Abstract
Background:
Elbow flexion contracture is a well-known complication of brachial plexus birth palsy that adversely affects upper-extremity function. The prevalence, risk factors, and rate of progression of elbow flexion contracture associated with brachial plexus birth palsy have not been established, and the effectiveness of nonoperative treatment involving nighttime splinting or serial casting has not been well studied.
Methods:
The medical records of 319 patients with brachial plexus birth palsy who had been seen at our institution between 1992 and 2009 were retrospectively reviewed to identify patients with an elbow flexion contracture (≥10°). The chi-square test for trend and the Kaplan-Meier estimator were used to evaluate risk factors for contracture, including age, sex, and the extent of brachial plexus involvement. Longitudinal models were used to estimate the rate of contracture progression and the effectiveness of nonoperative treatment.
Results:
An elbow flexion contracture was present in 48% (152) of the patients with brachial plexus birth palsy. The median age of onset was 5.1 years (range, 0.25 to 14.8 years). The contracture was ≥30° in 36% (fifty-four) of these 152 patients and was accompanied by a documented radial head dislocation in 6% (nine). The prevalence of contracture increased with increasing age (p < 0.001) but was not significantly associated with sex or with the extent of brachial plexus involvement. The magnitude of the contracture increased by 4.4% per year before treatment (p < 0.01). The magnitude of the contracture decreased by 31% when casting was performed (p < 0.01) but thereafter increased again at the same rate of 4.4% per year. The magnitude of the contracture did not improve when splinting was performed but the rate of increase thereafter decreased to <0.1% per year (p = 0.04).
Conclusions:
The prevalence of elbow flexion contracture in children with brachial plexus birth palsy may be greater than clinicians perceive. The prevalence increased with patient age but was not significantly affected by sex or by the extent of brachial plexus involvement. Serial casting may initially improve severe contractures, whereas nighttime splinting may prevent further progression of milder contractures.
Level of Evidence:
Therapeutic Level IV. See Instructions for Authors for a complete description of levels of evidence.
Brachial plexus birth palsy occurs at a rate of 0.38 to 4.6 per 1000 live births1-5. Although full recovery has been reported in up to 92% of cases6, recent studies have reported more modest recovery rates of 66% to 80%7,8. Children whose neurological deficit does not resolve may experience soft-tissue contractures and osseous deformities that can significantly impair upper-extremity function and appearance9-12.
Most long-term sequelae of brachial plexus birth palsy have been well studied. The prevalence of shoulder contracture, glenoid deformity, posterior shoulder dislocation, and forearm supination contracture have been reported to be 56%, 33%, 9%, and 6.9%, respectively13-15. The prevalence of elbow flexion contracture, however, has yet to be established, with the prevalence in previous reports ranging widely from 4.6% to 89.5%15-17. Furthermore, there has been limited study of the prevalence of contractures of ≥30°. The clinical importance of elbow flexion contracture in a pediatric population has received limited attention. Morrey et al. reported the functional elbow range of motion in an adult population to be 30° to 130°, which suggests that a contracture of >30° in a population of children with brachial plexus birth palsy may impair their ability to perform activities of daily living including self-care18.
The management of elbow flexion contracture associated with brachial plexus birth palsy may involve surgical or nonoperative techniques. Severe contractures may be treated surgically by releasing the soft-tissue structures surrounding the elbow or by gradually increasing elbow motion with a recently reported novel technique of gradual closed arthrodiatasis19,20. Nonoperative treatments for elbow flexion contracture associated with brachial plexus birth palsy include serial casting and nighttime splinting, although the indications for these two nonoperative treatments have not been well established.
A recently published study of nineteen patients indicated that nonsurgical treatment (serial casting or nighttime splinting) improved elbow motion but that noncompliance with the splinting regimen significantly impacted long-term maintenance of treatment gains21.
The first aim of the present study was to determine the prevalence of, rate of progression of, and risk factors associated with elbow flexion contracture in children with brachial plexus birth palsy. The second aim was to describe the efficacy of serial casting and of nighttime splinting for the treatment of the elbow flexion contracture.
