Abstract
Glutamate excitotoxicity is a major pathogenic process implicated in many neurodegenerative conditions, including AD (Alzheimer's disease) and following traumatic brain injury. Occurring predominantly from over-stimulation of ionotropic glutamate receptors located along dendrites, excitotoxic axonal degeneration may also occur in white matter tracts. Recent identification of axonal glutamate receptor subunits within axonal nanocomplexes raises the possibility of direct excitotoxic effects on axons. Individual neuronal responses to excitotoxicity are highly dependent on the complement of glutamate receptors expressed by the cell, and the localization of the functional receptors. To enable isolation of distal axons and targeted excitotoxicity, murine cortical neuron cultures were prepared in compartmented microfluidic devices, such that distal axons were isolated from neuronal cell bodies. Within the compartmented culture system, cortical neurons developed to relative maturity at 11 DIV (days in vitro) as demonstrated by the formation of dendritic spines and clustering of the presynaptic protein synaptophysin. The isolated distal axons retained growth cone structures in the absence of synaptic targets, and expressed glutamate receptor subunits. Glutamate treatment (100 μM) to the cell body chamber resulted in widespread degeneration within this chamber and degeneration of distal axons in the other chamber. Glutamate application to the distal axon chamber triggered a lesser degree of axonal degeneration without degenerative changes in the untreated somal chamber. These data indicate that in addition to current mechanisms of indirect axonal excitotoxicity, the distal axon may be a primary target for excitotoxicity in neurodegenerative conditions.
Keywords: α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA), distal axons, microfluidic, NMDA
Abbreviations: AD, Alzheimer's disease; AMPA, α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid; DIV, days in vitro; GFP, green fluorescent protein; MAP2, microtubule-associated protein-2; NMDA, N-methyl-d-aspartate; NR1, NMDA receptor subunit 1
INTRODUCTION
Glutamate excitotoxicity has been implicated as a major pathogenic process in many neurodegenerative conditions, including AD (Alzheimer's disease), motor-neuron disease and following traumatic brain injury (Arundine and Tymianski, 2004; Hynd et al., 2004). Excitotoxicity occurs by over-stimulation of excitatory amino acid receptors, resulting in calcium influx and consequential pathological changes triggering neuronal loss (Carriedo et al., 1996; Doble 1996; Van Den Bosch et al., 2000). The response of an individual neuron to excitotoxicity is dependent on a number of factors, including the intensity and duration of the excitatory insult, and the profile of excitatory receptors or downstream signalling molecules expressed by the cell (Arundine and Tymianski, 2003). Pathologically, excitotoxicity can result in apoptotic cell death that may be preceded by dendritic beading. Excitotoxicity has also been implicated in axon degeneration, and in cultured cortical neurons degeneration is preceded by neurofilament loss (Chung et al., 2005). Furthermore, excitotoxicity has been shown to result in a distal axonopathy in retinal ganglion cells (Saggu et al., 2008) and cultured motor neurons (King et al., 2007). It is currently unknown as to how excitotoxicity results in axonal degeneration.
Neuronal glutamate receptors, the mediators of excitotoxicity, are found on postsynaptic densities where they are involved in synaptic transmission. However, immunohistochemical techniques have demonstrated the presence of glutamate receptors at numerous extrasynaptic sites including the soma, dendrites and spines (reviewed in Newpher and Ehlers, 2008) and presynaptically (Tovar and Westbrook, 2002). Importantly, electrophysiological techniques have also indicated that these receptors can be functional (Andrasfalvy and Magee, 2001; Bardoni et al., 2004). Dendritic extrasynaptic receptors, and specifically NMDA (N-methyl-d-aspartate) receptors, have been particularly implicated in excitotoxicity (Sattler et al., 2000). Current evidence supports the notion that synaptic receptor activation promotes neuroprotection through activation of survival genes and suppression of apoptotic genes, whereas extrasynaptic stimulation promotes cell death (Hardingham et al., 2002), although this may be due to differences in receptor subunits (Liu et al., 2007). Thus, the distribution of synaptic and extrasynaptic NMDA receptors, rather than total calcium load, influence neuronal susceptibility and responses to excitotoxicity (reviewed in Hardingham and Bading, 2010).
Although excitotoxicity is considered primarily a neuronal somatodendritic insult, glutamate toxicity has also been demonstrated to occur in white matter tracts lacking neuronal cell bodies. This type of toxicity, often associated with brain injury, ischaemia and glaucoma (Stys and Li, 2000; Saggu et al., 2008), has been attributed to glial cells known to express functional glutamate receptors (Micu et al., 2006; Matute, 2007). Oligodendrocytes are directly vulnerable to AMPA (α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid) mediated Ca2+ excitotoxicity resulting in demyelination and secondary axonal pathology (Yoshioka et al., 1995; Bannerman et al., 2007). However, the recent identification of axonal glutamate receptor subunits within axonal internodal nanocomplexes, raises the possibility of direct axonal excitotoxicity (Ouardouz et al., 2009a, 2009b). The functional status of these receptors remains disputed; they may be involved in local regulatory mechanisms within the internodal nanocomplexes they reside in.
Investigation of the expression of glutamate receptors in different neuronal compartments and their role in excitotoxicity is difficult under standard culture conditions due to the inability to specifically target excitatory agonists. Similarly, in vivo investigations are complicated by the presence of glial cells. To overcome this, we have utilized microfluidic devices (Taylor et al., 2005) to establish compartmented embryonic cortical neuron cultures. Such devices allow fluidic isolation of distal axons from cell bodies, thus allowing focal exposure of the axon or soma to excitotoxins. In this study, we have examined the maturation of primary mouse cortical neurons within a microfluidic device, in addition to immunocytochemical and Western blot analysis of the expression of glutamate receptor subunits in both the somal and distal axon compartments. To determine if excitotoxin-induced axon degeneration can result from somal or axonal exposure to excitotoxin, we also examined the effect of a chronic (24 and 72 h) exposure of glutamate to either the somal or axonal chamber.
MATERIALS AND METHODS
All animals experiments used were reviewed and approved by the Animal Ethics Committee of the University of Tasmania.
Primary cell culture
Primary cortical neurons were dissected from the superficial layers of cerebral cortex of gestational day 14 embryos, obtained from pregnant C57Bl/6 mice and prepared as previously described (Dickson et al., 2000; King et al., 2006). Cells were dissociated in ‘initial’ plating medium: Neurobasal medium (Gibco BRL, Life Technologies), 2% B27 supplement (Gibco BRL, Life Technologies), 10% foetal bovine serum, 0.5 mM glutamine, 25 μM glutamate and penicillin/streptomycin. Cell density and viability was assessed using a Trypan Blue dye exclusion assay, and the volume adjusted to achieve a density of 8×106 cells/ml. Neurons were plated into prepared microfluidic devices (450 nm barrier grooves, Xona Microfluidics, Figure 1A) as outlined below. In contrast to Campenot compartmented chambers utilizing Teflon divisions and scratched substrate to guide axonal growth, microfluidic devices are fabricated from PDMS using a photoresist template to create microchannels for axonal growth between compartments (Taylor et al., 2005). Devices were attached to 22 cm2 glass coverslips (Livingstone), coated with 0.001% poly-l-lysine and incubated overnight. Microfluidics were rinsed with initial plating medium, which was removed immediately prior to addition of cells into the somal chamber. Plated neurons were incubated for 10 min to facilitate adhesion, followed by addition of initial plating medium. Cultures were incubated under standard conditions (37°C, 5% CO2), with the medium changed to 'subsequent' growth medium (Neurobasal medium, 2% B27 supplement, 0.5 mM glutamine and penicillin/streptomycin), at 1 and 7 DIV (days in vitro).
