Abstract
Non-technical summary
When vascular volume is expanded, atrial natriuretic peptide (ANP) released from the heart acts to restore plasma volume by increasing renal water excretion, causing vasodilatation and increasing vascular permeability to water and macromolecules. Previous experiments using mice with selective deletion of ANP receptors in vascular endothelial cells emphasized the importance of vascular permeability regulation by ANP in plasma volume restoration because this genetic manipulation of ANP action on vascular permeability limited the restoration of vascular volume after an acute increase in plasma volume. Here we demonstrate retention of intravenously infused fluid in wild-type mice in which the response to endogenous ANP was attenuated by the pharmacological agent rolipram that stabilized the endothelial barrier by tightening adhesion between adjacent endothelial cells. The strategy may provide novel approaches to the clinical problem of maintenance of vascular volume after acute intravenous fluid infusion.
Abstract
We tested the hypothesis that inhibition of phosphodiesterase 4 (PDE4) with rolipram to increase vascular endothelial cAMP and stabilize the endothelial barrier would attenuate the action of endogenous atrial natriuretic peptide (ANP) to increase vascular permeability to the plasma protein albumin after an acute plasma volume expansion. After rolipram pretreatment (8 mg (kg body wt)−1, intraperitoneal, 30 min) more than 95% of the peak increase in plasma volume after volume expansion (4.5% bovine serum albumin, 114 μl (g body wt)−1 h−1, 15 min) remained in the vascular space 75 min after the end of infusion, whereas only 67% of the fluid was retained in volume-expanded animals with no rolipram pretreatment. Rolipram significantly decreased 30 min fluorescently labelled albumin clearance (μl (g dry wt)−1) relative to untreated volume-expanded controls in skin (e.g. back, 10.4 ± 1.6 vs. 19.5 ± 3.6, P = 0.04), muscle (e.g. hamstring, 15.0 ± 1.9 vs. 20.8 ± 1.4, P = 0.04) and in colon, caecum, and rectum (average reduction close to 50%). The mass of muscle and skin tissue accounted for 70% of volume-expansion-dependent albumin shifts from plasma to interstitium. The results are consistent with observations that the PDE4 inhibitor rolipram attenuates ANP-induced increases in vascular permeability after infusion of exogenous ANP and observations of elevated central venous pressure after a similar volume expansion in mice with selective deletion of the endothelial ANP receptor. These observations may form the basis for new strategies to retain intravenous fluid containing macromolecules.
Introduction
Atrial natriuretic peptide (ANP) is known to have multiple actions to regulate blood pressure and blood volume. When vascular volume is expanded, ANP is released from specialized cells in the heart to increase circulating concentrations. The resulting actions of ANP to restore plasma volume include those on the kidney to increase water excretion, on vascular smooth muscle to cause vasodilatation and increased surface area for fluid exchange, and on endothelium to increase vascular permeability to water and macromolecules (de Bold et al. 1981; Brenner et al. 1990; Renkin & Tucker, 1996; Baxter, 2004). Renal water excretion clearly contributes to long term net water loss after volume expansion. However, the results of experiments in the 1990's to measure ANP-dependent increases in vascular permeability in normal and nephrectomized animals (Almeida et al. 1986; Fluckiger et al. 1986; Huxley et al. 1987; Tucker et al. 1992), and more recent experiments using mice with selective deletion of endothelial-specific ANP receptors (endothelial cell guanylyl cyclase-A knock-out (EC GC-A KO) mice) (Sabrane et al. 2005; Schreier et al. 2008; Curry et al. 2010) emphasize the importance of increased peripheral vascular permeability to plasma proteins including albumin, as well as vasodilatation, as contributions of ANP-dependent restoration of plasma volume after an acute increase. For example, in EC GC-A KO mice, a 4.5% serum albumin solution was infused to increase plasma volume by more than 50%. In these animals there was no ANP-dependent increase in vascular permeability (Sabrane et al. 2005; Curry et al. 2010) and plasma volume remained expanded as measured by elevated central venous pressure for a least 1 h (Schreier et al. 2008). A similar infusion into control littermates resulted in rapid restoration of normal plasma volume and central venous pressure (Schreier et al. 2008). An important question was whether similar retention of intravenously infused fluid would be observed in wild-type mice in which the response to endogenous ANP was attenuated by stabilizing the endothelial barrier against the physiological actions of ANP to increase vascular permeability. If infused fluid was retained under these conditions, the strategy may provide new approaches to the important clinical problem of maintenance of vascular volume after intravenous fluid infusion when plasma volume is compromised (Pearse et al. 2004; Kinsky et al. 2008).
