Abstract
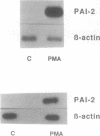
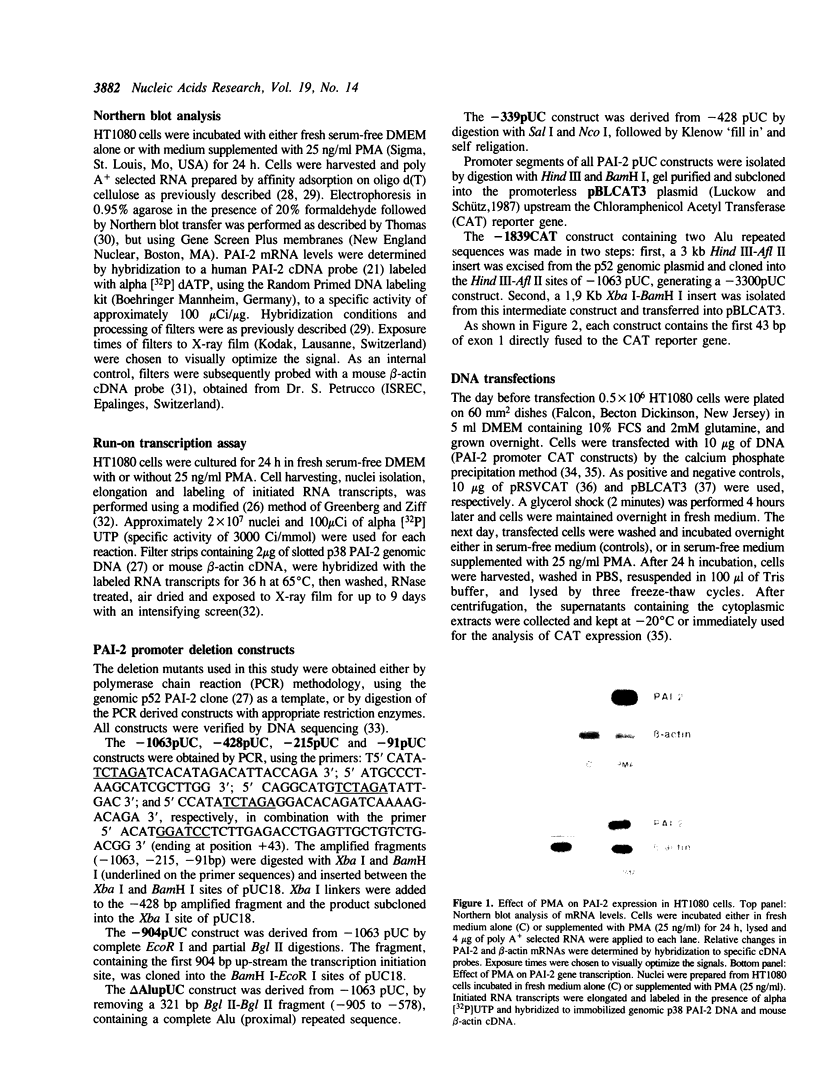
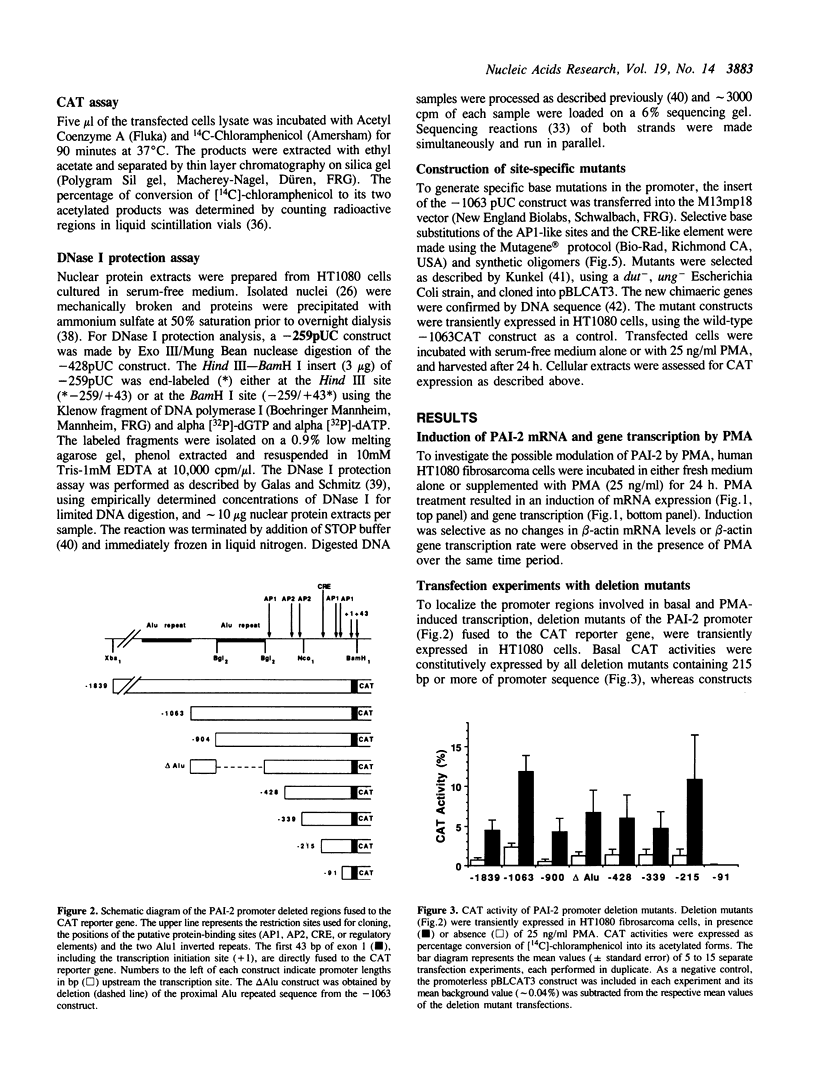
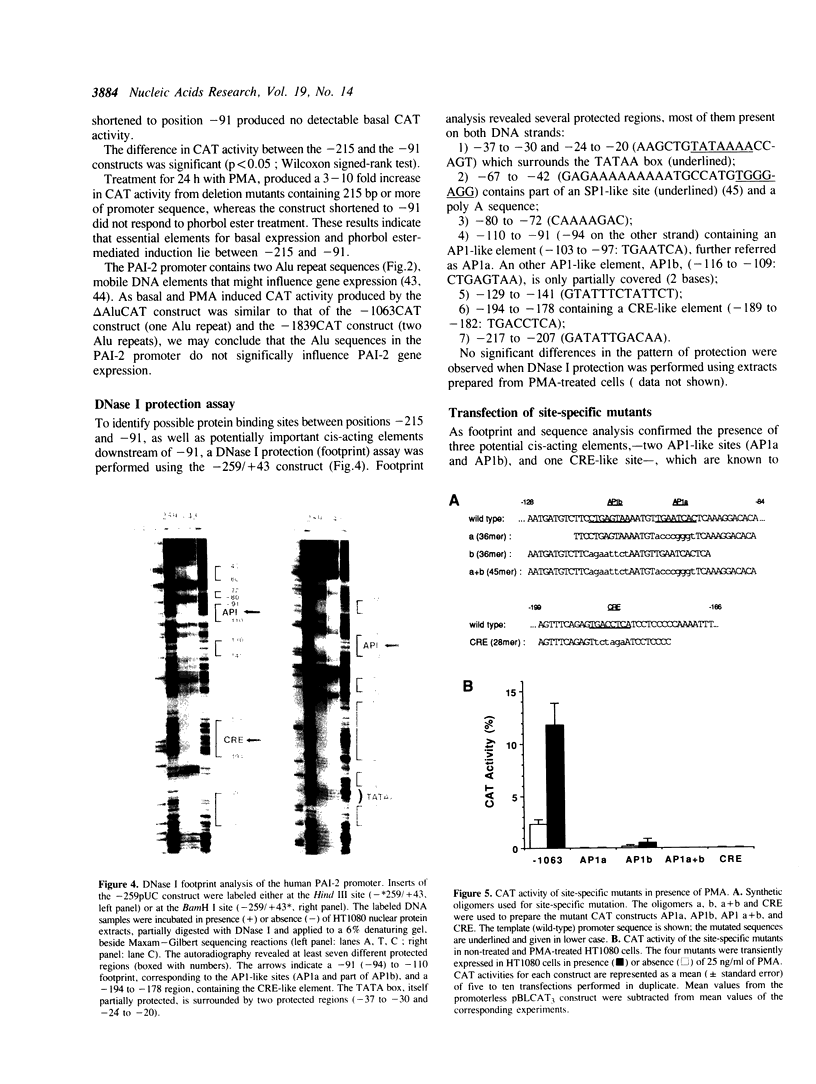
Gene transcription rates and mRNA levels of plasminogen activator inhibitor type 2 (PAI-2) are markedly induced by the tumor promoting agent phorbol 12-myristate 13-acetate (PMA) in human HT1080 fibrosarcoma cells. To identify promoter elements required for basal-, and phorbol ester-inducible expression, deletion mutants of the PAI-1 promoter fused to the chloramphenicol acetyl transferase (CAT) reporter gene, were transiently expressed in HT1080 cells. Constitutive CAT activity was expressed from constructs containing more than 215 bp of promoter sequence, whereas deletion to position -91 bp abolished CAT gene expression. Treatment of transfected cells with PMA resulted in a three- to ten-fold increase in CAT expression from all constructs except from the construct shortened to position -91. DNAse1 protection analysis of the promoter region between -215 and the transcription initiation site revealed numerous protected regions, including two AP1-like binding sites (AP1a and AP1b) and one CRE-like element. Site-directed mutagenesis of the AP1a site or of the CRE-like site resulted in the loss of basal CAT activity and abolished the PMA effect, whereas mutagenesis of AP1b only partially inhibited basal and PMA-mediated expression. Our results suggest that the PAI-2 promoter contains at least two elements required for basal gene transcription and PMA-mediated induction.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreasen P. A., Georg B., Lund L. R., Riccio A., Stacey S. N. Plasminogen activator inhibitors: hormonally regulated serpins. Mol Cell Endocrinol. 1990 Jan 2;68(1):1–19. doi: 10.1016/0303-7207(90)90164-4. [DOI] [PubMed] [Google Scholar]
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Blasi F., Vassalli J. D., Danø K. Urokinase-type plasminogen activator: proenzyme, receptor, and inhibitors. J Cell Biol. 1987 Apr;104(4):801–804. doi: 10.1083/jcb.104.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs M. R., Kadonaga J. T., Bell S. P., Tjian R. Purification and biochemical characterization of the promoter-specific transcription factor, Sp1. Science. 1986 Oct 3;234(4772):47–52. doi: 10.1126/science.3529394. [DOI] [PubMed] [Google Scholar]
