Abstract
Chemicals can interact with the genetic material giving rise to the formation of covalent adducts. These alterations can lead to adverse consequences, including cancer, reproductive impairment, development anomalies, or genetic diseases. In search for an assay allowing identification of hazardous compounds that might form covalent adducts with nucleic acids, electrochemistry (EC)/liquid chromatography (LC)/mass spectrometry (MS) is presented. EC/LC/MS is a purely instrumental approach. EC is used for oxidative activation, LC for the fractionation of the reaction mixture, and MS for the detection and characterization of the reaction products. To test the system capabilities, we investigated the formation of covalent adducts produced by guanosine and acetaminophen (APAP). Electrochemical activation of mixtures of guanosine and APAP gave rise to the formation of four isomers of (guanosine + APAP-2H). Mass voltammograms as well as dose–response-curves were used to obtain insights in the mechanism of adduct formation. These experiments revealed that a mechanism involving radical intermediates is favored. The initial step of adduct formation is the conversion of both APAP and guanosine into radicals via one-electron–one-proton reactions. Among different competing reaction pathways, the generated radical intermediates undergo intermolecular reactions to form covalent adducts between guanosine and APAP.
Key words: Electrochemistry, Mass spectrometry, Nucleic acids, Liquid chromatography, DNA adduct, DNA damage
1. Introduction
Humans are constantly being exposed to increasing numbers of mutagenic, genotoxic or carcinogenic chemicals in their everyday lives. These chemicals such as or activated via oxidative processes can react with nucleic acids giving rise to the formation of monoadducts, intrastrand cross-links, interstrand cross-links, and/or interhelical cross-links. These modifications can alter sequence, structure, function or segregation of the genetic material, and may lead to adverse consequences, including cancer, reproductive impairment, development anomalies, or genetic diseases.
A number of assays have been developed to identify potentially hazardous chemicals. The standard regulatory test battery generally includes an assessment of genotoxicity in bacterial and mammalian cells in vitro together with rodent assays for chromosomal and/or DNA damage [1,2]. Despite considerable success of these assays, there is still a need for tests that are quicker, more easily automatable, and less expensive than the existing assays. A particular demand for novel technologies allowing reactivity assessment of chemicals exists in drug development or large scale chemical evaluation programs such as European REACH (The Registration, Evaluation, Authorisation and Restriction of Chemical substances) legislation. In a workshop recently organized by the “In Vitro Genetic Toxicity Testing Project Committee” sixteen assays/technologies were reviewed which either could replace or complement the existing standard test battery [3]. One of the methods discussed was DNA adduct screening by liquid chromatography (LC)/mass spectrometry (MS) [4].
LC/MS is a commonly used method for the characterization of modified nucleic acids species including DNA adducts [5–13]. LC enables the separation of modified and unmodified species and is particularly helpful in the detection of isomers. MS provides sufficient sensitivity and specificity and the ability to elucidate modified DNA structures. With LC/MS adducts are usually detected at the nucleobase, nucleoside, or nucleotide level after enzymatic digestion (typically DNase I or nuclease P1). A critical part of the developed workflows is activation of the adduct-forming agents. In most cases bioactivation includes oxidation by cytochrome P450 (CYP) enzymes. Induced rodent liver S9 represents the exogenous activation system of choice. Alternatively, the use of human liver S9, genetically modified bacteria and mammalian cells, as well as recombinant human CYPs has been proposed. Reported problems of available bioactivation assays include: (i) due to the substrate-specificity of enzymes a large variety of oxidizing enzymes is needed for exhaustive activation; (ii) cofactors are necessary for efficient transformation reactions; (iii) organic solvents necessary to dissolve lipophilic molecules can decrease enzyme activity; (iv) highly concentrated test compound solutions induce substrate saturation or enzyme inhibition; (v) active metabolites can react with a number of alternative targets (e.g. proteins, lipids). In search of an alternative activation system the use of electrochemistry (EC) was proposed [14–16]. EC represents a versatile tool to mimic metabolic oxidation reactions [17,18]. EC provides a means to generate reactive metabolites without the need of any kind of chemical or biological oxidizer in an environment where only selected biological nucleophiles (i.e. proteins, peptides, small molecules) are present [19–21].
