Abstract
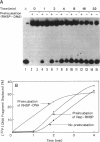
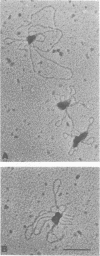
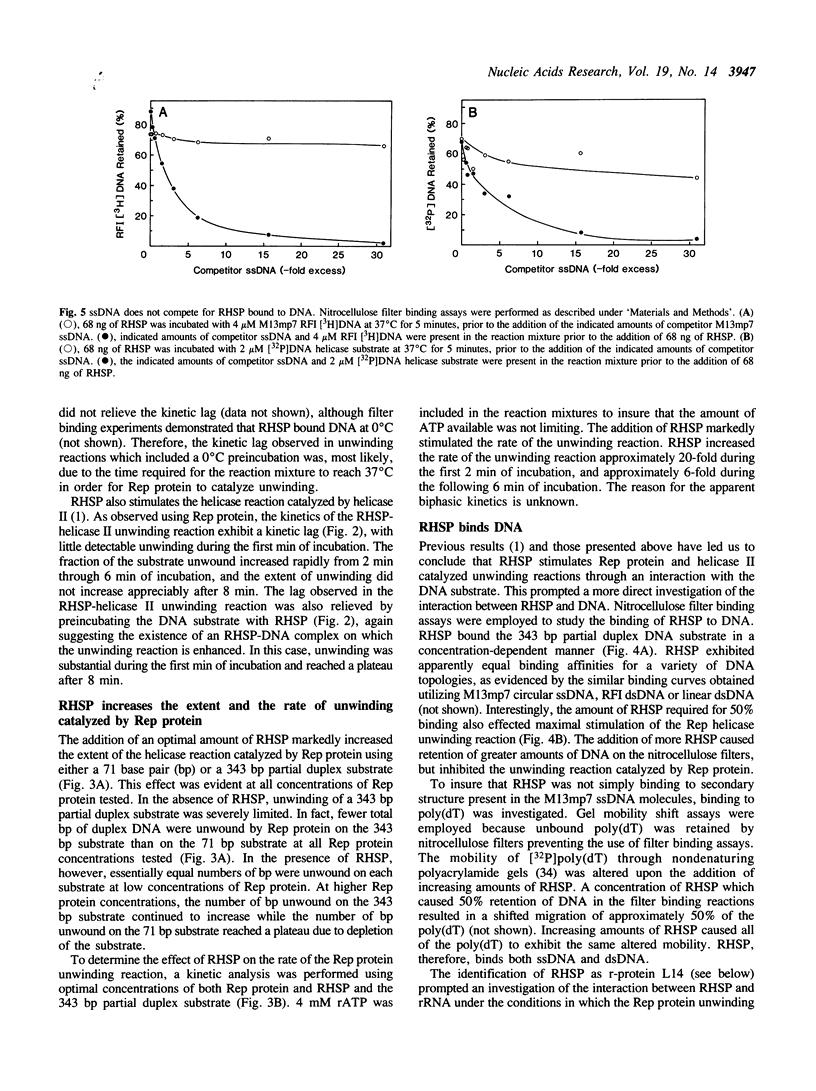
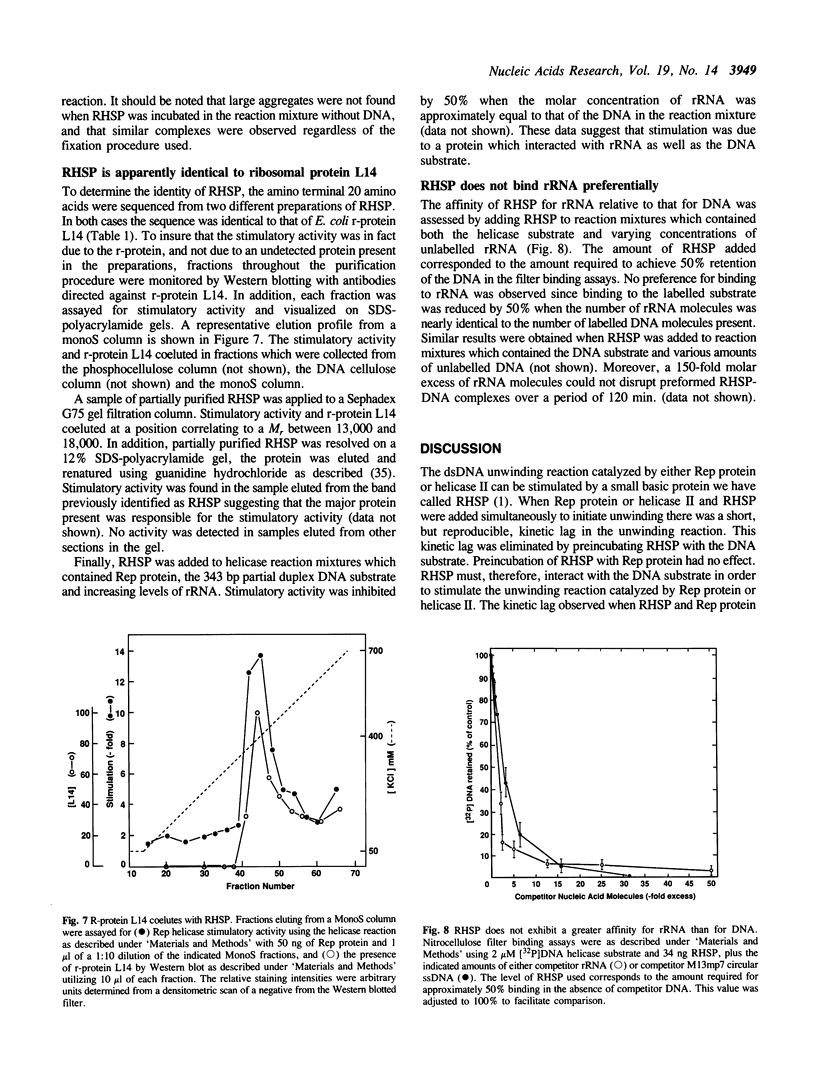
The unwinding reaction catalyzed by the Escherichia coli Rep protein is stimulated by a small 15 kDa protein called Rep helicase stimulatory protein (RHSP)(1). The RHSP-stimulated unwinding reaction catalyzed by Rep protein proceeded at a rapid rate after a time lag of 1-2 min at 37 degrees C. This time lag was eliminated by preincubating RHSP with the DNA substrate, indicating that stimulation resulted from an interaction between RHSP and DNA. RHSP was shown to increase the rate as well as the extent of the unwinding reaction catalyzed by Rep protein. RHSP bound both single- and double-stranded DNA with apparent equal affinity, forming an unusually stable complex. Electron microscopy illustrated that the RHSP-DNA complex consisted of large protein aggregates bound to DNA forming a highly condensed, aggregated DNA-protein complex. The protein aggregates were not observed in the absence of DNA and appeared to form cooperatively in the presence of DNA. NH2-terminal amino acid sequence analysis suggested that RHSP was identical to E. coli ribosomal-protein L14. Binding assays showed that the interaction between RHSP and rRNA was similar to the RHSP-DNA interaction. Several models are put forth to explain the stimulation of the unwinding reaction catalyzed by Rep protein. In addition, the potential physiological significance of the RHSP-stimulated Rep protein unwinding reaction is discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Caron P. R., Kushner S. R., Grossman L. Involvement of helicase II (uvrD gene product) and DNA polymerase I in excision mediated by the uvrABC protein complex. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4925–4929. doi: 10.1073/pnas.82.15.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colasanti J., Denhardt D. T. The Escherichia coli rep mutation. X. Consequences of increased and decreased Rep protein levels. Mol Gen Genet. 1987 Sep;209(2):382–390. doi: 10.1007/BF00329669. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T., Dressler D. H., Hathaway A. THE ABORTIVE REPLICATION OF PhiX174 DNA IN A RECOMBINATION-DEFICIENT MUTANT OF Escherichia coli. Proc Natl Acad Sci U S A. 1967 Mar;57(3):813–820. doi: 10.1073/pnas.57.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T., Iwaya M., Larison L. L. The rep mutation. II. Its effect on Escherichia coli and on the replication of bacteriophage phi X174. Virology. 1972 Aug;49(2):486–496. doi: 10.1016/0042-6822(72)90500-4. [DOI] [PubMed] [Google Scholar]