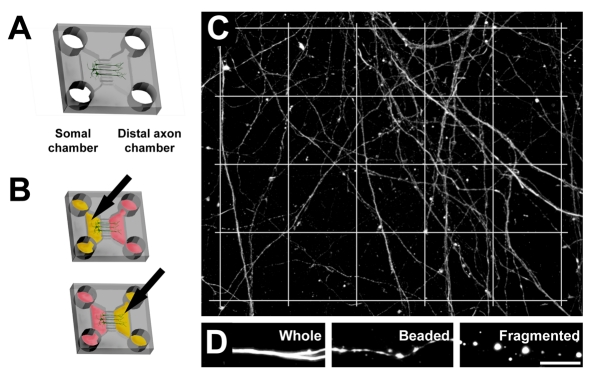
Figure 1. Schematic diagram of neuronal growth within the microfluidic culture device.
Cell bodies are restricted to the somal chamber, and axons extend into the distal axonal chamber (A). Arrows indicate selective glutamate treatment (yellow) of either the somal or distal axonal chamber, fluidic isolation is maintained by manipulating fluid levels (B). Quantification of axon degeneration following excitotoxicity was achieved by analysing axonal segments in each square of a superimposed grid (C); axonal morphology was scored as whole, beaded or fragmented (D). Scale bar = 5 μm.
Labelling of live cells in microfluidic chambers
Not all neurons extended axons through to the distal chamber, therefore, to identify neuronal soma with isolated distal axons, the lipophilic membrane stain CM-DiI (1 μg/ml, Molecular Probes) was added to the distal axon chamber. Microfluidic isolation between the treated and untreated sides was achieved by maintaining a lower fluid volume on the treated side (Taylor et al., 2005). Microfluidic devices were incubated for 4 h under standard growth conditions, followed by media change. Subsequent experimentation was performed the next day, as required.
In some experiments, neurons were transfected with a plasmid expressing GFP (green fluorescent protein; pmax GFP, Lonza) to allow morphological analysis of neuronal processes. Briefly, 800 ng plasmid DNA was applied to the somal chamber using the LipofectamineTM 2000 reagent (Invitrogen) for 6 h, under standard growth conditions, according to the manufacturer's instructions. This was followed by complete media change. Transfected neurons were visualized by fluorescence microscopy after 24 h.
Excitotoxicity
Excitotoxicity was initiated in mature (11 DIV) compartmented cultures (n = 5 repeats) by a single application of 100 μM glutamate in culture medium (Chung et al., 2005) to either the distal axon or somal chamber of the microfluidic device for 24 h (Figure 1B). Microfluidic isolation was performed as above. Additional cultures were treated with 100 μM glutamate for 72 h to the axonal chamber, beginning at 10 DIV. Treated cultures were maintained under standard growth conditions for 24 or 72 h, followed by fixation.
Inhibition of caspase activity
The pan-caspase inhibitor Z-VAD-FMK (R&D Systems) was added to either the distal axon or somal chambers to inactivate caspase activity. Cultures were pre-incubated in 10 μM Z-VAD-FMK in culture medium for 2 h and excitotoxicity induced as described above.
Cell fixation and immunocytochemical labelling
Cortical neuron cultures were fixed with 4% PFA (paraformaldehyde) for 30 min at room temperature, then permeabilized with 0.3% Triton X-100 for 2–5 min. For NR1 (NMDA receptor subunit 1) visualization, coverslips were fixed in ice-cold methanol for 10 min at −20°C, omitting permeabilization (note: AMPA receptor immunoreactivity was not affected by the method of fixation). This was followed by incubation with primary antibodies diluted in PBS, for 1 h at room temperature followed by 4°C overnight. Antibodies used in this study include those directed to GluR1 (1:100 Chemicon), GluR4 (1:500 Chemicon), MAP2 (microtubule-associated protein-2; 1:1000 Millipore), NF-M (1:1000 Serotec), NR1 (1:10,000 BD Pharmingen), synaptophysin (1:200 Millipore). All antibodies are commercially available and optimum concentrations were individually determined for each antibody. Species and isotype appropriate fluorescent AlexaFluor secondary antibodies (1:1000, Molecular Probes) were applied for 2 h at room temperature. The filamentous actin dye, phalloidin (1:200, AlexaFluor 488, Molecular Probes), was applied for 30 min during the secondary antibody incubation. All antibodies have been used in our previous studies (King et al., 2006, 2011). No cross reactivity for the primary antibody's isotype or secondary antibody non-specific binding have been observed. Following antibody labelling, coverslips were stained with Nuclear Yellow (0.0001%, Sigma) to identify nuclei.
Western blot
For Western blot analysis, the somal or distal axon chambers were harvested from 10 microfluidic devices (n = 2 repeats) in ice-cold Tris–Trion buffer (10 mM Tris, pH 7.4; 100 mM NaCl; 1 mM EDTA; 1 mM EGTA; 1% Triton X-100; 10% glycerol; 0.1% SDS and 0.5% deoxycholate) supplemented with CompleteTM (protease inhibitor cocktail, Roche). Samples were separated by SDS/12.5% PAGE. Coomassie Brilliant Blue staining was also performed on a gel from each experiment. Protein gels were transferred to a PVDF membrane. Membranes were blocked overnight in 5% non-fat dried skimmed milk powder, followed by an overnight incubation in primary antibodies, including a combination of GluR1 (1:200, Chemicon), GluR2 (1:500, Chemicon), GluR3 (1:500, Chemicon) and GluR4 (1:500, Chemicon) for AMPA receptors and NR1 (1:1000, BD Pharmingen) for NMDA receptors. Membranes were washed in TBS-Tween. Species appropriate HRP (horseradish-peroxidase)-conjugated secondary antibodies (1:1000, Dako) were applied and visualized with chemilluminescent peroxidase substrate (Sigma).
Quantification of axon degeneration
Treated cultures were immunolabelled for neurofilaments and images for analysis were obtained using a Leica (DM LB2) fluorescence microscope fitted with a cooled CCD camera (Magnafire Optronics). Image acquisition and subsequent analysis was performed blinded to experimental conditions. Four ×400 images were randomly captured from each distal axon chamber. A 4×5 50 μm2 grid (Figure 1C) was superimposed on each image using Adobe Photoshop (CS5). Axons in each square of the grid were scored as either whole, beaded (distinguishable swellings connected by sections of axon) or fragmented (disconnected swellings) (Figure 1D). Overall degeneration was calculated as the sum of beaded and fragmented axons. Values were expressed as a percentage of total axons. Total values from each square were averaged for each coverslip and analysed using Student's t-test. P-values <0.05 were considered significant. A minimum of five separate culture repeats were analysed. Data are represented as means±SEM.