In this work an integrated EC-LC–MS system for studying adduct formation processes is presented. EC/LC/MS is a purely instrumental approach that is capable to automatically produce, detect and characterize covalent DNA adducts. To test the system capabilities, we investigated the formation of covalent adducts produced by guanosine and acetaminophen (APAP). Mechanistic details of the electrochemical adduct formation process are discussed.
2. Materials and methods
2.1. Chemicals
Acetonitrile, guanosine, APAP (acetaminophen, paracetamol, N-acetyl-p-aminophenol, CAS no. 103-90-2) and water were obtained from Sigma–Aldrich (St. Louis, MO, USA). Ammonium hydroxide and formic acid were purchased from Fluka (Buchs, Switzerland). All chemicals were used in the highest quality available.
2.2. The EC-μLC–MS system
All experiments were performed on a modified ROXY EC/LC system (Antec, Zoeterwoude, The Netherlands) online hyphenated to a quadrupole–quadrupole-time-of-flight mass spectrometer (QqTOF; QSTAR XL, AB Sciex, Foster City, CA, USA). A schematic drawing of the setup is provided in Fig. 1.
Fig. 1.
Schematic drawing of the EC-LC-MS system. (1) Solvent reservoirs, (2a, 2b) high pressure gradient pumps, (3) degasser unit, (4a, 4b) pressure pulsation dampers, (5) gradient mixer, (6) splitting tee-piece and restriction capillary, (7) autosampler, (8) injection valve, (9) needle, (10) EC flow cell, (11) potentiostat, (12) capillary column, (13) ESI source, (14) mass spectrometer.
Electrochemical conversions were accomplished in an electrochemical thin layer cell (ReactorCell, Antec). The reactor cell consisted of a three electrode arrangement including a working electrode, a counter electrode and a reference electrode. As working electrode a conductive diamond electrode (Magic Diamond, Antec) was used. Conductive diamond is an ideal electrode material for the oxidation of nucleic acids due to its chemical inertness, high electrical conductivity, and extraordinarily low catalytic activity for both, hydrogen and oxygen evolution. The effective area of the working electrode was 15.1 mm2. The inlet block of the cell was employed as counter electrode and a Pd/H2 electrode (HyREF, Antec) was used as reference electrode. The working electrode and the counter electrode inlet block were separated by a 50 μm spacer giving a cell volume of approx. 750 nl. Electrochemical potentials (E, 0–4000 mV) were applied using a potentiostat (ROXY Potentiostat, Antec).
For automated sample delivery, the reactor cell was integrated into the autosampler system [22]. The reactor cell was placed between the injection capillary and the injection valve. Sample solutions containing 300–350 μM guanosine and 0–350 μM APAP dissolved in 10 mM ammonium formate (pH 7.3) were delivered through the electrochemical cell at a flow rate of 2.5 μL/min to the 2 μL sample loop.
For all experiments a miniaturized chromatographic system was used. Chromatographic separations were accomplished on a micro-column (200 mm × 0.2 mm i.d., Eurospher C18, 5 μm) prepared according to the published protocol [23–25]. A primary flow of 250 μL/min was split by a ratio of 1:100 to a final flow of 2.5 μL/min with the help of a tee-piece and a 50 μm i.d. fused-silica restriction capillary. The connection line between the gradient mixer and the splitting tee-piece was made of a 100 μm i.d. Peek tubing; all other transfer lines had an i.d. of 20 μm to minimize extra-column peak broadening. Chromatographic separations were accomplished with linear gradients of 2.5–30% acetonitrile in 10 mM ammonium formate (pH 7.3) within 10 min. The EC/LC system was controlled by the Clarity Chromatography software (DataApex, Prague, Czech Republic).
