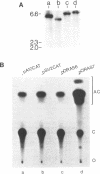
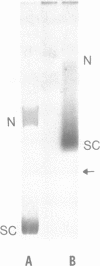
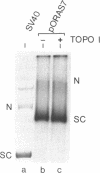
Abstract
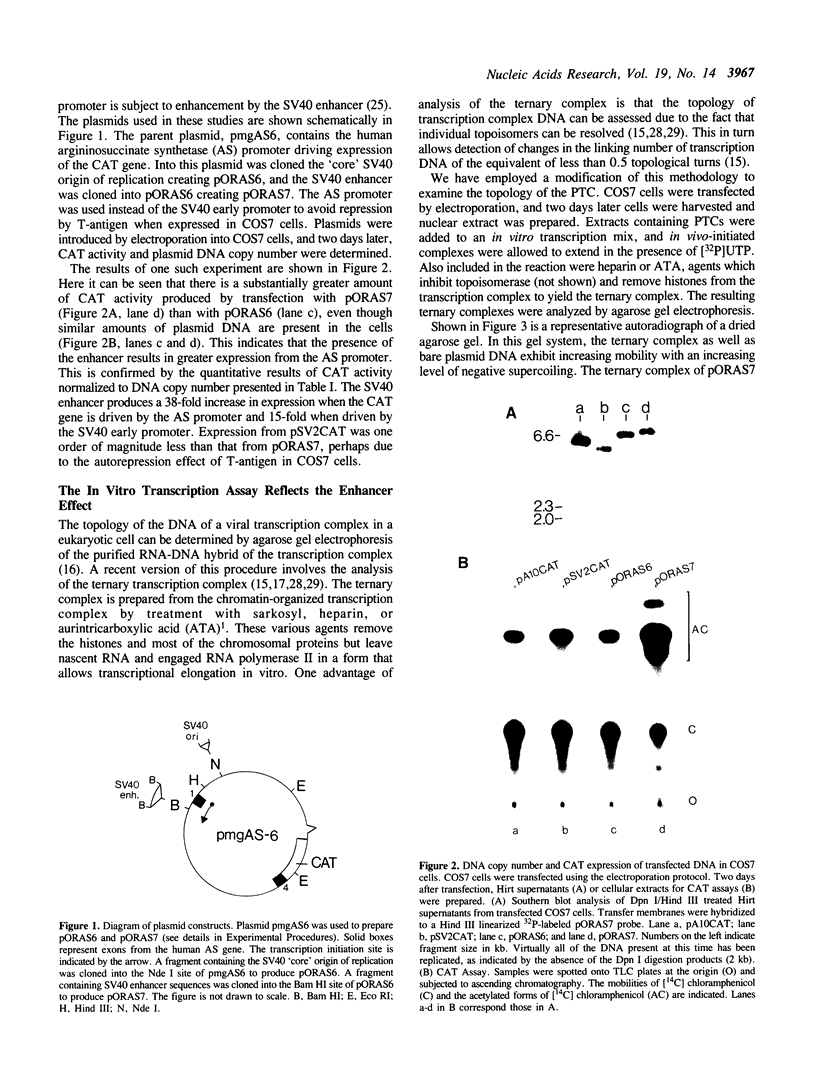
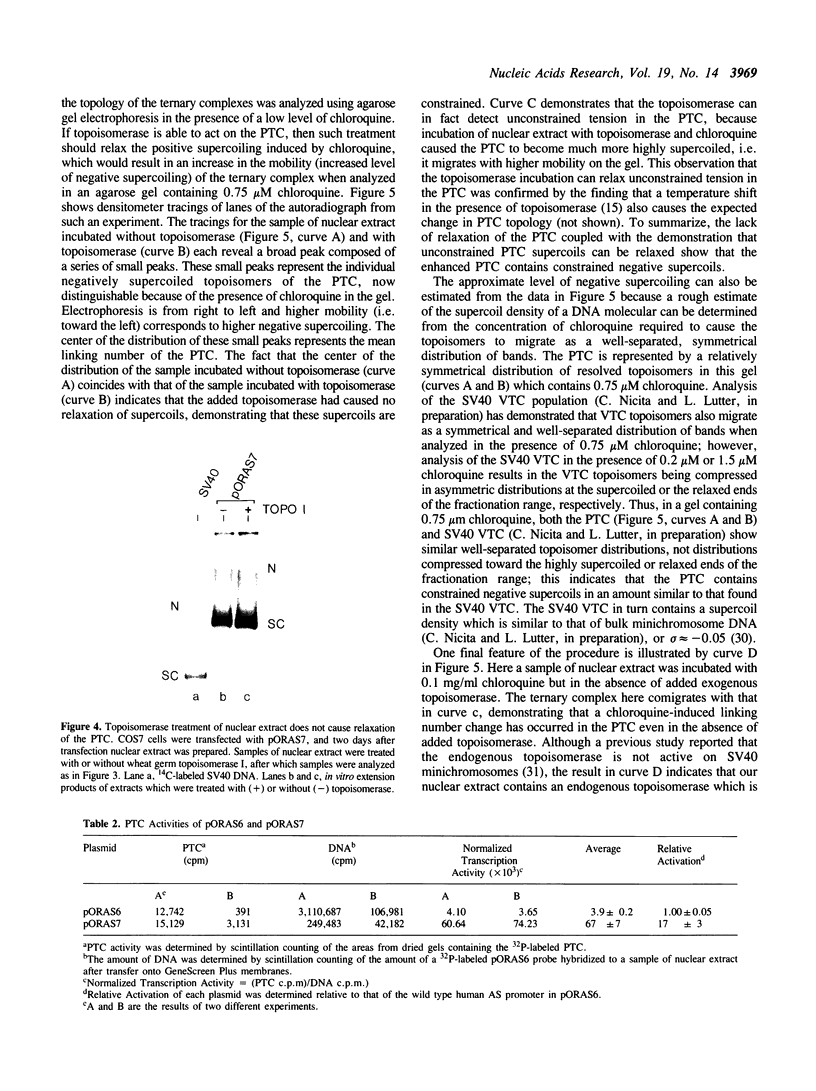
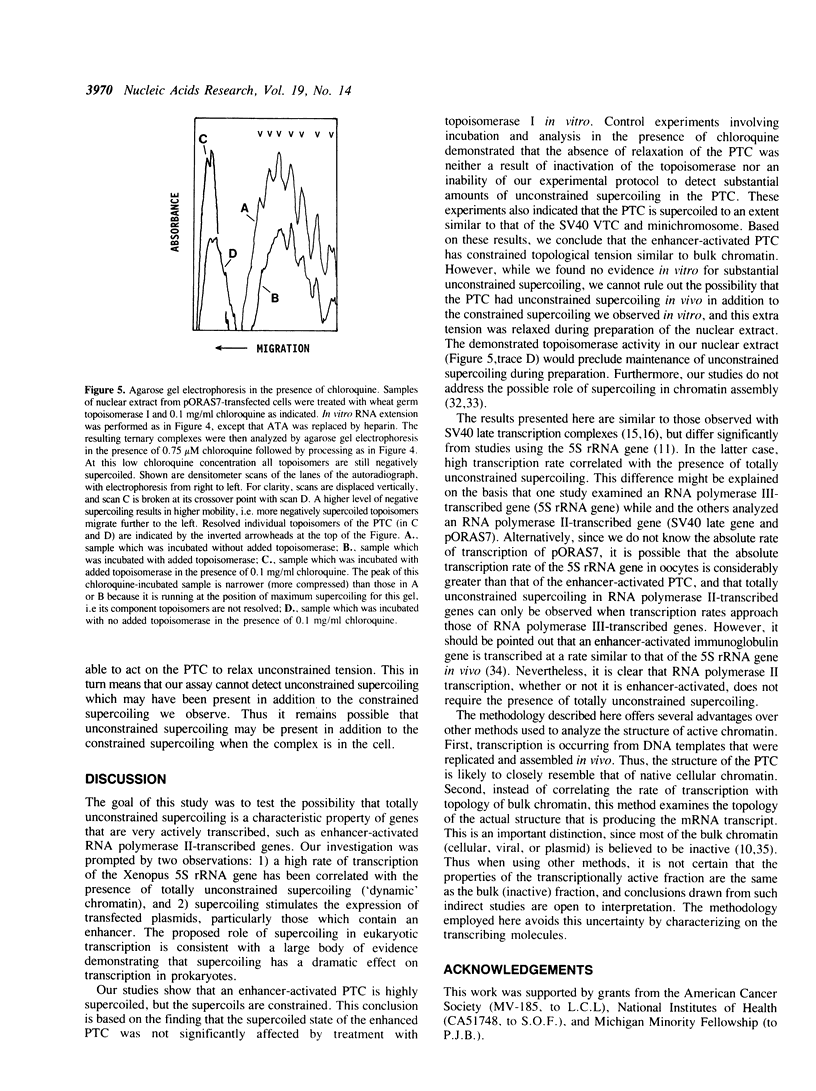
It has been proposed that transcriptionally active chromatin contains totally unconstrained supercoiling. The results of recent studies have raised the possibility that this topological state is the property of highly transcribed genes. Since the transcription rate of RNA polymerase II genes can be dramatically increased by the presence of an enhancer, we have determined if the transcription complex of an enhancer-activated plasmid contains totally unconstrained supercoils. Following transfection into COS7 cells, the topology of the transcription complex DNA was determined directly by agarose gel electrophoresis. We find that an enhancer-activated plasmid transcription complex is supercoiled, and these supercoils remain following topoisomerase I treatment. Thus the transcribing complexes contain constrained supercoils, and the level of supercoiling suggests a nucleosome-like organization. However, we cannot rule out the possibility that unconstrained supercoils exist in addition to these constrained supercoils in the transcription complex in the cell.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrose C., McLaughlin R., Bina M. The flexibility and topology of simian virus 40 DNA in minichromosomes. Nucleic Acids Res. 1987 May 11;15(9):3703–3721. doi: 10.1093/nar/15.9.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce F. M., Anderson G. M., Rusk C. D., Freytag S. O. Human argininosuccinate synthetase minigenes are subject to arginine-mediated repression but not to trans induction. Mol Cell Biol. 1986 Apr;6(4):1244–1252. doi: 10.1128/mcb.6.4.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choder M., Aloni Y. In vitro transcribed SV40 minichromosomes, as the bulk minichromosomes, have a low level of unconstrained negative supercoils. Nucleic Acids Res. 1988 Feb 11;16(3):895–905. doi: 10.1093/nar/16.3.