Abstract
Non-technical summary
Cochlear inner hair cells transform acoustic signals into electric signals. Another type of hair cells, outer hair cells, actively amplifies sensory signals and thus contributes to the exquisite sensitivity of the human auditory system. Cochlear amplification is based on length oscillations of outer hair cells that are mediated by a membrane-bound motor protein, prestin. Prestin transforms electrical signals directly in conformational changes and in extremely fast length changes of outer hair cells. Prestin belongs to a family of proteins that mainly function as anion transporters or channels. We here demonstrate that – under certain anionic conditions – all branches of this family can function as anion channels. These results show that prestin shares important functions with other family members that are not motor proteins. Knowledge of how prestin can switch between motor protein and channel mode will increase our understanding of molecular mechanisms underlying cochlear amplification.
Abstract
Prestin is a member of the SLC26 solute carrier family and functions as a motor protein in cochlear outer hair cells. While other SLC26 homologues were demonstrated to transport a wide variety of anions, no electrogenic transport activity has been assigned so far to mammalian prestin. We here use heterologous expression in mammalian cells, patch clamp recordings and measurements of expression levels of individual cells to study anion transport by rat prestin. We demonstrated that cells expressing rat prestin exhibit SCN− currents that are proportional to the number of prestin molecules. Variation of the SCN− concentration resulted in changes of the current reversal potential that obey the Nernst equation indicating that SCN− transport is not stoichiometrically coupled to other anions. Application of external SCN− causes large increases of anion currents, but only minor changes in non-linear charge movements suggesting that only a very small percentage of prestin molecules function as SCN− transporters under these conditions. Unitary current amplitudes are below the resolution limit of noise analysis and thus much smaller than expected for pore-mediated anion transport. A comparison with a non-mammalian prestin from D. rerio – recently shown to function as Cl−/SO42− antiporter – and an SLC26 anion channel, human SLC26A7, revealed that SCN− transport is conserved in these distinct members of the SLC26 family. We conclude that mammalian prestin is capable of mediating electrogenic anion transport and suggest that SLC26 proteins converting membrane voltage oscillations into conformational changes and those functioning as channels or transporters share certain transport capabilities.
The solute carrier 26 (SLC26) gene family encodes multifunctional membrane proteins in numerous tissues and organs (Mount & Romero, 2004). Eleven human members have been identified including one non-coding pseudogene. The physiological importance of these proteins is highlighted by several inherited human diseases caused by mutations in SLC26 genes (Everett & Green, 1999; Liu et al. 2003), such as diastrophic dysplasia, congenital chloride diarrhoea, and inner ear deafness. The symptomatic diversity of these diseases illustrates the variety of cellular functions relating to this class of transport proteins.
Most mammalian SLC26 proteins operate as anion exchangers with distinct substrate specificities (Bissig et al. 1994; Moseley et al. 1999; Scott & Karniski, 2000; Soleimani et al. 2001; Jiang et al. 2002; Xie et al. 2002). However, there are also family members that exhibit additional transport properties. SLC26A7 and SLC26A9 have been reported to function as anion carriers (Petrovic et al. 2004; Xu et al. 2005), but there is also experimental evidence for anion channel activity (Kim et al. 2005; Dorwart et al. 2007; Chang et al. 2009). SLC26A5/prestin represents a motor protein that allows outer hair cells in the mammalian cochlea to change length in response to acoustic signals (Zheng et al. 2000; Dallos & Fakler, 2002). This function results in mechanical sound amplification and enhances the hearing sensitivity by more than 40 dB. The basis for these length changes are voltage-dependent conformational changes that result in voltage-dependent capacitances of cells expressing prestin (Santos-Sacchi, 1991; Zheng et al. 2000). Non-mammalian prestin orthologues from chicken and zebrafish (Weber et al. 2003; Albert et al. 2007; Tan et al. 2011) function as electrogenic chloride/sulfate antiporters, suggesting that the mechanism by which prestin generates electromotility is evolutionarily derived from a transport cycle (Schaechinger & Oliver, 2007). At present, it is unclear whether mammalian prestin can mediate anion transport. Whereas Bai et al. (2009) recently demonstrated that mammalian cells expressing gerbil prestin were capable of accumulating radioactive formate and oxalate, a subsequent study by Tan et al. (2011) did not observe a statistically significant difference between mammalian prestin and negative controls.
Using electrophysiological techniques, we studied here the transport of another anion, the pseudohalide thiocyanate (SCN−), by a mammalian prestin and compared it to SCN− transport by zebrafish prestin and human SLC26A7. The aim of our study was to define the similarities and differences in anion transport between the three functional branches of the SLC26 family, anion channels, transporters and motor proteins, through the functional evaluation of SCN− currents by human SLC26A7, Slc26a5 (DANRE)/prestin from Danio rerio (zebrafish) and SLC26A5 (RAT)/prestin from Rattus norvegicus.
Methods
Construction of expression plasmids and heterologous expression
pcDNA3.1(+)Zeo-(RAT) Slc26a5-His and pcDNA5/FRT/TO-(RAT) Slc26a5-YFP encoding rat prestin (GenBank accession number NM_030840), pcDNA5FRT/TO-(DANRE) slc26a5-YFP encoding prestin from Danio rerio (zebrafish) (GenBank accession number BX571796) (Weber et al. 2003), and pcDNA5/FRT/TO-(HUMAN) SLC26A7-YFP encoding human SLC26A7 (GenBank accession number BC094730.1) (Kim et al. 2005) were created by PCR-based techniques, as described (Detro-Dassen et al. 2008). All fluorescent tags were added to the carboxy-terminus of the coding region. We expressed SLC26A5 (RAT)/prestin transiently in HEK293T cells, as described (Melzer et al. 2005). Moreover, we generated four inducible stable cell lines by selecting Flp-In T-REx cells (Invitrogen, Carlsbad, CA, USA) transfected with pcDNA5/FRT/TO-(RAT) Slc26a5-YFP, pcDNA5/FRT/TO-(DANRE) slc26a5-YFP, pcDNA5/FRT/TO-(HUMAN) SLC26A7-YFP or pcDNA5 /FRT/TO-YFP. Cells expressing rat or zebrafish prestin were used after 24–48 h incubation with 1 μg ml-1 tetracycline, whereas inducible cells expressing human SLC26A7 or yellow fluorescent protein (YFP) alone were incubated only for 8 h prior to electrophysiological characterization (Trapani & Korn, 2003). Mammalian cells were usually cultivated in media containing fetal serum. To minimize background expression of SLC26s, we used tetracycline-free sera (fetal bovine serum (FBS) certified, Invitrogen). For all inducible cell lines expressing SLC26 fusion proteins, current amplitudes were markedly increased by induction with tetracycline. However, there was also a faint fluorescent membrane staining in the absence of tetracycline (data not shown), suggesting that expression of SLC26s also occurs in the absence of tetracycline. For control experiments, we created pcDNA3.1(+)YFP for transient expression in HEK293T cells and pcDNA5/FRT/TO-YFP for generating an inducible stable cell line expressing YFP alone.
Functional properties of YFP fusion proteins were unaltered for SLC26A5 (RAT)/prestin (Detro-Dassen et al. 2008), Slc26a5 (DANRE)/prestin (Detro-Dassen et al. 2008) and human SLC27A7 (Supplementary Fig. 1). Comparison of SCN− currents in cells expressing YFP-tagged (Figs 3 and 4) and untagged (Figs 1 and 2) proteins demonstrate that these tags do not modify SLC26A5/prestin anion currents.
Figure 3. SCN− currents in cells stably expressing mammalian prestin, teleost prestin and human SLC26A7.
