Abstract
Astroglial excitability operates through increases in Ca2+cyt (cytosolic Ca2+), which can lead to glutamatergic gliotransmission. In parallel fluctuations in astrocytic Na+cyt (cytosolic Na+) control metabolic neuronal-glial signalling, most notably through stimulation of lactate production, which on release from astrocytes can be taken up and utilized by nearby neurons, a process referred to as lactate shuttle. Both gliotransmission and lactate shuttle play a role in modulation of synaptic transmission and plasticity. Consequently, we studied the role of the PMCA (plasma membrane Ca2+-ATPase), NCX (plasma membrane Na+/Ca2+ exchanger) and NKA (Na+/K+-ATPase) in complex and coordinated regulation of Ca2+cyt and Na+cyt in astrocytes at rest and upon mechanical stimulation. Our data support the notion that NKA and PMCA are the major Na+ and Ca2+ extruders in resting astrocytes. Surprisingly, the blockade of NKA or PMCA appeared less important during times of Ca2+ and Na+ cytosolic loads caused by mechanical stimulation. Unexpectedly, NCX in reverse mode appeared as a major contributor to overall Ca2+ and Na+ homoeostasis in astrocytes both at rest and when these glial cells were mechanically stimulated. In addition, NCX facilitated mechanically induced Ca2+-dependent exocytotic release of glutamate from astrocytes. These findings help better understanding of astrocyte-neuron bidirectional signalling at the tripartite synapse and/or microvasculature. We propose that NCX operating in reverse mode could be involved in fast and spatially localized Ca2+-dependent gliotransmission, that would operate in parallel to a slower and more widely distributed gliotransmission pathway that requires metabotropically controlled Ca2+ release from the ER (endoplasmic reticulum).
Keywords: astrocyte, calcium, calcium signalling, glutamate release, sodium, sodium-calcium exchanger
Abbreviations: AMPA, α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid; BAPTA-AM, 1,2-bis-(o-aminophenoxy)ethane-N,N,N′,N′-tetra-acetic acid tetrakis-acetoxymethyl ester; Ca2+cyt, cytosolic Ca2+; [Ca2+]cyt, Ca2+cyt, concentration; CNS, central nervous system; ECS, extracellular space; ER, endoplasmic reticulum; GDH, glutamate dehydrogenase; InsP3, inositol 1,4,5-trisphosphate; KB-R7943, 2-[2-[4-(4- nitrobenzyloxy)phenyl]ethyl]isothiourea methane sulfonate; LSD, least significant difference; mGluR, metabotropic glutamate receptor; Na+cyt, cytosolic Na+; [Na+]cyt, Na+cyt, concentration; NCX, Na+/Ca2+ exchanger; NCKX, K+-dependent NCX; NKA, Na+/K+-ATPase; PEI, polyethyleneimine; PMCA, plasma membrane Ca2+-ATPase; SERCA, sarcoplasmic/endoplasmic reticulum Ca2+-ATPase; TRPC, canonical transient receptor potential
INTRODUCTION
Multiple pathways are utilized for bi-directional astrocyte-neuron signalling in various regions of the CNS (central nervous system) particularly at the synaptic level (Araque et al., 1999a; Haydon and Carmignoto, 2006; Ni et al., 2007; Theodosis et al., 2008; Perea and Araque, 2009). It is at these locations that astrocytes by using their ionotropic and metabotropic receptors listen to neurotransmission. Here, activation of astrocytic receptors leads to dynamic changes in Ca2+cyt and Na+cyt (cytosolic Ca2+and cytosolic Na+) (Lalo et al., 2011). It is also at the tripartite synapse that astrocytes, via their Na+-dependent metabolic changes (Magistretti, 2006) and by utilizing their Ca2+-dependent exocytotic machinery (Parpura et al., 1995; Mothet et al., 2005; Parpura and Zorec, 2010), metabolically support and signal to neurons. Hence, the release of gliotransmitters, such as glutamate and metabolic products, such as lactate, can lead to modulation of synaptic transmission and plasticity (Araque et al., 1999a, 1999b; Perea and Araque, 2007; Suzuki et al., 2011). Thus, studying Ca2+cyt and Na+cyt dynamics is important for understanding the role of astrocytes in physiology of the CNS.
The vast majority of astrocytes in the brain grey matter, together with neurons, endothelial cells and pericytes, represent the neurovascular unit. It is at this interface with blood vessels, which dynamically change their diameter, that astrocytes undergo large morphological changes and mechanical strains associated with changes in their Ca2+cyt (Zonta et al., 2003; Filosa et al., 2004; Mulligan and MacVicar, 2004). Indeed, mechanical stimulation of astrocytes leads to an increase of Ca2+cyt and subsequent release of glutamate (Innocenti et al., 2000; Hua et al., 2004; Montana et al., 2004). Sources of Ca2+ for this mechanically induced exocytosis of glutamate from astrocytes have been recently reviewed (Parpura et al., 2011). Briefly, the major source of Ca2+ in this process resides within the ER (endoplasmic reticulum) endowed with InsP3 (inositol 1,4,5-trisphosphate) and ryanodine receptors. Activation of these receptors provides a conduit for the release of Ca2+ into the cytosol. The ER store depletion and refilling, the latter accomplished by the activity of the store-specific Ca2+-ATPase of SERCA (sarcoplasmic/endoplasmic reticulum Ca2+-ATPase) type, additionally draws Ca2+ from the ECS (extracellular space) via store-operated Ca2+ entry. An alternative, albeit less studied conduit for Ca2+ entry is associated with the plasma membrane NCX (Na+/Ca2+ exchanger). Activation of ionotropic receptors leading to increases in Na+cyt and depolarization was reported to stimulate Ca2+ entry through the reverse mode of the NCX (Kirischuk et al., 1997). Mild depolarization of astrocytes isolated from adult, but not neonatal, brains led to the reverse mode of NCX operation causing Ca2+ entry into astrocytic cytosol and consequential glutamate release (Paluzzi et al., 2007). In this process, however, neither Na+cyt changes have been investigated, nor the ability of neonatal astrocytes to utilize the reverse mode of NCX due to mechanical stimulation identified.
Astrocytes express the PMCA (plasma membrane: Ca2+-ATPase), which extrudes Ca2+cyt; NCX which depending of ion concentrations and the plasma membrane potential either extrudes or delivers Ca2+ and Na+ to the cytosol; and NKA (Na+/K+-ATPase) that extrudes Na+cyt. PMCAs are expressed throughout the plasma membrane of astrocytes (Mata and Fink, 1989; Blaustein et al., 2002), while NCXs are enriched at distal processes of astrocytes surrounding synapses (Minelli et al., 2007). A subtype of NKA (type α2) colocalizes with NCX in astrocytes at plasma membrane–ER junctions, a site of 'sodium microdomains' (Juhaszova and Blaustein, 1997; Blaustein et al., 2002).
We studied the contribution of PMCA, NCX and NKA to Ca2+ and Na+ homoeostasis in astrocytes isolated from neonatal rat visual cortex, both at rest and when these cells were mechanically stimulated. Our data reveal a complex interplay between these Ca2+-handling proteins, with NKA being the major Na+ extruder and PMCA the major Ca2+ extruder from astrocytes at rest. Surprisingly, the blockade of these pumps had minor effects on Ca2+cyt and Na+cyt levels in mechanically stimulated cells. Rather, NCX in the reverse mode was a major contributor to Ca2+ and Na+ homoeostasis in mechanically stimulated astrocytes, although it operated also in resting cells. In addition, NCX facilitated mechanically induced Ca2+-dependent exocytotic release of glutamate from astrocytes. We propose that the NCX reverse mode, due to location and turnover rate of this transporter, could be linked to the activation of plasmalemmal ionotropic glutamate receptors and glutamate transporters at the tripartite synapse to accomplish fast and spatially localized gliotransmission. Naturally, such intercellular signalling pathway would operate in parallel to a slower and more widely distributed pathway that requires activation of mGluR (metabotropic glutamate receptor) and Ca2+ release from the ER in perisynaptic astroglial compartments. Some of these data have appeared in preliminary form (Reyes and Parpura, 2009).
MATERIALS AND METHODS
Astrocyte cultures
We grew solitary astrocytes (individual astrocytes devoid of contact with other astrocytes) from visual cortices of 1- to 2- day-old Sprague Dawley rats as previously described (Hua et al., 2004; Reyes and Parpura, 2008). Briefly, visual cortices were dissected and enzymatically treated with papain (20 IU/ml, 1 h at 37°C) in the presence of L-cysteine (0.2 mg/ml); digestion was terminated by trypsin inhibitor (10 mg/ml; type II-O; 5 min at room temperature). Tissue was mechanically dissociated and neural cells were seeded into culture flasks containing culture medium composed of α-MEM (α-minimum essential medium, without phenol red; Invitrogen) supplemented with fetal bovine serum (10% (v/v); Thermo Scientific HyClone), glucose (20 mM), L-glutamine (2 mM), sodium pyruvate (1 mM), sodium bicarbonate (14 mM), penicillin (100 IU/ml), and streptomycin (100 μg/ml) (pH 7.35). After allowing cells to adhere to the bottom of the flasks for 1 h, they were washed and provided with fresh medium. Cells were then maintained at 37°C in a 95% air/ 5% CO2 incubator for 5–7 days to obtain cell growth and proliferation to approx. 60% confluency. At that juncture, the cell cultures were purified for astrocytes using a previously described procedure (McCarthy and de Vellis, 1980). Purified astrocytes were detached from the flasks using trypsin (10,000 Nα-benzoyl-arginine ethyl ester hydrochloride units/ml; Sigma–Aldrich). After inhibition of trypsin activity by addition of complete culture medium, cells were pelleted using centrifugation (100×g for 10 min), resuspended and plated onto round (12 mm in diameter) glass coverslips (Fisher Scientific, cat. no. 12-545-82-12CIR-1D) pre-coated with PEI (polyethyleneimine, 1 mg/ml; Sigma). Purified astrocytes were kept in culture medium at 37°C in a 95% air/5% CO2 incubator for 5–8 days when used in experiments. The purity of astrocytic culture (>99%) was confirmed: (i) by indirect immunocytochemistry using anti-glial fibrillary acidic protein antibody (Supplementary Figure S1 at http://www.asnneuro.org/an/004/an004e075add.htm) and (ii) by visualization of accumulation of a dipeptide, β-Ala-Lys, conjugated to 7-amino-4-methylcoumarin-3-acetic acid as previously described (Hua et al., 2004; Montana et al., 2004; Malarkey et al., 2008). Astrocytes in our culture system are flat polygonal cells having simplified morphological appearance when compared with astrocytes in situ (Supplementary Figure S1) (Hua et al., 2004; Montana et al., 2004; Malarkey et al., 2008).