Materials and Methods
The medical records of all 319 children and adolescents with brachial plexus birth palsy who had been seen at our institution between January 1992 and May 2009 were retrospectively reviewed to identify patients with an elbow flexion contracture (≥10°). The study protocol was approved by our institutional review board. The median age of the total patient population at the time of initial presentation to our brachial plexus birth palsy clinic was ten months (range, one month to eighteen years). Patients with brachial plexus birth palsy are typically asked to return for follow-up every six months. The patients in our study population were followed for a median of three years (range, zero to seventeen years) from the time of initial presentation. The right limb was affected in 52% of the patients, and 52% were boys. Demographic characteristics of the study population are shown in Table I.
TABLE I.
Patient Demographics
| All Patients | Patients with Elbow Flexion Contracture | Patients without Elbow Flexion Contracture | |
| No. | 319 | 152 | 167 |
| Age at initial presentation*(yr) | 0.8 (0-18) | 2.2 (0-18) | 0.3 (0-14) |
| Duration of follow-up*(yr) | 3 (0-17) | 5 (0-17) | 2 (0-13) |
| Sex (no. [%]) | |||
| Male | 167 (52) | 82 (54) | 85 (51) |
| Female | 152 (48) | 70 (46) | 82 (49) |
| Affected side (no. [%]) | |||
| Right | 165 (52) | 73 (48) | 92 (55) |
| Left | 154 (48) | 79 (52) | 75 (45) |
| Narakas classification (no. [%]) | |||
| Group I | 154 (48) | 67 (44) | 87 (52) |
| Group II | 99 (31) | 49 (32) | 50 (30) |
| Group III | 66 (21) | 36 (24) | 30 (18) |
The values are given as the median, with the range in parentheses.
Information regarding the severity of the neurological injury at the time of the initial evaluation was reviewed, and the Narakas classification was used to divide the patients into a C5-6 injury group (group I), a C5-7 injury group (group II), and a global C5-T1 injury group (group III) (see Appendix)22,23. Of the 319 patients included in the study, 154 were in group I, ninety-nine were in group II, and sixty-six were in group III. Occupational therapy documents were reviewed to collect information regarding elbow range of motion and other contractures that accompanied an elbow flexion contracture. Range of motion measurements were made with use of goniometry by the occupational therapist. If occupational therapy documents were unavailable, measurements recorded in the pediatric orthopaedic surgeon's notes were used. The medical records of the patients with an elbow flexion contracture, except for the thirty-nine patients who presented with a contracture at the initial visit, were reviewed to determine the age of onset of the contracture. To account for the potential bias that would have resulted if patients with a new onset of elbow flexion contracture were more likely to return to the clinic than patients who did not develop a contracture, we documented the frequency of follow-up visits between the initial visit and the onset of the elbow flexion contracture. The maximum magnitude of the contracture and the presence of other ipsilateral upper-extremity contractures and radial head dislocation in the patients with an elbow flexion contracture were also determined.
The prevalence of elbow flexion contracture was calculated for the entire study population and also for three age groups (zero to four years, five to eleven years, and twelve to twenty years) of approximately equal size, which permitted evaluation of contracture in an adolescent subgroup. The prevalence was also analyzed according to the extent of brachial plexus involvement (i.e., in Narakas groups I, II, and III). A chi-square test for trend was used to evaluate whether the prevalence of elbow flexion contracture increased with increasing age or with increasing extent of brachial plexus involvement. To estimate the effect of increasing extent of brachial plexus involvement on the odds of having an elbow flexion contracture, a logistic regression model was fitted with two indicator variables specified to estimate the increase in the odds of contracture; the first variable accounted for the increase in prevalence in patients with a Narakas group-II lesion compared with a group-I lesion, and the second accounted for the further increase in patients with a group-III lesion compared with a group-II lesion.
The Kaplan-Meier (product-limit) estimator for censored data was used to predict the probability of developing an elbow flexion contracture, according to age and sex24. A log-rank test was used to evaluate the association between sex and the probability of developing an elbow flexion contracture.
