Abstract
Non-technical summary
The isolated spinal cord contains networks capable of producing a locomotor rhythm. This can be evoked in vivo in decerebrate (unanaesthetised) rats, rabbits, cats and monkeys. The development of transgenic mice with mutations affecting these circuits have made it desirable to be able to now study this in the adult mouse in vivo. We demonstrate that a spinal network producing a rhythmic locomotor-like pattern can be pharmacologically activated in adult decerebrate and spinalised mice and that the output of this is roughly similar to that observed in other species.
Abstract
Recently, transgenic mice have been created with mutations affecting the components of the mammalian spinal central pattern generator (CPG) for locomotion; however, it has currently only been possible to evoke fictive locomotion in mice, using neonatal in vitro preparations. Here, we demonstrate that it is possible to evoke fictive locomotion in the adult decerebrate mouse in vivo using l-3,4-dihydroxyphenylalanine methyl ester hydrochloride (l-DOPA) and 5-hydroxytryptophan (5HTP) following injection of the monoaminoxiadase inhibitor Nialamide. We investigate the effects of afferent stimulation and spinalization as well as demonstrate the possibility of simultaneous intracellular recording of rhythmically active motoneurones. Our results demonstrate that several features of the mouse locomotor CPG are similar to those that have been observed in rat, cat, rabbit and monkey suggesting a fairly conserved organisation and allowing for future results in transgenic mice to be extrapolated to existing knowledge of CPG components and circuitry obtained in larger species.
Introduction
The adult mammalian spinal cord contains central pattern generators (CPGs) which can produce a rhythmic output resembling the patterns of muscle activation during locomotion (see Rossignol et al. 2006; Hultborn & Nielsen, 2007). The sequencing of the mouse genome and subsequent developments in the creation of various transgenic mice has recently revolutionised the field of locomotion (see Kiehn, 2011). The activity of genetically identified classes of neurones can be observed (electrophysiologically or using calcium imaging) in the mouse lumbar spinal cord during locomotion (Brownstone & Wilson, 2008; Dai et al. 2009; Zagoraiou et al. 2009; Dougherty & Kiehn, 2010; Hagglund et al. 2010; Wilson et al. 2010; Zhong et al. 2010) and their specific roles established by knocking-out the individual cell classes and observing the effects on the output of the CPG (Gosgnach et al. 2006; Crone et al. 2008).
One limitation to such investigations, however, is that the recording of the output of the CPG in mice has thus far been restricted to neonatal in vitro preparations. In such preparations recordings are obtained from ventral roots and the phase relationship between segments is taken to represent the activity of flexors and extensors during locomotion. These in vitro preparations, however, are limited by the age at which recording can be performed. In spinal cord preparations from mice older than postnatal day 2–3 (P2–3) adequate oxygenation is a problem and a hypoxic core develops which is exacerbated during metabolically demanding tasks such as activation of the locomotor CPG (Wilson et al. 2003). Although one group has had success with preparations up to P22 (Jiang et al. 1999), a time point at which they describe the spinal cord as ‘functionally mature’ (in that the animal can bear weight and walk freely), motor activity is more difficult to produce than in the younger neonatal mice preparations.
In other species the CPGs underlying locomotion can be activated in the adult, in vivo, using electrical stimulation of locomotor regions in the brainstem or pharmacologically with agents such as l-DOPA and recorded as fictive locomotion from electroneurograms in neuromuscularly blocked animals. This has been demonstrated in adult decerebrate (unanaesthetised) rats (Kinjo et al. 1990; Bem et al. 1993; Iles & Nicolopoulos-Stournaras, 1996), rabbits (Viala & Buser, 1969, 1971), cats (Orlovskii et al. 1966; Shik et al. 1966; Jankowska et al. 1967a,b; Grillner, 1975, 1981; Grillner & Zangger, 1975, 1979) and even monkeys (Fedirchuk et al. 1998). Fictive locomotion, however, has not yet been demonstrated in the adult mouse in vivo. The ability to do this is crucial not only to understand the effects of mutations in the adult spinal cord but also if one is to translate observations from the mutant mice to place them into the context of the extensive knowledge of the components and connections of CPG that have already been obtained in larger adult mammals in vivo.
Here we demonstrate that fictive locomotion can be evoked pharmacologically using l-DOPA and 5-hydroxytryptophan (5HTP) following injection of the monoaminoxiadase inhibitor Nialamide in the adult decerebrate mouse in vivo, recorded by electroneurograms from hind limb peripheral nerves and also, simultaneously, intracellularly in spinal motoneurones as locomotor drive potentials or rhythmic firing. We demonstrate that many of the features of the adult mouse locomotor CPG are similar to that which have been observed in rat, cat, rabbit and monkey, suggesting a fairly conserved organisation.
Methods
Ethical approval
The experimental procedure was approved by the Danish Animal Experiments Inspectorate and was conducted in accordance with EU regulations (Council Directive 86/609/EEC) and with National Institutes of Health guidelines for the care and use of laboratory animals (National Institutes of Health publication no. 86-23 revised 1985). Our experimental procedures also comply with polices set out by The Journal of Physiology (Drummond, 2009).
Experiments
Experiments were performed on 13 adult female C57BL/6J mice (12–20 weeks old). The monoamine oxidase inhibitor Nialamide (100 mg kg−1) was administered intraperitonally (i.p.) 2 h before the preparatory surgery commenced. In 10 mice anaesthesia was induced using isofluorane (Baxter A/S, Denmark) and maintained with 2% (in a 4:1 mix of air and oxygen). In three mice anaesthesia was induced and maintained with i.p. injections of Hypnorm (10 mg per 1 ml) and midazolam (1 mg per 1 ml) diluted in dH2O (1:1:2 parts, 0.15 ml of mixture for induction, 0.04 ml for maintenance). All mice received atropine (0.02 mg i.p.) to decrease tracheo-bronchial secretions. The following peripheral nerves were dissected: the main tibial branch (Tib) and the common peroneal (CP) bilaterally. In two mice the nerves to the gastrocnemii (including the branch to soleus) were separated from the main tibial branch leaving the rest of the main tibial nerve (taken as one bundle referred to as Tib in these experiments).