We have recently shown that stabilizing the endothelial barrier by pretreating mice with the phosphodiesterase 4 (PDE4) inhibitor rolipram attenuated the action of exogenous ANP to increase vascular permeability when ANP was infused to reach levels measured during volume expansion (Lin et al. 2011). Therefore one aim of the present experiments was to compare the retention of infused fluid in the vascular space in mice with and without pretreatment with rolipram to stabilize the endothelial barrier when exposed to endogenous ANP released due to vascular volume expansion. A second aim in the same animals was to measure the blood-to-tissue clearance of serum albumin in multiple organs with and without pretreatment with rolipram.
To do this we extended the approaches developed in previous experiments from our own and other laboratories. The plasma volume expansion protocol was identical to that used to expand vascular volume in the EC GC-A KO mice (Schreier et al. 2008). In these mice ANP levels increased from low levels (0.1–0.3 ng ml−1, i.e. <0.1 nm) to levels that approach 1 ng ml−1 (approx. 0.3 nm) (Kishimoto et al. 1996). The protocol to measure changes in vascular permeability using a two tracer method to measure the clearance of fluorescently labelled bovine serum albumin (BSA) was that described in our investigation of the attenuation of ANP-dependent increases in vascular permeability by rolipram when exogenous ANP was infused at a rate designed to reach the same circulating levels as those described above (Lin et al. 2011). Here, we predicted that, if rolipram attenuated the endogenous ANP-dependent increases in vascular permeability as shown for exogenous ANP, then we would measure increased retention of intravenously infused fluid containing albumin as the plasma colloid, and the extent of retention would be comparable to that when the same protocol was used for volume expansion in EC GC-A KO mice. If this was the case, we expected that pharmacological modulation of the vascular permeability actions of ANP using inhibitors of PDE4 such as rolipram to modify endothelial barrier permeability might be a useful tool for the manipulation of plasma volume during surgery.
Methods
Ethical approval
The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). Animal protocol 13052 was approved by the Institutional Animal Care and Use Committee of the University of California, Davis. The authors have read, and the experiments comply with, the policies and regulations of The Journal of Physiology given by Drummond (2009).
Animal preparation and tissue collection
Male mice, (C57BL/6J, 25–35 g, The Jackson Laboratory) were anaesthetized with isoflurane (1.5–2.0%) and both the jugular vein and carotid artery were cannulated with heparinized cannulae (very low dead volume, 10 μl). The jugular vein cannula was connected to an infusion pump for delivery of fluorescently labelled BSA (BSA-dye) tracers and infusion of 4.5% BSA. The carotid cannula was clamped and left untouched until blood collection at the end of the experiment. All animals were stabilized with a 30 min baseline infusion of 4.5% BSA at 4.3 μl (g body weight (BW))−1 h−1 (starting at t =−30 min). At t = 0 min, 40 μl of Alexa Fluor 647-labelled BSA (BSA-647) at concentrations of 60–100 mg ml−1 was injected via the jugular cannula and the cannula was quickly flushed with 10 μl of phosphate-buffered saline (PBS) followed by continued infusion of 4.5% BSA at 114 μl (g BW)−1 h−1 for 15 min. At t = 15 min, the infusion rate was switched back to 4.3 μl (g BW)−1 h−1. At t = 30 min, 40 μl of Alexa Fluor 555-labelled BSA (BSA-555) at the same concentration as the BSA-647 was injected. The cannula was flushed with 10 μl of PBS, and the 4.3 μl (g BW)−1 h−1 infusion was continued for 5 min to allow the BSA-555 (as a marker of local intravascular volume) to mix completely in the blood. At t = 35 min, blood samples were quickly drawn from the carotid cannula and the animal was killed with saturated KCl injected into the jugular cannula. Total volume of fluid injection was carefully controlled to enable distinction between haematocrit changes due to fluid infusion and due to fluid redistribution.