- Chapman H. A., Jr, Stone O. L. Characterization of a macrophage-derived plasminogen-activator inhibitor. Similarities with placental urokinase inhibitor. Biochem J. 1985 Aug 15;230(1):109–116. doi: 10.1042/bj2300109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman H. A., Jr, Vavrin Z., Hibbs J. B., Jr Macrophage fibrinolytic activity: identification of two pathways of plasmin formation by intact cells and of a plasminogen activator inhibitor. Cell. 1982 Mar;28(3):653–662. doi: 10.1016/0092-8674(82)90220-3. [DOI] [PubMed] [Google Scholar]
- Cubellis M. V., Andreasen P., Ragno P., Mayer M., Danø K., Blasi F. Accessibility of receptor-bound urokinase to type-1 plasminogen activator inhibitor. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4828–4832. doi: 10.1073/pnas.86.13.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danø K., Andreasen P. A., Grøndahl-Hansen J., Kristensen P., Nielsen L. S., Skriver L. Plasminogen activators, tissue degradation, and cancer. Adv Cancer Res. 1985;44:139–266. doi: 10.1016/s0065-230x(08)60028-7. [DOI] [PubMed] [Google Scholar]
- Del Rosso M., Fibbi G., Dini G., Grappone C., Pucci M., Caldini R., Magnelli L., Fimiani M., Lotti T., Panconesi E. Role of specific membrane receptors in urokinase-dependent migration of human keratinocytes. J Invest Dermatol. 1990 Mar;94(3):310–316. doi: 10.1111/1523-1747.ep12874442. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis V., Wun T. C., Behrendt N., Rønne E., Danø K. Inhibition of receptor-bound urokinase by plasminogen-activator inhibitors. J Biol Chem. 1990 Jun 15;265(17):9904–9908. [PubMed] [Google Scholar]
- Estreicher A., Mühlhauser J., Carpentier J. L., Orci L., Vassalli J. D. The receptor for urokinase type plasminogen activator polarizes expression of the protease to the leading edge of migrating monocytes and promotes degradation of enzyme inhibitor complexes. J Cell Biol. 1990 Aug;111(2):783–792. doi: 10.1083/jcb.111.2.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galas D. J., Schmitz A. DNAse footprinting: a simple method for the detection of protein-DNA binding specificity. Nucleic Acids Res. 1978 Sep;5(9):3157–3170. doi: 10.1093/nar/5.9.