Mice were then placed in a Narashige stereotactic frame with the head secured in a head holder. Two vertebral clamps (vertebral levels T11 and L1) secured the stability of the spine and spinal cord. The skin flaps around the exposed areas of the spinal cord and the hindlimb were sewn and retracted to form pools that were filled with warm paraffin oil. The nerves of the hindlimbs were mounted on bipolar silver chloride hook electrodes. It shall be noted that the hindlimbs were left in a rather extended position with this procedure (see below).
The temperature was monitored using a rectal probe and maintained at 37°C using a heat pad underneath and a heat lamp above the mouse controlled by the output from the temperature probe. The electrocardiogram was monitored using clips placed on the ear and rear foot. A tracheal cannula was inserted to allow for artificial ventilation during the initial surgery. Once moved to the stereotactic frame the mice were connected to a ventilator (SAR-83 CWE) and artificially ventilated at 70 breaths min-1 (and a tidal volume of approximately 0.2 ml). Expired carbon dioxide levels were measured using a Capstar CO2 analyser (IITC Life Science).
The decerebration
The surface of the skull was cleared and blood vessels cauterised. A craniotomy was then performed and the brain separated from the brainstem (just rostral to the superior colliculus) and removed using a small curved spatula. The void was then filled with an absorbable haemostat (Surgicel, Ethicon Inc.) with a small piece of dry ice in the centre. Once bleeding had subsided the Surgicel was removed to confirm the completeness of the decerebration. The isofluorane was then removed from the ventilation flow (or top-up injections of drugs ceased for the three mice anaesthetised with Hypnorm and midazolam). Following decerebration the expired pCO2 often dropped considerably, but returned to normal values after around 30 min, remaining remarkably stable for a considerable time after this.
Evoking fictive locomotion
Once decerebration was confirmed to be complete and isofluorane removed from the ventilation flow mice were neuromuscularly blocked with Pavulon (diluted 1:10 with saline then 0.1 ml dose i.p. initially followed by 0.05 ml doses every hour). In those mice anaesthetised with Hypnorm and midazolam, subsequent top-up doses of anaesthesia now ceased. The electrodes, onto which the nerves were mounted to record the electroneurogram (ENG) activity, were connected to a preamplifier and then further amplified and filtered using custom-made amplifiers. Finally the signals were digitised using the 1401 analog-to-digital converter (Cambridge Electronic Design, UK) and recorded using the Spike 2 software (Cambridge Electronic Design, UK). l-DOPA (80 mg kg−1) was then administered subcutaneously followed by 5HTP (100 mg kg−1) and later, once locomotion had commenced, Naloxone (0.025 mg) was also given in some mice to improve the locomotor rhythm (Schomburg & Steffens, 1995).
Intracellular recording
In mice in which intracellular recording was performed, a hemi-laminectomy was performed at vertebral level T12–L1 after the tracheal cannula was inserted and prior to the mice being put in the frame and ventilated. In the recording frame vertebral clamps were attached on the vertebrae above and below the laminectomy to secure the spinal column in this region. Intracellular recording was performed as previously described (Meehan et al. 2010). Briefly, using an electronic micro-drive, a glass microelectrode was inserted into the spinal cord and antidromic field potentials from stimulation of the peripheral nerves were used as guides to locate the motoneurones. Following successful penetrations of motoneurones, the peripheral nerve electrodes were switched back to recording mode to correlate the activity of the neurones with the ENG activity of the peripheral nerves during fictive locomotion. Some intracellular recordings were made after administration of l-DOPA but prior to the onset of spontaneous rhythmic activity (or after it had ceased). In these cases rhythmic activity was induced by stimulation of the peripheral nerves.
Results
The mice survived the decerebration remarkably well with minimal blood loss (with an almost 100% survival rate once the technique had been perfected). The pCO 2 level dropped drastically immediately following decerebration but returned to normal levels (and usually improved) within 1/2 h, then remaining stable (along with heart rate) often for many hours (up to 5 h) post-decerebration, although experiments were usually terminated when locomotor activity ceased.
Effects of afferent stimulation prior to locomotion: rhythmic activity and latency reflexes
In seven of the mice after administration of l-DOPA, prior to the commencement of fictive locomotion, the peripheral nerves were stimulated. In four mice short high-frequency (80–115 Hz) trains of stimulation of the Tib nerve resulted in a long-latency (100–200 ms) long-lasting flexor reflex response in the ipsilateral CP nerve (Fig. 1A) as has been observed following l-DOPA administration in spinalised cats (Andén et al. 1966). There was no discharge during the train, suggesting that there was an inhibition of the reflex transmission. The long-latency flexor reflexes had a fixed latency from the end of the train (regardless of the duration of the preceding train of stimuli; Fig. 1B), thus suggesting that the discharge was triggered by a disinhibitory rebound following the end of the stimulus train. Long-latency contralateral flexor reflexes were also observed (not shown). In the seven mice tested, longer lasting stimulus trains at lower frequencies (5–16 Hz) evoked rhythmic activity prior to the development of spontaneous (l-DOPA-evoked) locomotion (Fig. 1C). This was most effective when stimulating the Tib nerves. Stimulation of a single nerve resulted in rhythmic alternating activity between flexor and extensor nerves on the same side (Fig. 1D). Simultaneous stimulation of both the ipsilateral and contralateral Tib nerves resulted in rhythmic activity alternating between the ipsilateral and contralateral CP nerves (Fig. 1E). The ability to evoke this rhythmic activity depended upon the frequency of stimulation (being most effective between 5–16 Hz, mean 7.8 Hz) and the cycle duration of the rhythmic activity was dependent upon the frequency of stimulation (although it was never time-locked to the stimuli; Fig. 1F).
Figure 1. Effects of afferent stimulation following l-DOPA administration.