Pharmacological agents
Concentration and pre-incubation times for each pharmacological agent were adapted from literature (Goldman et al., 1994; Chaudhary et al., 2001; Benz et al., 2004; Rojas et al., 2007): caloxin 2A1 (peptide sequence VSNSNWPSFPSSGGG-NH2; 2 mM, 5 min; Synthetic Biomolecules), benzamil hydrochloride (benzamil; 100 μM, 5 min; Alexis Biochemical), KB-R7943 (2-[2-[4-(4- nitrobenzyloxy)phenyl]ethyl]isothiourea methane sulfonate; 30 μM, 10 min; EMD Chemicals, Inc.), and ouabain (1 mM, 10 min; Sigma). These agents were delivered to astrocytes in external solution (pH 7.35) containing (in mM): sodium chloride (140), potassium chloride (5), calcium chloride (2), magnesium chloride (2), HEPES (10) and glucose (5). In experiments using mechanical stimulation, and prior to imaging, astrocytes were pre-incubated with solutions containing a pharmacological agent or a combination of them at room temperature (22–25°C) at prescribed times above, and were then kept bathed in the agent(s) during the entire imaging procedure lasting approx. 250 s for ion measurements; for glutamate measurements, inherent to a dual run approach (see below), the imaging procedure lasted twice as long.
Intracellular Ca2+ imaging
Ca2+cyt levels in somata of cultured solitary astrocytes were assessed using the Ca2+ indicator fluo-3 as described earlier (Hua et al., 2004; Montana et al., 2004; Lee et al., 2008). Briefly, astrocytes were loaded with AM (acetoxymethyl) ester of fluo-3 (10 μg/ml; Invitrogen) in external solution containing pluronic acid (0.025% (w/v)) for 30 min at room temperature. To allow de-esterification of fluo-3 AM, cells were subsequently kept in external solution for 30 min at room temperature. Cells were then incubated with pharmacological agents described above. Coverslips were mounted onto a recording chamber, and astrocytes were visualized with a standard FITC filter set (Chroma). Fluorescence intensities obtained from somata of indicator-loaded astrocytes were corrected (digital subtraction) for the background fluorescence measured from regions of coverslips containing no cells. Fluorescence data were expressed as ΔF/Fo (%) with the cell baseline fluorescence (Fo) representing the average of the first five images before mechanical stimulation, while ΔF represents the change in fluorescence emission. The [Ca2+]cyt (Ca2+cyt concentration) was determined using calibration of fluo-3 as described elsewhere (Parpura and Haydon, 2000; Malarkey et al., 2008). In experiments studying the effect of agents on basal Ca2+cyt, we expressed fluorescence emission as a ratio of the average background subtracted fluorescence at testing time [F; five consecutive images obtained at the end of the incubation time with the agent(s)] over the average baseline fluorescence of cells at rest [Fo; five consecutive images obtained just prior to the addition of the agent(s)].
Intracellular Na+ imaging
Na+cyt levels in somata of cultured solitary astrocytes were assessed using the Na+ indicator CoroNaTMGreen AM (10 μM; Invitrogen) (Meier et al., 2006). Astrocytes were loaded with the indicator, imaged, and data collected and processed as described above for Ca2+ imaging. Because CoroNaTMGreen tends to leak out of the cell, its intracellular fluorescence intensity substantially decays over time (Meier et al., 2006). Consequently, using a linear regression and extrapolation of the baseline fluorescence of individual traces, we corrected them for the leak of the dye. To estimate the concentration of intracellular Na+ concentration, we used a calibration protocol in situ. Here, astrocytes were imaged in external solution containing various concentrations of Na+ (in mM: 140, 100, 50 or 10). Following approx. 4 min exposure to a particular extracellular Na+ concentration, astrocytes were treated with the Na+ ionophore gramicidin (50 μM; Sigma). On equilibration of the intracellular milieu with the extracellular Na+ concentration by using the peak of CoroNaTMGreen ΔF/Fo and by applying an exponential fit (r = 0.97) to the data, we obtained the relationship between [Na+]cyt, (Na+cyt, concentration) and CoroNaTMGreen ΔF/Fo, which can be formulated as: [Na+]cyt = 16.597 mM × e (0.0131×ΔF/Fo).
Buffering of intracellular Ca2+
To buffer Ca2+cyt in astrocytes, during the loading procedure with either fluo-3 AM or CoroNaTMGreen AM, we co-loaded astrocytes with the Ca2+ chelator BAPTA-AM [1,2-bis-(o-aminophenoxy)ethane-N,N,N′,N′-tetra-acetic acid tetrakis-acetoxymethyl ester]; 100 μM; Invitrogen, followed by a 30 min de-esterification period.
Extracellular glutamate imaging
Ca2+-dependent glutamate release from cultured solitary astrocytes was measured using the L-GDH (glutamate dehydrogenase)-linked assay as previously described (Hua et al., 2004; Montana et al., 2004; Lee et al., 2008). PEI-coated coverslips containing cultured astrocytes were mounted onto a recording chamber and bathed in external solution. A set of images containing the cell of interest were taken in a sham run and used to correct for reduction of fluorescence due to photobleaching in the follow-up experimental run, for which the external solution was exchanged with fresh external solution supplemented with 1 mM of NAD+ (Sigma) and 55–61 IU/ml of GDH (Sigma). Visualization was achieved using a standard DAPI (4′6-diamidino-2-phenylindole) filter set (Nikon). When released, glutamate is oxidized to α-ketoglutarate by GDH, while bath-supplied NAD+ is reduced to NADH, a fluorescent product when excited by UV light. Fluorescence data were expressed as ΔF/Fo (%) with the baseline fluorescence (Fo) being the fluorescence of the medium surrounding the solitary astrocyte, immediately and laterally of its soma, before mechanical stimulation. To account for the possibility that KB-R7943 has an effect on the activity of the GDH in the medium surrounding the astrocytes, a spectrophotometer assay (Genequant Pro) was performed using NADH absorbance as a measure of GDH activity (Reyes and Parpura, 2008; Reyes et al., 2011). The assay solution contained NAD+ (1 mM), glutamate (100 μM), and approx. 59 IU/ml of GDH. The NADH produced from a 5-min reaction was monitored by its absorbance at 320 nm. There was no significant difference (Student's t-test, P = 0.09) in NADH absorbance in assay solution either containing KB-R7943 (30 μM; n = 7; 0.35±0.03) or lacking this agent [control; n = 7; 0.43±0.03 (mean±SEM)]. It should be noted that due to its fluorescence when excited with UV light, benzamil is not amenable for use in our glutamate release assay.
Image acquisition and processing
An inverted microscope (TE 300; Nikon), equipped with DIC (differential interference contrast), wide-field fluorescence illumination and oil-immersion objectives, was used in all experiments. For glutamate imaging we used a 40× SFluor objective (1.3 NA; Nikon), whereas all other experiments were done using a 60× Plan Apo objective (1.4 NA; Nikon). Images were acquired using a CoolSNAP-HQ cooled CCD (charge-coupled device) camera (Roper Scientific Inc.) driven by V++ imaging software (Digital Optics Ltd.). All raw data/images had their pixel intensities within the camera's dynamic range (0–4095). For glutamate release analysis, the ΔF/Fo of the treatment groups were ranked and normalized to accommodate for variations in enzyme-based method and culture conditions, and to allow comparisons between experimental batches, as we previously described (Reyes and Parpura, 2008). Similar rankings of intracellular Na+ and Ca2+ dynamics were done for consistency.
Mechanical stimulation
To stimulate a solitary astrocyte of interest, we employed mechanical contact using a glass pipette filled with external solution as we described in detail elsewhere (Hua et al., 2004). Briefly, this approach allows spatio-temporal control of the stimulus application without affecting plasma membrane integrity. The establishment of the patch pipette contact with the plasma membrane was determined by an increase in pipette resistance monitored using a patch-clamp amplifier (PC-ONE) that delivered –20 mV, 10 ms square pulses at 50 Hz. Once established, cell contact was maintained for approx. 1 s. The strength of the stimulus, expressed as ΔR/Ro (%), where Ro represents the pipette resistance (1.4–9.3 MΩ) prior to the establishment of a pipette-astrocyte contact, and ΔR represents the increase in the resistance (0.02–1.3 MΩ) during the contact, had comparable intensities, under all conditions tested (Mann–Whitney U-test, P = 0.092–0.934).
Statistical analysis
All reported effects were tested using data originating from at least three independent cultures. The comparison of the pipette resistance increases (ΔR/Ro) in different conditions were tested by Mann–Whitney U-test. Effects of the pharmacological agents on basal and mechanically evoked intracellular Ca2+ and Na+ levels were tested using one-way ANOVA, followed by Fisher's LSD (least significant difference) test. Effects of mechanical stimulation on intracellular Ca2+ and Na+ levels were assessed with paired t-tests. Effects of BAPTA/AM on mechanically induced intracellular Ca2+ and Na+ load, as well as that of KB-R7943 on Ca2+ accumulation and glutamate release were tested using Mann–Whitney U-test. Data are expressed as means±SEM.
