Abstract
Liver repair is key to resuming homeostasis and preventing fibrogenesis as well as other liver diseases. Farnesoid X receptor (FXR, NR1H4) is an emerging liver metabolic regulator and cell protector. Here we show that FXR is essential to promote liver repair after carbon tetrachloride (CCl4)-induced injury. Expression of hepatic FXR in wild-type mice was strongly suppressed by CCl4 treatment, and bile acid homeostasis was disrupted. Liver injury was induced in both wild-type and FXR−/− mice by CCl4, but FXR−/− mice had more severe defects in liver repair than wild-type mice. FXR−/− livers had a decreased peak of regenerative DNA synthesis and reduced induction of genes involved in liver regeneration. Moreover, FXR−/− mice displayed increased mortality and enhanced hepatocyte deaths. During the early stages of liver repair after CCl4 treatment, we observed overproduction of TNFα and a strong decrease of phosphorylation and DNA-binding activity of signal transducer and activator of transcription 3 in livers from FXR−/− mice. Exogenous expression of a constitutively active signal transducer and activator of transcription 3 protein in FXR−/− liver effectively reduced hepatocyte death and liver injury after CCl4 treatment. These results suggest that FXR is required to regulate normal liver repair by promoting regeneration and preventing cell death.
FXR regulates the process of liver repair by promoting regeneration and preventing cell death.
The liver is the major organ to manage the detoxification of both exogenous and endogenous insults and therefore is constantly exposed to injury agents. Liver repair is an intrinsic defense mechanism to protect liver from injury. Impaired liver repair will lead to fibrogenesis and cirrhosis, which may eventually result in either liver failure or hepatocellular carcinoma. Many genes and pathways have been identified to regulate liver repair.
Farnesoid X receptor (FXR) belongs to the nuclear hormone receptor superfamily and is highly expressed in liver, intestine, kidney, and adrenal glands (1). FXR is the primary bile acid (BA) receptor that acts as a master regulator of BA homeostasis (2,3,4,5,6,7). BAs are end products of cholesterol catabolism and are essential for normal absorption of cholesterol, lipids, and fat-soluble vitamins by the intestine (8). BAs are synthesized in the liver and stored in the gall bladder. They are secreted into the intestine after food intake, but most BAs (95%) are reabsorbed and transported back to the liver through the portal vein. This system is known as enterohepatic circulation. The levels of BAs need to be tightly controlled due to their surfactant properties and hepatotoxicity at high doses. Accumulation of BAs in the liver, which may occur during intrahepatic cholestasis of pregnancy (9), or inherent diseases such as progressive familial intrahepatic cholestasis 1 and 2 (PFIC1 and PFIC2) (10,11,12), results in hepatocellular apoptosis and necrosis, thereby promoting liver fibrosis and cirrhosis (13).
To prevent the BA hepatotoxicity, liver has an intrinsic mechanism with which to sense and control BA levels by FXR. In PFIC1 patients, the decreased FXR activity is regarded as one of the primary reasons of the pathogenesis (10). Functional variants of FXR are detected in patients with intrahepatic cholestasis of pregnancy (9). Function of FXR in cholestasis is further clarified by the synthetic FXR agonist, GW4064, which prevents cholestatic liver diseases induced by bile duct ligation (BDL) through down-regulating BA synthetic genes and increasing expression of BA transport genes (14). Another FXR agonist, 6-ethyl-chenodeoxycholic acid, effectively suppresses the liver fibrosis caused by BDL in rats (13). Knockout of small heterodimer partner (SHP), the primary target gene of FXR, leads to increased liver damage by BDL (15), which also supports the roles of FXR in liver protection. In a recent study, FXR−/− mice were shown to be more susceptible to α-naphthyl isothiocyanate-induced acute intrahepatic cholestatic liver injury (16). However, the role of FXR in liver repair after injury is still not very clear.
We previously showed that FXR was required for normal liver regeneration after injury in a 70% partial hepatectomy (PH) model by promoting proliferation of liver cells (17). Rodents treated with CCl4 are widely used to study the mechanisms of toxin-induced liver injury (18,19). CCl4-induced hepatic injury is characterized by centrilobular necrosis and followed by hepatic fibrosis, which recapitulates drug-induced acute liver failure. Compared with 70% PH model, which leaves the remaining 30% of the liver intact, CCl4 injection model of liver injury and repair is closer to the clinical liver injury cases with simultaneous severe hepatocyte deaths and acute cholestasis. Therefore, we use this model to ask: 1) whether FXR is also required for normal liver repair in response to CCl4-induced hepatic cell deaths as it does in the 70% PH surgical model; 2) whether CCl4-induced hepatic necrosis and apoptosis potentially disrupt BA homeostasis, and whether this disruption will interfere with the normal liver repair process.
Results
Defective liver regeneration in FXR−/− mice after CCl4 treatment
Wild-type and FXR−/− mice were injected ip with CCl4 in corn oil (750 μl/kg body weight) or the same volume of corn oil alone as vehicle control. First we determined the expression levels of CYP2E1, which is the primary cytochrome P450 that catalyzes CCl4 metabolism. We did not observe a significant difference in the levels of CYP2E1 mRNA between wild-type and FXR−/− mice after CCl4 injection (Supplemental Fig. 1A published on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org). However, FXR−/− mice exhibited greater mortality (∼20%, three of 17 mice) 3 d after CCl4 treatment (Supplemental Fig. 1B), suggesting that FXR may be important for preventing liver failure-induced death.
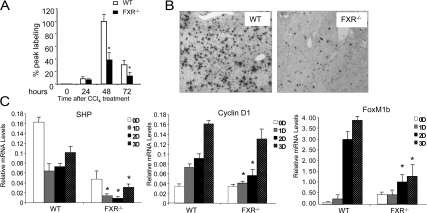
Liver repairs itself by regenerating the survived cells. Therefore, we compared the abilities of FXR−/− and wild-type hepatocytes to proliferate after CCl4-induced liver injury. FXR−/− mice had a severe reduction of hepatocyte proliferation compared with the wild-type controls (Fig. 1). There were significantly fewer 5-bromo-2′-deoxy-uridine (BrdU) positive hepatocytes in the FXR−/− livers compared with the wild-type controls, starting from the second day after CCl4 injection (Fig. 1, A and B). Consistent with these data, there were also significantly fewer mitotic indexes in the FXR−/− livers (data not shown). These results confirm an essential role of FXR in promoting liver regeneration after toxin-induced injury.
Figure 1.
Defective liver regeneration in FXR−/− mice after CCl4-induced liver injury. A, The BrdU-positive liver cells were counted as described previously (17). *, P < 0.05. B, Representative images of BrdU staining of liver tissue from wild-type (WT) and FXR−/− mice on the second day after CCl4 treatment. C, Gene expression analysis of SHP, FoxM1b, and CyclinD1 by quantitative real-time PCR. *, P < 0.05.
