Background
Electrochemically reduced water (ERW) contains a lot of hydrogen molecule (H2) and scavenges reactive oxygen species (ROS) to protect DNA from oxidative damage [1]. ERW also contains small amounts of Pt nanoparticles (NPs) and elongates the lifespan of C. elegans [2]. Pt NPs are newly recognized multi-functional ROS scavengers [3]. ERW exhibits anti-diabetes effects in vitro and in vivo [4-6][7]. We proposed mineral nanoparticle active hydrogen reduced water hypothesis to explain the activation mechanism of H2 to hydrogen atom (H)[4]. Recently, H2 has been reported to scavenge ROS and suppress a variety of oxidative stress-related diseases [8], however, the action mechanism of H2 has not been clarified thoroughly. Here, we examined anti-diabetes effects of H2 and Pt NPs.
Materials and methods
Pt NPs of 2-3 nm sizes were synthesized from H2PtCl6 by the citrate reduction method. L6 rat myoblast cells (1.2 x 105 cells) were inoculated into a 35 mm culture dish and a day later, the cells were treated with or without 25mM N-acetylcystein in the presence of BES-H2O2, a H2O2-specific detection reagent in DMEM for 2 h. After washing the cells, molecular hydrogen treatment was performed in a dark condition by cultivating cells in a fresh DMEM medium in a mixed gas incubator under an atmosphere of 75%N2/20%O2/5%CO2 or 75%(H2 and N2 mixed gas)/20%O2/5%CO2 for 1.5 h, followed by flowcytometric analysis. In this condition, culture medium contained maximum 0.4-0.5 ppm of dissolved hydrogen. Glucose uptake of differentiated myotube L6 cells was examined after treating the cells with 3H-2-deoxyglucose for 10 min. Gene expression of catalase (CAT), glutathione peroxidase (GPx) and hemoxoigenase (HO-1) was examined using RT-PCR method. Three weeks old type 2 diabetes model mice (KK-Ay) were fed H2 and/or Pt Nps-containing water ad lib for 6 weeks.
Results
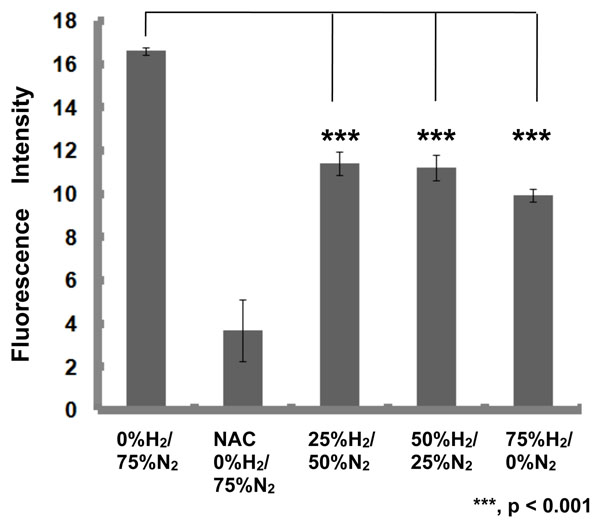
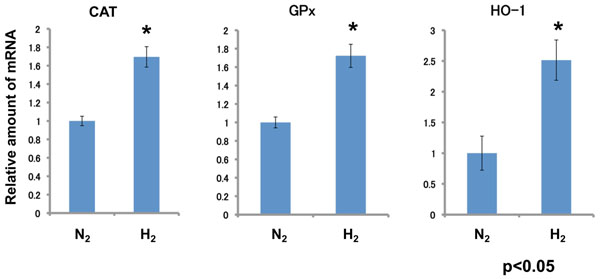
H2 stimulated glucose uptake into L6 cells. Pt NPs catalyzed the activation of H2 to hydrogen atom (H) to scavenge DPPH radical in vitro. The combined use of molecular hydrogen and Pt NPs resulted in extremely stimulated glucose uptake into L6 cells, suggesting that H produced from H2 by catalyst action of Pt NPs regulated glucose uptake signal transduction. As oppose to the paper by Ohsawa et al.[8], H2 of 25 to 75% concentration in the mixed gas significantly scavenged intracellular H2O2 in rat fibroblast L6 cells (Figure 1) and induced the gene expression of antioxidative enzymes such as CAT, GPx and HO-1 via activation of Nrf2 (Figure 2). H2, Pt NPs and their combination significantly suppressed the levels of fasting blood glucose and improved the impaired sugar tolerance abilities of obese insulin-resistant type 2 diabetic KK-Ay mice.