RESULTS
PMCA, NCX and NKA regulate basal Ca2+cyt and Na+ levels in cultured cortical astrocytes
PMCA, NCX and NKA have been shown to jointly regulate Ca2+cyt and Na+cyt homoeostasis at neuronal presynaptic terminals (Regehr, 1997; Zhong et al., 2001). Since astrocytes exhibit spatio-temporal changes in [Ca2+]cyt (Verkhratsky et al., 1998) and [Na+]cyt (Kirischuk et al., 1997; Rose and Ransom, 1997; Kirischuk et al., 2007), it has been proposed that combined PMCA, NCX and NKA also contribute to the regulation of astrocytic [Ca2+]cyt and [Na+]cyt (Goldman et al., 1994; Blaustein et al., 2002). Consequently, we evaluated the contribution of PMCA, NCX and NKA in maintaining the basal Ca2+cyt and Na+cyt levels in cultured astrocytes using specific pharmacological inhibitors previously tested in non-neuronal/glial cell types.
We tested the role of the PMCA in preserving basal [Ca2+]cyt by incubating cortical astrocytes with caloxin 2A1 (2 mM; 5 min), a peptide blocker that has affinity for the second extracellular domain sequence of the PMCA (Chaudhary et al., 2001; De Luisi and Hofer, 2003; Kawano et al., 2003). To assess the NCX contribution to basal Na+cyt and Ca2+cyt regulation, we incubated astrocytes with either the general NCX blocker, benzamil (100 μM; 5 min) or the NCX reverse mode blocker KB-R7943 (30 μM; 10 min) (Benz et al., 2004; Rojas et al., 2004; Rojas et al., 2007). To test the role of NKA in the regulation of basal [Na+]cyt we used the NKA blocker ouabain (1 mM; 10 min) (Goldman et al., 1994).
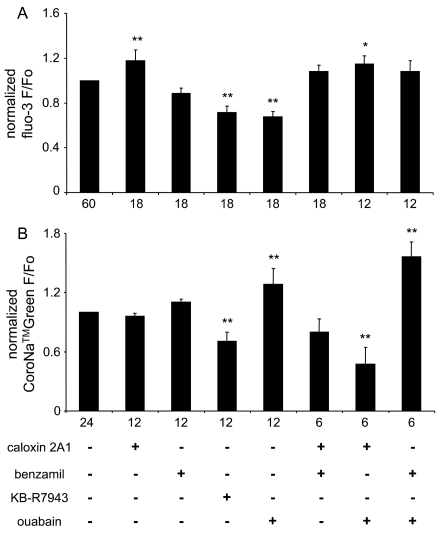
We monitored fluo-3 fluorescence to assess changes in basal [Ca2+]cyt in solitary astrocytes. We acquired the resting Ca2+cyt level from astrocytes bathed in external solution, which was then replaced by a pharmacological agent(s) containing solution. In control, astrocytes were sham treated by exchanging the plain external solution. We found that astrocytes treated with caloxin 2A1 showed a significant increase in the basal fluo-3 fluorescence when compared with control, sham-treated, astrocytes (Figure 1A; one-way ANOVA, followed by Fisher's LSD test; P<0.01). In contrast astrocytes treated with KB-R7943 or ouabain showed a significant decrease in fluo-3 fluorescence (Figure 1A; one-way ANOVA, followed by Fisher's LSD test; P<0.01). These changes in fluo-3 fluorescence corresponded to rather subtle variations in calculated [Ca2+]cyt from approx. 73 nM at rest, before the treatment with an agent, to approx. 85 nM in caloxin 2A1, approx. 61 nM in KB-R7943 and approx. 58 nM in ouabain; the exchange of external solution alone in sham-treated astrocytes resulted in basal [Ca2+]cyt of approx. 77 nM, which was essentially unchanged from the resting level. In addition, astrocytes treated with benzamil did not exhibit significant change in fluorescence when compared with control. Furthermore, we incubated astrocytes with various agents in tandem. We found that astrocytes treated with the caloxin 2A1/benzamil or benzamil/ouabain combination did not exhibit significant changes in fluo-3 fluorescence, while astrocytes treated with caloxin 2A1/ouabain showed a significant increase in fluorescence, corresponding to [Ca2+]cyt of approx. 82 nM, when compared with control (Figure 1A; one-way ANOVA, followed by Fisher's LSD test; P<0.05).
Figure 1. PMCA, NCX and NKA modulate basal Ca2+cyt and Na+ cyt levels in neonatal solitary cortical astrocytes at rest.
(A) Normalized fluo-3 fluorescence, reporting on Ca2+cyt levels, in astrocytes at rest. Astrocytes treated with caloxin 2A1 (PMCA blocker) alone or in conjunction with ouabain (NKA blocker) showed a significant increase in basal Ca2+cyt levels, which were decreased when cells were treated with KB-R7943 (NCX reverse mode blocker) or ouabain. (B) Normalized CoroNaTMGreen fluorescence, reporting on Na+cyt levels, in astrocytes at rest. A significant increase in basal Na+cyt levels was recorded from astrocytes treated with ouabain alone or in combination with benzamil (general NCX blocker), while astrocytes treated with KB-R7943 or caloxin 2A1/ouabain combination exhibited a decrease in basal Na+cyt levels. Bars represent average (±SEM) of fluorescence ratio before (Fo) and after (F) incubation of astrocytes in various agents. Statistical comparisons were made between time-matched sham treated (control) and agent treated astrocytes (one-way ANOVA, followed by Fisher's LSD test; *P<0.05, **P<0.01), whose numbers in each group are given below abscissas.
Plasmalemmal NCX regulates both [Ca2+]cyt and [Na+]cyt depending on the relative concentration of these two ions and the plasma membrane potential (Goldman et al., 1994; Rojas et al., 2007). Similar to their differential effect on [Ca2+]cyt, benzamil and KB-R7943 had a differential effect on the [Na+]cyt. Astrocytes treated with benzamil did not show a significant difference in CoroNaTMGreen fluorescence, corresponding to approx. 19.0 mM of [Na+]cyt, when compared with levels (approx.16.6 mM) recorded in astrocytes at rest or sham-treated. Treatment of these cells with KB-R7943 caused a decrease in CoroNaTMGreen fluorescence corresponding to approx. 11.3 mM of [Na+]cyt (Figure 1B; one-way ANOVA, followed by Fisher's LSD test; P<0.01). Since PMCA does not transport Na+, as predicted, astrocytes treated with caloxin 2A1 did not exhibit a change in [Na+]cyt, as evident from the lack of change in CoroNaTMGreen fluorescence compared with the sham-treated control. Furthermore, since the NKA transports Na+ extracellularly, astrocytes treated with ouabain exhibited significant increase in [Na+]cyt, corresponding to approx. 24.1 mM, when compared with control (Figure 1B; one-way ANOVA, followed by Fisher's LSD test; P<0.01). Astrocytes treated with benzamil in conjunction with ouabain exhibited a significant increase in CoroNaTMGreen fluorescence (corresponding to approx. 34.7 mM of [Na+]cyt), while astrocytes treated with caloxin 2A1 in conjunction with ouabain exhibited a significant decrease in fluorescence (corresponding to approx. 8.3 mM of [Na+]cyt) when compared with control (Figure 1B; one-way ANOVA, followed by Fisher's LSD test; P<0.01).
Taken together, at resting conditions it appears that the PMCA is the primary astrocytic Ca2+ extruder, since blockage by caloxin 2A1 caused a significant increase in [Ca2+]cyt. In contrast, at resting conditions the NCX appears to facilitate Ca2+ entry to the astrocytic cytosol, thus operating in the reverse mode, detected as the reduction of fluo-3 fluorescence in astrocytes treated with KB-R7943. The primary astrocytic Na+ extruder appears to be NKA, since its inhibition by ouabain caused significant increase in astrocytic [Na+]cyt.
Mechanical stimulation causes both Ca2+ and Na+ cytosolic increases in astrocytes
Solitary astrocytes are known to respond to mechanical stimulation, which does not compromise the plasma membrane integrity, by increasing their intracellular Ca2+ levels (Hua et al., 2004). The majority of mechanically induced Ca2+cyt accumulation originates from the ER internal store, although plasmalemmal Ca2+ entry contributes to this excitability and is ultimately required for the (re)filling of ER Ca2+ stores, a process that involves store-operated Ca2+ entry via TRPC (canonical transient receptor potential) 1 channels (Hua et al., 2004; Malarkey et al., 2008); these channels are permeable for both Ca2+ and Na+, albeit with much higher Ca2+ permeability (Clapham, 2003; Rychkov and Barritt, 2007). Additionally, mitochondria modulate the magnitude of this mechanically induced excitability (Reyes and Parpura, 2008). However, whether mechanical stimulus can also affect Na+cyt loads that should be inherently linked to Ca2+cyt changes has not been determined yet.
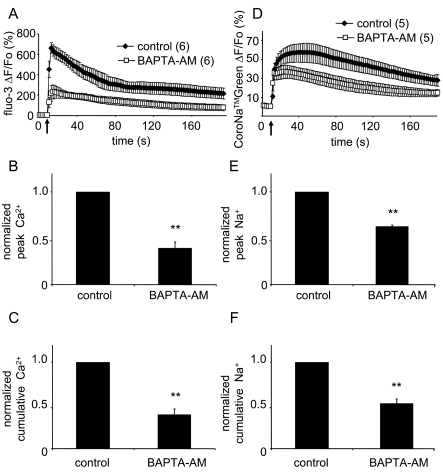
To address this issue, we loaded astrocytes, in parallel, with either fluo-3 or CoroNaTMGreen. As expected, mechanical stimulus caused a typical increase in Ca2+cyt levels in solitary astrocytes characterized by an initial transient Ca2+ elevation followed by a slowly decaying response (Figure 2A, paired t-test, P<0.01). Two aspects of the Ca2+cyt kinetics in astrocytes due to mechanical stimulation were measured and analysed: the peak and cumulative Ca2+ responses. The peak response represents the maximum Ca2+cyt in the stimulated astrocyte as a result of Ca2+ entry into the cytosol from the ER store and ECS, while the cumulative response additionally represents the declining Ca2+cyt, as free Ca2+ is removed from cytosol by pumps, such as the PMCA and SERCA (Kim et al., 2005; Reyes and Parpura, 2008). Mitochondria modulate the peak of [Ca2+]cyt transient as they immediately sequester Ca2+, while during the decay phase they slowly release Ca2+. In addition to inducing the Ca2+ response, mechanical stimulation caused an increase in intracellular Na+ levels (Figure 2D; paired t-test, P<0.01). Similar to the Ca2+ response, the mechanically induced Na+ response included an initial transient increase followed by a slow decline, albeit the overall response had a slower time-course than that of mechanically induced [Ca2+]cyt transient.