Gene expression in the liver after CCl4-induced injury was examined by quantitative real-time PCR. Expression of SHP, the major FXR target gene in BA metabolism, was suppressed by CCl4 treatment in the first 2 d, indicating that FXR activation may be affected by CCl4 treatment. In the FXR−/− mice, the SHP mRNA level was even more decreased, but recovered on the third day as the wild-type controls, indicating that expression of SHP during the liver injury was also modulated by FXR-independent pathways. Expression of the cell cycle gene, Cyclin D1, was reduced in the FXR−/− livers. Similarly, the induction of FoxM1b, a previously identified target gene regulated by FXR during liver regeneration, was significantly reduced in the FXR−/− mice after CCl4-induced liver injury (Fig. 1C).
Increased hepatocyte death and liver injury in FXR−/− mice after CCl4 treatment
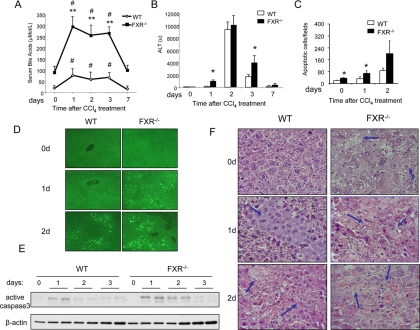
BAs are synthesized specifically from cholesterol in the liver and subsequently excreted into bile. We observed a significant increase of serum BA levels after CCl4 injection in the wild-type mice, which suggests that liver injury caused by CCl4 may impair enterohepatic circulation and BA homeostasis (Fig. 2A). Compared with the wild-type controls, the levels of serum BAs in the FXR−/− mice were 2-fold higher at all time points. Consistently, 1 d after injection of CCl4, there was significantly higher level of serum alanine aminotransferase (ALT) in the FXR−/− mice, but both genotypes reached a peak of similar ALT levels on the second day after injection (Fig. 2B). CCl4-induced liver injury is usually accompanied by increased hepatocyte apoptosis. Therefore, we compared the amount of apoptotic hepatocytes in the wild-type and the FXR−/− livers. TUNEL staining indicated that significantly greater numbers of apoptotic hepatocytes were present in the FXR−/− mice as compared with the wild-type controls (Fig. 2, C and D), which was further confirmed by higher levels of cleaved Caspase 3 in the FXR−/− livers (Fig. 2E). CCl4 also directly leads to severe necrosis in the liver. Hematoxylin and eosin (H&E) staining showed more necrotic areas in the FXR−/− livers than in the wild-type controls (Fig. 2F). These results demonstrate that liver injury disrupts BA homeostasis, and FXR−/− mice are more susceptible to CCl4-induced liver injury.
Figure 2.
Increased hepatocyte apoptosis and liver injury in FXR−/− livers after CCl4 treatment. A, Serum BA levels in wild-type (WT) or FXR−/− mice after CCl4 treatment. **, FXR−/−vs. WT, P < 0.01; #, serum BAs of FXR−/− mice treated with CCl4vs. serum BAs of FXR−/− mice at time zero. B, Serum ALT levels in WT or FXR−/− mice after CCl4 treatment. *, P < 0.05. C, Quantification of apoptotic hepatocytes. *, P < 0.05. D, Terminal deoxynucleotide transferase-mediated dUTP nick end labeling staining of liver sections from WT and FXR−/− mice after CCl4 treatment (0, 1, or 2 d after treatment). E, Caspase 3 activation after CCl4 injection in WT and FXR−/− mice. F, H&E staining of liver tissue sections from WT and FXR−/− mice after CCl4 treatment. Arrows indicate necrosis areas.
Intrahepatic cholestasis of FXR−/− mice after CCl4 treatment
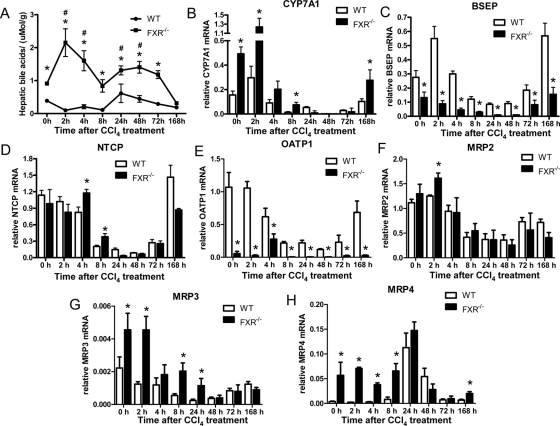
To further understand the effects of FXR loss on the increased BA flux, we evaluated the hepatic total BAs in the wild-type and the FXR−/− mice. Hepatic BAs began to accumulate in liver 24 h after the CCl4 treatment and were lowered to normal levels on the third day in the wild-type mice (Fig. 3A). In contrast, the intrahepatic BA level was sharply increased in the FXR−/− mice during the acute phase and kept at a much higher level than that in wild-type controls until 7 d after the treatment. The expression of the major BA synthetic enzyme CYP7A1 was significantly increased in the FXR−/− mice at the second hour whereas the CYP7A1 in the wild-type mice was not greatly increased compared with the nontreated control (Fig. 3B). BSEP is responsible for the transport of taurocholate and other cholate conjugates from hepatocytes to the bile, and its expression is activated by FXR (20). The early activation of BSEP transcription was not seen in the FXR−/− mice, and the expression levels were less than 50% of those in the wild-type controls at most of the time points (Fig. 3C). FXR reduces the BAs inside the hepatocytes also by suppressing NTCP expression. This suppression was slightly impaired in the FXR−/− mice in the acute phase after liver injury (Fig. 3D). The expression of OATP1, the organic anion-transporting peptide that helps hepatocytes uptake BAs together with NTCP at the basolateral membrane of hepatocytes, was decreased by CCl4 treatment in wild-type mice. The FXR−/− mice showed much lower levels of OATP1 expression in liver both before CCl4 treatment and during liver repair (Fig. 3E). Intrahepatic cholestasis also changes expression of ATP-binding cassette transporters [multidrug-resistant proteins (MRPs)]. Although MRP2 was reported as a FXR target gene (21), its expression in the FXR−/− mice was not very different from that of the wild-type controls (Fig. 3F). Similar to the BDL cholestasis model of FXR−/− mice, MRP3 and MRP4 were up-regulated in the FXR−/− mice via a FXR-independent manner (Fig. 3, G and H). In summary, due to the differentiated expression of CYP7A1, BSEP, and NTCP, FXR−/− mice developed more severe intrahepatic cholestasis despite their lower levels of OATP1 and adaptive up-regulation of MRP3/4.