For rolipram (8 mg (kg BW)−1) pretreatment, the rolipram (stock 50 mm in EtOH) in 100 μl total volume (prepared in PBS) was injected intraperitoneally after the cannulations were completed and just prior to the first 30 min stabilization period (t =−30 min). In this way, the rolipram was allowed to absorb for 30 min before tracer albumin was injected and either control or test infusions were started. The rolipram dose used here has been shown to block a range of inflammatory responses (Teixeira et al. 1994; Elwood et al. 1995; Alvarez et al. 2001). For non-rolipram control experiments, 100 μl of PBS with vehicle was injected before the stabilization period.
Renal water excretion was determined by measuring urine weight changes over the entire experimental period. A 3 ml syringe with 30 gauge needle was used to empty the bladder before pretreatment (t =−30 min). At the end of the experiment, a pre-weighed 3 ml syringe with 30 gauge needle was used to collect the urine produced during the entire period of the experiment (120 min consisting of 30 min pretreatment and 90 min of infusion and measurement; or 65 min consisting of 30 min pretreatment and 35 min of infusion and measurement). Urine excretion in animals with no volume expansion was control.
Analysis of blood and tissue samples for fluorescence
Plasma samples were separated from blood after centrifugation and measurement of haematocrit. The exact amount of plasma was determined by weighing. Tissues were dissected, placed into pre-weighed vials, minced with scissors, and then reweighed. The labelled albumin was extracted from the tissue samples by adding 1 ml of 1 mg ml−1 unlabelled albumin in PBS to each vial, vortexing and storing them overnight at 4°C. After this, the extract was separated from the tissue by centrifugation and the fluorescence from each tracer (BSA-647 and BSA-555) in both plasma samples and tissue extracts was determined by fluorometry. The tissues were then oven dried to constant weight.
To remove particulate matter which could interfere with the fluorescence measurements, the tissue extracts were filtered through a 5 μm syringe filter, and then through a 1.2 μm syringe filter, before fluorometric analysis. Standard curves were also prepared in the same way with solutions having comparable tissue extracts; both BSA-dyes were added at equal concentrations to non-fluorescent tissue extract and the extracts were filtered before fluorescence intensity measurement. Control experiments, using a known amount of labelled albumin added to non-fluorescent tissue and extracted using the same process, showed that BSA-dye recovery by extraction was better than 95%.
Tracer preparation
BSA (Sigma A0281) was labelled with either Alexa Fluor 647 (emission max at 671 nm) or Alexa Fluor 555 (emission max at 570 nm) according to the instructions from the manufacturer (Invitrogen/Molecular Probes) to produce BSA-647 and BSA-555, respectively. Briefly, 40 mg of albumin was labelled using 5 mg of dye in 11 ml of bicarbonate-buffered PBS for 2.5 h. The reaction was terminated with hydroxylamine and the albumin purified by running through a size exclusion column (Bio-Gel P-30, Bio-Rad). A labelling efficiency of 3–5 moles of dye per mole of albumin was determined by spectrophotometry per manufacturer's instructions. Fluorophore labelling of serum albumin can alter the physical and chemical properties of the protein, including size and net charge, potentially altering the permeability characteristics of the tracer with respect to the native protein. While the present studies did not directly address these issues, the 3–5 moles of dye per mole of albumin would yield a tracer molecule having a higher molecular mass by 4–7 kDa and a more negative net charge than albumin. Both changes could be expected to decrease the permeability of the tracer relative to that of the native protein (Adamson et al. 1988; Rumbaut et al. 1999; Bingaman et al. 2003). Nonetheless, the use of a consistent well-characterized tracer enabled comparison of macromolecule transport in control versus test protocols.