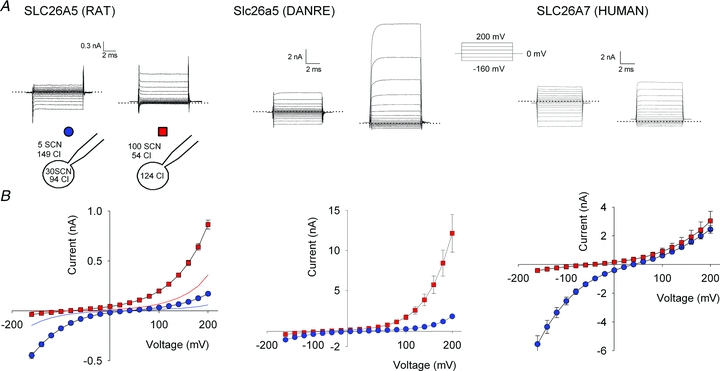
A, representative whole-cell SCN− currents in Flp-In T-REx cells stably expressing YFP-tagged SLC26A5 (RAT)/prestin, Slc26a5 (DANRE)/prestin or human SLC26A7 for two different anion distributions shown in the cartoon. The dotted lines indicate the zero current reference level. B, current–voltage relationships for YFP-tagged SLC26A5 (RAT)/prestin, Slc26a5 (DANRE)/prestin or human SLC26A7 determined by current recordings as shown in A. Means ± SEM from more than 7 cells. Protein expression was induced with tetracycline 24 h before electrophysiological characterization for the two prestins and 8 h before characterization of SLC26A7.
Figure 4. Comparison of SCN− transport by different SLC26s.
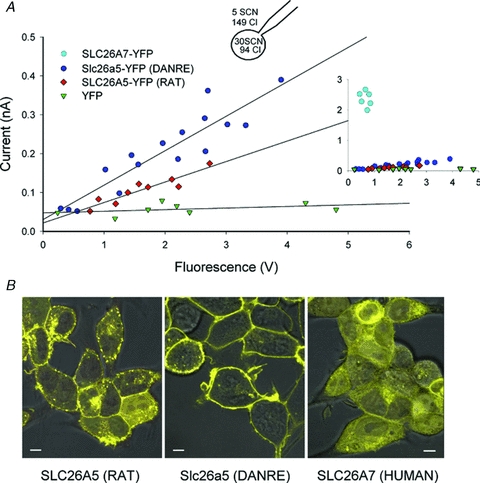
A, plot of whole-cell current amplitudes at –110 mV versus whole-cell fluorescence for Flip-In T-REx cells expressing YFP-tagged SLC26A5 (RAT)/prestin, Slc26a5 (DANRE)/prestin or human SLC26A7 (inset). An inducible stable cell line expressing only YFP was used as negative control. B, confocal images of Flp-In T-REx cells expressing the indicated YFP-tagged proteins superimposed on differential interference contrast images. Cells were incubated with tetracycline for either 24–48 h (SLC26A5 (RAT), Slc26a5 (DANRE)) or 8 h (YFP, human SLC26A7). Bar = 5 μm.
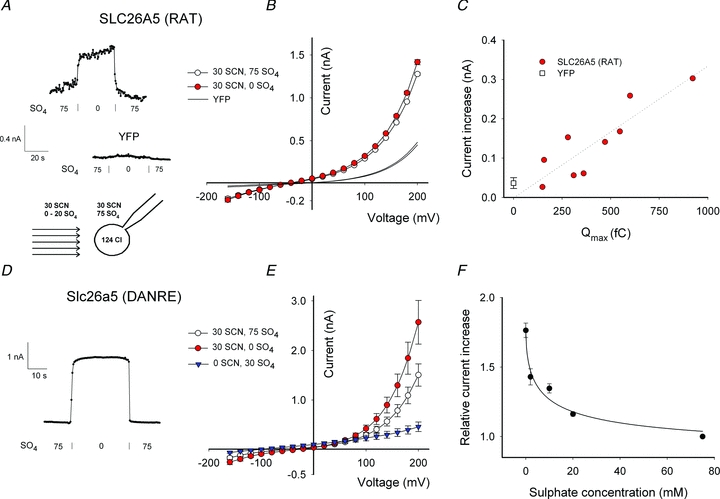
Figure 1. SCN− currents are conducted by mammalian prestin.
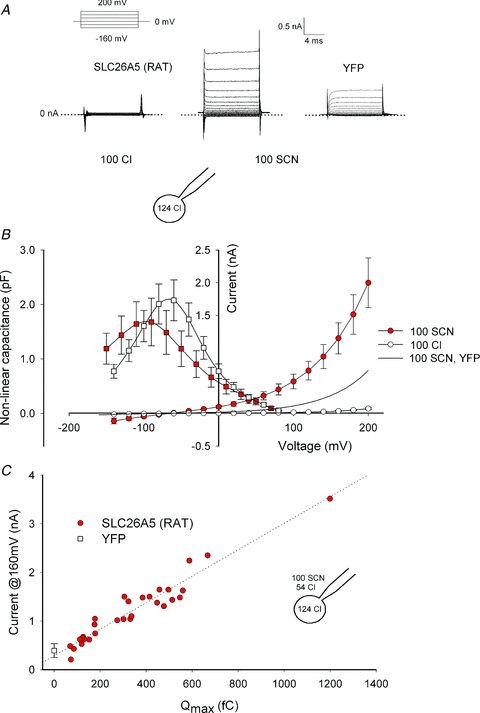
A, representative whole-cell currents in HEK293T cells transiently expressing SLC26A5 (RAT)/prestin with Cl− or SCN− as main extracellular anion. Membrane voltage was driven by a simple step protocol from –160 mV to +200 mV (upper left). The cartoon depicting the whole cell configuration illustrates the relevant ionic constituents. HEK293T cells expressing YFP were used as negative controls. B, voltage dependence of whole-cell capacitance (▪) and current–voltage relationships (•) for SCN−- or Cl−-based external solutions. Means ± SEM, n = (8, 10). C, plot of current amplitudes at +160 mV versus Qmax for 30 different cells. Qmax is proportional to the total number of active prestin molecules and was obtained by fitting the measured voltage-dependent capacitance with eqn (1). Cells expressing YFP do not exhibit non-linear capacitance and control current amplitudes are therefore given as means ± standard deviation at Qmax= 0 fC. The slope of the linear regression line is 2.6 pA fC-1.
Figure 2. Noise analysis of SCN− currents mediated by SLC26A5 (RAT)/prestin.
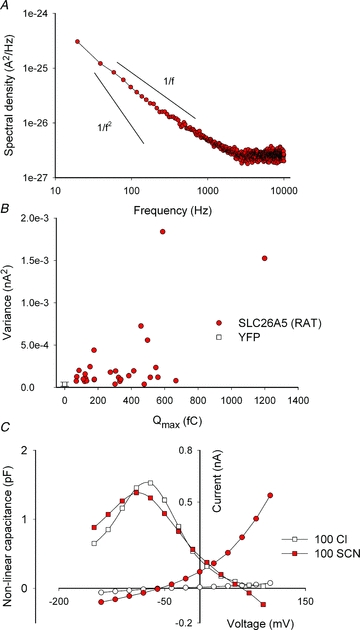
A, averaged power spectrum of SCN− currents from cells expressing rat prestin. More than 30 individual current responses to +200 mV were averaged and corrected for background noise. Arbitrary continuous lines represent the expected slopes for 1/f noise or Lorentzian 1/f2 noise for comparison. B, variance from 10 ms long current traces at +160 mV membrane potential versus Qmax. Data were generated from the same experiments used for Fig. 1C. C, representative voltage dependence of the whole-cell capacitance (▪) and SCN− currents (•) before and following perfusion with external solutions containing 100 mm Cl− or 100 mm SCN−. All experiments used HEK293T cells transiently transfected with SLC26A5 (RAT)/prestin or YFP.