RESULTS
Developmental characteristics of compartmented cortical neuronal culture
Mouse cortical neuron cultures were established within compartmented microfluidic culture devices (Taylor et al., 2005) and their growth characteristics were examined over a time course (Figures 2A–2E). Initial neuronal development (1–3 DIV) was restricted to the somal chamber (Figure 2A). By 5 DIV, the neurons had extended multiple neurites within the somal chamber (Figure 2C) and axons were present within the microchannels (Figure 2D). At 7 DIV long, relatively unbranched axons were present extending from the microchannels into the distal axon chamber, forming an extensively branched network at 11 DIV (Figure 2F). Isolated axonal health declined from 14 DIV, with extensive distal axon degeneration present at 15 DIV (Figure 2H). Degeneration of axons within the distal axon chamber from 15 DIV was not accompanied by degeneration within the somal chamber (Figure 2G).
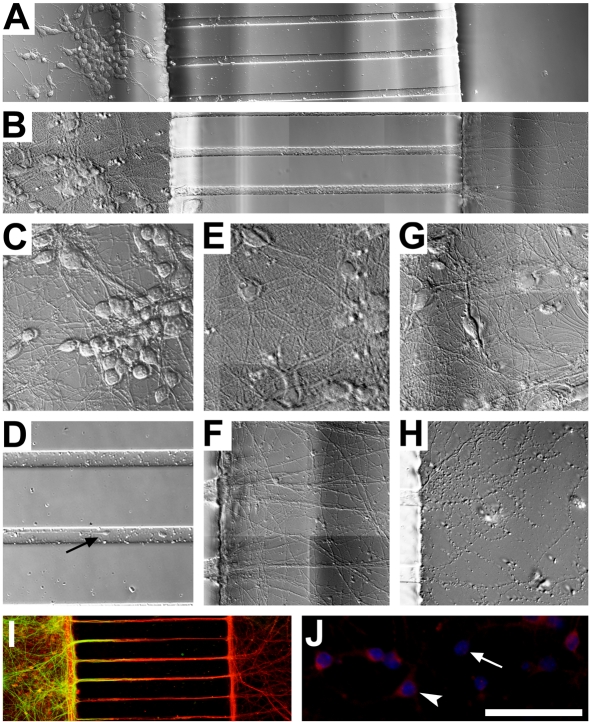
Figure 2. Neuronal growth in compartmented culture.
Initial neuronal growth in compartmented culture (2 DIV) (A) is restricted to the somal chamber with axons extending into the distal chamber to form a branched network at 11 DIV (B). At 5 DIV neuronal growth is characterized by branched neurites within the somal chamber (C) and neurites visible within the microchannels (arrow) (D). At 11 DIV neurites within somal chamber (E) were densely branched, and axons within the distal axon chamber (F) had formed extensive branched networks. Neurons retained normal healthy morphology within the somal chamber at 15 DIV (G) despite severe deterioration of isolated axons (H). Immunolabelling of 11 DIV neurons (I) demonstrates cell bodies (MAP2, green) restricted to the somal chamber and axons (NF-M, red) present within the somal chamber and extending to the distal axon chamber. Neurons (Nuclear Yellow, blue) with isolated axons were identified by CM-DiI (red) retrograde labelling from the distal axon chamber (J). Arrowhead indicates a neuron positive for CM-DiI uptake, arrow indicates a neuron negative for CM-DiI. Scale bars A = 100 μm, B–D = 50 μm, E = 30 μm, F = 50 μm.
Double immunolabelling verified that axons (NF-M immunoreactivity) were present in the distal axon chamber, with neuronal cell bodies and dendrites (MAP2 immunoreactivity) restricted to the somal chamber (Figure 2I), as previously described by Taylor et al. (2005). NF-M immunoreactive axons were also present in the somal chamber. Neurons with axons extending into the distal chamber were identified by incubating the distal axon chamber with CM-DiI prior to treatment. CM-DiI was taken up by the axons and transported to the cell body (Perlson et al., 2009) (Figure 2J). DiI retrograde labelling from the distal axon chamber indicated that approximately 30% of neurons extended axons to the distal chamber.
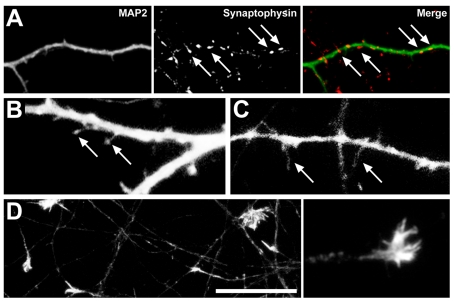
Neuronal maturity in compartmented cultures was determined by examining the presence of growth cones, synapses and spines. Previous investigations have demonstrated that under standard conditions, immature neurons prior to the development of synapses have numerous growth cones (Haas et al., 2004). As neurons mature, punctate synapses and mushroom-shaped spines are formed on the dendrites accompanied by the loss of growth cones (King et al., 2006). In the current study, the presence of growth cones in compartmented cultures was examined by phalloidin staining for filamentous actin and synaptic puncta were visualized by immunolabelling with the presynaptic marker synaptophysin in addition to the dendritic marker MAP2. Dendritic spine morphology was determined by examination of neurons transiently transfected with a GFP expression construct. In the somal chamber at 11 DIV, few growth cones were present and synaptophysin positive puncta were immunolabelled along MAP2 immunopositive dendrites (Figure 3A), with the number and density of puncta varying between cells, congruent with previous investigations (King et al., 2006). Furthermore, GFP expression revealed short, mushroom-shaped spines (Figure 3B) on the dendrites in addition to a number of long filopodial spines (Figure 3C). These data indicate that, in the somal chamber, synapses were present in accordance with neuronal maturity in standard cultures (King et al., 2006). However, in the distal axonal chamber at this developmental stage, large growth cones were present at the ends of axons (Figure 3D), indicating a stage of immaturity not usually seen in standard cultures at this time point in vitro.
Figure 3. Markers of neuronal maturity in compartmented culture.
At 11 DIV, synaptophysin expression is localized to puncta (arrows) along MAP2 immunoreactive dendrites (A) within the somal chamber. GFP expression indicates short, mushroom-shaped spines (arrows) along dendrites (B) in addition to long filopodial spines (arrows) (C). Axons within the distal axon chamber (D) retain growth cones, immunoreactive for filamentous actin; inset shows higher power of growth cone morphology. Scale bars A = 25 μm, B–C = 4 μm, D = 20 μm.
Glutamate receptor expression in compartmented cultures
To examine whether components of the machinery required for functional glutamatergic signalling, and therefore excitotoxicity, were present, the expression of glutamate receptor subunits was determined. Expression of both NMDA and AMPA receptors was examined within the somal and distal axon chambers of mature (11 DIV) cultures by immunocytochemistry using glutamate subunit specific antibodies. The expression profiles of the receptors varied between neurons within each culture. Within the somal chamber, immunoreactivity for AMPA (GluR1) and NMDA (NR1) receptor subunits was present throughout the soma, with punctate expression along the dendrites (Figures 4A and 4B). Within the distal chamber, AMPA subunit labelling (GluR4) was frequently present and punctate along distal axons and within the growth cone (Figure 4C). Immunoreactivity for NMDA receptors was occasionally present within distal axon growth cones (Figure 4D).