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genton C., Kruithof E. K., Schleuning W. D. Phorbol ester induces the biosynthesis of glycosylated and nonglycosylated plasminogen activator inhibitor 2 in high excess over urokinase-type plasminogen activator in human U-937 lymphoma cells. J Cell Biol. 1987 Mar;104(3):705–712. doi: 10.1083/jcb.104.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda T. J., Sheiness D. K., Bishop J. M. Transcripts from the cellular homologs of retroviral oncogenes: distribution among chicken tissues. Mol Cell Biol. 1982 Jun;2(6):617–624. doi: 10.1128/mcb.2.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Hearing V. J., Law L. W., Corti A., Appella E., Blasi F. Modulation of metastatic potential by cell surface urokinase of murine melanoma cells. Cancer Res. 1988 Mar 1;48(5):1270–1278. [PubMed] [Google Scholar]
- Kirchheimer J. C., Remold H. G. Endogenous receptor-bound urokinase mediates tissue invasion of human monocytes. J Immunol. 1989 Oct 15;143(8):2634–2639. [PubMed] [Google Scholar]
- Kirchheimer J. C., Remold H. G. Functional characteristics of receptor-bound urokinase on human monocytes: catalytic efficiency and susceptibility to inactivation by plasminogen activator inhibitors. Blood. 1989 Sep;74(4):1396–1402. [PubMed] [Google Scholar]
- Krishnamurti C., Wahl L. M., Alving B. M. Stimulation of plasminogen activator inhibitor activity in human monocytes infected with dengue virus. Am J Trop Med Hyg. 1989 Jan;40(1):102–107. doi: 10.4269/ajtmh.1989.40.102. [DOI] [PubMed] [Google Scholar]
- Kruithof E. K., Cousin E. Plasminogen activator inhibitor 2. Isolation and characterization of the promoter region of the gene. Biochem Biophys Res Commun. 1988 Oct 14;156(1):383–388. doi: 10.1016/s0006-291x(88)80852-0. [DOI] [PubMed] [Google Scholar]
- Kruithof E. K. Plasminogen activator inhibitors--a review. Enzyme. 1988;40(2-3):113–121. doi: 10.1159/000469153. [DOI] [PubMed] [Google Scholar]