Functional characterization of SLC26A5 and SLC26A7
Transfected HEK293 or HEK293T cells were studied through whole-cell patch clamping using an Axopatch 200B amplifier (Torres-Salazar & Fahlke, 2007). Two different standard solutions with different anion current reversal potentials were used. Solution A contained (mm): 100 NaSCN, 20 CsCl, 20 TEA-Cl, 2 KCl, 2 CaCl2, 2 MgCl2, 2 CoCl2, 5 Hepes (extracellular); and 120 CsCl, 2 MgCl2, 5 EGTA, 10 Hepes (intracellular). Solution B contained (mm): 5 NaSCN, 100 NaCl, 15 CsCl, 20 TEA-Cl, 2 KCl, 2 CaCl2, 2 MgCl2, 2 CoCl2, 5 Hepes (extracellular); and 30 NaSCN, 90 CsCl, 2 MgCl2, 5 EGTA, 10 Hepes (intracellular). To compare non-linear capacitances in the presence of external Cl− or SCN− (Fig. 1B), external solution A was modified by replacement of external NaSCN with NaCl and sodium gluconate so that concentrations of SCN− and Cl− were comparable. For the experiments shown in Fig. 5, solutions were modified equimolarly by replacing NaCl or NaSCN with sodium gluconate. For experiments studying block by SO42−, a bath solution containing (mm) 75 Na2SO4, 30 NaSCN, 2 potassium gluconate, 2 Ca(gluconate)2, 2 Mg(gluconate)2, 5 Hepes, and pipette solution A were used. Na2SO4 was replaced with sodium gluconate at a ratio of 2:3 to determine the concentration dependence of block, and NaSCN was equimolarly replaced by sodium gluconate to study isolated Cl−/SO42− antiport. Calculated osmolarities were 274 mosmol l-1 for all pipette solutions and between 310 and 312 mosmol l-1 for external solutions. The pH was adjusted to 7.4 for all solutions. For all experiments, absence of cell swelling was visually tested during the different phases of each experiment. The initial pipette resistances were 1–2.5 MΩ. Stray pipette capacitance was neutralized before establishing the whole-cell configuration. Agar salt bridges were used and the recorded data for Fig. 4 was additionally corrected for liquid junction potentials.
Figure 5. Current reversal potentials are independent of [Cl−] and obey the Nernst equation for [SCN−].
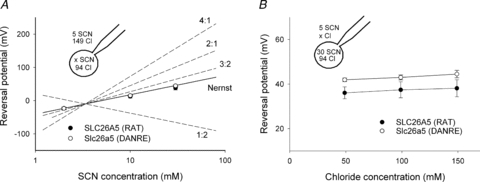
A, reversal potentials from cells expressing SLC26A5 (RAT)/prestin or Slc26a5 (DANRE)/prestin at different internal SCN− concentrations. Symbols are means ± SEM from 5 or more experiments, whereas continuous and dashed lines provide predictions for different Cl−/SCN− exchange stoichiometries or SCN− diffusion potentials calculated using eqns (3) and (4), respectively. The external solution contained 5 mm SCN− and 149 mm Cl−. B, reversal potentials from cells expressing SLC26A5 (RAT)/prestin or Slc26a5 (DANRE)/prestin at varying external [Cl−] and [SCN−]i/[SCN−]o= 30/5 versus external [Cl−]. Means ± SEM from 5 or more cells. The Nernst potential for SCN− is +45 mV.
In experiments determining transmembrane currents, pCLAMP9 (Molecular Devices, Sunnyvale, CA, USA) was used for data acquisition and analysis (Detro-Dassen et al. 2008). To measure voltage-dependent capacitance, data acquisition and analysis were performed with the Windows-based patch-clamp program, jClamp (SciSoft, Ridgefield, CT, USA). Non-linear charge movement was calculated using a continuous high-resolution (2.56 ms sampling) two-sine stimulus protocol (10 mV peak at both 390.6 and 781.2 Hz) superimposed onto voltage steps (150 ms duration) from –160 mV to +100 mV (Santos-Sacchi et al. 1998). The voltage dependence of the non-linear capacitance was fit to the first derivative of a two-state Boltzmann function (Santos-Sacchi, 1991).
| (1) |
with
| (2) |
Qmax is the maximum charge transferred across the membrane; V1/2 is the potential at half-maximal charge transfer; z is the number of elementary charges, eo, displaced across a fraction, δ, of the membrane dielectric; kB is Boltzmann's constant, and T is the absolute temperature.
For experiments correlating currents and the number of SLC26-YFP fusion proteins, cells were cultivated on collagen coated glass coverslips and mounted in a perfusion chamber on an inverted IX71 microscope with UPlanSApo 60×/1.35 oil immersion objective (Olympus, Hamburg, Germany). YFP was excited at 500 nm using a Polychrome V fast switching monochromator and emitted fluorescence light was detected at 530 nm using a photomultiplier tube equipped ViewFinder III (Till Photonics, Gräfelfing, Germany) (Alekov & Fahlke, 2009). Fluorescence excitation and detection were software-controlled by the Photometry extension of PATCHMASTER. Fluorescence was measured in the linear range of the photomultiplier and detected as voltage. Unless otherwise stated all values are given as means ± SEM.
Confocal imaging
Live cell confocal imaging was carried out with Flp-In T-REx cells cultured in μ-Dishes (ibidi GmbH, Munich, Germany) (Riazuddin et al. 2009) on an Olympus IX81 inverted microscope. Image acquisition was done using the Fluoview FV1000 system (Olympus, Tokyo, Japan) with 515 nm excitation wavelength and fluorescence detection at 535–565 nm. Confocal images were superimposed on differential interference contrast (DIC) images taken simultaneously.
Results
Mammalian prestin can mediate SCN− currents
SCN− was recently shown to uncouple SLC26A3 and SLC26A6 anion exchangers (Shcheynikov et al. 2006; Ohana et al. 2011). To test whether SCN− exerts similar actions on the motor protein prestin, we studied ionic currents in HEK293T cells transiently expressing SLC26A5(RAT)/prestin (Detro-Dassen et al. 2008) using whole-cell patch clamp recordings under conditions that have been shown to minimize endogenous current amplitudes in HEK293 cells (Santos-Sacchi, 2002; Farrell et al. 2006). With Cl− as predominant internal and external anions, only negligible whole-cell currents could be observed (Fig. 1A, Zheng et al. 2000). Currents were very small in external NO3− (data not shown). After substitution of external Cl− by SCN− outwardly rectifying currents could be observed (Fig. 1A and B). Whole cell currents reversed at negative potentials as expected of anion selective currents with higher permeability for SCN− than for Cl−. In control cells transfected with a vector encoding YFP alone, anion current amplitudes in SCN− are significantly smaller than those of prestin-expressing cells and negligible in external Cl− (Fig. 1A and B).
The electrophysiological signature of prestin is voltage-dependent charge movements that report on voltage-dependent conformational changes of these proteins (Zheng et al. 2000). Figure 1B shows mean values for charge–voltage relationships from HEK293T cells transiently expressing SLC26A5 (RAT)/prestin in Cl− or SCN−-based solutions. Exchange of external Cl− to SCN− slightly modifies prestin-associated charge movement. It shifts the operating range to more negative voltages and decreases the slope of the C–V curve. Non-linear capacitance of prestin can be described with eqn (1), the first derivative of a two-state Boltzmann probability distribution, from which the parameters Qmax, V1/2 and β can be obtained by least square fitting. Qmax is the total amount of charge moved if all active prestin molecules transition into its alternate state (Rybalchenko & Santos-Sacchi, 2008). V1/2 is the membrane potential resulting in a uniform distribution of prestin molecules between the two states and, as a direct consequence, the voltage at peak capacitance. The slope factor β specifies the apparent charge of the translocatable entity. Replacement of Cl- by SCN− decreases β and shifts the midpoint V1/2 to more negative voltages, but does not reduce Qmax (Table 1).