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choder M., Bratosin S., Aloni Y. A direct analysis of transcribed minichromosomes: all transcribed SV40 minichromosomes have a nuclease-hypersensitive region within a nucleosome-free domain. EMBO J. 1984 Dec 1;3(12):2929–2936. doi: 10.1002/j.1460-2075.1984.tb02234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg J. C., Cartwright I. L., Thomas G. H., Elgin S. C. Selected topics in chromatin structure. Annu Rev Genet. 1985;19:485–536. doi: 10.1146/annurev.ge.19.120185.002413. [DOI] [PubMed] [Google Scholar]
- Ernoult-Lange M., Omilli F., O'Reilly D. R., May E. Characterization of the simian virus 40 late promoter: relative importance of sequences within the 72-base-pair repeats differs before and after viral DNA replication. J Virol. 1987 Jan;61(1):167–176. doi: 10.1128/jvi.61.1.167-176.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher L. M. DNA supercoiling and gene expression. Nature. 1984 Feb 23;307(5953):686–687. doi: 10.1038/307686a0. [DOI] [PubMed] [Google Scholar]
- Fromm M., Berg P. Deletion mapping of DNA regions required for SV40 early region promoter function in vivo. J Mol Appl Genet. 1982;1(5):457–481. [PubMed] [Google Scholar]
- Futcher B. Supercoiling and transcription, or vice versa? Trends Genet. 1988 Oct;4(10):271–272. doi: 10.1016/0168-9525(88)90166-7. [DOI] [PubMed] [Google Scholar]
- Gardiner-Garden M., Frommer M. CpG islands in vertebrate genomes. J Mol Biol. 1987 Jul 20;196(2):261–282. doi: 10.1016/0022-2836(87)90689-9. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross D. S., Garrard W. T. Nuclease hypersensitive sites in chromatin. Annu Rev Biochem. 1988;57:159–197. doi: 10.1146/annurev.bi.57.070188.001111. [DOI] [PubMed] [Google Scholar]
- Hadlock K. G., Lutter L. C. T-antigen is not bound to the replication origin of the simian virus 40 late transcription complex. J Mol Biol. 1990 Sep 5;215(1):53–65. doi: 10.1016/S0022-2836(05)80094-4. [DOI] [PubMed] [Google Scholar]
- Hadlock K. G., Quasney M. W., Lutter L. C. Immunoprecipitation of the simian virus 40 late transcription complex with antibody against T-antigen. J Biol Chem. 1987 Nov 15;262(32):15527–15537. [PubMed] [Google Scholar]