We followed a standard treatment protocol when patients and parents requested treatment of an elbow flexion contracture, and this protocol remained uniform throughout the study period. A contracture of <30° was treated with nighttime splinting with the elbow in maximum extension, usually with use of a drop-lock hinge that allowed the wearer to adjust the amount of flexion permitted by the brace. A contracture of >30° was treated with serial casting with the elbow in increasing extension; the casts were changed weekly or biweekly until the contracture measured <30°, and the contracture was then treated with nighttime splinting as described above. An increase of approximately 10° of extension was achieved per cast change, and two or three cast changes were usually needed. Two patients with a contracture of >30° declined to undergo casting and were treated with splinting instead.
Spaghetti plots of the magnitude of the contracture in each patient over time demonstrated linear trajectories. We fitted longitudinal models to this data in order to investigate the effects of age, sex, and the extent of brachial plexus involvement on the initial magnitude of the contracture and in order to separate these effects from the effects of the same variables on progression of the contracture over time. The length of time between follow-up visits was accounted for in the estimation of the rate of progression. We added terms to estimate both the immediate response to initiation of treatment and the effect of treatment on the subsequent rate of contracture progression during the follow-up period. We also assessed whether the type of treatment or the extent of brachial plexus involvement modified the short-term or long-term impact of treatment. We allowed for differences in the initial magnitude of the contracture that were not accounted for by known patient characteristics by including a random effect for the magnitude of the initial contracture. We analyzed the residuals to assess the fit of linear models that assumed a normal distribution of the contracture magnitude; this analysis indicated that a logarithmic transformation of the contracture magnitude was necessary because of a skewed distribution that contained some outlying values. All models were therefore fitted on a logarithmic scale, and regression coefficients were interpreted in terms of the percentage difference or change in the magnitude of the contracture. All calculations were performed with use of SAS software (SAS Institute, Cary, North Carolina) and Stata software (release 11; StataCorp, College Station, Texas). A p value of <0.05 was considered significant.
Source of Funding
This study was made possible by grant number UL1 RR024146 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research.
Results
The prevalence of elbow flexion contracture (≥10°) was 48% (152 of 319). The left elbow was affected in 52% of the 152 patients (Table I). Thirty-nine patients had a documented elbow contracture at the time of the initial visit, which occurred at a median age of 10.8 years (range, one to eighteen years) in this group. The median age of onset of the elbow flexion contracture in the patients who developed a contracture after the initial visit was 5.1 years (range, 0.25 to 14.8 years). The median magnitude of the maximum documented contracture was 20° (range, 10° to 90°) (Table II). Of the 113 patients who developed an elbow flexion contracture after the initial clinic visit, thirty developed the contracture less than a year after the initial clinic visit, sixty-nine were followed regularly at least once per year before the onset of the contracture, seven were diagnosed with the contracture when they returned to the clinic after a hiatus of more than two years, and the timing of follow-up of the remaining seven patients could not be determined because of missing data.
TABLE II.
Characteristics of the Patients with Elbow Flexion Contracture
| Elbow flexion contracture of ≥10° (no.) | 152 |
| Age at onset*(yr) | 5.1 (0.25-14.8) |
| Maximum contracture* | 20° (10°-90°) |
| Contracture of ≥30° (no. [%]) | 54 (36) |
| Additional ipsilateral upper-limb joint contracture of ≥10° (no. [%]) | 48 (32) |
| Shoulder internal rotation only | 17 (11) |
| Supination only | 13 (9) |
| Shoulder abduction only | 7 (5) |
| Other contracture only | 5 (3) |
| >1 joint contracture | 6 (4) |
| Radial head dislocation (no. [%]) | 9 (6) |
The values are given as the median, with the range in parentheses.