Table 1.
Fit parameters of the voltage dependences of non-linear charge movement of rat prestin in external Cl− or SCN−
| β (mV-1) | V1/2 (mV) | Qmax (fC) | |
|---|---|---|---|
| Cl− (n = 10) | 0.0308 ± 0.00052 | −69 ± 1.4 | 266 ± 46 |
| SCN− (n = 8) | 0.0231 ± 0.00089 | −102 ± 3.2 | 290 ± 72 |
Heterologous expression of prestin might cause upregulation of endogenous anion channels, and the observed currents might be conducted by such channels rather than by prestin itself. SCN− currents in transfected cells are small, and background currents are not insignificant under these conditions, making additional tests necessary to demonstrate that SLC26A5 (RAT)/prestin itself conducts SCN−. Figure 1C plots the current amplitude at +160 mV versus Qmax for 30 cells. Qmax is proportional to the total number of functional prestin molecules in the membrane. Anion current amplitudes change linearly with the non-linear capacitance over a more than 20-fold variation in the number of SLC26A5 (RAT)/prestin, demonstrating that SCN− is transported by mammalian prestin and not by endogenous channels/transporters up-regulated by overexpression of prestin.
Noise analysis of macroscopic currents often provides information about underlying unitary current events (DeFelice, 1981). In ion channels, high unitary transport rates result in macroscopic currents whose variance is dominated by the opening and closing of individual channels. Such Lorentzian type noise is characterized by 1/f2 frequency dependence of the spectral density (Anderson & Stevens, 1973; DeFelice, 1981). We determined the power spectra of SLC26A5 (RAT)/prestin-mediated SCN− currents and found them not to resemble Lorentzian noise, but rather 1/f frequency dependence (Fig. 2A). Since 1/f noise can be also observed in untransfected cells (Alekov & Fahlke, 2009), separation of prestin-associated noise from background is not possible. Moreover, no correlation between the number of prestin proteins and the whole cell current variance could be observed. Figure 2B plots the current variance at +160 mV versus the maximum voltage-dependent capacitances for 30 different cells expressing largely different amounts of SLC26A5 (RAT)/prestin. Expression of SLC26A5 (RAT)/prestin therefore does not add significant current noise above background levels, and unitary current amplitudes of SLC26A5 (RAT)/prestin cannot be determined by noise analysis.
Ion channels that never close do not generate Lorentzian noise (Alvarez et al. 2002). The observed lack of prestin-specific noise might therefore be either due to very low unitary current amplitudes or to very high absolute open probabilities. To estimate the probability that SLC26A5 (RAT)/prestin assumes the anion conducting mode, we compared non-linear capacitances from individual cells that were consecutively perfused with external Cl−- and SCN−-based solution (Fig. 2C). SCN− resulted in a dramatic increase of macroscopic anion currents, but did not reduce non-linear capacitances. We rather observed in all cells a slight increase by 16 ± 7% after perfusion with SCN (n = 6), indicating that SCN− converts only a very small number of prestin molecules into an SCN− conducting state. Transported anions cannot stably bind to ion channels or transporters, and SCN− is thus likely to induce frequent transitions between non-transporting and transporting states of SLC26A5 (RAT)/prestin. In this case, the percentage of conducting proteins equals the probability of this state, and we can thus conclude that the probability of SLC26A5 (RAT)/prestin being in a transporting/conducting mode is very low in the presence of SCN−. The observed low current variances are therefore not due to very high open probabilities, but rather to unitary current amplitudes of SLC26A5 (RAT)/prestin that are too small to be resolved by macroscopic current noise measurements.
SCN− currents in SLC26A5 and SLC26A7
To compare the observed prestin-mediated currents with SCN− currents by other SLC26 proteins, we engineered inducible cell lines for fusion proteins of YFP with SLC26A5 (RAT)/prestin, Slc26a5 (DANRE)/prestin or human SLC26A7, and compared SCN− currents by these SLC26 proteins (Fig. 3). SLC26A7 was the first SLC26 for which anion channel activity was demonstrated (Kim et al. 2005), and Slc26a5 (DANRE)/prestin is firmly established to function as coupled anion exchanger (Schaechinger & Oliver, 2007). Figure 3A shows representative whole-cell currents from cells stably and inducibly expressing SLC26A5 (RAT)/prestin, Slc26a5 (DANRE)/prestin and human SLC26A7 for two different anion compositions. All tested SLC26s displayed whole-cell currents with reversal potentials that depend on the SCN− distribution across the membrane (Fig. 3B, Table 2). SCN− currents by Slc26a5 (DANRE)/prestin or by human SLC26A7 were larger than those of rat prestin. With external solutions containing high concentrations of thiocyanate ([SCN−]o= 100 mm and SCN−-free internal solutions), SLC26A5 (RAT)/prestin currents were usually below 1 nA at +200 mV after 24 h induction with tetracycline, but exceeded 10 nA in cells expressing zebrafish prestin. For human SLC26A7, currents were too large under these induction conditions, and we therefore used cells after induction for only 8 h.
Table 2.
Current reversal potentials for rat prestin, zebrafish prestin and human SLC26A7 at two different anion compositions
| [SCN−]o/[SCN−]i (mm) | 5/30 | 100/0 |
|---|---|---|
| SLC26A5 (RAT) | 35 ± 1.5 mV (n = 7) | −83 ± 2 mV (n = 9) |
| SLC26A5 (DANRE) | 37 ± 3 mV (n = 8) | −50 ± 5 mV (n = 8) |
| SLC26A7 (HUMAN) | 39 ± 1 mV (n = 7) | −48 ± 8 mV (n = 8) |
In all cases, currents rose instantaneously upon voltage steps, indicating that the tested SLC26s are indeed active at the holding potential of 0 mV. Current–voltage relationships were bidirectionally rectifying with [SCN−]o= 5 mm and [SCN−]i= 30 mm and outwardly rectifying for [SCN−]o= 100 mm (Fig. 3B). The voltage dependence of current rectification was distinct for each studied protein. Moreover, whereas currents conducted by SLC26A5 (RAT)/prestin or human SLC26A7 were time independent, Slc26a5 (DANRE)/prestin whole-cell currents exhibited time- and voltage-dependent activation that depended on ionic conditions (Fig. 3A). These findings illustrate specific conduction/transport properties of the different SLC26 proteins and argue against up-regulated endogenous anion channels as the basis of the observed currents.
Plotting non-linear charge movements versus SCN− current amplitudes allowed a comparison of protein amounts and SCN− currents with SLC26A5 (RAT)/prestin (Fig. 1C). Since non-linear charge movement is a unique property of SLC26A5/prestin (Zheng et al. 2000; Albert et al. 2007), we used another approach to include all three proteins in such comparison. SLC26 proteins were expressed as YFP fusion proteins, and YFP fluorescence thus provides a measure for the number of SLC26 proteins. Figure 4A plots whole-cell current versus whole-cell fluorescence for the three SLC26 proteins. For rat and zebrafish prestin, a linear correlation was observed, in agreement with the notion that SCN− currents are mediated by the SLC26-YFP fusion protein. Current/fluorescence ratios are larger for Slc26a5 (DANRE)/prestin than for SLC26A5 (RAT)/prestin. Currents associated with human SLC26A7 were very large even at small fluorescence amplitudes, precluding measurements of current amplitudes over a wide variety of expression levels. However, at a given fluorescence, these currents were much larger than for the two prestins (Fig. 4A, inset).