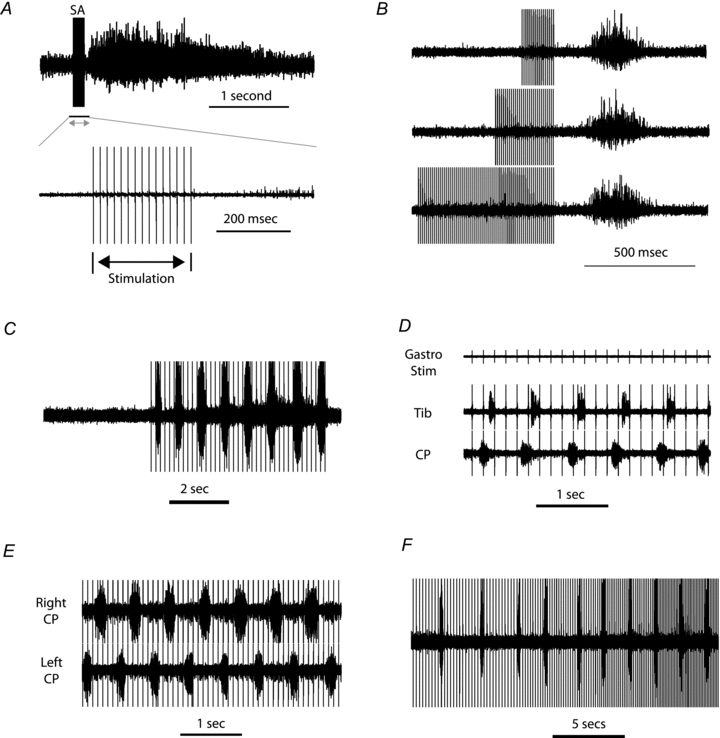
A, long-latency flexor reflex after administration of l-DOPA. Stimulation of the Tib nerve (SA indicates the stimulus artefacts) evokes a long-latency reflex in the CP nerve. No short-latency responses are evoked (more obvious in the expanded lower trace) as the short-latency flexor reflexes are suppressed by l-DOPA (Anden et al. 1966). B, the latency of the long-latency reflex from the end of stimulation is consistent regardless of length of stimulus train. C, prior to the commencement of spontaneous fictive locomotion longer trains of stimulation of the Tib nerve (at lower frequencies than in A and B) evoked rhythmic activity on the CP nerve. D, stimulation of the Gast nerve resulted in rhythmic alternating activity between flexor (CP) and extensor (Tib) nerves on the same side. E, simultaneous stimulation of both the ipsilateral and contralateral Tib nerves resulted in rhythmic activity alternating between the ipsilateral and contralateral CP nerves. F, effects of the frequency of stimulation on the cycle duration of the rhythmic activity.
l-DOPA evoked fictive locomotion
Rhythmic activity after subcutaneous administration of l-DOPA followed by 5HTP (with or without afferent stimulation) was not observed in any of the three mice that had previously been anaesthetised with Hypnorm and midazolam. In contrast rhythmic activity did develop in 9/10 preparations that had been anaesthetised with isofluorane. This activity first appeared between 17 and 112 min (mean, 48 min) after administration of l-DOPA (or between 43 and 163 min; mean, 90 min) after decerebration and removal of anaesthetic.
Activity usually, but not always, started in flexor nerves (6/9 CP first, 1/9 Tib first, 2/9 both started together). When activity appeared in the antagonist nerves this often started as a co-activation of flexors and extensors (Fig. 2A), but as the pattern developed and established itself, an alternation developed between the two anatagonists for each limb, and the two sides (Fig. 2B). Two things are important to note from Fig. 2B. The first is that the extensor phase appears rather short and did not continue to the point at which the next flexor phase starts. This was at least partially due to the hyper-extended leg position (see Grillner & Rossignnol 1978 and Discussion) as by lowering the leg slightly to a more natural position resulted in a prolongation of the extensor phase (although this made the construction of the leg pool more difficult). Also, occasionally there was a slight overlap of the activity of the Tib and CP nerve at the transition between the two phases. This may be due to the Tib nerve including some flexor-related muscle branches. When the gastronemii branches were isolated from the main Tib bundle this overlap was greatly reduced (Fig. 2C), the remaining minimal overlap we assume to be due to the fact that CP itself is not a totally pure collection of flexor nerves in rodents during fictive locomotion (Iles & Nicolopoulos-Stournaras, 1996). The activity of flexors and extensors on one side also alternated with the activity of flexors and extensors on the contralateral sides. (Fig. 2B). Once spontaneous (l-DOPA-evoked) locomotion commenced, long-lasting trains of stimulation could enhance activity and change the cycle duration.
Figure 2. l-DOPA and 5HTP evoked fictive locomotion.
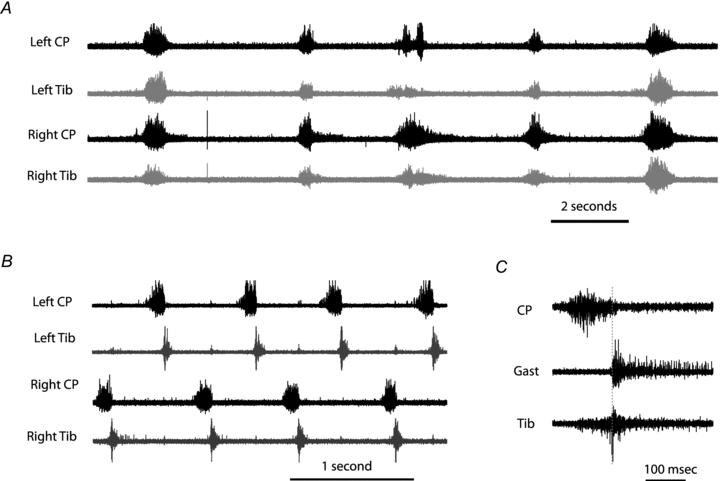
A, synchronous activity (co-activation) on both antagonist and bilateral nerves as rhythmic activity first begins to appear. B, the activity after the pattern establishes itself with alternation between ipsilateral antagonists and their contralateral counterparts. C, an example of slightly overlapping activity of the Tib and CP nerve at the transition between the 2 phases but a clear transition from CP activity to Gast activity.