Elimination of free dye
Because non-covalently bound dye (free dye) dissociating from the albumin would compromise measurements of tissue albumin uptake, great care was taken to ensure that the amount of free dye in the injectate was less than 0.3%. After labelling and column purification, the BSA-dye was stored for 3 weeks at 4°C in 2 mm sodium azide PBS to allow free dye to dissociate from the albumin. To increase the rate of dye dissociation, the free dye was removed each week by concentrating the labelled albumin using 30 kDa molecular mass cut-off centrifuge filters (Amicon Ultracel-4, 30 K, Millipore) and rediluting to the original volume with fresh PBS-azide. After 3 weeks the solution was concentrated again and then purified by cold ethanol precipitation. The precipitated albumin was re-dissolved in PBS alone to a concentration of about 60–100 mg ml−1, the concentration used in the experiments. We have confirmed that the potential presence of residual azide shows no measurable toxicity by the use of these stocks to measure stable permeability in single perfused vessels over much longer experiments. Free dye was checked by centrifuging a 2 mg ml−1 solution of BSA-dye through a 30 kDa molecular mass cut-off centrifuge filter (Centrifree YM30, Millipore) and checking the fluorescence of the ultrafiltrate in a fluorometer, comparing it to standards prepared from free dyes alone. Free dye concentration was always reduced to less than 0.3% before the labelled albumin was used for an experiment.
Expanded volume infusion
BSA (4.5%; BSA, Sigma A0281) was dissolved in lactated Ringer solution (BSA-LRS) and infused via the jugular vein at a dose of 114 μl (g BW)−1 h−1 using an infusion pump (World Precision Instruments SP120P). This rate, previously used in mice to study the effect of an endothelial cell-specific knockout of the GC-A receptor (Sabrane et al. 2005), was used to increase plasma volume and has previously been shown to increase ANP release to plasma (Kishimoto et al. 1996). Two periods of volume retention were investigated. First the volume of fluid retained 20 min after the end of the 15 min infusion was measured; second the volume retained 75 min after the end of volume expansion was measured.
Calculation of clearances
A 30 min albumin clearance was estimated as an extravascular plasma equivalent volume. This was calculated as the difference between the plasma equivalent distribution volume of BSA-647 at t = 35 min and the plasma equivalent volume of BSA-555 over the final 5 min (t = 30–35 min). The plasma equivalent distribution volume (μl (g dry wt)−1) for each tracer was calculated from the fluorescence intensity measurements as total BSA-dye content (μg (g dry wt)−1 divided by plasma BSA-dye concentration (μg μl−1). Assuming most of the BSA-555 was still in the plasma after 5 min, the BSA-555 equivalent volume gives a measure of local vascular volume in each tissue. All calculations were referenced to tissue blood-free dry weight as previously published (Nedrebo & Reed, 2002; Nedrebo et al. 2003; Curry et al. 2010).
Statistical analysis
Prism 4.03 (GraphPad Software., Inc.) was used for statistical analysis and all data were expressed as mean ± SEM. Groups were compared using one-way ANOVA and post hoc Bonferroni comparison to correct for multiple testing. Additional tests used two-tailed Student's t, unless otherwise specified. A difference was considered statistically significant when P < 0.05.
Results
The average plasma volume in male wild-type C57BL/6J mice was 1.05 ± 0.04 ml animal−1 (or 34.3 ± 1.3 μl (g body weight)−1, 3.5% body weight). It was not changed by rolipram pretreatment (1.07 ± 0.06 ml animal−1 or 34 ± 2 μl (g BW)−1, P > 0.05). In the first series of experiments we tested the action of rolipram pretreatment to modify the plasma volume after volume expansion. Fifteen minutes of infusion of 4.5% BSA-LRS at 114 μl g−1 h−1 reduced the haematocrit (%) measured 5 min after the end of infusion from an average of 44.9 ± 0.4 to 34.7 ± 0.3 (n = 12) in mice with no pretreatment, and by a similar amount in mice pretreated with rolipram (46.3 ± 0.3 to 36.1 ± 0.4, n = 12). The average increase of the plasma volume at 5 min after the end of the volume expansion (t = 20 min) was 560 μl in mice with no pretreatment, and 532 μl in rolipram-pretreated mice (P = 0.16, t test). Rolipram significantly attenuated the subsequent loss of plasma volume in animals with volume expansion. At 20 and 75 min after the end of volume expansion, mice with rolipram pretreatment had haematocrits that were not significantly different from those at the end the infusion. Specifically, after 75 min rolipram-treated mice retained 519 μl of fluid (compared with the initial expansion by 532 μl) while untreated mice retained only 376 μl compared to their initial expansion of 560 μl). Figure 1 summarizes these results at both 20 and 75 min after the end of fluid infusion. While plasma volume was initially expanded to similar amounts in treated and untreated mice, more than 95% of the fluid in the plasma space was retained in animals pretreated with rolipram after 75 min but only 67% of the fluid was retained in the absence of rolipram.