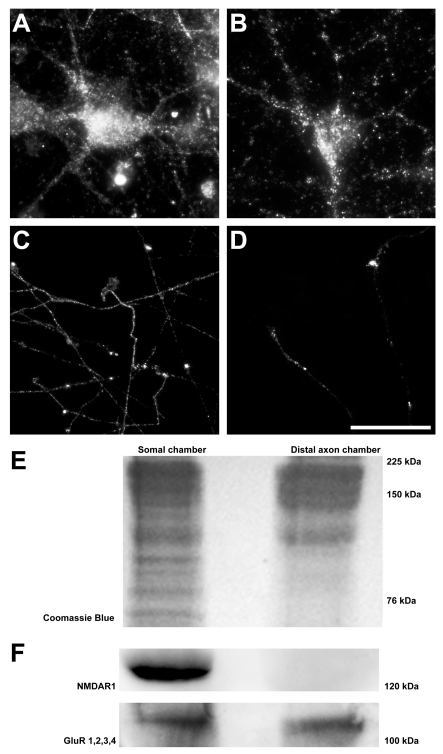
Figure 4. Expression of AMPA and NMDA glutamate receptor subunits in compartmented culture.
AMPA (GluR1) and NMDA (NR1) immunoreactivity were both present throughout the soma, with punctate expression along the dendrites (A, B). Distal axon AMPA (GluR4) immunoreactivity was frequently present, along distal axons and within the growth cone (C). Expression of NMDA receptors (NR1) was occasionally present within distal axon growth cones (D). Western blot analysis of somal and distal axonal expression (E) indicates AMPA subunits in both chambers; however, NMDA receptors were not detected in the distal axon chamber (F). Scale bars A–B = 20 μm, C–D = 50 μm.
To confirm the presence of glutamate receptor subunits in the somal and distal axon chambers, protein was harvested from each chamber and glutamate receptor subunit expression determined by Western blot analysis. Coomassie staining of SDS/PAGE gels demonstrated a good yield of protein from both chambers (Figure 4E). Western blots confirmed the presence of NMDA (NR1 labelling at 120 kDa) and AMPA (GluR1 GluR2, GluR3 and GluR4 labelling at 100 kDa) receptors in the somal chambers (Figure 4F). Interestingly, Western blot analysis also demonstrated the presence of AMPA receptors in protein harvested from the distal axon chamber (Figure 4F). NMDA receptors, however, were not detected in the distal axon chamber (Figure 4F). Together, these data indicate differential expression of glutamate receptors subunits on both the somatodendritic compartment and distal axon of the cortical neuron, with strong evidence for the presence of AMPA receptor subunits in the axon.
Functional contribution of expressed glutamate receptors to focal excitotoxicity
To determine the role of the axon in mediating excitotoxicity, mature (11 DIV) mouse cortical neurons were treated with 100 μM glutamic acid or vehicle control, applied to either the somal or the distal axon chamber of the microfluidic device, and maintained for 24 h post-treatment. Following treatment, distal axon degeneration was assessed based on neurofilament immunoreactivity for axonal integrity.
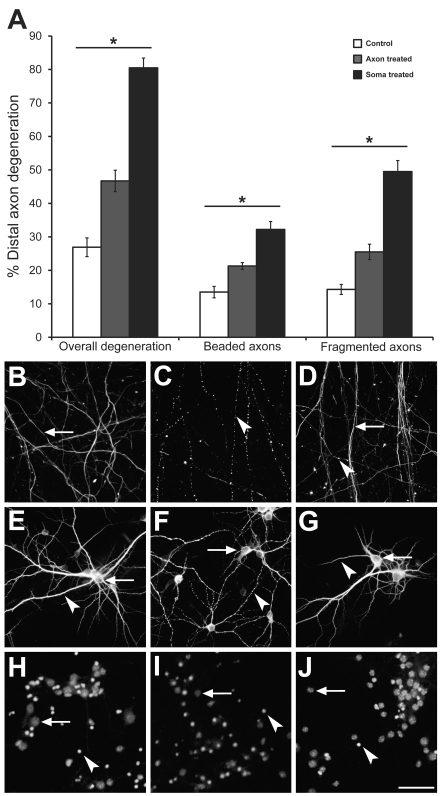
Glutamate applied to the somal chamber resulted in extensive neuronal degeneration, both within the treated chamber and at the unexposed distal axon. Specifically, axons in both chambers frequently showed a beaded morphology and additionally complete axon fragmentation was present in some axons. Quantitative analysis of neurofilament immunoreactivity in the untreated distal axon chamber demonstrated that somal glutamate treatment resulted in a significant (P<0.01) 3-fold increase in total axon degeneration, including the sum of both beaded and fragmented axons (80.5%±2.9). Beading (32.2%±2.4) and fragmentation (49.5%±3.3) were significantly (P<0.01) increased relative to untreated controls (26.9%±2.8 total damage; 13.5%±1.7 beading; 14.3%±1.5 fragmentation) (Figure 5A). Widespread axonal degeneration was also present in the treated somal chamber (not quantified) (Chung et al., 2005).
Figure 5. Axon degeneration following excitotoxicity.
Somatodendritic excitotoxicity resulted in a significant (P<0.01) 3-fold increase in distal axon degeneration (A). Distal axonal excitotoxicity resulted in a significant (P<0.01) 1.5-fold increase in distal axon degeneration (A). Immunocytochemical analysis of control and treated cultures (B–J). Distal axons demonstrated increased beading and fragmentation between control (B), somal-treated (C) and distal axon-treated (D) cultures, visualized with NF-M immunoreactivity. Arrows indicate whole axons, arrowheads indicate fragmented and beaded axons. Within the somal chamber, MAP2 immunoreactive dendrites demonstrated increased beading between control (E) and somal-treated (F) cultures. Distal axon-treated cultures (G) did not demonstrate changes to dendritic morphology. Arrows indicate soma, arrowheads indicate dendrites. Similarly, nuclear health (Nuclear Yellow) declined between control (H) and somal-treated cultures (I) as demonstrated by an increase in apoptotic nuclei (arrowhead) versus normal nuclei (arrow). Distal axon-treated cultures (J) showed no change in nuclear morphology compared with controls. Scale bar = 50 μm.
We next determined if specific targeting of excitotoxicity to the distal axon chamber could also result in degenerative changes. Twenty-four hour glutamate exposure also resulted in beading and fragmentation of the distal axon. Quantitative analysis of neurofilament immunoreactivity demonstrated a significant (P<0.01) 1.5-fold increase in total degeneration (46.7%±3.2), axonal beading (21.3%±1.0) and axonal fragmentation (25.5%±2.3) when compared with untreated controls (26.9%±2.8, total damage; 13.5%±1.7 beading; 14.3%±1.5 fragmentation) (Figure 5A). The degenerative changes in distally treated cultures was significantly (P<0.01) 1.5-fold less than the distal degeneration recorded from somal treatment, assessed as both axonal beading and fragmentation.