Figure 3.
Intrahepatic cholestasis of FXR−/− mice after CCl4 treatment. A, Hepatic BAs were measured as described in Materials and Methods. *, FXR−/−vs. wild type (WT), P < 0.05; #, hepatic BAs of FXR−/− mice treated with CCl4vs. hepatic BAs of FXR−/− mice at zero time. B–H, Quantitative real-time PCR analysis of CYP7A, BSEP, NTCP, OATP1, and MRP2/3/4 expression. The quantity of mRNA was normalized using the internal standard, m36B4. *, P < 0.05.
Exogenous overexpression of a constitutively active FXR suppressed liver injury after CCl4 treatment
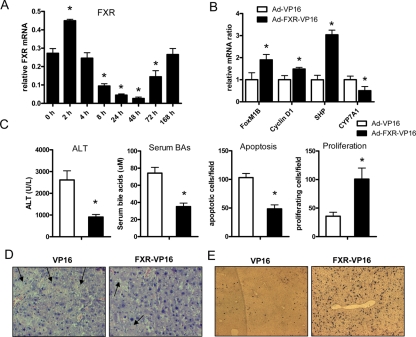
Of interest, the expression of FXR was strongly suppressed after CCl4 treatment (Fig. 4A), raising the question of whether it is the decreased FXR expression that contributes to liver injury. Therefore we infected the wild-type mice with an adenovirus expressing a constitutively active form of FXR, FXR-VP16, as described previously to test whether FXR overexpression rescues CCl4-induced liver injury (Supplemental Fig. 2A) (22). Compared with the controls infected with adenovirus expressing VP16 only, the mice infected with FXR-VP16 showed increased expression of SHP and reduced expression of CYP7A1 40 h after CCl4 treatment (Fig. 4B). Two genes regulating the cell cycle, FoxM1B and Cyclin D1, were increased by FXR-VP16 infection (Fig. 4B). Overexpression of FXR-VP16 effectively reduced serum BA levels and ALT levels and rescued apoptosis and necrosis of the hepatocyte after CCl4 administration (Fig. 4, C and D). The proliferation of the hepatocytes, as shown by BrdU staining, was simultaneously increased (Fig. 4, C and E).
Figure 4.
Overexpression of a constitutively active FXR (FXR-VP16)-suppressed liver injury caused by CCl4. A, mRNA levels of FXR at the different time points after CCl4 injection. *, One-way ANOVA test followed by a post hoc test (Dunnett’s Multiple Comparison Test) compared with control group, P < 0.05. B, Gene expression analysis in the wild-type mice infected by adenovirus (Ad)-expressing FXR-VP16 or VP16 alone 40 h after CCl4 treatment. C, Serum ALT, serum BAs, Terminal deoxynucleotide transferase-mediated dUTP nick end labeling, and BrdU staining in the mice treated with FXR-VP16 adenovirus or VP-16 adenovirus 40 h after CCl4 treatment. *, P < 0.05. D, Representative figures of H&E staining. Arrows indicate necrosis. E, Representative figures of BrdU staining.
Reduced signal transducer and activator of transcription 3 (STAT3) activities in FXR−/− mice during early time points after CCl4 treatment
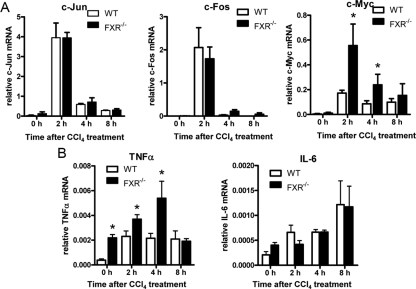
Hepatocytes normally exist in a highly differentiated quiescent G0 phase. Upon injury, the remaining hepatocytes are primed by protooncogenes such as c-fos, c-jun, and c-myc, the cytokines, TNFα and IL-6, and acute phase response factors including nuclear factor-κB (NF-κB) and STAT3 (23).
The induction of c-jun and c-fos is similar between the FXR−/− mice and the wild-type mice (Fig. 5A). However, the FXR−/− mice exhibited surprisingly high levels of c-myc induction (Fig. 5A). Although certain level of c-myc expression can promote the initiation of hepatocyte proliferation, the overactivation of c-myc may lead to cellular senescence and apoptosis in the liver (24).
Figure 5.
Expression analysis of early response genes in liver tissues from CCl4-treated wild-type (WT) and FXR−/− mice. Total hepatic RNA was prepared from wild-type and FXR−/− mice and subjected to quantitative real-time PCR analysis. A, Acute phase protooncogenes c-jun, c-fos, and c-myc. B, Inflammatory cytokines TNFα and IL-6. The quantity of mRNA was normalized using the internal standard, m36B4. *, P < 0.05.
CCl4 preferentially induces TNFα production in its early phase (25). In the wild-type mice, TNFα was induced and achieved a peak at the second hour, and its expression level was still maintained at the eighth hour. FXR−/− mice had preexisting high levels of TNFα and the expression was further enhanced by CCl4 and almost 2-fold higher than that in wild-type livers (Fig. 5B). The exaggerated TNFα might result in overexpression of c-myc and contribute to the increased necrosis and apoptosis in FXR−/− livers. IL-6 is a cytokine that plays a mainly protective role during liver injury. The induction of this cytokine was seen as early as the second hour, and the magnitude of induction could achieve a higher level on the eighth hour. However, no significant difference of IL-6 production in the acute phase was seen between the wild-type and the FXR−/− mice (Fig. 5B).