- Kruithof E. K., Vassalli J. D., Schleuning W. D., Mattaliano R. J., Bachmann F. Purification and characterization of a plasminogen activator inhibitor from the histiocytic lymphoma cell line U-937. J Biol Chem. 1986 Aug 25;261(24):11207–11213. [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W., Mitchell P., Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987 Jun 19;49(6):741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medcalf R. L., Kruithof E. K., Schleuning W. D. Plasminogen activator inhibitor 1 and 2 are tumor necrosis factor/cachectin-responsive genes. J Exp Med. 1988 Aug 1;168(2):751–759. doi: 10.1084/jem.168.2.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medcalf R. L., Richards R. I., Crawford R. J., Hamilton J. A. Suppression of urokinase-type plasminogen activator mRNA levels in human fibrosarcoma cells and synovial fibroblasts by anti-inflammatory glucocorticoids. EMBO J. 1986 Sep;5(9):2217–2222. doi: 10.1002/j.1460-2075.1986.tb04487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medcalf R. L., Rüegg M., Schleuning W. D. A DNA motif related to the cAMP-responsive element and an exon-located activator protein-2 binding site in the human tissue-type plasminogen activator gene promoter cooperate in basal expression and convey activation by phorbol ester and cAMP. J Biol Chem. 1990 Aug 25;265(24):14618–14626. [PubMed] [Google Scholar]
- Medcalf R. L., Van den Berg E., Schleuning W. D. Glucocorticoid-modulated gene expression of tissue- and urinary-type plasminogen activator and plasminogen activator inhibitor 1 and 2. J Cell Biol. 1988 Mar;106(3):971–978. doi: 10.1083/jcb.106.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minty A. J., Alonso S., Guénet J. L., Buckingham M. E. Number and organization of actin-related sequences in the mouse genome. J Mol Biol. 1983 Jun 15;167(1):77–101. doi: 10.1016/s0022-2836(83)80035-7. [DOI] [PubMed] [Google Scholar]
- Ossowski L. In vivo invasion of modified chorioallantoic membrane by tumor cells: the role of cell surface-bound urokinase. J Cell Biol. 1988 Dec;107(6 Pt 1):2437–2445. doi: 10.1083/jcb.107.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesler W. J., Vandenbark G. R., Hanson R. W. Cyclic AMP and the induction of eukaryotic gene transcription. J Biol Chem. 1988 Jul 5;263(19):9063–9066. [PubMed] [Google Scholar]