The neuron-wide effects of somal and axonal excitotoxin exposure were examined to determine the extent of neuronal damage. In addition to distal axon morphology (neurofilament immunoreactivity) (Figures 5B–5D), we examined changes to dendrites (MAP2 immunoreactivity) (Figures 5E–5G) and overall cell health, using Nuclear Yellow to assess nuclear integrity (Figures 5H–5J). Somal excitotoxicity resulted in severe dendritic beading at 24 h post-treatment (Figure 5E), as has been described previously (Park et al., 1996). There was a significant (20%±3.2, P<0.01) increase in apoptotic (condensed) nuclei following somatodendritic excitotoxicity (Figure 5I). In contrast with somal exposure, the unexposed somal chambers of distally treated cultures had no change to dendritic morphology relative to controls (Figure 5G). The nuclear morphology of CM-DiI-stained neurons was assessed; however, there was no difference in the percentage of apoptotic nuclei between axonal excitotoxicity at 24 h and untreated controls (25.4%±2.0 24 h excitotoxicity; 22.7%±1.3 control).
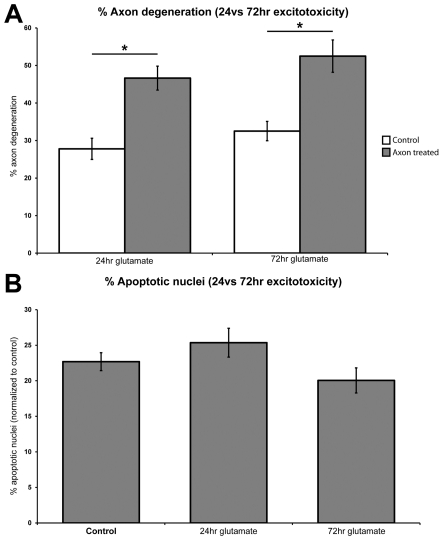
To investigate whether delayed apoptosis occurred, through dying-back from the axon, cultures were treated with 100 μM glutamate to the distal axon chamber for 72 h at 10 DIV and axon degeneration and cell death assessed. Seventy two hour-treated cultures demonstrated a significant (P<0.01) increase in total axon degeneration from untreated controls (52.5%±4.3 and 32.5%±2.6, respectively); however this was not significantly (P>0.05) different to cultures treated for 24 h (46.7%±3.2) (Figure 6A). Additional cultures were labelled with CM-DiI to the distal axon chamber prior to treatment to specifically label neuronal soma with axons in the distal chamber. Following treatment, the nuclear morphology of DiI positive soma was assessed with no significant (P>0.05) change to the percentage of apoptotic nuclei relative to controls (13.9%±1.9 72 h glutamate, 17.8%±1.4 controls) (Figure 6B).
Figure 6. Cell effects of long-term distal axonal excitotoxicity.
Application of glutamate to the distal axon compartment for 72 h significantly (P<0.01) increased percentage axon degeneration from untreated controls, however, it did not significantly (P>0.05) alter the percentage of axon degeneration from 24 h treated cultures (A). Application of glutamate to the distal axon chamber for 72 h did not significantly (P>0.05) alter the percentage of apoptotic nuclei between untreated, 24 h and 72 h treated cultures (B).
Mechanisms of focal excitotoxin-induced axonal degeneration
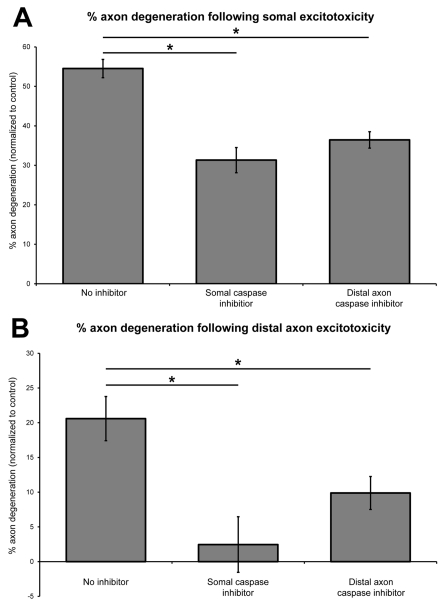
To investigate whether axon degeneration following soma or axonal excitotoxicity involved activation of caspases and apoptotic pathways, 11 DIV cultures were pre-incubated with 10 μM Z-VAD-FMK pan caspase inhibitor to either the somal or distal axon chambers. Cultures were subsequently treated with 100 μM glutamate for 24 h to induce excitotoxicity in the two compartments. The efficacy of the inhibitor was confirmed by a significant (P<0.05) decrease in apoptotic nuclei following somal excitotoxicity when compared with no inhibitor (16.06%±3.93 glutamate only; 3.93%±3.15 inhibition+glutamate).
Somal caspase inhibition prior to somal excitotoxicity significantly (P<0.01) decreased distal axon degeneration (31.3%±3.2) when compared with somal treatment alone (54.5%±2.3) (Figure 7A). Axonal caspase inhibition combined with somal excitotoxicity also significantly (P<0.01) reduced axon degeneration (36.4%±2.1) (Figure 7A). Somal caspase inhibition and axonal caspase inhibition similarly significantly (P<0.01) decreased axon degeneration in conjunction with axonal excitotoxicity (2.5%±4.0 and 9.9%±2.4, respectively) compared with axonal excitotoxicity alone (20.6%±3.2) (Figure 7B). For both axonal and somal excitotoxicity, there was no significant (P>0.05) difference between somal and axonal application of caspase inhibition.
Figure 7. Mechanisms of axon degeneration following focal excitotoxicity.
Inhibition of caspase activity, in either the somal or the axonal chamber, significantly (P<0.01) decreased axon degeneration following somal excitotoxicity (A), and following axonal excitotoxicity (B). Results expressed relative to untreated controls.
DISCUSSION
In this study, we have grown primary cortical neurons in a compartmentalized microfluidic device to determine the expression of glutamate receptors in specific neuronal compartments and to investigate degenerative responses following chronic targeted distal axon excitotoxicity. Of relevance to the current study, our previous investigations (King et al., 2006), and those of others (Choi et al., 1987; Liu et al., 1996) have demonstrated that neuronal vulnerability to excitotoxicity is dependent on neuronal maturity and expression of glutamate receptor subunits, which is variable between cell types. Thus, for the current study it was important to determine the maturity of neurons within the microfluidic culture chambers.
Under standard growth conditions, development of primary murine cortical neurons occurs via a sequence of predetermined steps that include neurite outgrowth, polarization and elongation followed by movement of glutamate receptors into the synapses and loss of immaturity markers such as growth cones (Dotti et al., 1988, Haas et al., 2004). The timing of these developmental stages is dependent on culture density (de Lima et al., 1997; Rao et al., 1998). Assessment of neuronal growth of cultures in microfluidic chambers indicates that development occurs in a similar manner to standard cortical culture within the somal chamber, including the presence of mature spines and punctate glutamate receptor subunits at 11 DIV. However, in the axonal chamber, even at relative culture maturity (11 DIV), numerous axonal growth cones remained present. The presence of growth cones is likely to be due to the inability of presynaptic neurons to find postsynaptic partners in this culture system and may affect the axonal expression of glutamate receptor subunits.
Our data indicate that, in addition to the well-documented expression of glutamate receptor subunits on the somatodendritic compartment of cultured primary cortical neurons (King et al., 2006), glutamate receptors, and in particular AMPA receptors, were also present on axons. Excitotoxic stimulation of axonal glutamate receptors resulted in axonal degeneration, which, unlike somatodendritic exposure, was confined to the exposed segment of the axon and did not cause retrograde degeneration or apoptotic cell death, even at extended time-points of 72 h. These data also suggest the presence of functional glutamate receptor complexes on the axons in this chamber.