Effects of spinalization
In five mice, once the pattern of locomotion had established itself, a spinalization was performed at the first cervical level. In all cases this resulted in an enhancement of activity in that the cycle period was shortened and had a similar effect on both extensor and flexors on both sides (Fig. 3A and B). Spinalisation did not appear to change the overall pattern, i.e. the phase relationship between ipsilateral antagonists and between ipsilateral nerves and their contralateral counterparts. The enhancement of activity by spinalisation was also effective when periods of locomotion were declining. In these cases, not only was the cycle period shortened but spinalisation also restored activity in nerves that had ceased firing (Fig. 3B).
Figure 3. Effects of spinalization.
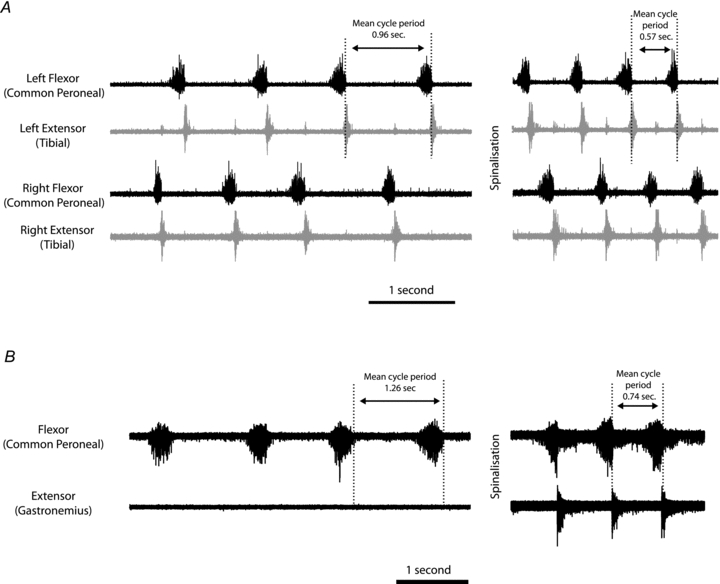
A, spinalization enhances the frequency, but does not change the overall pattern of rhythmic activity recorded on ipsilateral and contralateral ENGs. B, enhancement of activity by spinalization (shortening of cycle period) when periods of locomotion were declining. In this case not only was the cycle period shortened but spinalization also restored activity in a nerve (Gast) that had ceased firing.
Simultaneous intracellular recording
Intracellular recording was achieved in 4 motoneurones during periods of spontaneous fictive locomotion, recorded from ENGs and in 14 motoneurones after administration of l-DOPA but prior to the onset of spontaneous rhythmic activity or after it had ceased. All cells recorded during fictive locomotion showed firing patterns clearly related to the locomotor pattern (Fig. 4A and B), being either flexor or extensor related and displayed clear locomotor drive potentials ranging in magnitude from 7.5 to 13.2 mV (mean 10.57 mV). Figure 4A shows an intracellular recording of a CP motoneurone active during the flexor phase of the cycle. It is interesting to note that while the onset of activity in the neuron roughly mirrors the activity seen on the CP nerve, the intracellular activity ceases sharply, simultaneous with the onset of activity of the antagonist extensor nerve, in this example ipsilateral Tib. Furthermore, a hyperpolarization can be seen with a duration consistent with that of the activity on the Tib nerve. Stimulation of the Tib nerve produced an inhibitory postsynaptic potential in this motoneurone at a disynaptic latency, confirming that this neurone received disynaptic reciprocal inhibition from the tibial nerve (Fig. 4B). In this same motoneurone there were occasionally cycles in which the extensor phase failed (i.e. no activity was recorded in the tibial nerve, Fig. 4C). This was due to locomotion having just started when some nerves take longer to reach maximum consistent activity than others. The subsequent pattern upon full activity matched the patterns illustrated in the other figures with the exception of a longer extensor phase which we presume to be due to a deliberately less extended limb position hip leg angle for the oil pool and attempts to cut the hip tendons in this mouse to reduce the afferent signalling of hip extension (see Grillner and Rossignol, 1978). In cycles where the extensor phase failed, the activity on the flexor nerve (CP) was prolonged, although the full cycle length was shortened. The sharp hyperpolarization at the start of the inactive phase was then absent. We selected this particular cell to illustrate the consequences of the lack of antagonist activity on some cycles (even though it may be argued that if not all nerves are consistently active during every cycle then it may not be considered to be stable locomotion). Actually these observations are consistent with the concept of reciprocal inhibition (originating from the CPG) contributing to the hyperpolarisation of motoneurones during their inactive phases of locomotion cycles as we have previously demonstrated in the cat (Geertsen et al. 2011).
Figure 4. Intracellular recording during fictive locomotion and scratching.