Eluting compounds were detected by electrospray ionization (ESI)-MS in positive ion mode, which was performed on a QSTAR XL mass spectrometer (AB Sciex) equipped with a modified TurboIonSpray source [26,27]. The modifications included the replacements of the Peek tubing transfer line and of the stainless steel sprayer capillary by fused silica capillaries (transfer line: 375 μm o.d., 20 μm i.d., sprayer capillary: 90 μm o.d., 20 μm i.d., Polymicro Technologies Phoenix, AZ, USA). Mass spectrometric parameters were optimized using a 100 μM solution of guanosine in 10 mM ammonium formate (pH 7.3) containing 50% acetonitrile (v/v). The spray voltage was set to 4.5 kV. Gas flows of 2–5 arbitrary units (nebulizer gas) and 25–30 arbitrary units (turbo gas) were employed. The temperature of the turbo gas was adjusted to 200 °C. For MS/MS, the resolution of the first quadrupole was set to unit resolution. The collision gas (N2) flow was set to 5 arbitrary units, and a collision energy of 15 eV was applied. For MS3, up front fragmentation, which was induced by increasing the declustering potential from 55 V to 135 V, was combined with MS/MS at a collision energy of 30 eV. Accumulation time was set to 0.5 s, and a scan range 50–700 was used. Mass spectra were recorded on a personal computer with the Analyst QS software (version 1.0, service pack 8, AB Sciex).
3. Results and discussion
3.1. EC/LC/MS/MS of APAP-guanosine mixtures
As reported recently, on-line EC/MS can be used to study covalent adduct formation of nucleic acids with different reactants, such as small molecules [15], amino acids and peptides [16]. For such experiments, guanine-containing species represent the test compounds of choice since guanine has the lowest oxidation potential of all four DNA nucleobases. In both studies adducts of the form (guanine + reactant-2H) were observed. In one study (guanine + reactant)-species were detected as well [16]. These putative covalent adducts turned out to have the same molecular formula as the corresponding noncovalent adducts. As the proton-bound complexes are known to survive the ESI process, non-covalent forms can easily be misinterpreted as covalently bound species in MS. To enable the differentiation of covalent and noncovalent adducts, the hyphenation of LC and tandem mass spectrometry (MS/MS) was used for screening the reaction mixture. LC is intended to denature proton-bound adducts, and MS/MS allows structural characterization of any kind of adduct formed.
To test the capabilities of EC/LC/MS in studying adduct formation processes, mixtures of guanosine and APAP were selected as samples. APAP is a widely used over-the-counter analgesic and antipyretic, and is therefore a major ingredient in numerous cold and flu remedies. Several studies on cell cultures and rodents have demonstrated that acetaminophen can covalently bind to nucleic acids after metabolic activation [28]. Moreover, electrochemical oxidation of APAP is well understood [29], and was used to initiate adduct formation with nucleic acid species [15].
Mixtures of guanosine and APAP were electrochemically activated and screened for adducts with LC/MS/MS. Chromatographic separations were accomplished on a miniaturized reversed-phase column. Benefits of using such a setup include increased mass sensitivity with concentration-sensitive detectors and easier coupling with MS. Specific and sensitive detection of adducts was accomplished by MS/MS. Both adduct forms, (guanosine + APAP) and (guanosine + APAP-2H), were targeted. No evidence for the formation of (guanosine + APAP) was found; only (guanosine + APAP-2H)-adducts were detected.