Since the fluorescence value used does not distinguish between fusion proteins inserted into internal compartments or the surface membrane, the different slopes might be due to separate subcellular distributions of the distinct proteins. Confocal imaging of cells stably expressing SLC26A5 (RAT)/prestin or human SLC26A7 demonstrated that rat and zebrafish prestin show predominant surface staining (Fig. 4B). Although current/fluorescence ratios were much larger for human SLC26A7 than for prestin, intracellular fluorescence staining was more prominent in cells expressing human SLC26A7. SLC26A7 whole-cell currents were therefore larger than prestin currents, although a smaller percentage of protein was inserted into the surface membrane. The distinct SCN− currents by fluorescence ratios of the three SLC26 proteins thus must be caused either by separate unitary current amplitudes or separate percentages of protein functioning as SCN− transporters.
Concentration dependence of reversal potentials
The SLC26 family encompasses coupled anion transporters as well as anion channels (Mount & Romero, 2004, Kim et al. 2005; Dorwart et al. 2007). To distinguish between SLC26A5 functioning as coupled Cl−/SCN− exchanger – with a transport stoichiometry yet to be defined – or SCN− uniporter or channel, we measured reversal potentials for a variety of SCN− distributions across the membrane. In these experiments, the external [SCN−] was always 5 mm. Internal and external [Cl−] were adjusted to 94 mm and 149 mm, and internal [SCN−] was varied between 2 and 30 mm. Figure 5A gives the concentration dependence of the reversal potential from measurements in cells expressing SLC26A5 (RAT)/prestin (filled symbol) or Slc26a5 (DANRE)/prestin (open symbol). Measured current reversal potentials were compared with predicted SCN−-diffusion potentials calculated by the Nernst equation:
| (3) |
or with predictions of the equilibrium potential of coupled transporters
| (4) |
In this formula, the coupling ratio r is defined as the ratio of the stoichiometric numbers of SCN− (n(SCN)) and Cl− ions (m(Cl)) transported in one transport cycle (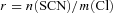 ).
).
The experimentally observed reversal potentials for SLC26A5 (RAT)/prestin and Slc26a5 (DANRE)/prestin are different from predictions for coupled transport with various coupling stoichiometries, but closely similar to predictions of the Nernst equation for SCN−. This finding supports the notion that SCN− transport is not coupled to transmembrane Cl− movement. As a further test, we studied whether reversal potentials are affected by the concentration of Cl− (Fig. 5B). In these experiments, [SCN−] was kept constant on both sides of the membrane, and external [Cl−] was modified between 49 mm and 149 mm. Again, Cl− did not exert any effect on the current reversal potential, providing further support for SCN− currents that are not coupled to Cl−. We conclude that prestin-mediated currents reverse at potentials closely similar to equilibrium potentials for SCN−, indicating that rat and zebrafish prestin do not mediate a coupled SCN−/Cl− transport. Prestin-mediated SCN− transport might thus either occur in a channel-mediated fashion as diffusion along an aqueous pore or by a uniporter that moves SCN− across the membrane via conformational changes.
Measured reversal potentials were not identical in cells expressing SLC26A5 (RAT)/prestin and in those expressing Slc26a5 (DANRE)/prestin (Fig. 5). A potential reason for this difference might be the lower current amplitudes of cells expressing SLC26A5 (RAT)/prestin (Fig. 3), resulting in a larger contribution of background currents. Alternatively, the channel/uniporter mode of prestin might also transport Cl− to some extent, and rat and zebrafish prestin might differ in SCN− over Cl− selectivity.
SCN− currents are reduced by external SO42−
A large number of SLC26 proteins transport SO42− (Bissig et al. 1994; Schaechinger & Oliver, 2007; Ohana et al. 2009). Assuming an alternating access transport mechanism coupled transport requires the existence of SO42− sites in the inward and as well as in the outward facing conformation. Moreover, a recent theoretical treatment of the function of mammalian prestin proposed an external high affinity SO42−-binding site (Muallem & Ashmore, 2006). We decided to study the effects of SO42− on rat and zebrafish prestin-mediated SCN− currents (Fig. 6). Ionic current measurements under uncoupling conditions has allowed characterization of substrate association for various transporters (Otis & Jahr, 1998; Watzke et al. 2001; Kovermann et al. 2010). We used a similar approach and determined currents first in the presence of SCN− and SO42− and thereafter at the same cell after switching to an external solution in which SO42− was replaced by gluconate-.
Figure 6. SCN− currents are blocked by external SO42−.
A and D, time course of current increases for rat prestin (A) and zebrafish prestin (D) upon removal of external SO42−. Current amplitudes were measured at repetitive voltage steps to +200 mV, and [SO42−] is given below the time dependence of the current amplitude. B, current–voltage relationships from cells transiently expressing SLC26A5 (RAT)/prestin in solutions with varying [SO42−] and [SCN−]. Means ± relative standard error (RSE) from 9 cells. continuous lines show data from cells expressing only YFP as negative control (n = 5). C, plot of SO42− -induced current amplitude reduction at +200 mV versus non-linear capacitance measured under the same ionic conditions for 9 different cells. In these experiments, transiently transfected HEK293T cells were used. Cells expressing YFP were accumulated into a single bin at Qmax= 0 fC and the current amplitude is shown as the mean ± standard deviation. E, current–voltage relationships from cells stably expressing Slc26a5 (DANRE)/prestin in solutions with varying [SO42−] and [SCN−]. Means ± SEM from 7 cells. F, dose–response relationship for sulphate block of SCN− currents mediated by Slc26a5 (DANRE)/prestin. Means ± SEM (n = 7). Currents were recorded at +200 mV membrane potential and then normalized to the current amplitude at [SO42−]= 75 mm.
SO42− blocked SCN− currents through SLC26A5 (RAT)/prestin and through Slc26a5 (DANRE)/prestin in a reversible manner (Fig. 6A and D). SO42− caused a small, but significant reduction of SLC26A5 (RAT)/prestin currents (170 ± 50 pA at +200 mV, n = 11, P < 0.005). We observed also a SO42− -induced reduction of currents in control cells expressing only YFP (37 pA ± 14 pA at +200 mV, n = 8, P < 0.01). However, the current reduction was significantly smaller in control cells than in cells expressing rat prestin (P < 0.01, one-tailed Mann–Whitney U test). We used Wilcoxon's signed-rank test to test statistical significance since current differences were not normal-distributed.
To further separate effects of SO42− on endogenous currents and on heterologously expressed SLC26A5 (RAT)/prestin we compared SO42−- induced current block and non-linear capacitance in transiently transfected HEK293T cells with differing expression levels (Fig. 6C). For a given cell, the non-linear capacitance is proportional to the number of prestin proteins in the surface membrane. If SO42− blocks SLC26A5 (RAT)/prestin, the relative block by SO42− has to be linearly related to the number of prestin molecules. We indeed observed a linear relationship between these two parameters supporting the notion that SO42− blocks SLC26A5 (RAT)/prestin. The small relative reduction of rat prestin-mediated SCN− currents is thus either due to a very low binding affinity for SO42− or to a very small percentage of SLC26A5 (RAT)/prestin being SO42− sensitive. At present, we cannot distinguish between these two possibilities. The relatively small reduction of SLC26A5 (RAT)/prestin currents prevented determination of its concentration dependence.
Figure 6E gives the voltage dependence of zebrafish prestin SCN− currents in the presence as well as in the absence of SO42−. With external SCN−, currents reversed at –39 mV ± 12 mV (n = 7) in the absence of SO42− and at –61 ± 15 mV (n = 7) in the presence of SO42−(Fig. 6E). The SO42− -induced current reduction was associated with a shift of the current reversal potential. After replacement of SCN− by gluconate the external solution contained SO42− as the sole transportable anion permitting observation of coupled Cl−/SO42− antiport. Under these conditions the reversal potential was more negative than in the presence of SCN− (–106 ± 12 mV, n = 4; Fig. 6E). This shift in the reversal potential suggests that in external SO42− and SCN− a certain percentage of zebrafish prestin proteins function as Cl−/SO42− antiporters, and that SO42− reduces the number of Slc26a5 (DANRE)/prestin functioning as SCN− uniporters/channels. In the presence of external SO42−, SCN− uncouples Slc26a5 (DANRE)/prestin to a lower extent than in its absence. SO42− at 75 mm blocked Slc26a5 (DANRE)/prestin currents by about 50%, and the pronounced block allowed determination of the SO42−-concentration dependence of Slc26a5 (DANRE)/prestin currents (Fig. 6F). The data were well fit with a Michaelis–Menten relationship providing an apparent dissociation constant, Kd, of 7.7 mm.