- Eisenberg S., Griffith J., Kornberg A. phiX174 cistron A protein is a multifunctional enzyme in DNA replication. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3198–3202. doi: 10.1073/pnas.74.8.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg S., Scott J. F., Kornberg A. An enzyme system for replication of duplex circular DNA: the replicative form of phage phi X174. Proc Natl Acad Sci U S A. 1976 May;73(5):1594–1597. doi: 10.1073/pnas.73.5.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassler J. S., Tessman I., Tessman E. S. Lethality of the double mutations rho rep and rho ssb in Escherichia coli. J Bacteriol. 1985 Feb;161(2):609–614. doi: 10.1128/jb.161.2.609-614.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geider K., Hoffmann-Berling H. Proteins controlling the helical structure of DNA. Annu Rev Biochem. 1981;50:233–260. doi: 10.1146/annurev.bi.50.070181.001313. [DOI] [PubMed] [Google Scholar]
- Griffith J. D., Christiansen G. Electron microscope visualization of chromatin and other DNA-protein complexes. Annu Rev Biophys Bioeng. 1978;7:19–35. doi: 10.1146/annurev.bb.07.060178.000315. [DOI] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Hinkle D. C., Chamberlin M. J. Studies of the binding of Escherichia coli RNA polymerase to DNA. I. The role of sigma subunit in site selection. J Mol Biol. 1972 Sep 28;70(2):157–185. doi: 10.1016/0022-2836(72)90531-1. [DOI] [PubMed] [Google Scholar]
- Husain I., Van Houten B., Thomas D. C., Abdel-Monem M., Sancar A. Effect of DNA polymerase I and DNA helicase II on the turnover rate of UvrABC excision nuclease. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6774–6778. doi: 10.1073/pnas.82.20.6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julin D. A., Riddles P. W., Lehman I. R. On the mechanism of pairing of single- and double-stranded DNA molecules by the recA and single-stranded DNA-binding proteins of Escherichia coli. J Biol Chem. 1986 Jan 25;261(3):1025–1030. [PubMed] [Google Scholar]
- Kuhn B., Abdel-Monem M., Hoffmann-Berling H. DNA helicases. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):63–67. doi: 10.1101/sqb.1979.043.01.011. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane H. E., Denhardt D. T. The rep mutation. IV. Slower movement of replication forks in Escherichia coli rep strains. J Mol Biol. 1975 Sep 5;97(1):99–112. doi: 10.1016/s0022-2836(75)80025-8. [DOI] [PubMed] [Google Scholar]
- LeBowitz J. H., McMacken R. The Escherichia coli dnaB replication protein is a DNA helicase. J Biol Chem. 1986 Apr 5;261(10):4738–4748. [PubMed] [Google Scholar]
- Lechner R. L., Richardson C. C. A preformed, topologically stable replication fork. Characterization of leading strand DNA synthesis catalyzed by T7 DNA polymerase and T7 gene 4 protein. J Biol Chem. 1983 Sep 25;258(18):11185–11196. [PubMed] [Google Scholar]