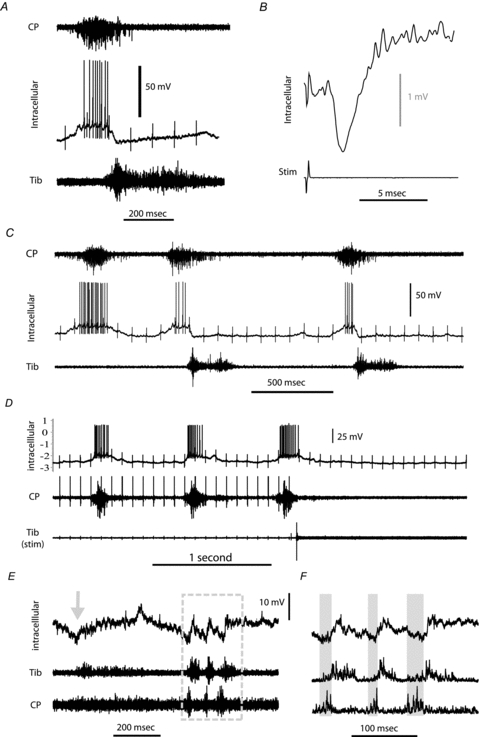
A, intracellular recording in a motoneurone (middle trace) active during the flexor phase of the locomotor cycle (indicated by activity on the CP nerve ENG, upper trace). The intracellular depolarisation ceases upon the onset of activity on the antagonist extensor nerve (Tib) ENG (lower trace). A hyperpolarisation can be observed on the intracellular recording with a duration consistent with that of the activity on the Tib nerve ENG. B, averaged intracellular recording from the same motoneurone as A after stimulating the Tib nerve, revealing a inhibitory post-synaptic potential with a latency consistent with being disynaptic, confirming that the motoneurone received disynaptic Ia reciprocal inhibition from the Tib nerve. C, an example with a few locomotor cycles from the same cell as A and B in which the extensor phase failed during one cycle (i.e. no activity was recorded in the Tib nerve). In this case the intracellular depolarisation is prolonged but the overall cycle length was shortened and the sharp hyperpolarization at the start of the inactive phase is absent. D, prior to the onset of spontaneous locomotion, stimulation of the Tib peripheral nerve (lower trace) induces intracellularly recorded rhythmic activity in a CP motoneurone (upper trace) matching the rhythmic activity seen on the CP ENG (middle trace). E, intracellular recording in a tibial motoneurone (upper trace) following mechanical stimulation of the ear. Resulting ENG resembling a scratch reflex and consists of an initial ‘aiming phase’ followed by rhythmic activity (boxed area) on the Tib and CP ENGs (middle and lower traces). F, expansion of the boxed area from E, with the ENGs rectified and smoothed to illustrate how the membrane potential of the Tib motoneurone is hyperpolarized during the active phase of the antagonist CP nerve (grey regions).
In the 14 cells recorded prior to the onset of spontaneous rhythmic activity or after it had ceased, stimulation of a peripheral nerve induced some form of rhythmic activity in the motoneurone matching the rhythmic activity seen in ENGs as described earlier (Fig. 4D). This was either rhythmic firing (in 9 of the cells) corresponding to the ENG activity of the nerve from which it was antidromically activated or in the remaining cells not firing action potentials it was seen as a depolarization in membrane potential during the active phase of the antidromic nerve and/or a hyperpolarization during ENG activity on the antagonist nerve. Thus, the intracellular activity was always as would be expected. In one case (during recording from a tibial motoneurone) mechanical stimulation of the ear resulted in a short burst of alternating rhythmic activity in the ENGs (Fig. 4E). We presume this activity represents a form of fictive scratch as has been seen in other animals, comprising of an initial ‘aiming’ phase followed by rapid short bursts. Scratch drive potentials could be seen intracellularly in the recorded motoneurone during the ENG activity, comprising a clear hyperpolarization during the active phase of the antagonist CP nerve (Fig. 4E and F).
Discussion
We have demonstrated the feasibility of an adult in vivo decerebrate mouse preparation in which to study pharmacologically evoked fictive locomotion, monitored by recording the activity of peripheral nerves and by intracellular recording from motoneurones. We observed rhythmic activity alternating between the nerves innervating flexors and extensors as well as alternation between the left and right sides. This makes this model useful for studying aspects of the CPG both controlling antagonistic muscles on one side of the cord and those organising left–right coordination in transgenic mice with various mutations affecting components of the CPG, in vivo. It also allows a comparison with the organisation of the locomotor CPG across different species. By spinalizing the mice we demonstrated that this output is indeed spinally generated, presumably by the same CPG that generates the locomotor activity seen in spinalized adult mice placed on treadmills (Leblond et al. 2003).
Comparisons with fictive locomotion in other species
Fictive locomotion has been evoked pharmacologically in decerebrate rats, rabbits, cats and monkeys and our present observations in the mouse are largely consistent with that which has been observed in these other species. The long-latency, long-duration reflexes after l-DOPA seem to reflect the facilitation of the spinal locomotor network as described in the cat (Anden et al. 1966), the rabbit (Viala et al. 1974) and the monkey (Fedirchuk et al. 1998). There was no discharge during the train suggesting that there was an inhibition of the reflex transmission. The long-latency flexor reflexes had a fixed latency from the end of the stimulus train regardless of the duration of the preceding train (Fig. 1B), thus suggesting that the discharge was triggered by a rebound excitation following the inhibition during the stimulus train.
The ability of single trains of afferent stimulation to evoke alternating rhythmic activity between flexors and extensors following l-DOPA was first demonstrated in the cat (see Lundberg, 1979). Afferent stimulation has also been demonstrated to produce a small increase in excitability in the rat during fictive locomotion (Iles & Nicolopoulos-Stournaras, 1996). Continuous nerve stimulation failed to evoke rhythmic activity following l-DOPA in the marmoset but could augment the locomotor activity initiated by other pharmacological agents (Fedirchuk et al. 1998).
The combinations of drugs required to evoke fictive locomotion differ between species. In the current experiment pre-treatment with Nialamide and administration of l-DOPA and 5HTP was effective in activating the locomotor CPG in almost 100% of the experiments. In the rat fictive locomotion was achieved with Nialamide pre-treatment and just methyl l-DOPA (successful in 9/13 rats) or 5HTP (successful in 3/4 rats) (Iles & Nicolopoulos-Stournaras, 1996). Nialamide pre-treatment followed by l-DOPA or 5HTP is also very effective in evoking fictive locomotion in the rabbit (Viala & Buser, 1969, 1971); however, l-DOPA, while enhancing extensor activity, actually reduces flexor activity, whereas 5HTP conversely enhances flexor activity and reduces extensor activity (Viala & Buser, 1969). In the cat administration of l-DOPA evoked fictive locomotion in 56% of the experiments (but in a further 24% activity could be evoked with afferent stimulation; Grillner & Zangger, 1979). In our recent series of cat experiments, the additional administration of 4AP and naloxone increased the success rate to close to 100% (Geertsen et al. 2011). In the marmoset monkey, although l-DOPA (and methyl l-DOPA) resulted in the long-latency reflexes, by itself it failed to evoke locomotion, which required Clonidine and Naloxone, 4AP or intrathecal administration of NMDA. 5HT administered intrathecally reinstated or enhanced locomotor behaviour (Fedirchuk et al. 1998).