The magnitude of the contracture was ≥30° in 36% (fifty-four) of the 152 patients with an elbow flexion contracture. An additional upper-extremity contracture was present in 32% (forty-eight) of the patients with an elbow flexion contracture; the most prevalent such contracture, in seventeen patients (11% of the 152), involved shoulder internal rotation (Table II). Radial head dislocation was documented in nine (6%) of the patients with an elbow flexion contracture. Surgical treatment for brachial plexus birth palsy was performed in 68% (103) of the patients with an elbow flexion contracture and included nerve exploration and grafting, external rotation tendon transfer at the shoulder, and external rotation humeral osteotomy. No patient underwent surgical release of the elbow flexion contracture.
Table III demonstrates the prevalence of elbow flexion contracture according to age and the extent of brachial plexus involvement (Narakas classification). The prevalence of elbow flexion contracture of ≥10° increased with increasing age (p < 0.001) (see Appendix). The association with the extent of brachial plexus involvement was not significant (p = 0.69) (see Appendix). The odds ratio of an elbow flexion contracture in patients with a Narakas group-I lesion was 0.77 (prevalence = 44%). The odds of an elbow flexion contracture in patients with a Narakas group-II lesion were not significantly greater than in patients with a group-I lesion (95% confidence interval [CI] for the relative change in the odds ratio, –23% to 111%; p = 0.35). Similarly, the odds in patients with a group-III lesion were not significantly greater than in patients with a group-II lesion (95% CI, –34% to 129%; p = 0.53).
TABLE III.
Prevalence of Elbow Flexion Contracture According to Age and Narakas Classification
| Total No. | No. with Elbow Flexion Contracture ≥10° | Prevalence (%) | P Value* | |
| Age in yr | <0.001 | |||
| 0 to 4 | 103 | 12 | 12 | |
| 5 to 11 | 132 | 73 | 55 | |
| 12 to 20 | 84 | 67 | 80 | |
| Narakas classification | 0.690 | |||
| Group I | 154 | 67 | 44 | |
| Group II | 99 | 49 | 49 | |
| Group III | 66 | 36 | 55 |
Chi-square test.
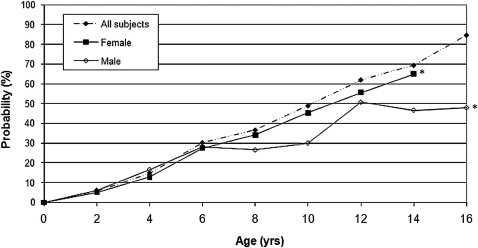
Use of the Kaplan-Meier (product-limit) estimator for censored data indicated that the predicted probability of developing an elbow flexion contracture by the age of skeletal maturity was 65% in the girls and 48% in the boys (Fig. 1). Data from the total population (male and female patients) to the age of sixteen years is provided in the figure. In the subpopulation analysis, the probability of developing an elbow flexion contracture by the age of skeletal maturity in male and female patients was evaluated. Since the age of skeletal maturity in girls is fourteen years, this excluded eight data points of female patients who were seen to the age of sixteen years. If the analysis had been carried out to the age of sixteen years in the female population, the probability of developing an elbow flexion contracture by the age of sixteen would be predicted to be 87%, which crosses the line for the total population. The association between sex and the probability of developing an elbow flexion contracture was not significant.
Fig. 1.
Probability of developing an elbow flexion contracture according to age at skeletal maturity and sex. The association between sex and the probability of elbow flexion contracture was not significant. *Denotes the age of skeletal maturity for boys and for girls.
Regression modeling was used to characterize the 107 patients who did not receive treatment for the elbow flexion contracture. The mean contracture in the untreated patients with a mild (Narakas group-I) lesion was estimated to be 11° at the time of entry into the study. The mean contracture was 13° (24% greater) in patients with a moderate (group-II) lesion and 14° (29% greater) in patients with a severe (group-III) lesion. These differences were not significant (p = 0.10 for both). Regression modeling was also used to estimate the rate of progression of the elbow flexion contracture to be 0.1% per year in the untreated patients, which was not significantly different from zero (p = 0.97).