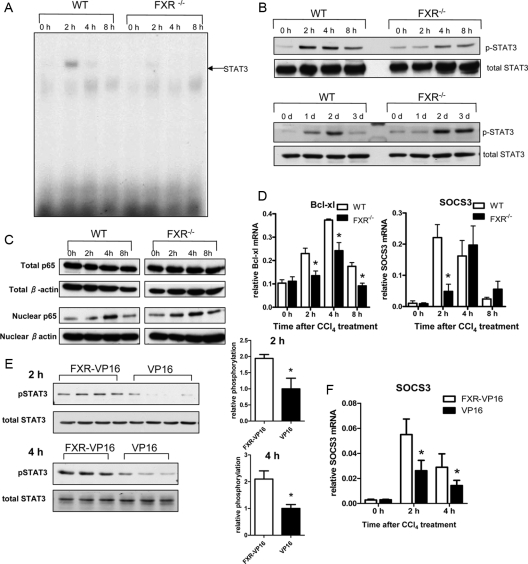
However, we observed a dramatically reduced DNA-binding activity of STAT3 in the FXR−/− livers (Fig. 6A). Whereas STAT3 activity was strongly up-regulated in the wild-type livers 2 h after CCl4 injection, this induction was much less in the FXR−/− livers. The result was further confirmed by Western blotting, which showed less STAT3 tyrosine phosphorylation in the FXR−/− livers (Fig. 6B). In addition, the STAT3 phosphorylation at later time points, which correlates with the initiation of the second round of the hepatocyte proliferation, was delayed in the FXR−/− livers until 2 d after the treatment, but FXR−/− mice showed greatly enhanced hepatic STAT3 phosphorylation at 3 d after the treatment. We also investigated NF-κB activation by measuring the nuclear translocation of p65, a catalytic subunit of NF-κB. There was a comparable increase in the levels of p65 in the nucleus of the wild-type and the FXR−/− livers after CCl4 injection (Fig. 6C). To further confirm the defective STAT3 activation in the FXR−/− livers, we measured the expression of two STAT3 target genes Bcl-xl and SOCS3 (26,27) in the acute responses after CCl4 treatment. Consistent with the STAT3 DNA-binding activity pattern, induction of Bcl-xl and SOCS3 was either decreased or delayed in the FXR−/− livers compared with induction of these two genes in the wild-type livers (Fig. 6D). The lower expression of the antiapoptotic gene, Bcl-xl, might contribute to the enhanced cell deaths in the FXR−/− livers.
Figure 6.
Impaired STAT3 activation after CCl4 treatment in FXR−/− mice. A, EMSA analysis of nuclear proteins (8 μg) extracted from pooled livers of four to six mice at the indicated times after CCl4 injections. Arrow indicates STAT3 homodimer. B, Western blot analysis of total protein extracts (30 μg) from the same liver samples as in panel A and the samples collected at later time points (1, 2, and 3 d). Blots were probed for STAT3 Tyr705 phosphorylation or total STAT3 protein levels using corresponding antibodies. C, Immunoblot analysis of total liver lysates or nuclear liver lysates (30 μg) from CCl4-treated mice at the indicated time points after treatment. Blots were probed with anti-p65 and anti-β-actin antibody. D, mRNA levels of STAT3 target genes Bcl-xl and SOCS3. *, P < 0.05. E, Immunoblot of phospho-STAT3 in the FXR−/− mice infected with adenovirus. F, Real-time PCR analysis of SOCS3 in the FXR−/− mice infected with adenovirus. *, P < 0.05. SOCS, Suppressor of cytokine signaling; WT, wild type.
We restored FXR activities in FXR−/− mice by introducing the adenovirus expressing FXR-VP16. Adenovirus expressing FXR-VP16 itself lowered basal levels of serum ALT and BAs of FXR−/− mice compared with control adenovirus that expressed VP16 alone (Supplemental Fig. 2B). The FXR-VP16 adenovirus restored the phosphorylation of STAT3 in the acute phases after CCl4 treatment (Fig. 6E) as well as promoted the expression of the STAT3 target gene SOCS3 (Fig. 6F) without changing hepatic IL-6 expression (Supplemental Fig. 3A). These results suggest a role of FXR for normal STAT3 activation in the acute phase of liver injury.
High levels of BAs suppress STAT3 phosphorylation during liver regeneration
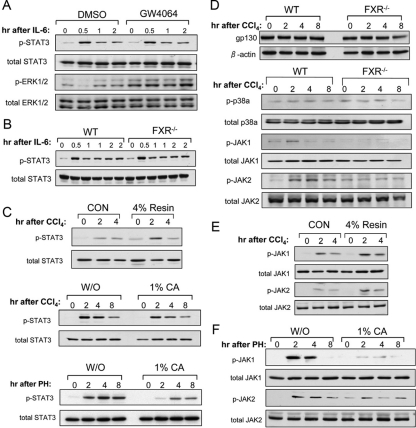
We then tested whether FXR activation directly affected the STAT3 phosphorylation in primary hepatocytes. FXR ligand GW4064 was not able to initiate STAT3 phosphorylation or prolong IL-6-induced STAT3 phosphorylation of the wild-type primary hepatocytes although the effectiveness of GW4064 on FXR activation was shown by the increased ERK1/2 phosphorylation (28) (Fig. 7A). Furthermore, no significant difference of IL-6-induced STAT3 phosphorylation was observed between the wild-type and the FXR−/− primary hepatocytes (Fig. 7B).
Figure 7.
High BA levels suppress STAT3 phosphorylation during liver regeneration. A, Primary hepatocytes from 8-wk-old wild-type mice were pretreated with 2 μm GW4064 or dimethylsulfoxide (DMSO) for 24 h and then treated with 10 ng/ml recombinant mouse IL-6. The total lysates from the hepatocytes were analyzed with Western blot. B, IL-6 induced STAT3 phosphorylation in wild-type (WT) and FXR−/− primary hepatocytes. C, Immunoblot analysis of STAT3 phosphorylation in total protein extracts pooled from livers of four to six FXR−/− mice prefed with a powdered diet supplemented with 4% cholestyramine (resin) or a control powdered diet (CON) for 5 d at the indicated time points after CCl4 treatment, STAT3 phosphorylation in total protein extracts pooled from livers of four to six WT mice either received 1% CA feeding or normal diet (W/O) for 5 d and terminated at the indicated time points after CCl4 treatment, and STAT3 phosphorylation in total liver protein extracts pooled from four WT mice that received the 1% CA-containing diet after 70% PH. D, Immunoblot analysis of gp130 protein levels, and phosphorylation of p38α, JAK1, and JAK2 in the liver of WT mice and FXR−/− mice after CCl4 treatment. E, Immunoblot analysis of JAK1 and JAK2 phosphorylation in the FXR−/− mice fed with the 4% Resin-containing diet after CCl4 treatment. F, Immunoblot analysis of phosphorylation of JAK1 and JAK2 in the livers of WT mice fed with normal diet (W/O) or 1% CA-containing diet after 70% PH.
A major deregulation in the FXR−/− mice was the sustained higher levels of BAs in serum and liver. Therefore, we tested whether high BA levels would affect STAT phosphorylation. We first fed the mice with a 4% cholestyramine (Resin) diet to reduce BA levels in FXR−/− mice as we previously described (17). Indeed, we observed a reduced suppression of STAT3 phosphorylation after CCl4 treatment by Resin feeding compared with the controls (Fig. 7C). On the other hand, when we fed the wild-type mice with a 1% cholic acid (CA) diet, we observed a suppression of STAT3 phosphorylation after CCl4 treatment (Fig. 7C and Supplemental Fig. 4A). This suppression of hepatic STAT3 phosphorylation by 1% CA feeding was even more prominent in the wild-type mice with 70% PH (Fig. 7C and Supplemental Fig. 4B). These results suggest that high levels of BAs contribute to the reduced STAT3 phosphorylation in the FXR−/− livers after CCl4 treatment. However, neither Resin- nor CA-containing diet altered hepatic IL-6 expression (Supplemental Fig. 3, B–D).