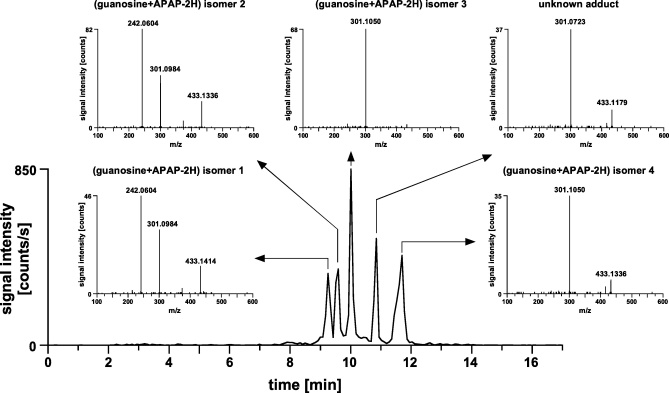
An extracted ion chromatogram obtained for adducts of the form (guanine + reactant-2H) is shown in Fig. 2. Due to chromatographic separation, five putative adducts were detected. Retention times (tr) were between 9.3 and 11.7 min. Mass spectrometric measurements were accomplished on a QqTOF instrument allowing accurate measurements of mass-to-charge ratios (m/z) in MS and MS/MS mode. The obtained mass spectrometric information was used to characterize the molecular structures of the adducts. For all five species, neutral loss of the ribose moiety was observed (433.1 > 301.1), which is a clear indicator that on the one hand guanosine is part of the molecule and that on the other hand the modification involved only the nucleobase and not the sugar. For four species detected, the measured m/z-values were within ±30 ppm of the calculated values proving the formation of (guanosine + APAP-2H). For one species (“unknown adduct”, tr = 10.8 min) the m/z value of the precursor ion as well as the m/z of the most intense fragment ion showed mass deviations of −107 and −66 ppm, respectively. This observation suggests that here an unknown compound, putatively some kind of impurity within the reaction mixture, might have formed a covalent adduct with guanosine.
Fig. 2.
Extracted ion chromatogram to identify putative (guanosine + APAP-2H)-adducts. E, 1750 mV; column, Eurospher C18, 5 μm, 200 mm × 0.2 mm i.d.; mobile phase (A) 10 mM ammonium formate, pH 7.3, (B) 10 mM ammonium formate containing 50% acetonitrile (v/v), pH 7.3; linear gradient, 5–60% B in 10 min; flow rate, 2.5 μL/min; MS/MS, precursor ion 433.1, scan 50–700; sample, 350 μM guanosine and 200 μM APAP dissolved in 10 mM ammonium formate, pH 7.3.
3.2. Dose–response-curves
As a further proof of adduct formation between guanosine and APAP, the amount of APAP added to the reaction mixture was varied. The remaining experimental parameters, including guanosine concentration (350 μM) and the electrochemical potential (1750 mV), were kept constant. Samples were screened with LC/MS/MS after electrochemical activation. The obtained “dose–response-curves” are depicted in Fig. 3. The four (guanosine + APAP-2H) isomers were only observed in solutions containing APAP. For the isomers 1–3 the normalized peak areas showed a steady increase over the concentration range studied (Fig. 3a). For isomer 4 maximum peak area was reached at 100 μM APAP; at higher concentration a decline of the peak area was observed (Fig. 3a). The unknown adduct eluting at 10.8 min was formed without the addition of APAP to the reaction mixture (Fig. 3b). The peak area remained almost constant over the concentration range studied. The relative standard deviation was 13.0% for the peak area. These two observations were taken as indication that the adduct-forming agent was an impurity part of the guanosine sample.
Fig. 3.
Dose–response-curves for different guanosine adducts. Sample, 350 μM guanosine and 0–350 μM APAP dissolved in 10 mM ammonium formate, pH 7.3. All other conditions as in Fig. 2.
3.3. Mechanistic details of electrochemically activated adduct formation
In a further set of experiments mass voltammograms were acquired to study mechanistic details of (guanosine + APAP-2H) formation by electrochemical activation (Fig. 4). Solutions of 350 μM guanosine and 200 μM APAP in 10 mM ammonium formate (pH 7.3) were used as samples. The potential was ramped from 0 mV to 4000 mV. Screening for oxidation products was accomplished by LC/MS/MS. Targeted species included (APAP-H)2, (guanosine-H)2, 8-hydroxyguanosine as well as the adducts.