Axon degeneration and excitotoxicity
Axon degeneration following glutamate receptor stimulation has been previously reported in a number of studies. In vitro studies have indicated that axon degeneration occurs as a result of chronic excitotoxicity in cultured motor (King et al., 2007) and cortical (Chung et al., 2005) neurons. In these studies, however, glutamate or other agonists were globally applied to the cells and so it is unclear if axon degeneration occurred from toxicity to somatodendritic glutamate receptors, or receptors present on axons, growth cones or presynaptic terminals. In vivo studies have allowed focal excitotoxin exposure of neuronal compartments. Somal glutamate exposure to retinal ganglion cells resulted in a degeneration of the distal axon (Saggu et al., 2008), confirming excitotoxic axonal degeneration in the unexposed axon segment, consistent with the current study in cortical neurons. In vivo glutamate exposure to myelinated axons also resulted in axonal damage to the optic nerve (Matute, 1998) and external capsule (Fowler et al., 2003). The vulnerability of myelinating oligodendrocytes to excitotoxicity has been well documented and myelin has been demonstrated to play a role in axonal excitotoxicity (Fowler et al., 2006). However, studies using myelin-deficient Shiverer mice show that compact myelin is not required for AMPA toxicity to axons (Pitt et al., 2010).
A study by Underhill and Goldberg (2007) utilized a Campenot style compartmentalized culture system to directly examine the role of glutamate receptor activation in axon degeneration in the absence of glial cells. Their data demonstrated that brief (2 h) axonal exposure to glutamate receptor agonists, NMDA or AMPA, did not result in significant axonal degeneration. Conversely, the current study using a compartmentalized microfluidic culture system, demonstrates the novel finding that chronic (24 h) glutamate exposure results in axonal blebbing and fragmentation in a proportion of axons. The reasons for these conflicting results may be the length of time of the exposure, the agonists used or differences in the culture systems, which may select for isolation of axons from specific cell types. Interestingly, in vivo studies also suggest difference in chronic versus acute excitotoxic axonal exposure (Matute, 1998). These data suggest that excitotoxicity can be mediated through glutamate receptors expressed on the axon.
Excitotoxicity and glutamate receptor expression
In the current study excitotoxicity could be mediated through glutamate receptors expressed either on the axon shaft or on the growth cones. Glutamate receptors can be trafficked to the axonal compartment and in particular their presence within growth cones during neuronal development has been well documented (King et al., 2006), with a proposed involvement in pathfinding (Zheng et al., 1996). Recently, subunits for AMPA and kainate receptors have been shown to be present on myelinated axons (Ouardouz et al., 2009a, 2009b), although the functional activity of these receptors is currently disputed. Our data confirm the presence of AMPA receptors in the axonal compartment of cultured axons by immunocytochemical labelling and Western blot analysis. The role of these axonal glutamate receptors is unclear; however, the expression of functional NMDA and non-NMDA receptors on glial cells (Gallo and Russell, 1995; Verkhratsky and Kirchhoff, 2007) raises the possibility that axonal glutamate receptor subunits may facilitate glutamatergic signalling between axons and myelinating oligodendrocytes or astrocytes.
A limitation of the current study is that cultured distal axons were unable to synapse on other neurons, preventing the formation of the presynaptic terminal. This excludes the possibility that excitotoxicity was mediated through presynaptic receptors. In vivo, both NMDA and AMPA receptors are found presynaptically, where they are thought to regulate glutamate release (recently reviewed in Pinheiro and Mulle, 2008) and could potentially be targets for excitotoxic stimulation. Although the expression pattern of axonal glutamate receptors may differ in vivo, the current study suggests that excitotoxicity can be mediated through extrasynaptic glutamate receptors expressed on the axon.
The demonstration of the extrasynaptic expression of glutamate receptor subtypes (Passafaro et al., 2001; Tovar and Westbrook, 2002; Kane-Jackson and Smith, 2003; van Zundert et al., 2004) suggests a wider role for glutamate than solely as an inter-neuronal excitatory transmitter (Araque and Perea, 2004). Glutamate receptor subunits at non-synaptic sites on dendrites and spines are thought to act as a reserve supply of synaptic receptors (for recent review see Newpher and Ehlers 2008). However, electrophysiological recordings indicate that these extrasynaptic receptors are functional (Andrasfalvy and Magee, 2001) and a modulatory role has been suggested, through activation by glutamate spillover from the synapse or glial derived glutamate (Diamond and Jahr, 2000; Jourdain et al., 2007). In terms of pathological stimulation of glutamate receptors, extrasynaptic and synaptic receptors have been reported to play a significantly different role in excitotoxicity. Stimulation of synaptic NMDA receptors is neuroprotective, whereas activation of extrasynaptic NMDA receptors triggers neuronal degeneration (Hardingham et al., 2002), although some authors demonstrate that preferential expression of receptor subunit types at extrasynaptic sites is responsible for this effect rather than localization itself (Liu et al., 2007).
Mechanisms of axonal glutamate excitotoxicity and implications for disease
Mechanism of axonal degeneration, including potential differences between axonal and somal glutamate stimulation, cannot be fully determined in the current study; however, the protective effect of a pan-caspase inhibitor suggests the involvement of pathways associated with apoptosis. It is of particular interest to note that application of inhibitors to either the soma or axon provided protection from degeneration. The role of axonal caspases in axonal degeneration is being increasingly recognized and has been reported in a number of models (Schoenmann et al., 2010, Smith et al., 2011). The study also suggests that retrograde signalling to the soma is involved in axon degeneration following axonal excitotoxicity, without inducing frank apoptosis. Further elucidating the mechanisms of excitotoxin-induced axon degeneration will be the subject of future studies using this model.
The findings of the current study have a number of implications for our understanding of neurodegenerative disease. Excitotoxicity within the white matter has been shown to occur in a number of degenerative conditions including glaucoma (Saggu et al., 2008) and multiple sclerosis (Pitt et al., 2003), and is also common following injury (reviewed in Lau and Tymianski, 2010). Furthermore, axonal excitotoxicity may be involved in any condition involving excitotoxic pathogenesis including, potentially, ALS (amyotrophic lateral sclerosis) (van Damme et al., 2005) and AD (Hynd et al., 2004), with local regions of excitotoxicity triggering axon degeneration and synaptic loss. At present, excitotoxic damage is attributed to neuronal soma and glial cells, and secondary axon degeneration via axon–glia signalling. The current study suggests that axon degeneration could occur from direct exposure of the axon to excitotoxins. However, axonal excitotoxicty did not result in a dying back, even 72 h following exposure. The relative immaturity of the axons through lack of presynaptic targets and therefore retrograde signals from postsynaptic cells may be a factor in the lack of die back in this study. It is currently unknown as to how axonally mediated excitotoxicity affects a cell's survival in mature cells in vivo in the long-term. However, this may have therapeutic implications as intervention may need to be directed specifically to induce axon protection.
Footnotes
The work was supported by the Wicking Dementia Research and Education Centre, National Health and Medical Research Council [grant number APP10039], Tasmanian Masonic Centenary Medical Research Foundation, and Alzheimer's Association Australia, Motor Neuron Disease Research Institute of Australia, University of Tasmania.