- Hartzell S. W., Byrne B. J., Subramanian K. N. Mapping of the late promoter of simian virus 40. Proc Natl Acad Sci U S A. 1984 Jan;81(1):23–27. doi: 10.1073/pnas.81.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Keller W. Determination of the number of superhelical turns in simian virus 40 DNA by gel electrophoresis. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4876–4880. doi: 10.1073/pnas.72.12.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llopis R., Perrin F., Bellard F., Gariglio P. Quantitation of transcribing native simian virus 40 minichromosomes extracted from CV1 cells late in infection. J Virol. 1981 Apr;38(1):82–90. doi: 10.1128/jvi.38.1.82-90.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchnik A. N., Bakayev V. V., Yugai A. A., Zbarsky I. B., Georgiev G. P. DNAaseI-hypersensitive minichromosomes of SV40 possess an elastic torsional strain in DNA. Nucleic Acids Res. 1985 Feb 25;13(4):1135–1149. doi: 10.1093/nar/13.4.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchnik A. N., Bakayev V. V., Zbarsky I. B., Georgiev G. P. Elastic torsional strain in DNA within a fraction of SV40 minichromosomes: relation to transcriptionally active chromatin. EMBO J. 1982;1(11):1353–1358. doi: 10.1002/j.1460-2075.1982.tb01322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutter L. C. Thermal unwinding of simian virus 40 transcription complex DNA. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8712–8716. doi: 10.1073/pnas.86.22.8712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North G. Eukaryotic topoisomerases come into the limelight. Nature. 1985 Aug 1;316(6027):394–395. doi: 10.1038/316394a0. [DOI] [PubMed] [Google Scholar]
- Petryniak B., Lutter L. C. Topological characterization of the simian virus 40 transcription complex. Cell. 1987 Jan 30;48(2):289–295. doi: 10.1016/0092-8674(87)90432-6. [DOI] [PubMed] [Google Scholar]