Fig. 4.
Mass voltammograms for oxidation products of APAP, guanosine as well as the (guanosine + APAP-2H)-adducts. E, 0–4000 mV. All other conditions as in Fig. 2.
The results obtained for (APAP-H)2 are depicted in Fig. 4a. (APAP-H)2 was included in the study because it can be used as indicator for oxidative activation of APAP. Due to the use of LC, two isomeric forms of the APAP dimer were discernible, eluting at 11.8 min and 14.6 min, respectively. The minimum potential necessary to induce APAP oxidation was 750 mV. Maximum peak areas were reached at 1000 mV. At higher potentials peak areas were declining.
Mass voltammograms of guanosine oxidation products are summarized in Fig. 4b. Guanosine oxidation started at 1250 mV. The primary oxidation product was (guanosine-H)2. LC separation enabled the detection of two isomeric forms eluting at 9.0 min and 11.5 min. Maximum peak areas were reached at 1750 mV and 2500 mV, respectively. The minimum voltage necessary to detect 8-hydroxyguanosine was 1500 mV; the maximum peak area was reached at 3500 mV.
Mass voltammograms of all five guanosine adducts detected are shown in Fig. 4c. Adduct formation started at 1250 mV. Maximum peak areas were obtained at 1750 mV. At higher voltages declining peak areas were observed. The mass voltammograms of the adducts were congruent with the mass voltammogram of the (guanosine-H)2 isomer eluting at 9.0 min.
APAP has a lower oxidation potential than guanosine. APAP oxidation started at 750 mV. A minimum potential of 1250 mV was necessary to initiate guanosine oxidation. For both compounds, initial oxidation occurred in a one-electron–one-proton step to give a free radical moiety. Due to their short lifetimes, the radicals were not detected with our setup; they were detected indirectly. The observed formation of the dimeric forms represented clear hints for the production of the radical intermediates. The dimers resulted from recombination of two radicals. As the radicals had tautomeric structures, several combinations were possible to give different isomeric forms.
A reaction mechanism similar to the formation of (guanosine-H)2 and (APAP-H)2 is proposed for adduct formation. The initial step of adduct formation is the conversion of both APAP and guanosine into the radical forms via one-electron–one-proton reactions. These radicals can react with each other to form (guanosine + APAP-2H). This hypothesis is supported by the fact that adducts were only detected at potentials sufficiently high to oxidize both compounds. Extensive APAP oxidation alone did not give rise to adduct formation. Simultaneous activation of guanosine was necessary to obtain detectable amounts of covalent adducts. Tautomerization of the radicals led to the formation of isomeric forms.
The mass voltammogram of the unknown adduct and of the isomeric forms (guanosine + APAP-2H) were congruent. This observation suggests that the proposed reaction mechanism for the formation of adducts via electrochemical coactivation might be transferable from APAP to other chemicals.