REFERENCES
- Andrasfalvy BK, Magee JC. Distance-dependent increase in AMPA receptor number in the dendrites of adult hippocampal CA1 pyramidal neurons. J. Neurosci. 2001;21:9151–9159. doi: 10.1523/JNEUROSCI.21-23-09151.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Perea G. Glial modulation of synaptic transmission in culture. Glia. 2004;47:241–248. doi: 10.1002/glia.20026. [DOI] [PubMed] [Google Scholar]
- Arundine M, Tymianski M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium. 2003;34:325–337. doi: 10.1016/s0143-4160(03)00141-6. [DOI] [PubMed] [Google Scholar]
- Arundine M, Tymianski M. Molecular mechanisms of glutamate-dependent neurodegeneration in ischemia and traumatic brain injury. Cell. Mol. Life Sci. 2004;61:657–668. doi: 10.1007/s00018-003-3319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman P, Horiuchi M, Feldman D, Hahn A, Itoh A, See J, Jia ZP, Itoh T, Pleasure D. GluR2-free alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptors intensify demyelination in experimental autoimmune encephalomyelitis. J. Neurochem. 2007;102:1064–1070. doi: 10.1111/j.1471-4159.2007.04612.x. [DOI] [PubMed] [Google Scholar]
- Bardoni R, Torsney C, Tong CK, Prandini M, MacDermott AB. Presynaptic NMDA receptors modulate glutamate release from primary sensory neurons in rat spinal cord dorsal horn. J. Neurosci. 2004;24:2774–2781. doi: 10.1523/JNEUROSCI.4637-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriedo SG, Yin HZ, Weiss JH. Motor neurons are selectively vulnerable to AMPA/kainate receptor-mediated injury in vitro. J. Neurosci. 1996;16:4069–4079. doi: 10.1523/JNEUROSCI.16-13-04069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW, Maulucci-Gedde M, Kriegstein AR. Glutamate neurotoxicity in cortical cell culture. J. Neurosci. 1987;7:357–368. doi: 10.1523/JNEUROSCI.07-02-00357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung RS, McCormack GH, King AE, West AK, Vickers JC. Glutamate induces rapid loss of axonal neurofilament proteins from cortical neurons in vitro. Exp. Neurol. 2005;193:481–488. doi: 10.1016/j.expneurol.2005.01.005. [DOI] [PubMed] [Google Scholar]
- de Lima AD, Merten MD, Voigt T. Neuritic differentiation and synaptogenesis in serum-free neuronal cultures of the rat cerebral cortex. J. Comp. Neurol. 1997;382:230–246. doi: 10.1002/(sici)1096-9861(19970602)382:2<230::aid-cne7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Diamond JS, Jahr CE. Synaptically released glutamate does not overwhelm transporters on hippocampal astrocytes during high-frequency stimulation. J. Neurophysiol. 2000;83:2835–2843. doi: 10.1152/jn.2000.83.5.2835. [DOI] [PubMed] [Google Scholar]
- Dickson TC, Adlard PA, Vickers JC. Sequence of cellular changes following localized axotomy to cortical neurons in glia-free culture. J. Neurotrauma. 2000;17:1095–1103. doi: 10.1089/neu.2000.17.1095. [DOI] [PubMed] [Google Scholar]
- Doble A. The pharmacology and mechanism of action of riluzole. Neurology. 1996;47:S233–241. doi: 10.1212/wnl.47.6_suppl_4.233s. [DOI] [PubMed] [Google Scholar]
- Dotti CG, Sullivan CA, Banker GA. The establishment of polarity by hippocampal neurons in culture. J. Neurosci. 1988;8:1454–1468. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JH, McCracken E, Dewar D, McCulloch J. Intracerebral injection of AMPA causes axonal damage in vivo. Brain Res. 2003;991:104–112. doi: 10.1016/j.brainres.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Fowler JH, Edgar JM, Pringle A, McLaughlin M, McCulloch J, Griffiths IR, Garbern JY, Nave KA, Dewar D. Alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid-mediated excitotoxic axonal damage is attenuated in the absence of myelin proteolipid protein. J. Neurosci. Res. 2006;84:68–77. doi: 10.1002/jnr.20859. [DOI] [PubMed] [Google Scholar]
- Gallo V, Russell JT. Excitatory amino acid receptors in glia: different subtypes for distinct functions? J. Neurosci. Res. 1995;42:1–8. doi: 10.1002/jnr.490420102. [DOI] [PubMed] [Google Scholar]
- Haas MA, Vickers JC, Dickson TC. Binding partners L1 cell adhesion molecule and the ezrin-radixin-moesin (ERM) proteins are involved in development and the regenerative response to injury of hippocampal and cortical neurons. Eur. J. Neurosci. 2004;20:1436–1444. doi: 10.1111/j.1460-9568.2004.03620.x. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat. Rev. Neurosci. 2010;11:682–696. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat. Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Hynd MR, Scott HL, Dodd PR. Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer's disease. Neurochem. Int. 2004;45:583–595. doi: 10.1016/j.neuint.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M, Domercq M, Matute C, Tonello F, Gundersen V, Volterra A. Glutamate exocytosis from astrocytes controls synaptic strength. Nat. Neurosci. 2007;10:331–339. doi: 10.1038/nn1849. [DOI] [PubMed] [Google Scholar]
- Kane-Jackson R, Smith Y. Presynaptic kainate receptors in GABAergic and glutamatergic axon terminals in the monkey globus pallidus. Neuroscience. 2003;122:285–289. doi: 10.1016/s0306-4522(03)00596-7. [DOI] [PubMed] [Google Scholar]
- King AE, Chung RS, Vickers JC, Dickson TC. Localization of glutamate receptors in developing cortical neurons in culture excitotoxicity. J. Comp. Neurol. 2006;294:277–294. doi: 10.1002/cne.21053. [DOI] [PubMed] [Google Scholar]
- King AE, Dickson TC, Blizzard CA, Foster SS, Chung RS, West AK, Chuah MI, Vickers JC. Excitotoxicity mediated by non-NMDA receptors causes distal axonopathy in long-term cultured spinal motor neurons. Eur. J. Neurosci. 2007;26:2151–2159. doi: 10.1111/j.1460-9568.2007.05845.x. [DOI] [PubMed] [Google Scholar]
- King AE, Dickson TC, Blizzard CA, Woodhouse A, Foster SS, Chung RS, Vickers JC. Neuron-glia interactions underlie ALS-like axonal cytoskeletal pathology. Neurobiol. Aging. 2011;32:459–469. doi: 10.1016/j.neurobiolaging.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Lau A, Tymianski M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch. 2010;460:525–542. doi: 10.1007/s00424-010-0809-1. [DOI] [PubMed] [Google Scholar]