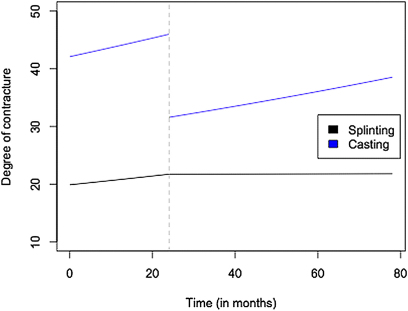
Forty-five of the 152 patients with an elbow flexion contracture received nonoperative treatment for the contracture (Table IV). This treatment consisted of nighttime splinting in thirty-six patients and serial elbow extension casting in nine. Patients in the treatment group were followed for a mean of 1.8 years (range, 0 to 14.4 years) before treatment and 2.2 years (range, 0.1 to 9.5 years) after treatment. The rate of contracture progression before treatment was 4.4% per year (p < 0.01 for the comparison with 0%) in both the patients who underwent casting and those who underwent splinting, and the difference in the rate of progression between the two groups was not significant (p = 0.87). On average, patients experienced a 31% decrease in the magnitude of the contracture during serial casting (p < 0.01). Patients treated with splinting did not experience a significant improvement during treatment (p = 0.93). The mean rate of contracture progression was 4.4% per year after casting and <0.1% per year after splinting. The difference between the rate of contracture progression before and after splinting was significant (p = 0.04) (Fig. 2).
TABLE IV.
Characteristics of the Forty-five Patients Who Received Nonoperative Treatment of an Elbow Flexion Contracture
| Mean (Range) | |
| Last elbow flexion contracture measurement before treatment (deg) | 32 (10-85) |
| Before splinting (n = 36) | 28 (10-50) |
| Before casting (n = 9) | 49 (30-85) |
| First elbow flexion contracture measurement after treatment (deg) | 25 (5-60) |
| After splinting (n = 36) | 24 (5-40) |
| After casting (n = 9) | 30 (15-60) |
| Length of time observed before treatment (mo) | 22 (0-173) |
| Length of time observed after treatment (mo) | 27 (1-114) |
Fig. 2.
Magnitude of the elbow flexion contracture for typical patients in the casting group and the splinting group as predicted by the final model estimates. Time zero represents the time of initial presentation for brachial plexus birth palsy. The vertical dashed line represents the start of treatment, and the trajectory for the patient in the casting group drops at this time.
Discussion
This study suggests that the prevalence of elbow flexion contracture (≥10°) in children with brachial plexus birth palsy may be higher than clinicians perceive and that the prevalence increases with age. The prevalence of elbow flexion contracture in the study group was not associated with sex or with the extent of brachial plexus involvement. A predictive model demonstrated that the probability of developing an elbow flexion contracture by the age of skeletal maturity was high in both girls (65%) and boys (48%). Nonoperative treatment of an elbow flexion contracture associated with brachial plexus birth palsy with serial casting may initially improve a severe elbow flexion contracture, whereas nighttime splinting may prevent further progression of a milder contracture.
The prevalence of elbow flexion contracture in the study was representative of the prevalence among the brachial plexus birth palsy patients treated at our institution, which provides specialized care for children with orthopaedic conditions. However, the observed prevalence does not take into account the six patients who were lost to follow-up before the median age of onset reported in the study (five years) or the 103 patients who were younger than this age at the time of the study. Thus, the observed prevalence of 48% in the study group may represent an underestimation of the true prevalence of elbow flexion contracture in our patient population as well as in the general population of children with brachial plexus birth palsy. It is also possible that the observed prevalence represents an overestimation if patients with an elbow flexion contracture were more likely to return to the clinic than patients without a contracture. However, only seven of the 113 patients who developed an elbow contracture after the initial visit returned to the clinic after more than a two-year hiatus and were diagnosed with an elbow contracture at that time, which suggests that our reported prevalence was only minimally biased by patients previously lost to follow-up who returned to the clinic because of a new-onset elbow flexion contracture. The prevalence of radial head dislocation observed in our population of children with an elbow flexion contracture (6%) was likely an underestimation of the true prevalence, as our population did not routinely undergo radiographic evaluation of the elbow and radial head dislocations may remain undiagnosed in children with brachial plexus birth palsy.