- Saffer J. D., Thurston S. J. A negative regulatory element with properties similar to those of enhancers is contained within an Alu sequence. Mol Cell Biol. 1989 Feb;9(2):355–364. doi: 10.1128/mcb.9.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksela O., Rifkin D. B. Cell-associated plasminogen activation: regulation and physiological functions. Annu Rev Cell Biol. 1988;4:93–126. doi: 10.1146/annurev.cb.04.110188.000521. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpati E. M., Sadler J. E. Regulation of endothelial cell coagulant properties. Modulation of tissue factor, plasminogen activator inhibitors, and thrombomodulin by phorbol 12-myristate 13-acetate and tumor necrosis factor. J Biol Chem. 1989 Dec 5;264(34):20705–20713. [PubMed] [Google Scholar]
- Schleuning W. D., Medcalf R. L., Hession C., Rothenbühler R., Shaw A., Kruithof E. K. Plasminogen activator inhibitor 2: regulation of gene transcription during phorbol ester-mediated differentiation of U-937 human histiocytic lymphoma cells. Mol Cell Biol. 1987 Dec;7(12):4564–4567. doi: 10.1128/mcb.7.12.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid C. W., Jelinek W. R. The Alu family of dispersed repetitive sequences. Science. 1982 Jun 4;216(4550):1065–1070. doi: 10.1126/science.6281889. [DOI] [PubMed] [Google Scholar]
- Schwartz B. S., Bradshaw J. D. Differential regulation of tissue factor and plasminogen activator inhibitor by human mononuclear cells. Blood. 1989 Oct;74(5):1644–1650. [PubMed] [Google Scholar]
- Stephens R. W., Pöllänen J., Tapiovaara H., Leung K. C., Sim P. S., Salonen E. M., Rønne E., Behrendt N., Danø K., Vaheri A. Activation of pro-urokinase and plasminogen on human sarcoma cells: a proteolytic system with surface-bound reactants. J Cell Biol. 1989 May;108(5):1987–1995. doi: 10.1083/jcb.108.5.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb A. C., Collins K. L., Snyder S. E., Alexander S. J., Rosenwasser L. J., Eddy R. L., Shows T. B., Auron P. E. Human monocyte Arg-Serpin cDNA. Sequence, chromosomal assignment, and homology to plasminogen activator-inhibitor. J Exp Med. 1987 Jul 1;166(1):77–94. doi: 10.1084/jem.166.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlwend A., Belin D., Vassalli J. D. Plasminogen activator-specific inhibitors in mouse macrophages: in vivo and in vitro modulation of their synthesis and secretion. J Immunol. 1987 Aug 15;139(4):1278–1284. [PubMed] [Google Scholar]