Figure 1. Rolipram prevents fluid loss from the plasma space in volume-expanded mice.
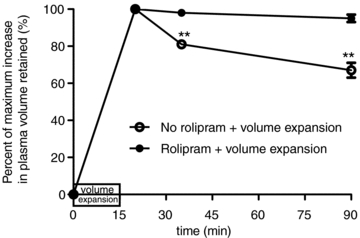
The increase in plasma volume after a 15 min infusion was measured 5, 20 and 75 min after the end of the volume expansion. The increase in plasma volume is expressed relative to the increased plasma volume measured 5 min after the end of volume expansion in each mouse. Twenty minutes (t = 35 min) after volume expansion, mice with rolipram pretreatment retained 98% of infused fluid while mice with no rolipram pretreatment retained 81% of the infused fluid. Seventy-five minutes (t = 90 min) after volume expansion, mice with rolipram pretreatment retained more than 95% of infused fluid while mice with volume expansion and no rolipram pretreatment retained 67% of the infused fluid (**P < 0.01, relative to 5 min volume).
Fluid can be lost from the plasma volume by shifting into the interstitial space and by renal fluid loss. The control urine flow in mice with no volume expansion was close to 1 μl min−1. Urine was collected over the entire period of the volume expansion experiments (120 min, i.e. 30 min of rolipram pretreatment, 15 min of infusion, and 75 min of observation). During volume expansion and no rolipram pretreatment, the total urine volume measured over 120 min was 184 ± 15 μl which is significantly increased compared to control (121 ± 12 μl). In rolipram-pretreated animals, the total urine volume over the same 120 min was 158 ± 16 μl which is also significantly increased compared to control. However, the difference between these two amounts in volume-expanded animals (26 μl) is far too small to account for the difference between the amount of fluid retained in the plasma in rolipram-treated animals compared with their untreated controls (519 – 376 = 143 μl). We conclude that most of the redistribution of fluid that takes place over a period of 75 min after volume expansion is due to fluid movements into the interstitium, and that rolipram significantly attenuates this fluid redistribution.
The second important finding in this study was that rolipram pretreatment significantly attenuated the 30 min clearance of albumin measured after plasma volume expansion in skin, muscle, and in the gastrointestinal (GI) tissues caecum, colon and rectum (P < 0.05; t test). These results, together with results in tissues where rolipram did not have a significant action are presented in detail below. We have previously demonstrated significant actions of exogenous ANP to increase albumin clearance in the microvessels of skin and muscle which together account for close to 60% of the body weight in a 30 g mouse (12 g muscle, 40% body weight and 6 g skin, 20% body weight) and these results are presented first.
The 30 min albumin clearances in skin and muscle for volume expansion with and without rolipram pretreatment are shown in Fig. 2A. Rolipram pretreatment significantly decreased the 30 min clearance of albumin measured after volume expansion. The 30 min albumin clearance in back skin was reduced to 53% of the clearance in volume-expanded animals without rolipram pretreatment. The corresponding values in hamstring muscle were 72%, in quadriceps muscle 67%, and in tail skin 33% (P < 0.05, t test). Table 1 summarizes values of local vascular volume in male volume-expanded animals with and without rolipram pretreatment. There was a tendency for local vascular volume to increase with rolipram pretreatment in volume-expanded mice, but, with the exception of quadriceps muscle, the increases in local vascular volume were not significantly increased in skin and muscle in relation to no-rolipram pretreatment. Because an increase in local vascular volume probably reflects increased local perfusion (due, for example to changes in arteriolar resistance) with associated increased surface area for exchange, the fact that rolipram pretreatment actually reduced albumin clearance in quadriceps muscle with volume expansion, even when local vascular volume was increased, clearly indicates that rolipram acts to attenuate increased permeability. This argument can be put on a more quantitative basis as follows. We have previously argued that the ratio of 30 min clearance in μl (g dry wt)−1 to local vascular volume in μl (g dry wt)−1 helps to distinguish increases or decreases in clearance due to changes in the vascular exchange surface from real changes in permeability (Curry et al. 2010; Lin et al. 2011). Figure 2B shows the ratio of 30 min clearance in μl (g dry wt)−1 to local vascular volume in μl (g dry wt)−1 for the clearance values in Fig. 2A. The result that the normalized clearances are all significantly reduced with rolipram pretreatment supports the conclusion above that the main action of rolipram is to attenuate the blood-to-tissue efflux of albumin during volume expansion by reducing vascular permeability in skin and muscle. We did not carry out additional control measurements of 30 min albumin clearance in mice with no volume expansion because we recently published these data with and without rolipram pretreatment (Lin et al. 2011). The normalized 30 min clearances for non-volume-expanded animals are included in Fig. 2B. There is no consistent difference between the normalized clearance in animals with volume expansion and rolipram, and in animals with no volume expansion, showing that in the presence of rolipram, albumin clearance in volume-expanded animals was returned towards the normal values in animals with no volume expansion.