Our study indicated that children with brachial plexus birth palsy are at risk of developing an elbow flexion contracture regardless of the extent of the initial neurological injury, which is inconsistent with previous reports of an association between lesion severity and other sequelae of brachial plexus birth palsy15,17,25-27. We had expected to see a greater prevalence of elbow flexion contracture in children with a weak triceps muscle (C7 involvement, or Narakas groups II and III) resulting in elbow flexor-extensor muscle imbalance. However, our results suggested that the etiology of elbow flexion contracture associated with brachial plexus birth palsy, unlike that of other soft-tissue contractures associated with neuromuscular conditions, may not involve muscle imbalance due to weak or paralyzed antagonist muscles. Although the prevalence of elbow flexion contracture in our population increased incrementally with increasing brachial plexus involvement, the trend was not significant. Retrospective Narakas classification may have biased our results in patients who had spontaneous resolution of affected nerve roots. A larger sample size may be needed to establish significance for the small effect size observed for the extent of brachial plexus involvement; however, the p values from the logistic regression model were within the range of chance, so the most likely explanation is that the extent of brachial plexus involvement, as assessed with use of the Narakas classification, is not a strong clinical predictor of the odds of developing an elbow flexion contracture.
Our study indicated the rate of contracture progression to be 0.1% per year for patients who never received treatment compared with 4.4% per year for patients who later received treatment involving serial casting or nighttime splinting. These rates were prone to selection bias, as patients who do not progress are unlikely to seek treatment and those with greater progression may be seen more frequently than those with slower progression. Thus, the progression rate observed in the treatment group prior to treatment may represent an overestimation. A larger sample of patients undergoing nonoperative treatment would predict the rate of progression more accurately; however, the results of the present study provide information to assist clinicians with patient and family education regarding the potential course of elbow flexion contracture associated with brachial plexus birth palsy.
Currently, the mainstays of treatment for elbow flexion contracture in children with brachial plexus birth palsy at our institution are serial casting and nighttime splinting. A recently published treatment algorithm recommends serial casting for contractures of ≥40° and nighttime splinting for contractures of 20° to 40°21. Although it was limited by the small number of patients who were available, the present study of the effectiveness of nonoperative treatment in forty-five patients with a mean follow-up of twenty-seven months since the start of treatment supported that treatment algorithm and suggested that serial casting may initially improve severe elbow flexion contractures whereas nighttime splinting may prevent further progression of milder contractures.
The etiology of elbow flexion contracture in children with brachial plexus birth palsy is unknown. Current hypotheses in the literature include a misdirection of regenerating neurons leading to cross-innervation of upper-extremity musculature, recovery of elbow flexor function before elbow extensor function, and imbalance between the elbow flexor and extensor muscles for another reason16,17,28. Other authors have questioned why elbow flexion contractures associated with brachial plexus birth palsy occur in the direction of muscle weakness. One possible explanation is that partial muscle denervation at birth alters the normal development of muscle contractile properties29. Others have hypothesized that a mild contracture may develop to assist in overcoming weak elbow flexors16. Indeed, limited elbow flexion in this population may be more functionally detrimental than limited elbow extension in tasks such as grooming and feeding. However, contractures that are more severe (≥30°), which were reported in 36% of our population, are likely to impair the overall upper-extremity function and appearance in children with brachial plexus birth palsy.
In summary, we reported the prevalence of elbow flexion contracture in a population of children with brachial plexus birth palsy and evaluated its association with age, sex, and the extent of brachial plexus involvement. Knowledge of the disease prevalence will allow clinicians to educate children and their parents regarding this diagnosis and its potential complications. Understanding the most prevalent sequelae of brachial plexus birth palsy may guide the development of treatment techniques to target the conditions that have the greatest impact on children with brachial plexus birth palsy.