BAs are known to directly modulate phosphorylation of STAT3 by affecting its upstream components (29,30). It was reported that certain hydrophobic BAs such as glycochenodeoxycholic acid (GCDCA) down-regulated IL-6-induced STAT3 phosphorylation through both gp130-dependent and p38α-dependent pathways. Treatment of GCDCA to cultured rat primary hepatocytes caused caspase-mediated gp130 degradation (30). Therefore, we compared gp130 protein levels between the 1% CA-containing diet feeding mice and the mice fed with the normal diet after 70% PH, and between the FXR−/− mice and the wild-type mice treated with CCl4. However, no significant difference was observed (Supplemental Figs. 4C and 7D). The levels of p38α phosphorylation between the two groups were comparable (Fig. 7D). Furthermore, there was no difference in the induction of p38α phosphorylation between 1% CA-containing diet-fed mice and the wild-type controls (Supplemental Fig. 4C).
STAT3 pathways are known to be directly regulated mainly through another two upstream components, Janus kinase (JAK)1 and JAK2, the phosphorylated forms of which, in turn, phosphorylate STAT3 (23,31). We found that phosphorylation of both JAK1 and JAK2 was impaired in the FXR−/− mice. Thus we speculated whether BAs affected phosphorylation of JAK1 and JAK2 through a gp130- and p38α-independent pathway. Indeed, 4% Resin-containing diet effectively restored phosphorylation of JAK1 and JAK2 in the FXR−/− mice after CCl4 treatment (Fig. 7E). In addition, 1% CA-containing diet greatly reduced phosphorylation of both JAK1 and JAK2 in the wild-type mice after 70% PH (Fig. 7F). Furthermore, we observed that the CA-containing diet would augment CCl4-induced liver injury and impair liver repair whereas the Resin-containing diet slightly reduced CCl4-induced liver injury and help liver repair (Supplemental Fig. 5, A–F).
Exogenous overexpression of a constitutively active STAT3 suppressed liver injury in FXR−/− mice after CCl4 treatment
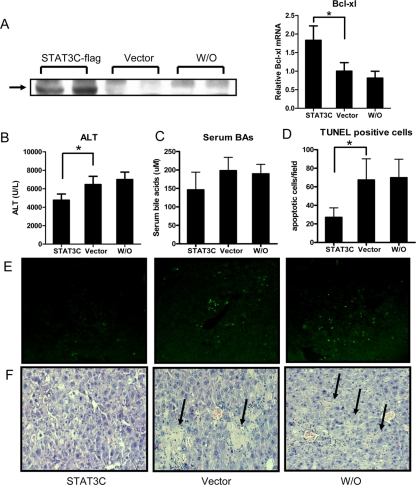
To better understand the specific roles of STAT3 in mediating the effects of FXR on hepatocyte survival, we expressed a constitutively active STAT3 (STAT3C) in FXR−/− livers via hydrodynamic tail vein injection (32). Hydrodynamic injection resulted in STAT3 protein expression in liver and enhanced expression of STAT3 target gene Bcl-xl (Fig. 8A). We then treated the mice with CCl4 (750 μl/kg) 1 d after the hydrodynamic transfection. Exogenous overexpression of STAT3C significantly reduced ALT levels, hepatocyte deaths, and liver injury after CCl4 treatment in FXR−/− mice, whereas FXR−/− mice with the treatment of a control plasmid did not show different responses to CCl4 compared with the mice receiving saline only (Fig. 8, B–F). These results demonstrate that diminished STAT3 activity in the FXR−/− livers after CCl4 treatment may contribute to the enhanced hepatocyte deaths.
Figure 8.
Suppression of CCl4-induced hepatocyte death by a constitutively active STAT3 in FXR−/− livers. A, Immunoblot analysis of ectopic STAT3 expression and real-time PCR analysis of STAT3 target gene Bcl-xl. Total protein extracts (30 μg) were pooled from livers of four mice that were hydrodynamically injected with constitutively active STAT3 (STAT3C). Liver samples were harvested 24 h after CCl4 injections. Blots were probed with anti-flag antibody. Vector indicates the mice injected with the pCMV/Rc plasmid without insertion of STAT3C sequence; W/O indicates the mice with mock saline injection. B and C, Serum ALT (B) and BAs (C) levels in the indicated groups of FXR−/− mice. *, P < 0.05. D, Quantification of terminal deoxynucleotide transferase-mediated dUTP nick end labeling (TUNEL)-positive cells in randomly chosen fields. *, P < 0.05. E, Representative TUNEL-stained liver sections. F, Representative H&E-stained liver sections. Arrows indicate necrosis areas.
Discussion
Liver repair is an intrinsic defense mechanism after injury. The impact of the liver metabolic pathways on liver injury and repair has not been well studied. We observe that CCl4-induced liver injury disrupts the BA enterohepatic circulation and causes the accumulation of BAs in the liver. This may result from the repression of FXR expression and other key components involved in BA circulation such as the BA transporters. The suppression of FXR expression may be due to the increase of proinflammatory cytokines induced by strong liver injury (33). Interestingly, we observed a transient increase of FXR mRNA levels shortly after CCl4 treatment. This could be due to the fact that FXR activation by increased BA flow up-regulates the transcription of FXR. BAs as well as FXR ligands, CDCA and GW4064, are shown to increase FXR mRNA levels (34,35).
The key role of FXR in controlling BA levels after CCl4 treatment was confirmed by FXR−/− mice that displayed severe cholestasis and exaggerated liver injury. Previous studies indicate that high levels of BAs can induce cell death and augment inflammatory cytokine production such as TNFα (Fig. 5B) (36). TNFα biosynthesis is required for liver repair after CCl4 treatment, but overproduction of TNFα can induce more apoptosis mediated by TNF receptor/Fas-associates death domain pathways (37). Therefore, the uncontrolled inflammatory cytokine production due to high levels of BAs may contribute to the overall liver injury.
In this study, we also identify a specific effect of BAs on STAT3 activation. STAT3 signaling pathway, usually activated in the acute phase of liver injury, is a previously identified important pathway for survival and repair of liver cells. Here we show that overload of BAs caused by FXR deficiency leads to delayed responses of STAT3 to liver injury. Tyrosine phosphorylation of STAT3 was severely repressed in the FXR−/− livers in the acute phase of CCl4-induced liver injury, although stronger responses of STAT3 were observed in the later stages of liver repair in FXR−/− mice. The compensatory hyperphosphorylation of STAT3 in the FXR−/− livers at the later stages was probably due to either the release of BA stress, such as compensatory exportation and conjugation of toxic hydrophobic BAs, or more aggravated inflammation caused by massive cell deaths in these mice, which may help to explain the accelerated recovery of FXR−/− mice after 3 d in the second round of hepatocyte cell cycling after injury (38).