- Liu Z, Stafstrom CE, Sarkisian M, Tandon P, Yang Y, Hori A, Holmes GL. Age-dependent effects of glutamate toxicity in the hippocampus. Brain Res. Dev. Brain Res. 1996;97:178–184. doi: 10.1016/s0165-3806(96)00141-1. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wong TP, Aarts M, Rooyakkers A, Liu L, Lai TW, Wu DC, Lu J, Tymianski M, Craig AM, Wang YT. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J. Neurosci. 2007;27:2846–2857. doi: 10.1523/JNEUROSCI.0116-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute C. Characteristics of acute and chronic kainate excitotoxic damage to the optic nerve. Proc. Natl. Acad. Sci. U.S.A. 1998;95:10229–10234. doi: 10.1073/pnas.95.17.10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute C. Interaction between glutamate signalling and immune attack in damaging oligodendrocytes. Neuron Glia Biol. 2007;3:281–285. doi: 10.1017/S1740925X08000033. [DOI] [PubMed] [Google Scholar]
- Micu I, Jiang Q, Coderre E, Ridsdale A, Zhang L, Woulfe J, Yin X, Trapp BD, McRory JE, Rehak R, Zamponi GW, Wang W, Stys PK. NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia. Nature. 2006;439:988–992. doi: 10.1038/nature04474. [DOI] [PubMed] [Google Scholar]
- Newpher TM, Ehlers MD. Glutamate receptor dynamics in dendritic microdomains. Neuron. 2008;58:472–497. doi: 10.1016/j.neuron.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouardouz M, Coderre E, Basak A, Chen A, Zamponi GW, Hameed S, Rehak R, Yin X, Trapp BD, Stys PK. Glutamate receptors on myelinated spinal cord axons: I. GluR6 kainate receptors. Ann. Neurol. 2009a;65:151–159. doi: 10.1002/ana.21533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouardouz M, Coderre E, Zamponi GW, Hameed S, Yin X, Trapp BD, Stys PK. Glutamate receptors on myelinated spinal cord axons: II. AMPA and GluR5 receptors. Ann. Neurol. 2009b;65:160–166. doi: 10.1002/ana.21539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Bateman MC, Goldberg MP. Rapid alterations in dendrite morphology during sublethal hypoxia or glutamate receptor activation. Neurobiol. Dis. 1996;3:215–227. doi: 10.1006/nbdi.1996.0022. [DOI] [PubMed] [Google Scholar]
- Passafaro M, Piech V, Sheng M. Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat. Neurosci. 2001;4:917–926. doi: 10.1038/nn0901-917. [DOI] [PubMed] [Google Scholar]
- Perlson E, Jeong GB, Ross JL, Dixit R, Wallace KE, Kalb RG, Holzbaur ELF. A switch in retrograde signaling from survival to stress in rapid-onset neurodegeneration. J. Neurosci. 2009;29:9903–9917. doi: 10.1523/JNEUROSCI.0813-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro PS, Mulle C. Presynaptic glutamate receptors: physiological functions and mechanisms of action. Nat. Rev. Neurosci. 2008;9:423–436. doi: 10.1038/nrn2379. [DOI] [PubMed] [Google Scholar]
- Pitt D, Nagelmeier IE, Wilson HC, Raine CS. Glutamate uptake by oligodendrocytes: Implications for excitotoxicity in multiple sclerosis. Neurology. 2003;61:1113–1120. doi: 10.1212/01.wnl.0000090564.88719.37. [DOI] [PubMed] [Google Scholar]
- Pitt D, Gonzales E, Cross AH, Goldberg MP. Dysmyelinated axons in shiverer mice are highly vulnerable to alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor-mediated toxicity. Brain Res. 2010;1309:146–154. doi: 10.1016/j.brainres.2009.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A, Kim E, Sheng M, Craig AM. Heterogeneity in the molecular composition of excitatory postsynaptic sites during development of hippocampal neurons in culture. J. Neurosci. 1998;18:1217–1229. doi: 10.1523/JNEUROSCI.18-04-01217.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggu SK, Chotaliya HP, Cai Z, Blumbergs P, Casson RJ. The spatiotemporal pattern of somal and axonal pathology after perikaryal excitotoxic injury to retinal ganglion cells: a histological and morphometric study. Exp. Neurol. 2008;211:52–58. doi: 10.1016/j.expneurol.2007.12.022. [DOI] [PubMed] [Google Scholar]
- Sattler R, Xiong Z, Lu WY, MacDonald JF, Tymianski M. Distinct roles of synaptic and extrasynaptic NMDA receptors in excitotoxicity. J. Neurosci. 2000;20:22–33. doi: 10.1523/JNEUROSCI.20-01-00022.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenmann Z, Assa-Kunik E, Tiomny S, Minis A, Haklai-Topper L, Arama E, Yaron A. Axonal degeneration is regulated by the apoptotic machinery or a NAD+-sensitive pathway in insects and mammals. J. Neurosci. 2010;30:6375–6386. doi: 10.1523/JNEUROSCI.0922-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B, Galbiati F, Castelvetri LC, Givogri MI, Lopez-Rosas A, Bongarzone ER. Peripheral neuropathy in the Twitcher mouse involves the activation of axonal caspase 3. ASN Neuro. 2011;3:213–222. doi: 10.1042/AN20110019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stys PK, Li S. Glutamate-Induced white matter injury: excitotoxicity without synapses. Neuroscientist. 2000;6:230–233. [Google Scholar]
- Taylor AM, Blurton-Jones M, Rhee SW, Cribbs DH, Cotman CW, Jeon NL. A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nat. Methods. 2005;2:599–605. doi: 10.1038/nmeth777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. Mobile NMDA receptors at hippocampal synapses. Neuron. 2002;34:255–264. doi: 10.1016/s0896-6273(02)00658-x. [DOI] [PubMed] [Google Scholar]
- Underhill SM, Goldberg MP. Hypoxic injury of isolated axons is independent of ionotropic glutamate receptors. Neurobiol. Dis. 2007;25:284–290. doi: 10.1016/j.nbd.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme P, Dewil M, Robberecht W, Van Den Bosch L. Excitotoxicity and amyotrophic lateral sclerosis. Neurodegener. Dis. 2005;2:147–159. doi: 10.1159/000089620. [DOI] [PubMed] [Google Scholar]
- Van Den Bosch L, Vandenberghe W, Klaassen H, Van Houtte E, Robberecht W. Ca2+-permeable AMPA receptors and selective vulnerability of motor neurons. J. Neurol. Sci. 2000;180:29–34. doi: 10.1016/s0022-510x(00)00414-7. [DOI] [PubMed] [Google Scholar]
- van Zundert B, Yoshii A, Constantine-Paton M. Receptor compartmentalization and trafficking at glutamate synapses: a developmental proposal. Trends Neurosci. 2004;27:428–437. doi: 10.1016/j.tins.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Kirchhoff F. NMDA Receptors in glia. Neuroscientist. 2007;13:28–37. doi: 10.1177/1073858406294270. [DOI] [PubMed] [Google Scholar]
- Yoshioka A, Hardy M, Younkin DP, Grinspan JB, Stern JL, Pleasure D. Alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptors mediate excitotoxicity in the oligodendroglial lineage. J. Neurochem. 1995;64:2442–2448. doi: 10.1046/j.1471-4159.1995.64062442.x. [DOI] [PubMed] [Google Scholar]
- Zheng JQ, Wan JJ, Poo MM. Essential role of filopodia in chemotropic turning of nerve growth cone induced by a glutamate gradient. J. Neurosci. 1996;16:1140–1149. doi: 10.1523/JNEUROSCI.16-03-01140.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]