Figure 2. Mechanical stimulation of astrocytes induces both Na+cyt and Ca2+cyt signals.
(A) Average kinetics of fluo-3 fluorescence, reporting on changes in Ca2+cyt, in solitary astrocytes that were mechanically stimulated. (B, C) Bar graphs showing normalized peak and cumulative Ca2+cyt responses, respectively. (D) Average kinetics of CoroNaTMGreen, reporting on changes in Na+cyt, in mechanically stimulated solitary astrocytes. (E, F) Bar graphs showing normalized peak and cumulative Na+cyt responses, respectively. Mechanical stimulation evoked both Ca2+cyt and Na+cyt increases (A and D, receptively). Peak and cumulative values of mechanically induced Ca2+cyt elevations were greatly reduced when cells were pre-incubated with the Ca2+ chelator BAPTA-AM (B and C, respectively). Similar reduction in peak and cumulative values of Na+cyt elevations was observed in astrocytes treated with BAPTA/AM (E and F, respectively). Points and bars represent means±SEM of measurements. Arrows in A and D indicate the time when the pipette-astrocyte contact occurred. Numbers in parentheses indicate the number of cells in each group. Asterisks denote significant change in comparison with control, untreated and stimulated, astrocytes (Mann–Whitney U-test; **P<0.01).
Such coordinated dynamics, where Na+cyt changes appear to follow those of Ca2+cyt, raised the question of whether the mechanically induced Na+ response involves NCX activity. Since the amount of Na+cyt extruded by NCXs is proportional to the amount of extracellular Ca2+ taken in, if the reverse operation of the NCX could play a role in mechanically induced Ca2+ and Na+ dynamics in astrocytes, we predict that the buffering of Ca2+cyt, an experimental manipulation that would facilitate the reverse mode (see Discussion), could result in a decrease of Na+ load in these cells. In contrast, a sole involvement of TRPC1 in mechanically induced Ca2+ and Na+ dynamics under conditions of Ca2+cyt buffering should not lead to a decrease, but rather to an increase in Na+ load. To test this hypothesis, we used the Ca2+ chelator BAPTA-AM (100 μM; 30 min) to co-load astrocytes with one of the above indicators, while cells loaded with indicators alone served as control. Mechanically stimulated astrocytes co-loaded with the Ca2+ chelator exhibited a decrease in peak and cumulative fluo-3 (Figures 2A–2C) and CoroNaTMGreen (Figure 2D–2F) fluorescence when compared with control cells (Mann–Whitney U-test, P<0.01). These data point to a role of NCX reverse mode in modulation of mechanically induced Ca2+ and Na+ dynamics in astrocytes, which we further studied using a pharmacological approach below.
PMCA, NCX and NKA modulate mechanically induced Ca2+cyt accumulations in astrocytes
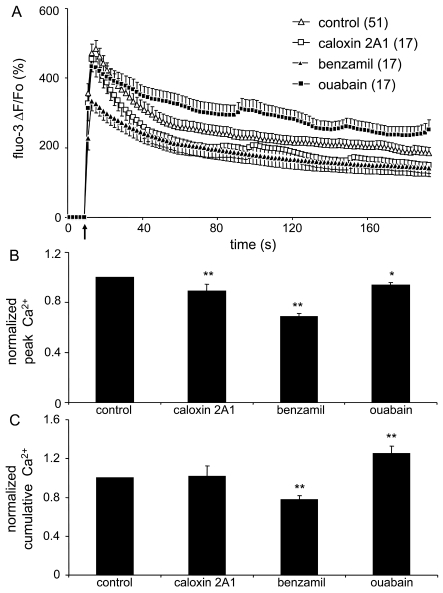
Having determined effects of pharmacological blockers of PMCA, NCX and NKA on Ca2+ and Na+ cytosolic levels of cells at rest along with finding that mechanical stimulation induced both Ca2+cyt and Na+cyt elevations, we studied the role of these pumps and the exchanger on cytosolic Ca2+ dynamics of mechanically stimulated astrocytes. As we described above, mechanical stimulation of control astrocytes caused a robust increase in [Ca2+]cyt, corresponding to approx. 3.8 μM (Figure 3A; paired t-test, P<0.01). We found that the treatment of astrocytes with either caloxin 2A1 (2 mM; 5 min) to block PMCAs, benzamil (100 μM; 5 min) to block NCX, or ouabain (1 mM; 10 min) to block NKA modulated the mechanically induced Ca2+ response as evident in the average kinetics of the fluorescence intensity (Figure 3A). We should be reminded that pharmacological agents affect resting Ca2+cyt levels bringing them to various post-treatment baselines (Figure 1A). Since we were interested in the astrocytic ability to handle Ca2+cyt after the treatments, we used post-treatment Ca2+cyt levels as a baseline (Fo) for further quantitative analysis. Here, pretreatment of astrocytes with either caloxin 2A1, benzamil or ouabain reduced the peak of mechanically induced Ca2+ response, when compared with control (Figure 3B; one-way ANOVA, followed by Fisher's LSD test; P<0.01 for caloxin 2A1 and benzamil, while P<0.05 for ouabain). In contrast, astrocytes treated with caloxin 2A1 did not exhibit significant difference in cumulative response, while cells treated with benzamil or ouabain exhibited attenuated or enhanced cumulative responses respectively (Figure 3C, one-way ANOVA, followed by Fisher's LSD test; P<0.01).
Figure 3. Ca2+cyt accumulation in astrocytes induced by mechanical stimulation is modulated by the plasmalemmal PMCA, NCX, and NKA.
(A) Average kinetics of fluo-3 fluorescence, reporting on changes in Ca2+cyt, in solitary astrocytes treated with pharmacological agents and in response to mechanical stimulation. Arrow represents the time when mechanical stimulation was applied to the cells. (B,C) Bar graphs showing normalized peak and cumulative Ca2+cyt responses, respectively. When treated with caloxin 2A1, benzamil and ouabain, mechanically stimulated astrocytes had a decrease in the peak Ca2+cyt response (B). Benzamil led to a decrease, while ouabain to an increase in the normalized cumulative Ca2+cyt response obtained from mechanically stimulated astrocytes (C). Points and bars represent means±SEM of measurements from solitary astrocytes (numbers in parentheses); SEM are shown in single directions for clarity. Asterisks denote significant change in comparison with control group (one-way ANOVA, followed by Fisher's LSD test; *P<0.05, **P<0.01).
Taken together it appears that PMCA, NCX and NKA all play a role in modulating the mechanically induced Ca2+ entry into the cytosol since these agents attenuated the peak Ca2+ response. In addition, it also appears that NCX and NKA play additional, but opposing roles in the removal of Ca2+ from the cytosol.
Na+cyt load induced by mechanical stimulation is mediated by NCX
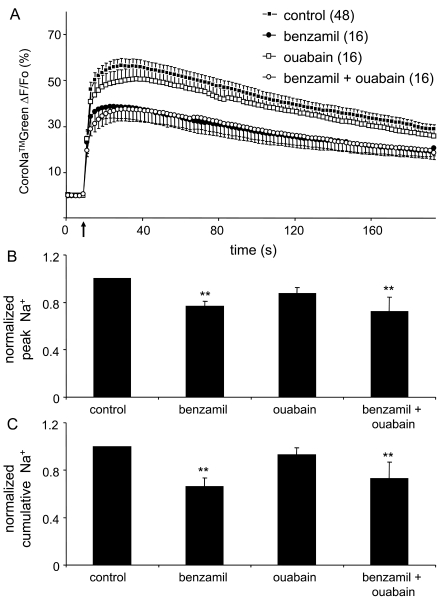
Mechanical stimulation of control astrocytes, in addition to Ca2+cyt response, caused an increase in [Na+]cyt corresponding to ∼36.8 mM (Figure 4A; paired t-test, P<0.01), which is about the same [Na+]cyt reached upon treatment of astrocytes at rest with the NKA blocker (Figure 1B). We tested whether mechanically induced Na+ load in astrocytes can be modulated by NCX and NKA. We loaded cortical astrocytes with CoroNaTMGreen, and then treated them with either benzamil (100 μM; 5 min) to block NCX, or ouabain (1 mM; 10 min) to block NKA. We were interested in the astrocytic ability to handle Na+cyt following pharmacological treatments, which affected resting [Na+]cyt levels bringing them to various post-treatment baselines (Figure 1B). Thus, we used post-treatment [Na+]cyt baselines for further quantitative analysis. Astrocytes pretreated with benzamil showed a reduced mechanically induced peak and cumulative CoroNaTMGreen fluorescence (Figures 4A and 4B; one-way ANOVA, followed by Fisher's LSD test; P<0.01), while ouabain did not significantly affect these parameters by comparison to control astrocytes. Additionally, when ouabain was added in combination with benzamil, it did not alter the effect caused by benzamil (Figures 4B–4C).
Figure 4. Na+cyt accumulation in astrocytes induced by mechanical stimulation is regulated by the plasmalemmal NCX.
(A) Average kinetics of CoroNaTMGreen fluorescence, reporting on changes in Na+cyt, in solitary astrocytes treated with pharmacological agents and in response to mechanical stimulation. Arrow represents the time when mechanical stimulation was applied to the cells. (B, C) Bar graphs showing normalized peak and cumulative Na+cyt values of mechanically stimulated astrocytes, respectively. Mechanically stimulated astrocytes pre-treated with benzamil alone or in combination with ouabain had a significantly lower peak Na+cyt values when compared with control cells (B). These treatments caused a similar decrease in normalized cumulative Na+cyt values (C). Ouabain had no effect on either a peak or cumulative response. Points and bars represent means±SEM of measurements from solitary astrocytes (numbers in parentheses); SEM are shown in single directions for clarity. Asterisks denote significant change in comparison with control group (one-way ANOVA, followed by Fisher's LSD test; **P<0.01).