We did not observe a direct effect of FXR on either basal or IL-6-induced STAT3 phosphorylation. Instead, we provide evidence that high levels of BAs in FXR−/− livers may result in reduced STAT3 phosphorylation. The BA levels are much higher in FXR−/− livers after CCl4-induced liver injury than after 70% PH. It may suggest why we observed reduced STAT3 phosphorylation only in CCl4 treatment. Indeed, an exogenous supplement of BAs suppresses STAT3 phosphorylation in 70% PH livers. It is well known that cholestatic livers have impaired liver regeneration and display higher grades of liver injury (39). A previous study reported that BA treatment on rat hepatocytes in vitro can inhibit IL-6-induced STAT3 phosphorylation via a gp130- and p38α-dependent manner (30). However, we did not observe the similar mechanisms in our FXR−/− mouse models or 1% CA-containing diet models of cholestasis. This is potentially because the dose of the hydrophobic BA (GCDCA) that the two groups used in vitro, 100 μm, was not easily achieved in our cholestasis models. In contrast, we observed that high levels of BAs would directly modulate phosphorylation of JAK1 and JAK2, two direct upstream components of STAT3 and well-accepted main transducers of STAT3 pathways in liver regeneration (40). Our results thus indicate that the interference with STAT3 activation by high levels of BAs in damaged liver contributes to the defective liver repair. It should be mentioned that loss of other IL-6 family cytokines, such as IL-11, oncostatin M, leukemia inhibitory factor, and complement C3 may also be involved in the deficient STAT3 activation in the FXR−/− mice after CCl4 treatment because of their documented roles in liver regeneration (41,42).
Our previous studies suggest a novel role of FXR in promoting liver regeneration after 70% PH (17). However, there are intrinsic differences of responses between 70% PH and CCl4-induced liver injury. In 70% PH, no significant apoptosis or necrosis is seen (17). In contrast, in CCl4-induced injury, there is strong cell death and liver necrosis. The response of hepatocytes to cytokine pathways, such as STAT3 phosphorylation, in these two different types of liver damage is different also. Although the transient increase of BA will promote liver regeneration by activating FXR, it may aggravate liver injury after CCl4 treatment. Therefore, we consider BA as a stress signal, and its function in liver repair depends on the status of FXR and the type of liver injury (43).
In summary, we demonstrate that FXR is essential to promote liver repair after injury. On one hand, FXR activation can directly transform the stress of BA overload into force of promotion of liver repair. For example, FXR promotes liver regeneration by regulating expression of genes involved in cell cycle progression such as FoxM1b. We also have evidence that FoxM1b is a FXR direct target gene (44). Therefore, FXR may direct a program of gene expression to promote liver regeneration after injury. On the other hand, FXR activation will help release the BA overload in liver, which can prevent BA-induced cell deaths and other deleterious effects on normal liver repair pathways such as STAT3 signaling. We propose that FXR is a novel liver protector (21). Therefore, FXR may represent a novel target of drug development to promote liver repair and to treat liver-injury related diseases.
Materials and Methods
Animal maintenance and treatments
Wild-type and FXR−/− mice were maintained as previously described (17). After an overnight fast, 8- to 12-wk-old mice were injected ip with a single dose of CCl4 (750 μl/kg in corn oil) or vehicle control as the zero time point. All procedures followed the National Institute of Health guidelines for the care and use of laboratory animals. The cholestyramine or the cholic acid feedings are described previously (17).
RNA preparation and quantitative real-time PCR
RNA preparation was described previously (22). mRNA was quantified by real-time quantitative PCR using an Applied Biosystems 7300 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA). The sequences of primers for each gene are provided in Supplemental Table 1.
EMSAs
Liver nuclear extract was prepared as described previously (22). Nuclear extracts (6 μg), polydeoxyinosinic deoxycytidylic acid (3 μg), and 32P-radiolabeled oligonucleotides were coincubated for 30 min and separated by electrophoresis. For signal transducer and activator of transcription (STAT) 3-DNA binding analysis, double-stranded radiolabeled oligonucleotide high-affinity serum inducible element (5′-ATCCTCCAGCATTTCCCGTAAATCCTC-3′) was added to the mixture. The mixture was subjected to 5% nondenaturing gel electrophoresis. The gel was dried on chromatography paper (Whatman, Florham Park, NJ) and exposed overnight at −80 C using BioMax MS film (Eastman Kodak, Rochester, NY).
Western blotting
Liver total protein was prepared and separated as described previously (22). After blocking in 5% nonfat milk, the membranes were exposed to specific primary antibodies [anti-STAT3, anti-p65, antiphospho-STAT3-tyrosine 705, anti-p38α, antiphospho-p38α, anti-JAK1, antiphospho-JAK1, anti-JAK2, antiphospho-JAK2, and anti-cleaved Caspase 3 were from Cell Signaling Technology (Beverly, MA); anti-gp130 were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA); anti-flag and anti-β-actin were from Sigma (St. Louis, MO) at a concentration of 1:1000]. Membranes were then washed and exposed to peroxidase-conjugated secondary antibodies (Amersham Bioscience, Little Chalfont, Buckinghamshire, UK). Immunoblots were imaged using a medical film processor (SRX-101A; Konica Minolta Medical & Graphic Inc., New York, NY).
Adenovirus injection
Adenovirus expressing FXR-VP16 or VP16 alone was prepared and purified as previously described (22). Eight days after tail vein injection with 1 × 109 plaque-forming units of the FXR-VP16 adenovirus or the control per mouse, the mice were euthanized. Livers and blood were collected for analysis.
Primary hepatocyte preparation
Primary hepatocytes were isolated and cultured as described previously (22). GW4064 (2 μm) (TOCRIS Bioscience, Ellisville, MO) was applied to the hepatocytes, and 24 h later 10 ng/ml mouse recombinant IL-6 (PeproTech, Rocky Hill, NJ) was used to induce STAT3 phosphorylation.
Hydrodynamic injection
The pRc/CMV-stat3C-flag and pRc/CMV plasmid vectors, as described previously (45), were transfected into 8-wk-old male FXR mice via hydrodynamic injection through the tail vein, as described previously (32). The volume of the plasmid solutions was 0.1 ml/g body weight with a concentration of 10 μg/ml. the mice were injected with CCl4 1 d after the infusion of the plasmids. Mice were euthanized and livers were removed for analysis 24 h later.