Figure 2. Thirty minute albumin clearances in skin and muscle and normalized clearances in skin and muscle.
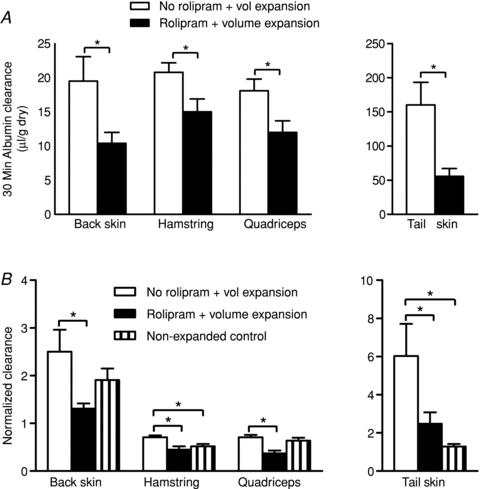
A, the 30 min albumin clearances are shown for skin tissue (back and tail) and muscle tissue (hamstring and quadriceps) under 2 conditions: volume expansion with no rolipram pretreatment and volume expansion with rolipram. In both skin and muscle, rolipram significantly reduced the 30 min clearance during volume expansion. B, the 30 min albumin clearances in A were normalized using local vascular volumes. The results demonstrate significant reductions in vascular permeability with rolipram pretreatment (*P < 0.05, Student's t test).
Table 1.
Local vascular volumes (μl (g dry wt)−1) and water content (ml (g dry wt)−1) in skin and muscle
| No rolipram + volume expansion | Rolipram + volume expansion | Non-expanded control (n = 24) | |
|---|---|---|---|
| Vascular volume | |||
| Back skin | 8.8 ± 0.9 (n = 8) | 8.7 ± 0.7 (n = 10) | 9.0 ± 0.8 |
| Tail skin | 23.8 ± 4.0 (n = 10) | 26.1 ± 2.2 (n = 11) | 24.9 ± 2.1 |
| Hamstring muscle | 30.7 ± 2.31 (n = 9) | 33.8 ± 2.21 (n = 11) | 22.9 ± 1.1 |
| Quadriceps muscle | 24.8 ± 1.01 (n = 9) | 32.7 ± 1.61,2 (n = 11) | 16.1 ± 0.9 |
| Water content | |||
| Back skin | 1.79 ± 0.03 (n = 10) | 1.79 ± 0.04 (n = 11) | 1.79 ± 0.12 |
| Tail skin | 1.78 ± 0.04 (n = 10) | 1.71 ± 0.03 (n = 11) | 1.84 ± 0.04 |
| Hamstring muscle | 4.26 ± 0.08 (n = 10) | 4.18 ± 0.07 (n = 11) | 4.01 ± 0.08 |
| Quadriceps muscle | 4.35 ± 0.091 (n = 10) | 4.40 ± 0.081 (n = 11) | 4.07 ± 0.04 |
P < 0.05
when compared to Non-expanded control with one-way ANOVA
when compared to No rolipram + volume expansion with one-way ANOVA.