Discussion
Mammalian SLC26A5/prestin is unique among the SLC26 family in its ability to convert changes of voltage into conformational changes that result in alteration of cell lengths (Zheng et al. 2000). Recently, non-mammalian prestin homologues were identified in zebrafish (Albert et al. 2007), as well as in chicken (Schaechinger & Oliver, 2007). These non-mammalian prestins exhibit non-linear capacitance; however, they also mediate stoichiometrically coupled transport of monovalent and divalent anions (Tan et al. 2011). Under the same conditions that resulted in coupled transport by non-mammalian homologues, no electrogenic transport activity could be determined for mammalian prestin (Schaechinger & Oliver, 2007).
We here demonstrate that SLC26A5 (RAT)/prestin mediates electrogenic SCN− transport. In transfected cells, SCN− current amplitudes were well above background currents determined with transfected cells heterologously expressing a cytoplasmic protein (Fig. 1A). Moreover, we could demonstrate that SCN− current amplitudes change linearly with expression levels of SLC26A5 (RAT)/prestin, either measured by non-linear capacitance (Fig. 1C) or whole-cell fluorescence (Fig. 4A). We observed such linear relationship over pronounced differences in the number of SLC26A5 (RAT)/prestin molecules as shown by a more than 20-fold variation in non-linear capacitance (Fig. 1C). This correlation provides strong evidence that SCN− transport is mediated by SLC26A5 (RAT)/prestin itself. Such behaviour would not be expected if expression of mammalian prestin only upregulated endogenous anion channels, since upregulation of endogenous channels does not follow a linear relationship to the expressed protein. Moreover, background anion channels have been extensively studied in cultured mammalian cells, and no endogenous channels with similar characteristics have been observed so far. Lastly, the functional similarity between rat and zebrafish prestin despite the clear difference in current amplitude further supports the notion of SLC26A5 (RAT)/prestin-mediated anion conduction (Figs 3 and 6). We did not test for electrogenic transport of formate and oxalate, but it appears likely that mammalian prestin is not perfectly selective and might also transport other anions (Bai et al. 2009). We observed measurable anion currents by prestin only for the non-physiological anion SCN−. Moreover, even for this anion, macroscopic current conductances were very small making high and non-physiological voltages necessary to obtain significant current amplitudes. Most likely, anion currents by mammalian prestin thus do not exert a physiological function.
Two SLC26 anion exchangers, SLC26A3 and SLC26A6, were recently shown to function as conductive anion pathways in the presence of uncoupling anions such as NO3− and SCN− (Shcheynikov et al. 2006; Ohana et al. 2011). Charge inverting mutations in a conserved glutamate residue, E367 in SLC26A3 and E357 in SLC26A6, inhibit coupled as well as uncoupled transport, indicating that NO3− and SCN− transport occur through the same conduction path as anion exchange (Ohana et al. 2011). In the case that SCN− transport by the related SLC26A5 occurs by a similar mechanism, SLC26A5 is expected to assume two conformational states and switch between these two states. In one state, the anion conduction pathway is open and can conduct SCN−. In the other state, no transport occurs, and voltage steps cause conformational changes that result in charge movements resembling an incomplete transport cycle in transporters (Loo et al. 1993; Mager et al. 1993). We interpret all our finding in the framework of this model and assume that SLC26A5 (RAT)/prestin exclusively functions as a motor protein in the absence of SCN− and that SCN− can switch the motor protein into an anion-conducting mode. In the presence of external SCN− the maximum capacitance is almost unaltered as compared to external Cl− (Fig. 1B), indicating that the majority of rat prestin proteins assume the functional state characterized by voltage-dependent conformational changes. SCN− thus seems to uncouple only a very small fraction of SLC26A5 (RAT)/prestin into anion channels. Since association and dissociation of SCN− is expected to be fast (Alekov & Fahlke, 2009), the fraction of uncoupled proteins exactly translates into the absolute probability of being in the conducting mode. For ion channels/transporters with very small open probability, the unitary current amplitude can be calculated as the ratio of the current variance by the macroscopic current (Alvarez et al. 2002). Noise analysis revealed SCN− current variances that were not different from background indicating that the unitary SCN− transport rate of SLC26A5 (RAT)/prestin is very small. Our data indicate that – in external SCN−– a very small fraction of SLC26A5 (RAT)/prestin assumes an anion conducting mode with very small transport rate.
Reversal potentials in mixtures of SCN− and Cl− are independent of the Cl− concentration (Fig. 5), indicating that SCN− transport is not thermodynamically coupled to Cl− movement across the membrane. This finding suggests that prestin operates as an anion channel in the presence of external SCN−. Alternatively, these currents might be mediated in a transporter mode that involves conformational changes mediating SCN− transport. Unitary transport rates are too low to unambiguously distinguish between these two transport processes. However, since SLC26 proteins encompass motor proteins, anion exchangers and anion channels, but no anion uniporter, and since channel-like transport has been shown for SLC26A3 and SLC26A6 (Ohana et al. 2011), we suggest that prestin assumes a channel mode of conduction in the presence of SCN−.
We compared SCN− transport by mammalian SLC26A5 (RAT)/prestin with Slc26a5 (DANRE)/prestin and human SLC26A7. All tested SLC26 proteins transported SCN−, in agreement with the notion that this transport function is conserved within different functional branches of the SLC26 family. However, at comparable whole-cell fluorescences and thus protein expression levels human SLC26A7-mediated SCN− current amplitudes were much bigger than for both prestins (Fig. 4). The different current amplitudes demonstrate that SCN− transport rates by a given number of proteins are different for SLC26A5 (RAT)/prestin, Slc26a5 (DANRE)/prestin and human SLC26A7. This might suggest different unitary transport rates or different percentages of proteins functioning as SCN− transporters, with very few SLC26A5 (RAT)/prestin, but a much larger percentage of Slc26a5 (DANRE)/prestin and human SLC26A7 functioning as SCN− channels. At present, accurate determination of unitary current amplitudes are available neither for SLC26A5 nor for SLC26A7, precluding discrimination between distinct unitary transport rates or transport probabilities for these SLC26 proteins. The notion that only a certain percentage of SLC26 proteins function as SCN− channels/uniporters is supported by experiments on Slc26a5 (DANRE)/prestin in mixtures of SCN− and SO42− (Fig. 6). SO42− shifted reversal potentials to more negative potentials, indicating that zebrafish prestin can still function as an anion antiporter in the presence of SCN−. Our results together with earlier findings (Shcheynikov et al. 2006; Ohana et al. 2011) suggest that all SLC26 proteins might be able to conduct polyatomic anions as a distinctive common feature of this family of transport proteins.