- Lee M. S., Marians K. J. Escherichia coli replication factor Y, a component of the primosome, can act as a DNA helicase. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8345–8349. doi: 10.1073/pnas.84.23.8345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson S. W., George J. W. DNA helicase II of Escherichia coli. Characterization of the single-stranded DNA-dependent NTPase and helicase activities. J Biol Chem. 1987 Feb 15;262(5):2066–2076. [PubMed] [Google Scholar]
- Matson S. W., Kaiser-Rogers K. A. DNA helicases. Annu Rev Biochem. 1990;59:289–329. doi: 10.1146/annurev.bi.59.070190.001445. [DOI] [PubMed] [Google Scholar]
- Matson S. W., Richardson C. C. Nucleotide-dependent binding of the gene 4 protein of bacteriophage T7 to single-stranded DNA. J Biol Chem. 1985 Feb 25;260(4):2281–2287. [PubMed] [Google Scholar]
- Morinaga T., Funatsu G., Funatsu M. Primary structure of protein L14 isolated from Escherichia coli ribosomes. FEBS Lett. 1978 Jul 1;91(1):74–77. doi: 10.1016/0014-5793(78)80020-9. [DOI] [PubMed] [Google Scholar]
- Ramstein J., Lavery R. Energetic coupling between DNA bending and base pair opening. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7231–7235. doi: 10.1073/pnas.85.19.7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. F., Eisenberg S., Bertsch L. L., Kornberg A. A mechanism of duplex DNA replication revealed by enzymatic studies of phage phi X174: catalytic strand separation in advance of replication. Proc Natl Acad Sci U S A. 1977 Jan;74(1):193–197. doi: 10.1073/pnas.74.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. F., Kornberg A. Purification of the rep protein of Escherichia coli. An ATPase which separates duplex DNA strands in advance of replication. J Biol Chem. 1978 May 10;253(9):3292–3297. [PubMed] [Google Scholar]
- Smith G. R., Amundsen S. K., Chaudhury A. M., Cheng K. C., Ponticelli A. S., Roberts C. M., Schultz D. W., Taylor A. F. Roles of RecBC enzyme and chi sites in homologous recombination. Cold Spring Harb Symp Quant Biol. 1984;49:485–495. doi: 10.1101/sqb.1984.049.01.055. [DOI] [PubMed] [Google Scholar]
- Smith K. R., Yancey J. E., Matson S. W. Identification and purification of a protein that stimulates the helicase activity of the Escherichia coli Rep protein. J Biol Chem. 1989 Apr 15;264(11):6119–6126. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezu K., Nakayama K., Nakayama H. Escherichia coli RecQ protein is a DNA helicase. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5363–5367. doi: 10.1073/pnas.87.14.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood E. R., Matson S. W. Purification and characterization of a new DNA-dependent ATPase with helicase activity from Escherichia coli. J Biol Chem. 1987 Nov 5;262(31):15269–15276. [PubMed] [Google Scholar]
- Yarranton G. T., Gefter M. L. Enzyme-catalyzed DNA unwinding: studies on Escherichia coli rep protein. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1658–1662. doi: 10.1073/pnas.76.4.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]