Thus, it appears that the NCX may contribute to Na+ entry into cytosol of mechanically stimulated astrocytes since its blockade with benzamil reduced the Na+ load in stimulated astrocytes. In contrast, the NKA appears to be a minor player in the extrusion of Na+ in mechanically stimulated astrocytes.
Mechanically induced glutamate release from astrocytes is modulated by the Ca2+ entry via the reverse mode of NCX
Benz et al. (2004), using benzamil, showed that the NCX participates in Ca2+ signalling, and in the subsequent release of homocysteic acid from cortical astrocytes stimulated by glutamate. Additionally, activation of astroglial ionotropic receptors causing increases in [Na+]cyt and depolarization engaged this exchanger in the reverse mode to cause Ca2+ entry (Kirischuk et al., 1997; Rojas et al., 2007). Similarly, mild depolarization, due to an increase in extracellular K+ (15 and 35 mM), of cortical astrocytes cultured from adult, but not neonatal, rats caused NCX to operate in the reverse mode and generated [Ca2+]cyt increases which triggered release of glutamate (Paluzzi et al., 2007); these effects were blocked by KB-R7943 implicating the reverse mode of NCXs. Consequently, using KB-R7943, we investigated whether the reverse mode of NCX can contribute to mechanically induced Ca2+-dependent exocytosis of glutamate from neonatal astrocytes.
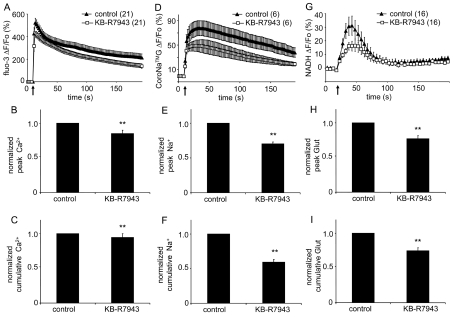
Astrocytes were loaded, in parallel, with either the Ca2+ indicator fluo-3 or the Na+ indicator CoroNaTMGreen. Once again the mechanical stimulus caused increases in [Ca2+]cyt and [Na+]cyt levels, corresponding to approx. 4.2 μM and 47.3 mM respectively (Figures 5A and 5D; paired t-test, P<0.01) When we blocked Ca2+ entry via the reverse mode of NCX with KB-R7943 (30 μM; 10 min), we saw a significant decrease in the mechanically evoked Ca2+ and Na+ responses (Figures 5B–5C and 5E–5F respectively; Mann–Whitney U-test, P<0.01).
Figure 5. Mechanically induced glutamate release from astrocytes is modulated by the Ca2+ entry via the reverse mode of the plasmalemmal NCX.
(A) Average kinetics of fluo-3 fluorescence, reporting on changes in Ca2+cyt, in solitary astrocytes treated with KB-R7943 in response to mechanical stimulation. (B, C) Bar graphs showing normalized peak and cumulative Ca2+cyt values of mechanically stimulated astrocytes, respectively. When treated with KB-R7943, astrocytes had significantly lower peak and cumulative Ca2+cyt responses to mechanical stimulation than those recorded in control. (D) Average kinetics of CoroNaTMGreen (CoroNaTMG) fluorescence, reporting on changes in Na+cyt, in solitary astrocytes treated with KB-R7943 in response to mechanical stimulation. (E, F) Bar graphs showing normalized peak and cumulative Na+cyt values of mechanically stimulated astrocytes, respectively. When treated with KB-R7943, astrocytes had significantly lower peak and cumulative Na+cyt responses to mechanical stimulation than those recorded in control. (H) Time lapse of extracellular NADH fluorescence, reporting on glutamate levels in the ECS surrounding somata of solitary astrocytes. Mechanical stimulation of solitary astrocytes caused glutamate release, which was affected by KB-R7943. (H, I) Normalized peak and cumulative extracellular glutamate (Glut) values of mechanically stimulated astrocytes, respectively. When treated with KB-R7943, astrocytes had both parameters significantly reduced. Points and bars represent means±SEM of measurements from solitary astrocytes (numbers in parentheses); SEM in A are shown in single directions for clarity. Arrows in A, D and G represent the time when mechanical stimulation was applied to the cells. Asterisks denote significant change in comparison with control group (Mann–Whitney U-test, ** P<0.01).
To test whether this decrease in mechanically induced Ca2+ elevations lead to a reduction of the consequent exocytotic glutamate release from solitary astrocytes we optically monitored the release of glutamate into the ECS surrounding cultured astrocytes using a GDH-linked assay based on accumulation of the fluorescent product, NADH (Hua et al., 2004). Mechanical stimulation of astrocytes caused glutamate release as indicated by a transient increase in NADH fluorescence, reporting on extracellular glutamate levels, corresponding to approx. 0.7 μM, around the astrocytes (Figure 5G; paired t-test; P<0.01). Incubating astrocytes with KB-R7943 (30 μM; 10 min) reduced the peak and cumulative amount of glutamate released from mechanically stimulated astrocytes (Figure 5H–5I; Mann–Whitney U-test, P<0.01), indicating that Ca2+ entry through NCX is important for the elevation of cytoplasmic [Ca2+]cyt necessary for mechanically induced Ca2+-dependent glutamate release from astrocytes.
DISCUSSION
We studied the role of PMCA, NCX and NKA in coordinated regulation of Ca2+cyt and Na+cyt in astrocytes at rest and upon mechanical stimulation. This stimulus activates major intracellular signalling pathways represented by intracellular Ca2+ increase and consequential glutamate release, which is mediated by exocytosis, since Rose Bengal, an inhibitor of vesicular glutamate uptake, abolishes it (Montana et al., 2004); additionally, this stimulus promotes vesicular fusions (Malarkey and Parpura, 2011). Ca2+-dependent exocytotic glutamatergic gliotransmission can modulate synaptic transmission and plasticity at the tripartite synapse (Araque et al., 1999c; Perea et al., 2009; Parpura and Zorec, 2010). Since TRPC1 channels, found to be a component of vertebrate mechanosensitive cation channels (Maroto et al., 2005), provide the conduit for Ca2+ entry from the ECS (Malarkey et al., 2008), mechanical stimulation could therefore mimic a physiological event, perhaps at astrocyte–blood vessel interface (Reyes et al., 2011). Astrocytes control microcirculation, a process that includes astrocytic Ca2+ dynamics occurring at their end feet and bodies (Gordon et al., 2007); it is at this interface that astrocytes undergo large mechanical dynamics.
We used a culturing method yielding solitary astrocytes which appear as flat polygonal cells, having less complex morphological features than astrocytes in situ which display elaborate processes and are in intimate contact with other neural cell types. While this system minimizes effects of intercellular astrocyte–astrocyte communications and thus eases the interpretation of our measurements, it brings some limitations. Hence, we were not able to study: (i) the difference in measurements at the somatic region compared with peripheral astrocytic processes, and (ii) the effects that the presence of other cell types, e.g., neurons, would have on our measurements. Nonetheless, using solitary astrocytes we are poised to study in detail spatio-temporal characteristics of Ca2+ and Na+ fluxes at mitochondrial, ER and plasma membranes, as well as to assess concentrations of these ions within mitochondria and ER. Indeed, some future studies would also need to cross-correlate our findings in culture with experiments done in more intact systems.
Our finding that mechanical stimulus leads to activation of another intracellular signalling pathway, an increase in [Na+]cyt, has important consequences to astrocytic metabolism. Na+ entry drives glutamate uptake through the plasma membrane glutamate transporters, and is used to counter transport H+ out of the cytosol via the Na+/H+ exchanger to regulate cytosolic pH (Deitmer, 2004). As such, maintenance of the Na+ gradient via the NKA is a major ATP expenditure in astrocytes. Na+cyt increases leading to depolarization due to activity of plasma membrane glutamate transporters (Rojas et al., 2007) and ionotropic glutamate receptors and/or purinoceptors (Benz et al., 2004; Lalo et al., 2006; Lalo et al., 2008) can trigger intracellular Ca2+ signalling mediated via NCX. Additionally, increases in [Na+]cyt can trigger aerobic glycolysis leading to lactate production (Magistretti, 2006), which shuttled to neurons appears to be important for synaptic transmission and plasticity (Suzuki et al., 2011).
Inhibition of the PMCA with caloxin 2A1 raised basal [Ca2+]cyt in cortical astrocytes (Figure 1A). This observation further supports the notion that the PMCA is an important extrusion mechanism to maintain resting levels of Ca2+ in cortical astrocytes, consistent with findings in other cell types, e.g. in mammalian photoreceptors (Morgans et al., 1998) and the calyx of Held (Kim et al., 2005), as well as an invertebrate model of squid giant axons (DiPolo and Beauge, 1979). The relative contribution of PMCA isoforms 1, 2 and 4, expressed by cortical astrocytes (Fresu et al., 1999), in this process is not presently understood and it should be investigated in future. Nonetheless, at stimulated conditions when the [Ca2+]cyt is increased due to Ca2+ mobilization from the ER and ECS, inhibition of the PMCA resulted in a significant reduction in the peak and with no effect on the cumulative Ca2+ response (Figures 3A and 3B). This apparent lack of the PMCA activity in the removal of Ca2+cyt during times of high [Ca2+]cyt indicates that this task is accomplished by other extrusion systems, perhaps the ER store-specific Ca2+-ATPase (Hua et al., 2004).