Figure 1.
The scavenging effect of hydrogen molecule on intracellular hydrogen peroxide in rat myotube L6 cells. ***, p<0.001.
Figure 2.
Induced gene expression of anitoxidative enzymes by hydrogen molecule. L6 myoblast cells were cultivated under an atmosphere of 75%N2 or H2/20%O2/5%CO2 for 2 h and gene expression was analyzed by RT-PCR. *, P<0.05
Conclusion
H2, Pt NPs, and their combined use resulted in activation of glucose uptake signal transduction pathways and stimulation of glucose uptake into L6 myotubes. In the groups of H2, Pt NPs and their combined use groups, blood sugar levels and impaired sugar tolerance of type 2 diabetes model mouse (KK-Ay) were significantly improved, suggesting that H2, Pt NPs and H are redox regulation factors in animal cells.
References
- Shirahata S, Kabayama S, Nakano M, Miura T, Kusumoto K, Gotoh M, Hayashi H, Otsubo K, Morisawa S, Katakura Y. Electrolyzed-reduced water scavenges active oxygen species and protects DNA from oxidative damage. Biophys Biochem Res Commun. 1997;234:269–274. doi: 10.1006/bbrc.1997.6622. [DOI] [PubMed] [Google Scholar]
- Yan H, Tian H, Kinjo T, Hamasaki T, Tomimatsu K, Nakamichi N, Teruya K, Kabayama S, Shirahata S. Extension of the lifespan of Caenorhabditis elegans by the use of electrolyzed reduced water. Biosci Biotech Biochem. 2010;74:2011–2015. doi: 10.1271/bbb.100250. [DOI] [PubMed] [Google Scholar]
- Hamasaki T, Kashiwagi T, Imada T, Nakamichi N, Aramaki S, Toh K, Morisawa S, Shimakoshi H, Hisaeda Y, Shirahata S. Kinetic analysis of superoxide anion radical-scavenging and hydroxyl radical-scavenging activities of platinum nanoparticles. Langmuir. 2008;24:7354–7364. doi: 10.1021/la704046f. [DOI] [PubMed] [Google Scholar]
- Shirahata S, Hamasaki H, Teruya K. Advanced research on the health benefit of reduced water. Trends Food Sci Tech. 2011. DOI 10.1016/j.tifs.2011.10.009.
- Li Y–P, Nishimura T, Teruya K, Maki T, Komatsu T, Hamasaki T, Kashiwagi T, Kabayama S, Shim S–Y, Katakura Y, Osada K, Kawahara T, Otsubo K, Morisawa S, Ishii Y, Gadek Z, Shirahata S. Protective mechanism of reduced water against alloxan-induced pancreatic β-cell damage: Scavenging effect against reactive oxygen species. Cytotechnology. 2002;40:139–149. doi: 10.1023/A:1023936421448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y–P, Hamasaki T, Nakamichi N, Kashiwagi T, Komatsu T, Ye J, Teruya K, Abe M, Yan H, Kinjo T, Kabayama S, Kawamura M, Shirahata S. Suppressive effects of electrolyzed reduced water on alloxan-induced apoptosis and type 1 diabetes mellitus. Cytotechnology. 2010. DOI 10.1007/s10616-010-9317-6. [DOI] [PMC free article] [PubMed]
- Kim M–J, Kim H–K. Anti-diabetic effects of electrolyzed reduced water in streptozotocin-induced and genetic diabetic mice. Life Sciences. 2006;79:2288–2292. doi: 10.1016/j.lfs.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S, Ohta S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radials. Nature Med. 2007;13:688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]