Supplementary Material
A table outlining the Narakas classification and figures showing the prevalence of elbow flexion contracture according to age and the Narakas classification
Footnotes
Disclosure: One or more of the authors received payments or services, either directly or indirectly (i.e., via his or her institution), from a third party in support of an aspect of this work. In addition, one or more of the authors, or his or her institution, has had a financial relationship, in the thirty-six months prior to submission of this work, with an entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. No author has had any other relationships, or has engaged in any other activities, that could be perceived to influence or have the potential to influence what is written in this work. The complete Disclosures of Potential Conflicts of Interest submitted by authors are always provided with the online version of the article.
References
- 1.Adler JB, Patterson RL., Jr Erb's palsy. Long-term results of treatment in eighty-eight cases. J Bone Joint Surg Am. 1967;49:1052-64 [PubMed] [Google Scholar]
- 2.Evans-Jones G, Kay SP, Weindling AM, Cranny G, Ward A, Bradshaw A, Hernon C. Congenital brachial palsy: incidence, causes, and outcome in the United Kingdom and Republic of Ireland. Arch Dis Child Fetal Neonatal Ed. 2003;88:F185-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hardy AE. Birth injuries of the brachial plexus: incidence and prognosis. J Bone Joint Surg Br. 1981;63:98-101 [DOI] [PubMed] [Google Scholar]
- 4.Hoeksma AF, Wolf H, Oei SL. Obstetrical brachial plexus injuries: incidence, natural course and shoulder contracture. Clin Rehabil. 2000;14:523-6 [DOI] [PubMed] [Google Scholar]
- 5.Jackson ST, Hoffer MM, Parrish N. Brachial-plexus palsy in the newborn. J Bone Joint Surg Am. 1988;70:1217-20 [PubMed] [Google Scholar]
- 6.Michelow BJ, Clarke HM, Curtis CG, Zuker RM, Seifu Y, Andrews DF. The natural history of obstetrical brachial plexus palsy. Plast Reconstr Surg. 1994;93:675-81 [PubMed] [Google Scholar]
- 7.Hoeksma AF, ter Steeg AM, Nelissen RG, van Ouwerkerk WJ, Lankhorst GJ, de Jong BA. Neurological recovery in obstetric brachial plexus injuries: an historical cohort study. Dev Med Child Neurol. 2004;46:76-83 [DOI] [PubMed] [Google Scholar]
- 8.Pondaag W, Malessy MJ, van Dijk JG, Thomeer RT. Natural history of obstetric brachial plexus palsy: a systematic review. Dev Med Child Neurol. 2004;46:138-44 [DOI] [PubMed] [Google Scholar]
- 9.Bae DS, Zurakowski D, Avallone N, Yu R, Waters PM. Sports participation in selected children with brachial plexus birth palsy. J Pediatr Orthop. 2009;29:496-503 [DOI] [PubMed] [Google Scholar]
- 10.Dedini RD, Bagley AM, Molitor F, James MA. Comparison of pediatric outcomes data collection instrument scores and range of motion before and after shoulder tendon transfers for children with brachial plexus birth palsy. J Pediatr Orthop. 2008;28:259-64 [DOI] [PubMed] [Google Scholar]
- 11.Huffman GR, Bagley AM, James MA, Lerman JA, Rab G. Assessment of children with brachial plexus birth palsy using the Pediatric Outcomes Data Collection Instrument. J Pediatr Orthop. 2005;25:400-4 [DOI] [PubMed] [Google Scholar]
- 12.Mosqueda T, James MA, Petuskey K, Bagley A, Abdala E, Rab G. Kinematic assessment of the upper extremity in brachial plexus birth palsy. J Pediatr Orthop. 2004;24:695-9 [DOI] [PubMed] [Google Scholar]