With respect to the patterns of locomotion that we observed in the mice, the most striking difference from the normal locomotion pattern in other species is the very brief extensor phase. Brief extensor phases were also seen in rat experiments in which a leg pool was used (Iles & Nicolopoulos-Stournaras, 1996). The authors cite various work to explain this, including the demonstration of the duration of hip extensor activity being reduced by limb restraint in neonatal rats (Atsuta et al. 1990). It was described early on, as the first example of the sensory control of the spinal CPG, that stretch of the hip caused termination of the extensor phase and initiation of the next swing phase (Grillner & Rossignol, 1978). Thus, the duration of the extensor phase that we observed in the mouse most probably reflects a too-extended position of the limb/hip joint necessary to make our oil pool. This could perhaps be resolved by use of buried electrodes, eliminating the need for an oil pool and by cutting the obturator nerve and hip tendons as has proved beneficial in cat experiments.
The CP and Tib nerves were selected for use in these experiments, mainly due to the ease of dissection. However, the results suggest that the common Tib nerve includes some flexor-related muscle branches and that the gastrocnemii nerve activity is a more accurate representation of extensor activity. Similarly, in the rat the tibial nerve and the CP nerve are not innervating a collection of pure extensors and flexors, respectively (Iles & Nicolopoulos-Stournaras, 1996). Clearly a systemic investigation requires recording from nerves innervating multiple different muscles to establish the overall central pattern of activity of the different muscles during fictive locomotion in the mouse.
When rhythmic activity first appeared in the mouse it began as co-contractions in all nerves before eventually alternating between flexors and extensors and left and right sides occurred. In decerebrate rabbits, in which no drugs have been given, rhythmic synchronized motor discharges have also been reported (Viala & Buser, 1971). These disappear following spinal cord transection but rhythmic activity then reappears following administration of l-DOPA or 5HTP but now with a clear alternation between the activity of flexors and extensors, suggesting that different mechanisms produce the two different patterns (Viala & Buser, 1971). In the cat, synchronous bursts do occur but they are infrequent and occur later in experiments (unpublished observation from Hans Hultborns’ laboratory), which is at least consistent with what we observe in the mouse when l-DOPA-induced locomotion begins to fail and residual discharges become synchronous again. Synchronous bursts of activity were also frequently observed in the monkey. These bursts could coexist with other fictive motor patterns, also consistent with their being generated by different independent rhythm generators (Fedirchuk et al. 1998).
Although routinely performed in in vitro rat and mouse preparations, intracellular recording during fictive locomotion has only been performed in vivo in the cat during l-DOPA-evoked locomotion (initially; Edgerton et al. 1976; Schomburg et al. 1977) and MLR evoked locomotion (initially; Jordan et al. 1981; Jordan, 1983; Shefchyk & Jordan, 1985) and in high (thalamic) and in high decerbrate (thalamic) cats (initially; Perret & Cabelguen, 1980). Our observations of rhythmic firing and a hyperpolarization/depolarization during the inactive/active phases of the corresponding nerve activity are as would be expected from earlier work in the cat. Our presently limited observations thus far regarding the detailed relationship between the activity of the antagonist nerve and the corresponding hyperpolarization seen intracellularly is particularly interesting to us given the consistency of this observation with our recent experiments in cat demonstrating the contribution of Ia inhibition to the hyperpolarization of motoneurones during their inactive phase (Geertsen et al. 2011). It is also highly relevent given recent findings from an in vitro preparation using a transgenic mouse in which glutamtergic neurotransmision is ablated and, in which case, flexor–extensor alternation appears to be sufficiently generated by Ia inhibitory interneurones (Talpalar et al. 2011). Our single observation of a presumed scratch response to mechanical stimulation of the ear demonstrates that this model may also be suitable to study this particular fictive motor pattern. The hyperpolarization of the membrane potential seen during the activity of the antagonist nerve is also in accordance with findings in adult turtles (in vitro) and in adult cats (in vivo) that motoneurones receive strong inhibition during their inactive phase of the scratch cycle compared to during their active phase (Robertson & Stein, 1988; Geertsen et al. 2011).
The behaviour in response to afferent stimulation and administration of 5HT and l-DOPA suggests a similar basic organisation of the CPG across species although there may be differences with respect the pharmacological agents necessary to activate it, suggesting differences between the monoaminergic innervation of the components of the CPG.
This model allows one to directly record the output from the adult mouse locomotor CPGs in vivo in the various transgenic mice now available with mutations affecting various components of the CPG. This constitutes a useful addition to the repertoire of tools that can be used to studying locomotion in mutants, complementing the current neonatal in vitro models and adult EMG studies. By comparing the patterns of locomotor activity recorded from EMGs in freely moving mutants (Pearson et al. 2005) with subsequent ENG recording during fictive locomotion in the same mice one may explore the extent to which compensatory plasticity targets the CPG circuit or the reflex adaptation of the locomotor pattern in the same mice.
Acknowledgments
This work was supported by a grant from the Lundbeck foundation. C.F.M. was supported by a Marie Curie Fellowship FP7.
Glossary
Abbreviations
- CP
common peroneal nerve
- CPG
central pattern generation
- ENG
electroneurogram
- 5HTP
5-hydroxytryptophan
- l-DOPA
l-3,4-dihydroxyphenylalanine methyl ester hydrochloride
- Tib
main tibial nerve
Author contributions
The experiments were performed at the University of Copenhagen. All authors were responsible for the conception and design of the experiments. C.F.M., L.G. & H.H. were responsible for the collection, analysis and interpretation of data. C.F.M. was responsible for drafting the article which was critically revised for intellectual content by H.H. & J.B.N. and all authors approved the final version of the manuscript.
