. [DOI] [PubMed] [Google Scholar]
- 24.Salengros JC, Velghe-Lenelle CE, Bollens R, Engelman E, Barvais L. Lactic acidosis during propofol-remifentanil anesthesia in an adult. Anesthesiology. 2004;101:241–243. doi: 10.1097/00000542-200407000-00036. [DOI] [PubMed] [Google Scholar]
- 25.Link RE, Desai K, Hein L, Stevens ME, Chruscinski A, Bernstein D, et al. Cardiovascular regulation in mice lacking alpha2-adrenergic receptor subtypes b and c. Science. 1996;273:803–805. doi: 10.1126/science.273.5276.803. [DOI] [PubMed] [Google Scholar]
- 26.Kobilka BK, Matsui H, Kobilka TS, Yang-Feng TL, Francke U, Caron MG, et al. Cloning, sequencing, and expression of the gene coding for the human platelet alpha 2-adrenergic receptor. Science. 1987;238:650–656. doi: 10.1126/science.2823383. [DOI] [PubMed] [Google Scholar]
- 27.Regan JW, Kobilka TS, Yang-Feng TL, Caron MG, Lefkowitz RJ, Kobilka BK. Cloning and expression of a human kidney cDNA for an alpha 2-adrenergic receptor subtype. Proc Natl Acad Sci U S A. 1988;85:6301–6305. doi: 10.1073/pnas.85.17.6301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Snapir A, Posti J, Kentala E, Koskenvuo J, Sundell J, Tuunanen H, et al. Effects of low and high plasma concentrations of dexmedetomidine on myocardial perfusion and cardiac function in healthy male subjects. Anesthesiology. 2006;105:902–910. doi: 10.1097/00000542-200611000-00010. quiz 1069-70. [DOI] [PubMed] [Google Scholar]
- 29.Lakhlani PP, MacMillan LB, Guo TZ, McCool BA, Lovinger DM, Maze M, et al. Substitution of a mutant alpha2a-adrenergic receptor via "hit and run" gene targeting reveals the role of this subtype in sedative, analgesic, and anesthetic-sparing responses in vivo. Proc Natl Acad Sci U S A. 1997;94:9950–9955. doi: 10.1073/pnas.94.18.9950. [DOI] [PMC free article] [PubMed] [Google Scholar]