BA and serum ALT analysis
Total BAs were measured using the BA L3K assay kit, according to the manufacturer’s instructions (Diagnostic Chemicals Limited, Oxford, CT). Serum ALT was measured at the City of Hope Helford Research Hospital.
Statistical analysis
Data are expressed as means ± sd. Two-tailed Student’s t test was used to determine differences between data groups. P < 0.05 was considered statistically significant, unless otherwise stated.
Supplementary Material
Acknowledgments
We thank Dr. Keely Walker at City of Hope for her help in proofreading the manuscript. We thank Dr. Hua Yu at City of Hope for kindly providing the constitutively active STAT3 construct and Dr. Peter A. Edwards (University of California, Los Angeles, CA) for providing adenovirus that expressed VP16 alone (Ad-VP16) or murine FXRα2 fused to VP16 (FXR-VP16).
Footnotes
W.H. is supported by the Pilot/Feasibility Grant P30DK48522 from the University of Southern California Research Center for Liver Diseases and Concern Foundation.
Current address for N.L.: Department of Hematology and Oncology, First Affiliated Hospital of Jilin University, 71 Xinmin Street, Changchun City, Jilin 130041, China.
Disclosure Summary: The authors do not have any conflict of interest in this work.
First Published Online March 8, 2010
Abbreviations: ALT, Alanine aminotransferase; BA, bile acid; BDL, bile duct ligatior BrdU, 5-bromo-2′-deoxy-uridine; CA, cholic acid; FXR, farnesoid X receptor; GCDCA, glycochenodeoxycholic acid; H&E, hematoxylin and eosin; JAK, Janus kinase; MRP, multidrug-resistant protein; NF-κB, nuclear factor-κB; PFIC, progressive familial intrahepatic cholestasis; PH, partial hepatectomy; SHP, small heterodimer partner; STAT3, signal transducer and activator of transcription 3.
References
- Forman BM, Goode E, Chen J, Oro AE, Bradley DJ, Perlmann T, Noonan DJ, Burka LT, McMorris T, Lamph WW, Evans RM, Weinberger C 1995 Identification of a nuclear receptor that is activated by farnesol metabolites. Cell 81:687–693 [DOI] [PubMed] [Google Scholar]
- Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B 1999 Identification of a nuclear receptor for bile acids. Science 284:1362–1365 [DOI] [PubMed] [Google Scholar]
- Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD, Lehmann JM 1999 Bile acids: natural ligands for an orphan nuclear receptor. Science 284:1365–1368 [DOI] [PubMed] [Google Scholar]
- Wang H, Chen J, Hollister K, Sowers LC, Forman BM 1999 Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell 3:543–553 [DOI] [PubMed] [Google Scholar]
- Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, Galardi C, Wilson JG, Lewis MC, Roth ME, Maloney PR, Willson TM, Kliewer SA 2000 A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell 6:517–526 [DOI] [PubMed] [Google Scholar]
- Lu TT, Makishima M, Repa JJ, Schoonjans K, Kerr TA, Auwerx J, Mangelsdorf DJ 2000 Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell 6:507–515 [DOI] [PubMed] [Google Scholar]
- Wang YD, Chen WD, Huang W 2008 FXR, a target for different diseases. Histol Histopathol 23:621–627 [DOI] [PubMed] [Google Scholar]
- Russell DW 2003 The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem 72:137–174 [DOI] [PubMed] [Google Scholar]
- Van Mil SW, Milona A, Dixon PH, Mullenbach R, Geenes VL, Chambers J, Shevchuk V, Moore GE, Lammert F, Glantz AG, Mattsson LA, Whittaker J, Parker MG, White R, Williamson C 2007 Functional variants of the central bile acid sensor FXR identified in intrahepatic cholestasis of pregnancy. Gastroenterology 133:507–516 [DOI] [PubMed] [Google Scholar]
- Chen F, Ananthanarayanan M, Emre S, Neimark E, Bull LN, Knisely AS, Strautnieks SS, Thompson RJ, Magid MS, Gordon R, Balasubramanian N, Suchy FJ, Shneider BL 2004 Progressive familial intrahepatic cholestasis, type 1, is associated with decreased farnesoid X receptor activity. Gastroenterology 126: 756–764 [DOI] [PubMed] [Google Scholar]
- Strautnieks SS, Bull LN, Knisely AS, Kocoshis SA, Dahl N, Arnell H, Sokal E, Dahan K, Childs S, Ling V, Tanner MS, Kagalwalla AF, Németh A, Pawlowska J, Baker A, Mieli-Vergani G, Freimer NB, Gardiner RM, Thompson RJ 1998 A gene encoding a liver-specific ABC transporter is mutated in progressive familial intrahepatic cholestasis. Nat Genet 20:233–238 [DOI] [PubMed] [Google Scholar]
- van Mil SW, van der Woerd WL, van der Brugge G, Sturm E, Jansen PL, Bull LN, van den Berg IE, Berger R, Houwen RH, Klomp LW 2004 Benign recurrent intrahepatic cholestasis type 2 is caused by mutations in ABCB11. Gastroenterology 127: 379–384 [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Antonelli E, Rizzo G, Renga B, Mencarelli A, Riccardi L, Orlandi S, Pellicciari R, Morelli A 2004 The nuclear receptor SHP mediates inhibition of hepatic stellate cells by FXR and protects against liver fibrosis. Gastroenterology 127:1497–1512 [DOI] [PubMed] [Google Scholar]
- Liu Y, Binz J, Numerick MJ, Dennis S, Luo G, Desai B, MacKenzie KI, Mansfield TA, Kliewer SA, Goodwin B, Jones SA 2003 Hepatoprotection by the farnesoid X receptor agonist GW4064 in rat models of intra- and extrahepatic cholestasis. J Clin Invest 112:1678–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YJ, Qatanani M, Chua SS, LaRey JL, Johnson SA, Watanabe M, Moore DD, Lee YK 2008 Loss of orphan receptor small heterodimer partner sensitizes mice to liver injury from obstructive cholestasis. Hepatology 47:1578–1586 [DOI] [PubMed] [Google Scholar]
- Cui YJ, Aleksunes LM, Tanaka Y, Goedken MJ, Klaassen CD 2009 Compensatory induction of liver efflux transporters in response to ANIT-induced liver injury is impaired in FXR-null mice. Toxicol Sci 110:47–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Ma K, Zhang J, Qatanani M, Cuvillier J, Liu J, Dong B, Huang X, Moore DD 2006 Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science 312:233–236 [DOI] [PubMed] [Google Scholar]