References
- Anden NE, Jukes MG, Lundberg A, Vyklicky L. The effect of DOPA on the spinal cord. 1. Influence on transmission from primary afferents. Acta Physiol Scand. 1966;67:373–386. doi: 10.1111/j.1748-1716.1966.tb03324.x. [DOI] [PubMed] [Google Scholar]
- Atsuta Y, Garcia-Rill E, Skinner RD. Characteristics of electrically induced locomotion in rat in vitro brain stem-spinal cord preparation. J Neurophysiol. 1990;64:727–735. doi: 10.1152/jn.1990.64.3.727. [DOI] [PubMed] [Google Scholar]
- Bem T, Orsal D, Cabelguen JM. Fictive locomotion in the adult thalamic rat. Exp Brain Res. 1993;97:301–304. doi: 10.1007/BF00228698. [DOI] [PubMed] [Google Scholar]
- Brownstone RM, Wilson JM. Strategies for delineating spinal locomotor rhythm-generating networks and the possible role of Hb9 interneurones in rhythmogenesis. Brain Res Rev. 2008;57:64–76. doi: 10.1016/j.brainresrev.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone SA, Quinlan KA, Zagoraiou L, Droho S, Restrepo CE, Lundfald L, Endo T, Setlak J, Jessell TM, Kiehn O, Sharma K. Genetic ablation of V2a ipsilateral interneurons disrupts left-right locomotor coordination in mammalian spinal cord. Neuron. 2008;60:70–83. doi: 10.1016/j.neuron.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Dai Y, Carlin KP, Li Z, McMahon DG, Brownstone RM, Jordan LM. Electrophysiological and pharmacological properties of locomotor activity-related neurons in cfos-EGFP mice. J Neurophysiol. 2009;102:3365–3383. doi: 10.1152/jn.00265.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty KJ, Kiehn O. Firing and cellular properties of V2a interneurons in the rodent spinal cord. J Neurosci. 2010;30:24–37. doi: 10.1523/JNEUROSCI.4821-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton VR, Grillner S, Sjöström A, Zangger P. Central generation of locomotion. In: Herman R, Grillner S, Stein P, Stuart D, editors. Control of Locomotion. New York: Plenum Press; 1976. pp. 439–464. [Google Scholar]
- Fedirchuk B, Nielsen J, Petersen N, Hultborn H. Pharmacologically evoked fictive motor patterns in the acutely spinalized marmoset monkey (Callithrix jacchus. Exp Brain Res. 1998;122:351–361. doi: 10.1007/s002210050523. [DOI] [PubMed] [Google Scholar]
- Geertsen SS, Stecina K, Meehan CF, Nielsen JB, Hultborn H. Reciprocal Ia inhibition contributes to motoneuronal hyperpolarisation during the inactive phase of locomotion and scratching in the cat. J Physiol. 2011;589:119–134. doi: 10.1113/jphysiol.2010.199125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosgnach S, Lanuza GM, Butt SJ, Saueressig H, Zhang Y, Velasquez T, Riethmacher D, Callaway EM, Kiehn O, Goulding M. V1 spinal neurons regulate the speed of vertebrate locomotor outputs. Nature. 2006;440:215–219. doi: 10.1038/nature04545. [DOI] [PubMed] [Google Scholar]
- Grillner S. Locomotion in vertebrates – central mechanisms and reflex interaction. Physiol Rev. 1975;55:247–304. doi: 10.1152/physrev.1975.55.2.247. [DOI] [PubMed] [Google Scholar]
- Grillner S. Control of locomotion in bipeds, tertapods and fish. In: Brookhart JM, Mountcastle VB, editors. Handbook of Physiology, section 1, The Nervous System. II. Bethesda, MD, USA: American Physiological Society; 1981. pp. 1179–1236. [Google Scholar]
- Grillner S, Rossignol S. On the initiation of the swing phase of locomotion in chronic spinal cats. Brain Res. 1978;146:269–277. doi: 10.1016/0006-8993(78)90973-3. [DOI] [PubMed] [Google Scholar]
- Grillner S, Zangger P. How detailed is central pattern generation for locomotion. Brain Res. 1975;88:367–371. doi: 10.1016/0006-8993(75)90401-1. [DOI] [PubMed] [Google Scholar]
- Grillner S, Zangger P. On the central generation of locomotion in the low spinal cat. Exp Brain Res. 1979;34:241–261. doi: 10.1007/BF00235671. [DOI] [PubMed] [Google Scholar]
- Hagglund M, Borgius L, Dougherty KJ, Kiehn O. Activation of groups of excitatory neurons in the mammalian spinal cord or hindbrain evokes locomotion. Nat Neurosci. 2010;13:246–252. doi: 10.1038/nn.2482. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Nielsen JB. Spinal control of locomotion – from cat to man. Acta Physiol (Oxf) 2007;189:111–121. doi: 10.1111/j.1748-1716.2006.01651.x. [DOI] [PubMed] [Google Scholar]
- Iles JF, Nicolopoulos-Stournaras S. Fictive locomotion in the adult decerebrate rat. Exp Brain Res. 1996;109:393–398. doi: 10.1007/BF00229623. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Jukes MG, Lund S, Lundberg A. The effect of DOPA on the spinal cord. 5. Reciprocal organization of pathways transmitting excitatory action to alpha motoneurones of flexors and extensors. Acta Physiol Scand. 1967a;70:369–388. doi: 10.1111/j.1748-1716.1967.tb03636.x. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Jukes MG, Lund S, Lundberg A. The effect of DOPA on the spinal cord. 6. Half-centre organization of interneurones transmitting effects from the flexor reflex afferents. Acta Physiol Scand. 1967b;70:389–402. doi: 10.1111/j.1748-1716.1967.tb03637.x. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Carlin KP, Brownstone RM. An in vitro functionally mature mouse spinal cord preparation for the study of spinal motor networks. Brain Res. 1999;816:493–499. doi: 10.1016/s0006-8993(98)01199-8. [DOI] [PubMed] [Google Scholar]
- Jordan LM. Factors determining motoneuron rhythmicity during fictive locomotion. Symp Soc Exp Biol. 1983;37:423–44. [PubMed] [Google Scholar]