3.4. Insights into the structures of the (guanosine + APAP-2H) isomers from MS3 experiments
LC–MS/MS is a sensitive tool to detect modified guanosine species. Four different isomers of (guanosine + APAP-2H) were identified by scanning the neutral loss of the ribose moiety (433.1 > 301.1). Such scanning experiments, however, do not provide any information on the structure of the modified nucleobases. To overcome this limitation, full scan MS/MS spectra were acquired and interpreted. The spectra obtained from the (guanosine + APAP-2H) isomers are depicted in Fig. 2. In all cases the number of spectral features observed was low; neutral loss of the ribose moiety was the most dominant fragmentation reaction. Only for the first two isomers abundant fragments characteristic for cleavage within the modified nucleobases (242.1) were observed within the MS/MS spectra. To get more detailed structural information, MS3 experiments were conducted. To convert the QqTOF instrument into a MS3 instrument up front fragmentation was combined with MS/MS [30]. Up front fragmentation was used to initiate loss of the ribose moiety (433.1 > 301.1). The obtained fragment ions, which represented protonated (guanine + APAP-2H) ions, were selected as precursor ions for consecutive MS/MS experiments. MS3 spectra obtained from the four isomeric forms are depicted in Fig. 5. In each spectrum a number of neutral losses were detected that were related to guanine and/or APAP fragmentation (Table 1). The obtained data was particularly useful to elucidate the site of modification within the APAP moiety. APAP-specific fragmentation included loss of water (−18.01), partial or complete loss of the acetamido group (−42.01, −59.04) as well as combinations thereof (−87.03), and suggested that guanosine was directly linked to the aromatic ring of APAP. To localize or at least to limit the number of possible reactive sites within the guanosine moieties, an equivalent amount of spectral features was missing. The cleavage of NH3 (−17.02) was the only guanosine-specific loss observed. Nevertheless, based on the occurrence of this fragment ion linkage of APAP to the exocyclic N2 was excluded.
Fig. 5.
MS3 spectra of (guanosine + APAP-2H)-adducts. Up front fragmentation inducing cleavage of the ribose was combined with MS/MS. MS/MS, precursor ion 301.1; sample, 300 μM guanosine and 300 μM APAP dissolved in 10 mM ammonium formate, pH 7.3. All other conditions as in Fig. 2.
Table 1.
Comparison of neutral losses characteristic for guanine, APAP as well as fragments obtained from up front fragmentation of the four (guanosine + APAP-2H) isomers.
| Guanine | [Guanosine + APAP-2H] isomer |
APAP | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| Precursor ion for MS/MS | 152.06 | 301.10 | 301.10 | 301.10 | 301.10 | 152.07 |
| Characteristic neutral losses | −17.02 | x | x | x | ||
| x | x | x | x | −18.01 | ||
| −24.01 | ||||||
| −42.02 | x | x | x | x | −42.01 | |
| −59.05 | x | x | x | x | −59.04 | |
| −70.02 | ||||||
| x | x | x | −87.03 | |||
| −97.03 | ||||||
EC/LC/MS represents a convenient tool for the identification of compounds reacting with nucleic acids species after electrochemical activation. EC/LC/MS can be regarded as fast, simple and automatable screening tool. The provided amount of structural information is usually sufficient to narrow down the number of possible reaction sites by excluding moieties from being modified. With mass spectrometric methods, however, only in rare cases unequivocal localization of the linkage sites is possible. More detailed structural information can be obtained from other types of spectroscopic methods [19]. Nuclear magnetic resonance (NMR) spectroscopy, for instance, clearly surpasses MS in terms of obtainable structural information. Due to its limited detection sensitivity, however, NMR can hardly be used as online detection method for EC/LC. The quantities needed for NMR even with most modern instrumentation are at least three orders of magnitude higher than those produced by EC, which renders EC/LC/NMR inappropriate for the development of any kind of screening tool.
4. Conclusions
EC/LC/MS represents an integrated system solution capable to simultaneously produce, resolve and characterize covalent adducts of small molecules with nucleic acids. In comparison to other in vitro assays developed to identify potentially hazardous chemicals EC/LC/MS is a purely instrumental assay; problems related to biological processes (i.e. activation, transport, side reactions) are not encountered. Oxidation reactions take place in a controlled environment. All kind of oxidizing enzymes or chemicals are substituted by an electrode with controlled electrochemical potential. LC/MS is used to characterize the reaction mixture leaving the electrochemical reactor cell. Chromatographic separation reduces the complexity of the sample introduced into the mass spectrometer. LC contributes to the sensitivity of the whole method as ion suppression effects are reduced and as analytes are concentrated on the column to chromatographic peaks before entering MS. Furthermore, isomeric species become discernible. Structural information can be obtained from MS and MS/MS experiments.