Astrocytes at rest treated with benzamil alone showed only a trend in a [Ca2+]cyt decrease, while application of this general NCX blocker in conjunction with caloxin 2A1 occluded the action of this PMCA blocker, as evidenced by the absence of significant change in [Ca2+]cyt from controls (Figure 1A). This finding suggests that the Ca2+ influx via NCX is opposed/balanced by the Ca2+ efflux via the PMCA (Figure 1A). This inference is supported by experiments using the specific antagonist of the NCX reverse mode, KB-R7943, which significantly decreased [Ca2+]cyt in cortical astrocytes (Figure 1A), confirming the apparent Ca2+ entry via NCX in astrocytes at rest. Additionally, KB-R7943 significantly decreased basal [Na+]cyt, which is likely an indirect effect due to an increased NKA activity. These data also imply that the resting membrane potential in our cultured cortical astrocytes is slightly depolarized from the equilibrium potential for the NCX (ENCX), as supported by the previously published work and the calculated ENCX. Hence, the membrane potential of cortical astrocytes was reported to have a bimodal distribution with peaks at −68 mV and −41 mV [see Figure 2A of (Kucheryavykh et al., 2007)]. Using our recorded [Na+]cyt of 16.6 mM and [Ca2+]cyt of 73 nM in astrocytes at rest, together with concentration of these ions in the external solution and presumed NCX 3:1 stoichiometry, we calculated ENCX to be approx.−98 mV at 25°C. Thus, the vast majority or astrocytes at rest should display the reverse mode of NCX operation in our experimental conditions. It should be noted that there are three NCX isoforms (1–3), with NCX1 being a predominant isoform, having three splice variants, in primary cultures of rat cortical astrocytes (He et al., 1998). Future experiments are needed to address relative contribution of each of these splice variants in Ca2+cyt regulation.
When cortical astrocytes were mechanically stimulated to raise [Ca2+]cyt, this stimulus also caused large increases in [Na+]cyt. As above by using our recorded peak [Na+]cyt of 36.8 mM and peak [Ca2+]cyt of 4 μM due to mechanical stimulation, we calculated ENCX to be approx.−57 mV at 25°C. Such shift in ENCX would be less favourable for the reverse mode of NCX operation in astrocytes. Consequently, to initially test a possible involvement of NCX in [Ca2+]cyt and [Na+]cyt regulation at elevated levels of these ions, we used BAPTA-AM to clamp the Ca2+cyt increase due to mechanical stimulation, corresponding to approx. 614 nM, which would substantially shift ENCX to hyperpolarization at approx. −105 mV assuming unchanged peak [Na+]cyt of 36.8 mM. Such experimental conditions would result in bettering of NCX activity that will be seen as a reduction of [Na+]cyt in astrocytes. Indeed, in BAPTA-AM treated and mechanically stimulated astrocytes we recorded a significantly lower increase of [Na+]cyt, approx. 26.8 mM, when compared with controls, at which juncture cells would settle for their ENCX at approx. −80 mV (Figure 2). Of course, large Ca2+ entry associated with the reverse mode of NCX operation was clamped down by the fast buffering capabilities of BAPTA, resulting in reduced Ca2+cyt levels. We tested this immediate conclusion based on thermodynamics considerations further by using NCX pharmacological blockers.
When cortical astrocytes were mechanically stimulated to raise [Ca2+]cyt, inhibition of the NCX with benzamil resulted in a significant reduction in peak and cumulative Ca2+cyt accumulation (Figure 3). This observation suggests that the NCX mediates Ca2+ entry and promotes Ca2+ excitability in astrocytes, consistent with earlier studies (Kirischuk et al., 1997; Benz et al., 2004; Rojas et al., 2007). Since calculated ENCX is approx.−57 mV during the initial phase of mechanical stimulation, depolarization of the majority of astrocytes would be required for the reverse mode of NCX operation. This seems a plausible scenario because mechanical stimulation leads to rather large Na+cyt load, which would lead to depolarization of astrocytes. Perhaps as in basal conditions, a decrease in [Na+]cyt that was recorded from mechanically stimulated astrocytes exposed to benzamil could be due to increased activity of NKA (Figure 4), although another alternative is possible (see below). Ca2+ entry through the NCX may modulate the InsP3 receptor-gated channel activation, since the NCX1 isoform can associate with plasma membrane–ER junctions, microdomains containing InsP3 receptors (Lencesova et al., 2004). Whether this notion could extend to a possible interplay between the Ca2+ entry via NCX and activity of ryanodine/caffeine-sensitive receptors of the ER is not clear due to lack of evidence for spatial association of these proteins; additionally, the role of ryanodine receptors in astrocytic Ca2+ excitability remains debatable (Parpura et al., 2011).
Unlike previous studies demonstrating that inhibition of NKA increased basal [Ca2+]cyt in cortical and in cerebellar type 1 astrocytes (Rojas et al., 2004), our treatment with ouabain significantly reduced the basal [Ca2+]cyt in cortical astrocytes (Figure 1A). This observation cannot be explained by an inadequate inhibition of the NKA since in parallel experiments ouabain significantly increased Na+cyt accumulation (Figure 1B). The likely explanation for these seemingly disparate findings might be lower temperature in our experiments. Namely, while we recorded at room temperature (22–25°C), above mentioned studies have done so at higher temperatures [32–34°C (Goldman et al., 1994) or 35–37°C (Rojas et al., 2004)]. If we compare calculated ENCX using our ionic conditions at 25°C (−98 mV) compared with 37°C (−123 mV), we find ENCX to be shifted towards less hyperpolarizing values at room temperature, which would lessen the operation of NCX in the reverse mode. Indeed, it has been reported that NCX activity is greatly inhibited in cultured Purkinje neurons at room temperature (Rojas et al., 2003). Thus, it is possible that reduction in Ca2+cyt during NKA blockade points to restrained operation of NCX in the reverse mode at room temperature, while PMCA activity would remain grossly uninterrupted, as one could expect from difference in turnover rates between the pump and the transporter; PMCA turnover rate is 30–250/s, while that of NCX is 2000–5000/s (Blaustein and Lederer, 1999). In addition, NCX activity requires Ca2+cyt to bind internal regulatory sites, which at the low [Ca2+]cyt and relatively low temperature conditions we tested, may not be optimally engaged (Blaustein and Lederer, 1999). Indeed, when Ca2+cyt was increased by addition of caloxin 2A1, which inhibited the PMCA along with the NKA blockade, the [Na+]cyt was greatly reduced revealing the operation of NCX in the reverse mode to extrude Na+cyt while facilitating Ca2+ entry (Figure 1). Furthermore, a decrease in basal [Ca2+]cyt due to ouabain treatment and via NCX modulation was occluded when astrocytes were co-treated by benzamil (Figure 1A).
At elevated [Ca2+]cyt induced by mechanical stimulation, the NKA indirectly affected Ca2+ homoeostasis as well. Inhibition of NKA decreased the peak but increased cumulative Ca2+ accumulation in response to mechanical stimulation (Figure 3). The reduced Ca2+cyt peak response suggests that elevated Na+cyt initially reduces the activity of the NCX (Blaustein and Lederer, 1999). However, as the NCX activity ramped up, the elimination of the NKA activity as the primary reducer of Na+cyt significantly increased the cumulative accumulation of Ca2+cyt (Figure 3C). This observation suggests that Na+ extrusion at elevated mechanically induced Na+cyt is in part mediated by the NCX in exchange for Ca2+ entry. It is possible that Na+ extrusion is also mediated by other Na+ extrusion systems, such as the NCKX (K+-dependent NCX) (Kim et al., 2005; Visser and Lytton, 2007), although neurons, but not astrocytes seem to preferentially express NCKX (Kiedrowski et al., 2002). Hence, treatment with ouabain alone or in combination with benzamil did not cause increase in peak and cumulative Na+cyt accumulation in mechanically stimulated astrocytes, but rather a significant decrease reaching levels similar to that seen in the stimulated astrocytes pre-treated with benzamil alone (Figure 4). This contradicts our previous consideration that decrease in [Na+]cyt seen in stimulated astrocytes pretreated with benzamil could be due to the NKA activity and might imply an involvement of NCKX in this process. Consequently, although a minor contribution of NCKX is expected (Kiedrowski et al., 2002), its possible role in the regulation of the Na+cyt and Ca2+cyt in astrocytes needs to be evaluated as it has been done in the calyx of Held (Kim et al., 2005).
The complex interplay between Na+cyt and Ca2+cyt dynamics and signalling in astrocytes warrants some further considerations. The NKA (type α2) have been found to colocalize with NCX in cortical astrocytes at plasma membrane–ER junctions where tightly regulated 'sodium microdomains’ may occur (Juhaszova and Blaustein, 1997; Blaustein et al., 2002). Inhibition of NKA has been shown to generate Ca2+ oscillations in cultured hippocampal astrocytes (Liu et al., 2007). In addition, subsets of mitochondria have been shown to interact with the ER (Rizzuto et al., 1998; Csordas et al., 2006). At higher [Na+]cyt near mitochondria–ER junctions, the driving force for Ca2+ efflux from mitochondria via the mitochondrial NCX would increase. Hypothetically, this Ca2+ efflux could in turn increase [Ca2+]cyt to activate ryanodine receptor of the ER. Indeed, it appears that the mitochondrial NCX plays a role in Ca2+cyt oscillations (Hernandez-SanMiguel et al., 2006).
Previous work showed that mild depolarization of cortical astrocytes cultured from adult, but not neonatal, rats caused NCX to operate in the reverse mode leading to [Ca2+]cyt increases and consequential exocytotic glutamate release (Paluzzi et al., 2007). Our data are consistent with this finding, yet they add an additional possibility: the involvement of the reverse mode of NCX in mechanically induced glutamate release from neonatal astrocytes. Mechanical stimulation drives Ca2+-dependent regulated exocytosis in astrocytes (Hua et al., 2004). The contribution of the NCX in these processes was seen as reduced peak and cumulative Ca2+cyt accumulations (Figures 5A–5C), as well as reduction in peak and cumulative release of glutamate from astrocytes to the ECS (Figures 5G–5I). It should be noted that, consistent with our calculated ENCX at rest and during mechanical stimulation, KB-R7943 blockade of Ca2+ entry via the reverse mode of NCX upon mechanical stimulation appeared smaller (85 and 90% of peak and cumulative response of control, respectively) than its effect on reduction of Ca2+ entry in astrocytes at rest (72% of basal level) (compare Figure 5A and Figure 1A). Additionally, KB-R7943 significantly reduced peak and cumulative Na+cyt accumulations (Figures 5D–5F), an indirect effect, likely due to an increased NKA activity, which is in agreement with data obtained using this blocker on astrocytes at rest.