Since prestin is closely related to SLC26 anion exchangers (Zheng et al. 2000; Mount & Romero, 2004), various complete and incomplete transport processes have been implicated to be the basis of its voltage-dependent conformational changes. Oliver and colleagues (2001) studied the effects of internal anions on the non-linear capacitance of prestin, and proposed that monovalent anions like Cl− or HCO3− bind to an intracellular binding site as an initial step in voltage-dependent conformational changes by prestin. An electric gradient translocates the anions across a fraction of the membrane and thereby triggers a conformational modification of the protein, resembling an incomplete transport cycle. Charge movement through the membrane dielectric manifests itself in non-linear capacitance, the distinct electrophysiological signature of prestin. Although the voltage-dependent capacitance predicted by this model is in excellent agreement with many experimental data, the model fails to correctly explain the observed effects of internal anion concentration and anion valence on non-linear charge movement. Subsequently, theoretical work by Muallem & Ashmore (2006) demonstrated that a transport model with an anion antiporter associated with intrinsic charge movement correctly predicts the anion and voltage dependence of the linear charge movement of prestin. The authors (Muallem & Ashmore, 2006) suggested that prestin functions as a Cl−/SO4− antiporter. This prediction was experimentally supported for non-mammalian prestin homologues from chicken and zebrafish (Schaechinger & Oliver, 2007). However, since zebrafish prestin differs from mammalian prestin in a charge movement with shifted voltage dependence and lower speed (Schaechinger & Oliver, 2007), these experiments were not sufficient to unequivocally support the Muallem–Ashmore model. Our results support many of the predictions of this model. There is one transport mode that is shared by SLC26A5 (RAT)/prestin, Slc26a5 (DANRE)/prestin and human SLC26A7. SCN− transport by SLC26A5 (RAT)/prestin is blocked by external SO42−, indicating that there is an external SO42− binding site. Our findings, together with recent results that internal SO42− supports the non-linear capacitance of prestin (Rybalchenko & Santos-Sacchi, 2003; Rybalchenko & Santos-Sacchi, 2008), indicate that mammalian prestin exhibits monovalent and also divalent anion binding sites on both membrane sides, as expected for a Cl−/SO42− antiporter.
In summary, we demonstrated a transport function of the motor protein prestin that is conserved in other members of the SLC26 family operating as channels or transporters. The observed transport process is unlikely to have any direct physiological function, but could be useful to further characterize mechanisms underlying the function of the motor protein prestin or related SLC26 proteins.
Acknowledgments
We would like to thank Dr B. Fakler for providing expression constructs for rat and zebrafish prestin and to the members of the Institut für Neurophysiologie, MHH, for helpful discussions. These studies were supported by the Deutsche Forschungsgemeinschaft (FA301/10 to Ch.F.).
Glossary
Abbreviations
- HEK293T
transformed human kidney cell line stably expressing an SV40 temperature-sensitive T antigen
- SLC26
solute carrier family 26
- SLC26A7 (HUMAN)
solute carrier family 26 member 7 from Homo sapiens
- Slc26a5 (DANRE)/prestin
solute carrier family 26 member 5 (prestin) from Danio rerio
- SLC26A5 (RAT)/prestin
solute carrier family 26 member 5 (prestin) from Rattus norvegicus
Author contributions
M.S. and Ch.F. designed research, M.S. performed the experiments and analysed the data, M.S. and Ch.F. wrote the manuscript.
Supplementary material
Supplementary Fig. 1
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors
References
- Albert JT, Winter H, Schaechinger TJ, Weber T, Wang X, He DZ, Hendrich O, Geisler HS, Zimmermann U, Oelmann K, Knipper M, Gopfert MC, Oliver D. Voltage-sensitive prestin orthologue expressed in zebrafish hair cells. J Physiol. 2007;580:451–461. doi: 10.1113/jphysiol.2007.127993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alekov A, Fahlke Ch. Channel-like slippage modes in the human anion/proton exchanger ClC-4. J Gen Physiol. 2009;133:485–496. doi: 10.1085/jgp.200810155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez O, Gonzalez C, Latorre R. Counting channels: a tutorial guide on ion channel fluctuation analysis. Adv Physiol Educ. 2002;26:327–341. doi: 10.1152/advan.00006.2002. [DOI] [PubMed] [Google Scholar]
- Anderson CR, Stevens CF. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973;235:655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai JP, Surguchev A, Montoya S, Aronson PS, Santos-Sacchi J, Navaratnam D. Prestin's anion transport and voltage-sensing capabilities are independent. Biophys J. 2009;96:3179–3186. doi: 10.1016/j.bpj.2008.12.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissig M, Hagenbuch B, Stieger B, Koller T, Meier PJ. Functional expression cloning of the canalicular sulfate transport system of rat hepatocytes. J Biol Chem. 1994;269:3017–3021. [PubMed] [Google Scholar]
- Chang MH, Plata C, Zandi-Nejad K, Sindic A, Sussman CR, Mercado A, Broumand V, Raghuram V, Mount DB, Romero MF. Slc26a9-anion exchanger, channel and Na+ transporter. J Membr Biol. 2009;228:125–140. doi: 10.1007/s00232-009-9165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P, Fakler B. Prestin, a new type of motor protein. Nat Rev Mol Cell Biol. 2002;3:104–111. doi: 10.1038/nrm730. [DOI] [PubMed] [Google Scholar]
- DeFelice LJ. Introduction to Membrane Noise. New York: Plenum Press; 1981. [Google Scholar]
- Detro-Dassen S, Schanzler M, Lauks H, Martin I, zu Berstenhorst SM, Nothmann D, Torres-Salazar D, Hidalgo P, Schmalzing G, Fahlke Ch. Conserved dimeric subunit stoichiometry of SLC26 multifunctional anion exchangers. J Biol Chem. 2008;283:4177–4188. doi: 10.1074/jbc.M704924200. [DOI] [PubMed] [Google Scholar]
- Dorwart MR, Shcheynikov N, Wang Y, Stippec S, Muallem S. SLC26A9 is a Cl− channel regulated by the WNK kinases. J Physiol. 2007;584:333–345. doi: 10.1113/jphysiol.2007.135855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett LA, Green ED. A family of mammalian anion transporters and their involvement in human genetic diseases. Hum Mol Genet. 1999;8:1883–1891. doi: 10.1093/hmg/8.10.1883. [DOI] [PubMed] [Google Scholar]
- Farrell B, Do Scope C, Brownell WE. Voltage-dependent capacitance of human embryonic kidney cells. Phys Rev E. 2006;73:041930. doi: 10.1103/PhysRevE.73.041930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Grichtchenko II, Boron WF, Aronson PS. Specificity of anion exchange mediated by mouse Slc26a6. J Biol Chem. 2002;277:33963–33967. doi: 10.1074/jbc.M202660200. [DOI] [PubMed] [Google Scholar]
- Kim KH, Shcheynikov N, Wang Y, Muallem S. SLC26A7 is a Cl− channel regulated by intracellular pH. J Biol Chem. 2005;280:6463–6470. doi: 10.1074/jbc.M409162200. [DOI] [PubMed] [Google Scholar]
- Kovermann P, Machtens JP, Ewers D, Fahlke Ch. A conserved aspartate determines pore properties of anion channels associated with excitatory amino acid transporter 4 (EAAT4) J Biol Chem. 2010;285:23676–23686. doi: 10.1074/jbc.M110.126557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XZ, Ouyang XM, Xia XJ, Zheng J, Pandya A, Li F, Du LL, Welch KO, Petit C, Smith RJ, Webb BT, Yan D, Arnos KS, Corey D, Dallos P, Nance WE, Chen ZY. Prestin, a cochlear motor protein, is defective in non-syndromic hearing loss. Hum Mol Genet. 2003;12:1155–1162. doi: 10.1093/hmg/ddg127. [DOI] [PubMed] [Google Scholar]