- Trudell JR, Bösterling B, Trevor AJ 1982 Reductive metabolism of carbon tetrachloride by human cytochromes P-450 reconstituted in phospholipid vesicles: mass spectral identification of trichloromethyl radical bound to dioleoyl phosphatidylcholine. Proc Natl Acad Sci USA 79:2678–2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Wang F, Jin C, Wu X, Chan WK, McKeehan WL 2002 Increased carbon tetrachloride-induced liver injury and fibrosis in FGFR4-deficient mice. Am J Pathol 161:2003–2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noé J, Stieger B, Meier PJ 2002 Functional expression of the canalicular bile salt export pump of human liver. Gastroenterology 123:1659–1666 [DOI] [PubMed] [Google Scholar]
- Wang YD, Chen WD, Moore DD, Huang W 2008 FXR: a metabolic regulator and cell protector. Cell Res 18:1087–1095 [DOI] [PubMed] [Google Scholar]
- Wang YD, Chen WD, Wang M, Yu D, Forman BM, Huang W 2008 Farnesoid X receptor antagonizes nuclear factor κB in hepatic inflammatory response. Hepatology 48:1632–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausto N, Campbell JS, Riehle KJ 2006 Liver regeneration. Hepatology 43:S45–S53 [DOI] [PubMed] [Google Scholar]
- Ladu S, Calvisi DF, Conner EA, Farina M, Factor VM, Thorgeirsson SS 2008 E2F1 inhibits c-Myc-driven apoptosis via PIK3CA/Akt/mTOR and COX-2 in a mouse model of human liver cancer. Gastroenterology 135:1322–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier A, Kim SK, Gerloff T, Dietrich CG, Lammert F, Karpen SJ, Stieger B, Meier PJ, Matern S, Gartung C 2002 Hepatobiliary organic anion transporters are differentially regulated in acute toxic liver injury induced by carbon tetrachloride. J Hepatol 37:198–205 [DOI] [PubMed] [Google Scholar]
- Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell Jr JE 1999 Stat3 as an oncogene. Cell 98:295–303 [DOI] [PubMed] [Google Scholar]
- Riehle KJ, Campbell JS, McMahan RS, Johnson MM, Beyer RP, Bammler TK, Fausto N 2008 Regulation of liver regeneration and hepatocarcinogenesis by suppressor of cytokine signaling 3. J Exp Med 205:91–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YD, Yang F, Chen WD, Huang X, Lai L, Forman BM, Huang W 2008 Farnesoid X receptor protects liver cells from apoptosis induced by serum deprivation in vitro and fasting in vivo. Mol Endocrinol 22:1622–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YH, Chuang JH, Yang YL, Huang CC, Wu CL, Chen CL 2009 Cholestasis downregulate hepcidin expression through inhibiting IL-6-induced phosphorylation of signal transducer and activator of transcription 3 signaling. Lab Invest 89:1128–1139 [DOI] [PubMed] [Google Scholar]
- Graf D, Kohlmann C, Haselow K, Gehrmann T, Bode JG, Häussinger D 2006 Bile acids inhibit interleukin-6 signaling via gp130 receptor-dependent and -independent pathways in rat liver. Hepatology 44:1206–1217 [DOI] [PubMed] [Google Scholar]
- Yu H, Jove R 2004 The STATs of cancer—new molecular targets come of age. Nat Rev Cancer 4:97–105 [DOI] [PubMed] [Google Scholar]
- Liu F, Song Y, Liu D 1999 Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther 6:1258–1266 [DOI] [PubMed] [Google Scholar]
- Kim MS, Shigenaga J, Moser A, Feingold K, Grunfeld C 2003 Repression of farnesoid X receptor during the acute phase response. J Biol Chem 278:8988–8995 [DOI] [PubMed] [Google Scholar]
- Xu G, Pan LX, Li H, Forman BM, Erickson SK, Shefer S, Bollineni J, Batta AK, Christie J, Wang TH, Michel J, Yang S, Tsai R, Lai L, Shimada K, Tint GS, Salen G 2002 Regulation of the farnesoid X receptor (FXR) by bile acid flux in rabbits. J Biol Chem 277:50491–50496 [DOI] [PubMed] [Google Scholar]
- Zhang Q, He F, Kuruba R, Gao X, Wilson A, Li J, Billiar TR, Pitt BR, Xie W, Li S 2008 FXR-mediated regulation of angiotensin type 2 receptor expression in vascular smooth muscle cells. Cardiovasc Res 77:560–569 [DOI] [PubMed] [Google Scholar]
- Greve JW, Gouma DJ, Buurman WA 1989 Bile acids inhibit endotoxin-induced release of tumor necrosis factor by monocytes: an in vitro study. Hepatology 10:454–458 [DOI] [PubMed] [Google Scholar]
- Liu H, Lo CR, Jones BE, Pradhan Z, Srinivasan A, Valentino KL, Stockert RJ, Czaja MJ 2000 Inhibition of c-Myc expression sensitizes hepatocytes to tumor necrosis factor-induced apoptosis and necrosis. J Biol Chem 275:40155–40162 [DOI] [PubMed] [Google Scholar]
- Dai G, He L, Bu P, Wan YJ 2008 Pregnane X receptor is essential for normal progression of liver regeneration. Hepatology 47:1277–1287 [DOI] [PubMed] [Google Scholar]
- Yokoyama Y, Nagino M, Nimura Y 2007 Mechanism of impaired hepatic regeneration in cholestatic liver. J Hepatobiliary Pancreat Surg 14:159–166 [DOI] [PubMed] [Google Scholar]
- Gao B 2005 Cytokines, STATs and liver disease. Cell Mol Immunol 2:92–100 [PubMed] [Google Scholar]
- Nakamura K, Nonaka H, Saito H, Tanaka M, Miyajima A 2004 Hepatocyte proliferation and tissue remodeling is impaired after liver injury in oncostatin M receptor knockout mice. Hepatology 39:635–644 [DOI] [PubMed] [Google Scholar]
- Omori N, Evarts RP, Omori M, Hu Z, Marsden ER, Thorgeirsson SS 1996 Expression of leukemia inhibitory factor and its receptor during liver regeneration in the adult rat. Lab Invest 75:15–24 [PubMed] [Google Scholar]
- Zhang L, Huang X, Meng Z, Dong B, Shiah S, Moore DD, Huang W 2009 Significance and mechanism of CYP7a1 gene regulation during the acute phase of liver regeneration. Mol Endocrinol 23:137–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WD, Wang YD, Zhang L, Shiah S, Wang M, Yang F, Yu D, Forman BM, Huang W 22 October, 2009 Farnesoid X receptor alleviates age-related proliferation defects in regenerating mouse livers by activating forkhead box m1b transcription. Hepatology 10.1002/hep23390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, Heller R, Ellis LM, Karras J, Bromberg J, Pardoll D, Jove R, Yu H 2002 Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene 21:2000–2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.