The cellular location of the NCX favours its role in the modulation of Ca2+-dependent glutamate release. NCXs are located on plasma membrane–ER junctions and are expressed near InsP3 receptor-gated channels (Lencesova et al., 2004) and NKA (Juhaszova and Blaustein, 1997). Additionally, NCX is co-localized with plasma membrane glutamate transporters in perisynaptic processes (Minelli et al., 2007). Thus, in terms of glutamatergic synaptic transmission, as glutamate is released from neurons, it acts upon the plasma membrane glutamate transporters and ionotropic receptors on astrocytes, which leads to an increase in astrocytic [Na+]cyt (Langer and Rose, 2009; Lalo et al., 2011). Such Na+cyt dynamics should lead to depolarization causing NCX to operate in reverse and allow Ca2+ entry from the ECS subsequently stimulating glutamatergic gliotransmission. However, the role of plasma membrane glutamate ionotropic receptors and transporters in exocytotic glutamate release from astrocytes is speculative at the moment. Exposure of cultured cortical astrocytes to AMPA (α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid) did not induce glutamate release, unless it was co-applied with an mGluR agonist (Bezzi et al., 1998); co-application had much greater effect than that of an mGluR agonist alone. While this finding is consistent with the expression of GluR2 subunit in astrocytes, leading to low Ca2+ permeability through their AMPA channels, it is at odds with findings that depolarization due to increases in Na+cyt following activation of kainate receptors was reported to stimulate Ca2+ entry through the reverse mode of the NCX in specialized astrocytes, the Bergmann glia, in acute slice (Kirischuk et al 1997). These ostensibly incongruent findings could perhaps be due to different spatial associations between NCX, glutamate receptor and secretory machinery in different regions of the brain (cortex against cerebellum), choice of the agonist (AMPA against kainate) and preparation (culture against acute slice). Nonetheless, when operable, this NCX-linked pathway should be faster and spatially more confined, than the parallel pathway engaging astrocytic mGluRs tapping mainly into recruitment of Ca 2+ from the ER store to cause glutamatergic gliotransmission. Of course, this possibility would need to be corroborated morphologically by studying the spatial relationship between exocytotic proteins, NCX, glutamate receptors and transporters, as well as using electrophysiological recordings from neurons while specifically manipulating NCX in nearby astrocytes.
ACKNOWLEDGEMENTS
We dedicate this paper to the late Professor Dale J. Benos. We thank Manoj K. Gottipati for comments on a previous version of this paper.
Footnotes
This work was supported by the National Institute of Mental Health [grant number MH 069791] and the National Science Foundation [grant number CBET 0943343].
REFERENCES
- Araque A, Sanzgiri RP, Parpura V, Haydon PG. Astrocyte-induced modulation of synaptic transmission. Can. J. Physiol. Pharmacol. 1999a;77:699–706. [PubMed] [Google Scholar]
- Araque A, Sanzgiri RP, Parpura V, Haydon PG. Calcium elevation in astrocytes causes an NMDA receptor-dependent increase in the frequency of miniature synaptic currents in cultured hippocampal neurons. J. Neurosci. 1999b;18:6822–6829. doi: 10.1523/JNEUROSCI.18-17-06822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999c;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- Benz B, Grima G, Do KQ. Glutamate-induced homocysteic acid release from astrocytes: possible implication in glia-neuron signaling. Neuroscience. 2004;124:377–386. doi: 10.1016/j.neuroscience.2003.08.067. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- Blaustein MP, Lederer WJ. Sodium/calcium exchange: its physiological implications. Physiol. Rev. 1999;79:763–854. doi: 10.1152/physrev.1999.79.3.763. [DOI] [PubMed] [Google Scholar]
- Blaustein MP, Juhaszova M, Golovina VA, Church PJ, Stanley EF. Na/Ca exchanger and PMCA localization in neurons and astrocytes: functional implications. Ann. NY Acad. Sci. 2002;976:356–366. doi: 10.1111/j.1749-6632.2002.tb04762.x. [DOI] [PubMed] [Google Scholar]
- Chaudhary J, Walia M, Matharu J, Escher E, Grover AK. Caloxin: a novel plasma membrane Ca2+ pump inhibitor. Am. J. Physiol. Cell Physiol. 2001;280:C1027–1030. doi: 10.1152/ajpcell.2001.280.4.C1027. [DOI] [PubMed] [Google Scholar]
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Csordas G, Renken C, Varnai P, Walter L, Weaver D, Buttle KF, Balla T, Mannella CA, Hajnoczky G. Structural and functional features and significance of the physical linkage between ER and mitochondria. J. Cell. Biol. 2006;174:915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luisi A, Hofer AM. Evidence that Ca(2+) cycling by the plasma membrane Ca(2+)-ATPase increases the ‘excitability’ of the extracellular Ca(2+)-sensing receptor. J. Cell. Sci. 2003;116:1527–1538. doi: 10.1242/jcs.00368. [DOI] [PubMed] [Google Scholar]
- Deitmer JW. pH regulation and acid/base-mediated transport in glial cells. In: Hatton G I, Parpura V, editors. Glial-Neuronal Signaling. Boston, MA: Kluwer Academic Publishers; 2004. pp. 263–277. [Google Scholar]
- DiPolo R, Beauge L. Physiological role of ATP-driven calcium pump in squid axon. Nature. 1979;278:271–273. doi: 10.1038/278271a0. [DOI] [PubMed] [Google Scholar]
- Filosa JA, Bonev AD, Nelson MT. Calcium dynamics in cortical astrocytes and arterioles during neurovascular coupling. Circ. Res. 2004;95:e73–81. doi: 10.1161/01.RES.0000148636.60732.2e. [DOI] [PubMed] [Google Scholar]
- Fresu L, Dehpour A, Genazzani AA, Carafoli E, Guerini D. Plasma membrane calcium ATPase isoforms in astrocytes. Glia. 1999;28:150–155. [PubMed] [Google Scholar]
- Goldman WF, Yarowsky PJ, Juhaszova M, Krueger BK, Blaustein MP. Sodium/calcium exchange in rat cortical astrocytes. J. Neurosci. 1994;14:5834–5843. doi: 10.1523/JNEUROSCI.14-10-05834.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon GR, Mulligan SJ, MacVicar BA. Astrocyte control of the cerebrovasculature. Glia. 2007;55:1214–1221. doi: 10.1002/glia.20543. [DOI] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol. Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- He S, Ruknudin A, Bambrick LL, Lederer WJ, Schulze DH. Isoform-specific regulation of the Na+/Ca2+ exchanger in rat astrocytes and neurons by PKA. J. Neurosci. 1998;18:4833–4841. doi: 10.1523/JNEUROSCI.18-13-04833.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-SanMiguel E, Vay L, Santo-Domingo J, Lobaton CD, Moreno A, Montero M, Alvarez J. The mitochondrial Na+/Ca2+ exchanger plays a key role in the control of cytosolic Ca2+ oscillations. Cell Calcium. 2006;40:53–61. doi: 10.1016/j.ceca.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Hua X, Malarkey EB, Sunjara V, Rosenwald SE, Li WH, Parpura V. Ca2+-dependent glutamate release involves two classes of endoplasmic reticulum Ca2+ stores in astrocytes. J. Neurosci. Res. 2004;76:86–97. doi: 10.1002/jnr.20061. [DOI] [PubMed] [Google Scholar]
- Innocenti B, Parpura V, Haydon PG. Imaging extracellular waves of glutamate during calcium signaling in cultured astrocytes. J. Neurosci. 2000;20:1800–1808. doi: 10.1523/JNEUROSCI.20-05-01800.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhaszova M, Blaustein MP. Na+ pump low and high ouabain affinity alpha subunit isoforms are differently distributed in cells. Proc. Natl. Acad. Sci. U.S.A. 1997;94:1800–1805. doi: 10.1073/pnas.94.5.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano S, Otsu K, Shoji S, Yamagata K, Hiraoka M. Ca(2+) oscillations regulated by Na(+)-Ca(2+) exchanger and plasma membrane Ca(2+) pump induce fluctuations of membrane currents and potentials in human mesenchymal stem cells. Cell Calcium. 2003;34:145–156. doi: 10.1016/s0143-4160(03)00069-1. [DOI] [PubMed] [Google Scholar]
- Kiedrowski L, Czyz A, Li XF, Lytton J. Preferential expression of plasmalemmal K-dependent Na+/Ca2+ exchangers in neurons versus astrocytes. Neuroreport. 2002;13:1529–1532. doi: 10.1097/00001756-200208270-00008. [DOI] [PubMed] [Google Scholar]
- Kim MH, Korogod N, Schneggenburger R, Ho WK, Lee SH. Interplay between Na+/Ca2+ exchangers and mitochondria in Ca2+ clearance at the calyx of Held. J. Neurosci. 2005;25:6057–6065. doi: 10.1523/JNEUROSCI.0454-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirischuk S, Kettenmann H, Verkhratsky A. Na+/Ca2+ exchanger modulates kainate-triggered Ca2+ signaling in Bergmann glial cells in situ. FASEB J. 1997;11:566–572. doi: 10.1096/fasebj.11.7.9212080. [DOI] [PubMed] [Google Scholar]
- Kirischuk S, Kettenmann H, Verkhratsky A. Membrane currents and cytoplasmic sodium transients generated by glutamate transport in Bergmann glial cells. Pflugers Arch. 2007;454:245–252. doi: 10.1007/s00424-007-0207-5. [DOI] [PubMed] [Google Scholar]
- Kucheryavykh YV, Kucheryavykh LY, Nichols CG, Maldonado HM, Baksi K, Reichenbach A, Skatchkov SN, Eaton MJ. Downregulation of Kir4.1 inward rectifying potassium channel subunits by RNAi impairs potassium transfer and glutamate uptake by cultured cortical astrocytes. Glia. 2007;55:274–281. doi: 10.1002/glia.20455. [DOI] [PubMed] [Google Scholar]