- Jordan LM, Pratt CA, Menzies JE. Intraspinal mechanisms for the control of locomotion. In: Szentagothai J, Palkovits M, Hamori J, editors. Advances in Physiological Sciences. Vol. 1. Budapest: Pergamon Press/Akademiai Kiado; 1981. pp. 183–186. [Google Scholar]
- Kiehn O. Development and functional organization of spinal locomotor circuits. Curr Opin Neurobiol. 2011;21:100–109. doi: 10.1016/j.conb.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Kinjo N, Atsuta Y, Webber M, Kyle R, Skinner RD, Garcia-Rill E. Medioventral medulla-induced locomotion. Brain Res Bull. 1990;24:509–516. doi: 10.1016/0361-9230(90)90104-8. [DOI] [PubMed] [Google Scholar]
- Leblond H, L’Espérance N, Orsal D, Rossignol S. Treadmill locomotion in the intact and spinal mouse. J Neurosci. 2003;23:11411–11419. doi: 10.1523/JNEUROSCI.23-36-11411.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg A. Multisensory control of spinal reflex pathways. Prog Brain Res. 1979;50:11–28. doi: 10.1016/S0079-6123(08)60803-1. [DOI] [PubMed] [Google Scholar]
- Meehan CF, Sukiasyan N, Zhang M, Nielsen JB, Hultborn H. Intrinsic properties of mouse lumbar motoneurons revealed by intracellular recording in vivo. J Neurophysiol. 2010;103:2599–2610. doi: 10.1152/jn.00668.2009. [DOI] [PubMed] [Google Scholar]
- Orlovskii GN, Severin FV, Shik ML. [Locomotion induced by stimulation of the mesencephalon] (in Russian) Dokl Akad Nauk SSSR. 1966;169:1223–1226. [PubMed] [Google Scholar]
- Pearson KG, Acharya H, Fouad K. A new electrode configuration for recording electromyographic activity in behaving mice. J Neurosci Methods. 2005;148:36–42. doi: 10.1016/j.jneumeth.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Perret C, Cabelguen JM. Main characteristics of the hindlimb locomotor cycle in the decorticate cat with special reference to bifunctional muscles. Brain Res. 1980;187:333–352. doi: 10.1016/0006-8993(80)90207-3. [DOI] [PubMed] [Google Scholar]
- Robertson GA, Stein PS. Synaptic control of hindlimb motoneurones during three forms of the fictive scratch reflex in the turtle. J Physiol. 1988;404:101–128. doi: 10.1113/jphysiol.1988.sp017281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol S, Dubuc R, Gossard JP. Dynamic sensorimotor interactions in locomotion. Physiol Rev. 2006;86:89–154. doi: 10.1152/physrev.00028.2005. [DOI] [PubMed] [Google Scholar]
- Shefchyk S, Jordan L. Excitatory and inhibitory postsynaptic potentials in alpha-motoneurons produced during fictive locomotion by stimulation of the mesencephalic locomotor region. J Neurophysiol. 1985;53:1345–1355. doi: 10.1152/jn.1985.53.6.1345. [DOI] [PubMed] [Google Scholar]
- Schomburg ED, Roesler J, Meinck HM. Phase-dependent transmission in the excitatory propriospinal reflex pathway from forelimb afferents to lumbar motoneurones during fictive locomotion. Neurosci Lett. 1977;4:249–252. doi: 10.1016/0304-3940(77)90187-2. [DOI] [PubMed] [Google Scholar]
- Schomburg ED, Steffens H. Influence of opioids and naloxone on rhythmic motor activity in spinal cats. Exp Brain Res. 1995;103:333–343. doi: 10.1007/BF00241493. [DOI] [PubMed] [Google Scholar]
- Shik ML, Severin FV, Orlovskii GN. [Control of walking and running by means of electric stimulation of the midbrain] (in Russian) Biofizika. 1966;11:659–666. [PubMed] [Google Scholar]
- Talpalar AE, Endo T, Löw P, Borgius L, Hägglund M, Dougherty KJ, Ryge J, Hnasko TS, Kiehn O. Identification of minimal neuronal networks involved in flexor-extensor alternation in the mammalian spinal cord. Neuron. 2011;71:1071–1084. doi: 10.1016/j.neuron.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Viala D, Buser P. The effects of DOPA and 5-HTP on rhythmic efferent discharges in hind limb nerves in the rabbit. Brain Res. 1969;12:437–443. doi: 10.1016/0006-8993(69)90011-0. [DOI] [PubMed] [Google Scholar]
- Viala D, Buser P. [Methods of obtaining locomotor rhythms in the spinal rabbit by pharmacological treatments (DOPA, 5-HTP, D-amphetamine)] (in French) Brain Res. 1971;35:151–165. doi: 10.1016/0006-8993(71)90601-9. [DOI] [PubMed] [Google Scholar]
- Viala D, Valin A, Buser P. Relationship between the “late reflex discharge” and locomotor movements in acute spinal cats and rabbits treated with DOPA. Arch Ital Biol. 1974;112:299–306. [PubMed] [Google Scholar]
- Wilson JM, Blagovechtchenski E, Brownstone RM. Genetically defined inhibitory neurons in the mouse spinal cord dorsal horn: a possible source of rhythmic inhibition of motoneurons during fictive locomotion. J Neurosci. 2010;30:1137–1148. doi: 10.1523/JNEUROSCI.1401-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RJ, Chersa T, Whelan PJ. Tissue PO2 and the effects of hypoxia on the generation of locomotor-like activity in the in vitro spinal cord of the neonatal mouse. Neuroscience. 2003;117:183–196. doi: 10.1016/s0306-4522(02)00831-x. [DOI] [PubMed] [Google Scholar]
- Zagoraiou L, Akay T, Martin JF, Brownstone RM, Jessell TM, Miles GB. A cluster of cholinergic premotor interneurons modulates mouse locomotor activity. Neuron. 2009;64:645–662. doi: 10.1016/j.neuron.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G, Droho S, Crone SA, Dietz S, Kwan AC, Webb WW, Sharma K, Harris-Warrick RM. Electrophysiological characterization of V2a interneurons and their locomotor-related activity in the neonatal mouse spinal cord. J Neurosci. 2010;30:170–182. doi: 10.1523/JNEUROSCI.4849-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]