- Potter H., Weir L., Leder P. Enhancer-dependent expression of human kappa immunoglobulin genes introduced into mouse pre-B lymphocytes by electroporation. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7161–7165. doi: 10.1073/pnas.81.22.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves R. Transcriptionally active chromatin. Biochim Biophys Acta. 1984 Sep 10;782(4):343–393. doi: 10.1016/0167-4781(84)90044-7. [DOI] [PubMed] [Google Scholar]
- Ryoji M., Worcel A. Chromatin assembly in Xenopus oocytes: in vivo studies. Cell. 1984 May;37(1):21–32. doi: 10.1016/0092-8674(84)90297-6. [DOI] [PubMed] [Google Scholar]
- Schibler U., Marcu K. B., Perry R. P. The synthesis and processing of the messenger RNAs specifying heavy and light chain immunoglobulins in MPC-11 cells. Cell. 1978 Dec;15(4):1495–1509. doi: 10.1016/0092-8674(78)90072-7. [DOI] [PubMed] [Google Scholar]
- Shaw P. E., Bohmann D., Sergeant A. The SV40 enhancer influences viral late transcription in vitro and in vivo but not on replicating templates. EMBO J. 1985 Dec 1;4(12):3247–3252. doi: 10.1002/j.1460-2075.1985.tb04073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinden R. R., Carlson J. O., Pettijohn D. E. Torsional tension in the DNA double helix measured with trimethylpsoralen in living E. coli cells: analogous measurements in insect and human cells. Cell. 1980 Oct;21(3):773–783. doi: 10.1016/0092-8674(80)90440-7. [DOI] [PubMed] [Google Scholar]
- Tabuchi H., Hirose S. DNA supercoiling facilitates formation of the transcription initiation complex on the fibroin gene promoter. J Biol Chem. 1988 Oct 25;263(30):15282–15287. [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases. Annu Rev Biochem. 1985;54:665–697. doi: 10.1146/annurev.bi.54.070185.003313. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Cheng P. F., Conrad K. Expression of transfected DNA depends on DNA topology. Cell. 1986 Jul 4;46(1):115–122. doi: 10.1016/0092-8674(86)90865-2. [DOI] [PubMed] [Google Scholar]
- Weintraub H. Formation of stable transcription complexes as assayed by analysis of individual templates. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5819–5823. doi: 10.1073/pnas.85.16.5819. [DOI] [PMC free article] [PubMed] [Google Scholar]