- Loo DD, Hazama A, Supplisson S, Turk E, Wright EM. Relaxation kinetics of the Na+/glucose cotransporter. Proc Natl Acad Sci U S A. 1993;90:5767–5771. doi: 10.1073/pnas.90.12.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager S, Naeve J, Quick M, Labarca C, Davidson N, Lester HA. Steady states, charge movements, and rates for a cloned GABA transporter expressed in Xenopus oocytes. Neuron. 1993;10:177–188. doi: 10.1016/0896-6273(93)90309-f. [DOI] [PubMed] [Google Scholar]
- Melzer N, Torres-Salazar D, Fahlke Ch. A dynamic switch between inhibitory and excitatory currents in a neuronal glutamate transporter. Proc Natl Acad Sci U S A. 2005;102:19214–19218. doi: 10.1073/pnas.0508837103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley RH, Hoglund P, Wu GD, Silberg DG, Haila S, de la CA, Holmberg C, Kere J. Downregulated in adenoma gene encodes a chloride transporter defective in congenital chloride diarrhea. Am J Physiol Gastrointest Liver Physiol. 1999;276:G185–G192. doi: 10.1152/ajpgi.1999.276.1.G185. [DOI] [PubMed] [Google Scholar]
- Mount DB, Romero MF. The SLC26 gene family of multifunctional anion exchangers. Pflugers Arch. 2004;447:710–721. doi: 10.1007/s00424-003-1090-3. [DOI] [PubMed] [Google Scholar]
- Muallem D, Ashmore J. An anion antiporter model of prestin, the outer hair cell motor protein. Biophys J. 2006;90:4035–4045. doi: 10.1529/biophysj.105.073254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohana E, Shcheynikov N, Yang D, So I, Muallem S. Determinants of coupled transport and uncoupled current by the electrogenic SLC26 transporters. J Gen Physiol. 2011;137:239–251. doi: 10.1085/jgp.201010531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohana E, Yang D, Shcheynikov N, Muallem S. Diverse transport modes by the solute carrier 26 family of anion transporters. J Physiol. 2009;587:2179–2185. doi: 10.1113/jphysiol.2008.164863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D, He DZ, Klocker N, Ludwig J, Schulte U, Waldegger S, Ruppersberg JP, Dallos P, Fakler B. Intracellular anions as the voltage sensor of prestin, the outer hair cell motor protein. Science. 2001;292:2348–2343. doi: 10.1126/science.1060939. [DOI] [PubMed] [Google Scholar]
- Otis TS, Jahr CE. Anion currents and predicted glutamate flux through a neuronal glutamate transporter. J Neurosci. 1998;18:7099–7110. doi: 10.1523/JNEUROSCI.18-18-07099.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic S, Barone S, Xu J, Conforti L, Ma L, Kujala M, Kere J, Soleimani M. SLC26A7: a basolateral Cl−/HCO3− exchanger specific to intercalated cells of the outer medullary collecting duct. Am J Physiol Renal Physiol. 2004;286:F161–F169. doi: 10.1152/ajprenal.00219.2003. [DOI] [PubMed] [Google Scholar]
- Riazuddin S, Anwar S, Fischer M, Ahmed ZM, Khan SY, Janssen AG, Zafar AU, Scholl U, Husnain T, Belyantseva IA, Friedman PL, Riazuddin S, Friedman TB, Fahlke Ch. Molecular basis of DFNB73: mutations of BSND can cause nonsyndromic deafness or Bartter syndrome. Am J Hum Genet. 2009;85:273–280. doi: 10.1016/j.ajhg.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybalchenko V, Santos-Sacchi J. Anion control of voltage sensing by the motor protein prestin in outer hair cells. Biophys J. 2008;95:4439–4447. doi: 10.1529/biophysj.108.134197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybalchenko V, Santos-Sacchi J. Cl− flux through a non-selective, stretch-sensitive conductance influences the outer hair cell motor of the guinea-pig. J Physiol. 2003;547:873–891. doi: 10.1113/jphysiol.2002.036434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J. Reversible inhibition of voltage-dependent outer hair cell motility and capacitance. J Neurosci. 1991;11:3096–3110. doi: 10.1523/JNEUROSCI.11-10-03096.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J. Functional motor microdomains of the outer hair cell lateral membrane. Pflugers Arch. 2002;445:331–336. doi: 10.1007/s00424-002-0928-4. [DOI] [PubMed] [Google Scholar]
- Santos-Sacchi J, Kakehata S, Takahashi S. Effects of membrane potential on the voltage dependence of motility-related charge in outer hair cells of the guinea-pig. J Physiol. 1998;510:225–235. doi: 10.1111/j.1469-7793.1998.225bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaechinger TJ, Oliver D. Nonmammalian orthologs of prestin (SLC26A5) are electrogenic divalent/chloride anion exchangers. Proc Natl Acad Sci U S A. 2007;104:7693–7698. doi: 10.1073/pnas.0608583104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DA, Karniski LP. Human pendrin expressed in Xenopus laevis oocytes mediates chloride/formate exchange. Am J Physiol Cell Physiol. 2000;278:C207–C211. doi: 10.1152/ajpcell.2000.278.1.C207. [DOI] [PubMed] [Google Scholar]
- Shcheynikov N, Wang Y, Park M, Ko SB, Dorwart M, Naruse S, Thomas PJ, Muallem S. Coupling modes and stoichiometry of Cl−/HCO3− exchange by slc26a3 and slc26a6. J Gen Physiol. 2006;127:511–524. doi: 10.1085/jgp.200509392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleimani M, Greeley T, Petrovic S, Wang Z, Amlal H, Kopp P, Burnham CE. Pendrin: an apical Cl−/OH− exchanger. Am J Physiol Renal Physiol. 2001;280:F356–F364. doi: 10.1152/ajprenal.2001.280.2.F356. [DOI] [PubMed] [Google Scholar]
- Tan X, Pecka JL, Tang J, Okoruwa OE, Zhang Q, Beisel KW, He DZ. From zebrafish to mammal: functional evolution of prestin, the motor protein of cochlear outer hair cells. J Neurophysiol. 2011;105:36–44. doi: 10.1152/jn.00234.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Salazar D, Fahlke Ch. Neuronal glutamate transporters vary in substrate transport rate but not in unitary anion channel conductance. J Biol Chem. 2007;282:34719–34726. doi: 10.1074/jbc.M704118200. [DOI] [PubMed] [Google Scholar]
- Trapani JG, Korn SJ. Control of ion channel expression for patch clamp recordings using an inducible expression system in mammalian cell lines. BMC Neurosci. 2003;4:15. doi: 10.1186/1471-2202-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watzke N, Bamberg E, Grewer C. Early intermediates in the transport cycle of the neuronal excitatory amino acid carrier EAAC1. J Gen Physiol. 2001;117:547–562. doi: 10.1085/jgp.117.6.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T, Gopfert MC, Winter H, Zimmermann U, Kohler H, Meier A, Hendrich O, Rohbock K, Robert D, Knipper M. Expression of prestin-homologous solute carrier (SLC26) in auditory organs of nonmammalian vertebrates and insects. Proc Natl Acad Sci U S A. 2003;100:7690–7695. doi: 10.1073/pnas.1330557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q, Welch R, Mercado A, Romero MF, Mount DB. Molecular characterization of the murine Slc26a6 anion exchanger: functional comparison with Slc26a1. Am J Physiol Renal Physiol. 2002;283:F826–F838. doi: 10.1152/ajprenal.00079.2002. [DOI] [PubMed] [Google Scholar]
- Xu J, Henriksnas J, Barone S, Witte D, Shull GE, Forte JG, Holm L, Soleimani M. SLC26A9 is expressed in gastric surface epithelial cells, mediates Cl−/HCO3− exchange, and is inhibited by NH4+ Am J Physiol Cell Physiol. 2005;289:C493–C505. doi: 10.1152/ajpcell.00030.2005. [DOI] [PubMed] [Google Scholar]
- Zheng J, Shen W, He DZ, Long KB, Madison LD, Dallos P. Prestin is the motor protein of cochlear outer hair cells. Nature. 2000;405:149–155. doi: 10.1038/35012009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