EC/LC/MS is particularly useful to study experimental and mechanistic details of the adduct formation process. The effect of reactant concentration on adduct formation can be deduced from dose–response-curves. Insights into the mechanism of adduct formation can be obtained from mass voltammograms. We found that electrochemically activated mixtures of APAP and guanosine seem to favor a mechanism involving radical intermediates. Both APAP and guanosine are converted into radical forms via one-electron–one-proton reactions. These radicals can react with each other to form (guanosine + APAP-2H). Further experiments are necessary to decide whether the radical mechanism is limited to APAP or common to other adduct-forming species as well. For validation of the assay, the transferability of the results from electrochemical oxidation to in vitro and also in vivo systems will have to be shown since the generally accepted mechanism of adduct formation includes activation of the adduct-forming agent to a reactive electrophile that can bind to nucleophilic sites of nucleic acids.
Acknowledgement
This work was funded by the Austrian Science Fund (FWF): P 22526-B11.
Footnotes
This paper is part of the special issue “LC–MS/MS in Clinical Chemistry”, Edited by Michael Vogeser and Christoph Seger.
References
- 1.Kirkland D.J., Henderson L., Marzin D., Muller L., Parry J.M., Speit G., Tweats D.J., Williams G.M. Mutat. Res. 2005;588:88. doi: 10.1016/j.mrgentox.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Thybaud V., Aardema M., Clements J., Dearfield K., Galloway S., Hayashi M., Jacobson-Kram D., Kirkland D., MacGregor J.T., Marzin D., Ohyama W., Schuler M., Suzuki H., Zeiger E. Mutat. Res. 2007;627:41. doi: 10.1016/j.mrgentox.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Lynch A.M., Sasaki J.C., Elespuru R., Jacobson-Kram D., Thybaud V., De Boeck M., Aardema M.J., Aubrecht J., Benz R.D., Dertinger S.D., Douglas G.R., White P.A., Escobar P.A., Fornace A., Jr., Honma M., Naven R.T., Rusling J.F., Schiestl R.H., Walmsley R.M., Yamamura E., van Benthem J., Kim J.H. Environ. Mol. Mutagen. 2011;52:205. doi: 10.1002/em.20614. [DOI] [PubMed] [Google Scholar]
- 4.Kanaly R.A., Hanaoka T., Sugimura H., Toda H., Matsui S., Matsuda T. Antioxid. Redox Signal. 2006;8:993. doi: 10.1089/ars.2006.8.993. [DOI] [PubMed] [Google Scholar]
- 5.Harsch A., Vouros P. Anal. Chem. 1998;70:3021. doi: 10.1021/ac9713823. [DOI] [PubMed] [Google Scholar]
- 6.Andrews C.L., Vouros P., Harsch A. J. Chromatogr. A. 1999;856:515. doi: 10.1016/s0021-9673(99)00779-7. [DOI] [PubMed] [Google Scholar]
- 7.Singh R., Farmer P.B. Carcinogenesis. 2006;27:178. doi: 10.1093/carcin/bgi260. [DOI] [PubMed] [Google Scholar]
- 8.Farmer P.B., Brown K., Tompkins E., Emms V.L., Jones D.J., Singh R., Phillips D.H. Toxicol. Appl. Pharmacol. 2005;207:S293. doi: 10.1016/j.taap.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 9.Farmer P.B., Singh R. Mutat. Res. 2008;659:68. doi: 10.1016/j.mrrev.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Mohamed D., Linscheid M. Anal. Bioanal. Chem. 2008;392:805. doi: 10.1007/s00216-008-2236-0. [DOI] [PubMed] [Google Scholar]