- Lalo U, Pankratov Y, Parpura V, Verkhratsky A. Ionotropic receptors in neuronal-astroglial signalling: what is the role of 'excitable' molecules in non-excitable cells. Biochim. Biophys. Acta. 2011;1813:992–1002. doi: 10.1016/j.bbamcr.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Lalo U, Pankratov Y, Kirchhoff F, North RA, Verkhratsky A. NMDA receptors mediate neuron-to-glia signaling in mouse cortical astrocytes. J. Neurosci. 2006;26:2673–2683. doi: 10.1523/JNEUROSCI.4689-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalo U, Pankratov Y, Wichert SP, Rossner MJ, North RA, Kirchhoff F, Verkhratsky A. P2×1 and P2×5 subunits form the functional P2X receptor in mouse cortical astrocytes. J. Neurosci. 2008;28:5473–5480. doi: 10.1523/JNEUROSCI.1149-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer J, Rose CR. Synaptically induced sodium signals in hippocampal astrocytes in situ. J. Physiol. 2009;587:5859–5877. doi: 10.1113/jphysiol.2009.182279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Malarkey EB, Reyes RC, Parpura V. Micropit: a new cell culturing approach for characterization of solitary astrocytes and small networks of these Glial cells. Front Neuroeng. 1:2. 2008 doi: 10.3389/neuro.16.002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencesova L, O'Neill A, Resneck WG, Bloch RJ, Blaustein MP. Plasma membrane–cytoskeleton–endoplasmic reticulum complexes in neurons and astrocytes. J. Biol. Chem. 2004;279:2885–2893. doi: 10.1074/jbc.M310365200. [DOI] [PubMed] [Google Scholar]
- Liu XL, Miyakawa A, Aperia A, Krieger P. Na,K-ATPase generates calcium oscillations in hippocampal astrocytes. Neuroreport. 2007;18:597–600. doi: 10.1097/WNR.0b013e3280b07bc9. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ. Neuron-glia metabolic coupling and plasticity. J. Exp. Biol. 2006;209:2304–2311. doi: 10.1242/jeb.02208. [DOI] [PubMed] [Google Scholar]
- Malarkey EB, Parpura V. Temporal characteristics of vesicular fusion in astrocytes: examination of synaptobrevin 2-laden vesicles at single vesicle resolution. J. Physiol. 2011;589:4271–4300. doi: 10.1113/jphysiol.2011.210435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malarkey EB, Ni Y, Parpura V. Ca2+ entry through TRPC1 channels contributes to intracellular Ca2+ dynamics and consequent glutamate release from rat astrocytes. Glia. 2008;56:821–835. doi: 10.1002/glia.20656. [DOI] [PubMed] [Google Scholar]
- Maroto R, Raso A, Wood TG, Kurosky A, Martinac B, Hamill OP. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat. Cell Biol. 2005;7:179–185. doi: 10.1038/ncb1218. [DOI] [PubMed] [Google Scholar]
- Mata M, Fink DJ. Ca++-ATPase in the central nervous system: an EM cytochemical study. J. Histochem. Cytochem. 1989;37:971–980. doi: 10.1177/37.7.2525142. [DOI] [PubMed] [Google Scholar]
- McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J. Cell. Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier SD, Kovalchuk Y, Rose CR. Properties of the new fluorescent Na+ indicator CoroNa Green: comparison with SBFI and confocal Na+ imaging. J. Neurosci. Methods. 2006;155:251–259. doi: 10.1016/j.jneumeth.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Minelli A, Castaldo P, Gobbi P, Salucci S, Magi S, Amoroso S. Cellular and subcellular localization of Na+–Ca2+ exchanger protein isoforms, NCX1, NCX2, and NCX3 in cerebral cortex and hippocampus of adult rat. Cell Calcium. 2007;41:221–234. doi: 10.1016/j.ceca.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Montana V, Ni Y, Sunjara V, Hua X, Parpura V. Vesicular glutamate transporter-dependent glutamate release from astrocytes. J. Neurosci. 2004;24:2633–2642. doi: 10.1523/JNEUROSCI.3770-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgans CW, El Far O, Berntson A, Wassle H, Taylor WR. Calcium extrusion from mammalian photoreceptor terminals. J. Neurosci. 1998;18:2467–2474. doi: 10.1523/JNEUROSCI.18-07-02467.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothet JP, Pollegioni L, Ouanounou G, Martineau M, Fossier P, Baux G. Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter D-serine. Proc. Natl. Acad. Sci. U.S.A. 2005;102:5606–5611. doi: 10.1073/pnas.0408483102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431:195–199. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- Ni Y, Malarkey EB, Parpura V. Vesicular release of glutamate mediates bidirectional signaling between astrocytes and neurons. J. Neurochem. 2007;103:1273–1284. doi: 10.1111/j.1471-4159.2007.04864.x. [DOI] [PubMed] [Google Scholar]
- Paluzzi S, Alloisio S, Zappettini S, Milanese M, Raiteri L, Nobile M, Bonanno G. Adult astroglia is competent for Na+/Ca2+ exchanger-operated exocytotic glutamate release triggered by mild depolarization. J. Neurochem. 2007;103:1196–1207. doi: 10.1111/j.1471-4159.2007.04826.x. [DOI] [PubMed] [Google Scholar]
- Parpura V, Haydon PG. Physiological astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons. Proc. Natl. Acad. Sci. U.S.A. 2000;97:8629–8634. doi: 10.1073/pnas.97.15.8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V, Zorec R. Gliotransmission: exocytotic release from astrocytes. Brain Res. Rev. 2010;63:83–92. doi: 10.1016/j.brainresrev.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V, Grubisic V, Verkhratsky A. Ca2+ sources for the exocytotic release of glutamate from astrocytes. Biochim. Biophys. Acta. 2011;1813:984–991. doi: 10.1016/j.bbamcr.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Parpura V, Fang Y, Basarsky T, Jahn R, Haydon PG. Expression of synaptobrevin II, cellubrevin and syntaxin but not SNAP-25 in cultured astrocytes. FEBS Lett. 1995;377:489–492. doi: 10.1016/0014-5793(95)01401-2. [DOI] [PubMed] [Google Scholar]
- Perea G, Araque A. Astrocytes potentiate transmitter release at single hippocampal synapses. Science. 2007;317:1083–1086. doi: 10.1126/science.1144640. [DOI] [PubMed] [Google Scholar]
- Perea G, Araque A. Synaptic information processing by astrocytes. In: Parpura V, Haydon P G, editors. Astrocytes in (patho)physiology of the Nervous System. New York, NY: Springer; 2009. pp. 287–300. [Google Scholar]
- Perea G, Navarrete M, Araque A. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci. 2009;32:421–431. doi: 10.1016/j.tins.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Regehr WG. Interplay between sodium and calcium dynamics in granule cell presynaptic terminals. Biophys. J. 1997;73:2476–2488. doi: 10.1016/S0006-3495(97)78276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes RC, Parpura V. Mitochondria modulate Ca2+-dependent glutamate release from rat cortical astrocytes. J. Neurosci. 2008;28:9682–9691. doi: 10.1523/JNEUROSCI.3484-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes RC, Parpura V. Modulation of Ca2+-dependent glutamate release in rat cortical astrocytes by the Na+/Ca2+ exchanger. J. Neurochem. 2009;108:116–117. [Google Scholar]
- Reyes RC, Perry G, Lesort M, Parpura V. Immunophilin deficiency augments Ca2+-dependent glutamate release from mouse cortical astrocytes. Cell Calcium. 2011;49:23–34. doi: 10.1016/j.ceca.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- Rojas H, Ramos M, Dipolo R. A genistein-sensitive Na+/Ca2+ exchange is responsible for the resting [Ca2+]i and most of the Ca2+ plasma membrane fluxes in stimulated rat cerebellar type 1 astrocytes. Japan. J. Physiol. 2004;54:249–262. doi: 10.2170/jjphysiol.54.249. [DOI] [PubMed] [Google Scholar]
- Rojas H, Ramos M, Mijares A, DiPolo R. Role of Na+/Ca2+ exchange in [Ca2+]i clearance in rat culture Purkinje neurons requires reevaluation. Japan. J. Physiol. 2003;53:259–269. doi: 10.2170/jjphysiol.53.259. [DOI] [PubMed] [Google Scholar]
- Rojas H, Colina C, Ramos M, Benaim G, Jaffe EH, Caputo C, DiPolo R. Na+ entry via glutamate transporter activates the reverse Na+/Ca2+ exchange and triggers Ca(i)2+-induced Ca2+ release in rat cerebellar Type-1 astrocytes. J. Neurochem. 2007;100:1188–1202. doi: 10.1111/j.1471-4159.2006.04303.x. [DOI] [PubMed] [Google Scholar]
- Rose CR, Ransom BR. Gap junctions equalize intracellular Na+ concentration in astrocytes. Glia. 1997;20:299–307. doi: 10.1002/(sici)1098-1136(199708)20:4<299::aid-glia3>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Rychkov G, Barritt GJ. TRPC1 Ca(2+)-permeable channels in animal cells. Handb. Exp. Pharmacol. 2007;179:23–52. doi: 10.1007/978-3-540-34891-7_2. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144:810–823. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodosis DT, Poulain DA, Oliet SH. Activity-dependent structural and functional plasticity of astrocyte-neuron interactions. Physiol. Rev. 2008;88:983–1008. doi: 10.1152/physrev.00036.2007. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Orkand RK, Kettenmann H. Glial calcium: homeostasis and signaling function. Physiol. Rev. 1998;78:99–141. doi: 10.1152/physrev.1998.78.1.99. [DOI] [PubMed] [Google Scholar]
- Visser F, Lytton J. K+ -dependent Na+/Ca2+ exchangers: key contributors to Ca2+ signaling. Physiology (Bethesda) 2007;22:185–192. doi: 10.1152/physiol.00001.2007. [DOI] [PubMed] [Google Scholar]
- Zhong N, Beaumont V, Zucker RS. Roles for mitochondrial and reverse mode Na+/Ca2+ exchange and the plasmalemma Ca2+ ATPase in post-tetanic potentiation at crayfish neuromuscular junctions. J. Neurosci. 2001;21:9598–9607. doi: 10.1523/JNEUROSCI.21-24-09598.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signalling is central to the dynamic control of brain microcirculation. Nat. Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]