- 13.Hoeksma AF, Ter Steeg AM, Dijkstra P, Nelissen RG, Beelen A, de Jong BA. Shoulder contracture and osseous deformity in obstetrical brachial plexus injuries. J Bone Joint Surg Am. 2003;85:316-22 [DOI] [PubMed] [Google Scholar]
- 14.Wickstrom J, Haslam ET, Hutchinson RH. The surgical management of residual deformities of the shoulder following birth injuries of the brachial plexus. J Bone Joint Surg Am. 1955;37:27-36; passim [PubMed] [Google Scholar]
- 15.Yam A, Fullilove S, Sinisi M, Fox M. The supination deformity and associated deformities of the upper limb in severe birth lesions of the brachial plexus. J Bone Joint Surg Br. 2009;91:511-6 [DOI] [PubMed] [Google Scholar]
- 16.Ballinger SG, Hoffer MM. Elbow flexion contracture in Erb's palsy. J Child Neurol. 1994;9:209-10 [DOI] [PubMed] [Google Scholar]
- 17.Sibinski M, Sherlock DA, Hems TE, Sharma H. Forearm rotational profile in obstetric brachial plexus injury. J Shoulder Elbow Surg. 2007;16:784-7 [DOI] [PubMed] [Google Scholar]
- 18.Morrey BF, Askew LJ, Chao EY. A biomechanical study of normal functional elbow motion. J Bone Joint Surg Am. 1981;63:872-7 [PubMed] [Google Scholar]
- 19.Haerle M, Gilbert A. Management of complete obstetric brachial plexus lesions. J Pediatr Orthop. 2004;24:194-200 [DOI] [PubMed] [Google Scholar]
- 20.Vekris MD, Pafilas D, Lykissas MG, Soucacos PN, Beris AE. Correction of elbow flexion contracture in late obstetric brachial plexus palsy through arthrodiatasis of the elbow (Ioannina method). Tech Hand Up Extrem Surg. 2010;14:14-20 [DOI] [PubMed] [Google Scholar]
- 21.Ho ES, Roy T, Stephens D, Clarke HM. Serial casting and splinting of elbow contractures in children with obstetric brachial plexus palsy. J Hand Surg Am. 2010;35:84-91 [DOI] [PubMed] [Google Scholar]
- 22.Al-Qattan MM, El-Sayed AA, Al-Zahrani AY, Al-Mutairi SA, Al-Harbi MS, Al-Mutairi AM, Al-Kahtani FS. Narakas classification of obstetric brachial plexus palsy revisited. Hand Surg Eur Vol. 2009;34:788-91 [DOI] [PubMed] [Google Scholar]
- 23.Narakas A. Obstetrical brachial plexus injuries. : Lamb DW, editor The paralysed hand: the hand and upper limb. Edinburgh, Churchill-Livingstone; 1987. p 116-35 [Google Scholar]
- 24.Pritchett JW. Growth and predictions of growth in the upper extremity. J Bone Joint Surg Am. 1988;70:520-5 [PubMed] [Google Scholar]
- 25.Chuang DC, Ma HS, Wei FC. A new strategy of muscle transposition for treatment of shoulder deformity caused by obstetric brachial plexus palsy. Plast Reconstr Surg. 1998;101:686-94 [DOI] [PubMed] [Google Scholar]
- 26.Kambhampati SB, Birch R, Cobiella C, Chen L. Posterior subluxation and dislocation of the shoulder in obstetric brachial plexus palsy. J Bone Joint Surg Br. 2006;88:213-9 [DOI] [PubMed] [Google Scholar]
- 27.Smith NC, Rowan P, Benson LJ, Ezaki M, Carter PR. Neonatal brachial plexus palsy. Outcome of absent biceps function at three months of age. J Bone Joint Surg Am. 2004;86:2163-70 [PubMed] [Google Scholar]
- 28.Chuang DC, Ma HS, Wei FC. A new evaluation system to predict the sequelae of late obstetric brachial plexus palsy. Plast Reconstr Surg. 1998;101:673-85 [DOI] [PubMed] [Google Scholar]
- 29.Stefanova-Uzunova M, Stamatova L, Gatev V. Dynamic properties of partially denervated muscle in children with brachial plexus birth palsy. J Neurol Neurosurg Psychiatry. 1981;44:497-502 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A table outlining the Narakas classification and figures showing the prevalence of elbow flexion contracture according to age and the Narakas classification