- 11.Mohamed D., Mowaka S., Thomale J., Linscheid M.W. Chem. Res. Toxicol. 2009;22:1435. doi: 10.1021/tx900123r. [DOI] [PubMed] [Google Scholar]
- 12.Matter B., Wang G., Jones R., Tretyakova N. Chem. Res. Toxicol. 2004;17:731. doi: 10.1021/tx049974l. [DOI] [PubMed] [Google Scholar]
- 13.Matter B., Guza R., Zhao J., Li Z.Z., Jones R., Tretyakova N. Chem. Res. Toxicol. 2007;20:1379. doi: 10.1021/tx7001146. [DOI] [PubMed] [Google Scholar]
- 14.Mazerska Z., Zon A., Stojek Z. Electrochem. Commun. 2003;5:770. [Google Scholar]
- 15.Pitterl F., Chervet J.P., Oberacher H. Anal. Bioanal. Chem. 2010;397:1203. doi: 10.1007/s00216-010-3674-z. [DOI] [PubMed] [Google Scholar]
- 16.Looi D.W., Eyler J.R., Brajter-Toth A. Electrochim. Acta. 2011;56:2633. [Google Scholar]
- 17.Jurva U., Wikstrom H.V., Bruins A.P. Rapid Commun. Mass Spectrom. 2000;14:529. doi: 10.1002/(SICI)1097-0231(20000331)14:6<529::AID-RCM904>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 18.Jurva U., Wikstrom H.V., Weidolf L., Bruins A.P. Rapid Commun. Mass Spectrom. 2003;17:800. doi: 10.1002/rcm.978. [DOI] [PubMed] [Google Scholar]
- 19.Jurva U., Holmen A., Gronberg G., Masimirembwa C., Weidolf L. Chem. Res. Toxicol. 2008;21:928. doi: 10.1021/tx700400c. [DOI] [PubMed] [Google Scholar]
- 20.Lohmann W., Hayen H., Karst U. Anal. Chem. 2008;80:9714. doi: 10.1021/ac801699g. [DOI] [PubMed] [Google Scholar]
- 21.Lohmann W., Karst U. Anal. Bioanal. Chem. 2009;394:1341. doi: 10.1007/s00216-008-2586-7. [DOI] [PubMed] [Google Scholar]
- 22.H.J. Brouwer, J.P. Chervet, A. Kraj, Analytical Apparatus Comprising an Electrochemical Flow Cell and a Structural Elucidation Spectrometer, PCT/NL2010/050309.
- 23.Oberacher H., Krajete A., Parson W., Huber C.G. J. Chromatogr. A. 2000;893:23. doi: 10.1016/s0021-9673(00)00731-7. [DOI] [PubMed] [Google Scholar]
- 24.Schubert B., Pavlic M., Libiseller K., Oberacher H. Anal. Bioanal. Chem. 2008;392:1299. doi: 10.1007/s00216-008-2447-4. [DOI] [PubMed] [Google Scholar]
- 25.Schubert B., Oberacher H. J. Chromatogr. A. 2011;1218:3413. doi: 10.1016/j.chroma.2011.03.051. [DOI] [PubMed] [Google Scholar]
- 26.Oberacher H., Niederstatter H., Parson W. J. Mass Spectrom. 2005;40:932. doi: 10.1002/jms.870. [DOI] [PubMed] [Google Scholar]
- 27.Pavlic M., Libiseller K., Oberacher H. Anal. Bioanal. Chem. 2006;386:69. doi: 10.1007/s00216-006-0634-8. [DOI] [PubMed] [Google Scholar]
- 28.Bergman K., Muller L., Teigen S.W. Mutat. Res. 1996;349:263. doi: 10.1016/0027-5107(95)00185-9. [DOI] [PubMed] [Google Scholar]
- 29.Lohmann W., Karst U. Anal. Bioanal. Chem. 2006;386:1701. doi: 10.1007/s00216-006-0801-y. [DOI] [PubMed] [Google Scholar]
- 30.Tuytten R., Lemiere F., Van Dongen W., Esmans E.L., Witters E., Herrebout W., Van Der Veken B., Dudley E., Newton R.P. J. Am. Soc. Mass Spectrom. 2005;16:1291. doi: 10.1016/j.jasms.2005.03.026. [DOI] [PubMed] [Google Scholar]