Figure 2B also shows that the normalized clearances in hamstring muscle and tail skin in volume-expanded animals are significantly increased when compared with the previously published data from animals without volume expansion. There is also a trend for the normalized clearance to increase in back skin and quadriceps muscle with volume expansion. These increases occur in spite of a clear tendency for volume expansion to increase local vascular volume in both hamstring and quadriceps muscle. The increased normalized clearances with volume expansion are consistent with previously published results that ANP, released endogenously during volume expansion, causes real increases in permeability in skin and muscle.
Table 2 summarizes measurements of 30 min albumin clearances in GI tissues. Clearances in all these tissues were 10–30 times larger than those in skin and muscle. The clearances in volume-expanded animals were also significantly increased relative to values in animals with no volume expansion published previously (Lin et al. 2011). Table 3 shows that local vascular volumes were also significantly increased by volume expansion alone. For example, in jejunum, local vascular volume was almost 2 times values in the absence of volume expansion. As described above, increased surface area for exchange probably contributes to the increased clearance. Thus, to interpret the action of rolipram in these tissues it was essential to distinguish real changes in permeability from changes associated with volume expansion using normalized clearances shown in Fig. 3. The action of rolipram to cause a real reduction in albumin permeability could be demonstrated only in ileum, colon and rectum because these were the only GI segments where the reduction in normalized clearance with rolipram pretreatment was significant (P < 0.05). It follows that rolipram may not exert a significant action in other GI segments if the increase in albumin clearance with volume expansion is mainly the result of increased surface area for exchange. This is confirmed, at least for duodenum and jejunum in Fig. 3. Specifically, the normalized albumin clearance in these tissues with volume expansion was not increased compared to values in animals with no volume expansion. Further the normalized albumin clearances in these tissues in rolipram-treated animals were not different from the volume-expanded animals. The results suggest that the increase in 30 min clearance in duodenum and jejunum with volume expansion is due, in large part, to local increased area for exchange. Thus at least part of the reason for the failure of pretreatment with rolipram to significantly change the normalized clearance in jejunum and duodenum is that changes in local vascular volume (and associated changes in surface area) are a more important determinant of albumin clearance than real increases in permeability.
Table 2.
Thirty minute albumin clearances (μl (g dry wt)−1) in GI tissues
| No rolipram + volume expansion | Rolipram + volume expansion | Non-expanded control (n = 6) | |
|---|---|---|---|
| Clearance | |||
| Duodenum | 816.4 ± 55.21 (n = 10) | 660.3 ± 46.9 (n = 9) | 494.5 ± 81.1 |
| Jejunum | 533.3 ± 39.61 (n = 10) | 465.6 ± 60.41 (n = 9) | 256.8 ± 49.2 |
| Ileum | 407.7 ± 49.61 (n = 10) | 325.9 ± 41.31 (n = 11) | 135.1 ± 38.3 |
| Colon | 432.7 ± 38.31 (n = 10) | 242.2 ± 48.42 (n = 10) | 99.8 ± 29.6 |
| Caecum | 452.4 ± 31.71 (n = 9) | 303.7 ± 44.2 1,2 (n = 11) | 120.7 ± 36.7 |
| Rectum | 250.6 ± 281 (n = 10) | 107.4 ± 152 (n = 7) | 70.9 ± 27.6 |
P < 0.05
when compared to Non-expanded control with one-way ANOVA
when compared to No rolipram + volume expansion with one-way ANOVA.
Table 3.
Local vascular volumes (μl (g dry wt)−1) and water content (ml (g dry wt)−1) in GI tissues
| No rolipram + volume expansion | Rolipram + volume expansion | Non-expanded control (n = 6) | |
|---|---|---|---|
| Vascular volume | |||
| Duodenum | 274.2 ± 48.81 (n = 10) | 265.8 ± 36.51 (n = 11) | 140.4 ± 17.7 |
| Jejunum | 162.0 ± 36.9 (n = 10) | 174.8 ± 33.31 (n = 11) | 78.6 ± 11.4 |
| Ileum | 95.4 ± 14.9 (n = 10) | 114.2 ± 14.51 (n = 11) | 65.2 ± 11.3 |
| Colon | 94.3 ± 14.21 (n = 10) | 91.0 ± 12.71 (n = 11) | 51.0 ± 16.5 |
| Caecum | 90.4 ± 13.51 (n = 10) | 84.4 ± 121 (n = 11) | 50.5 ± 15.8 |
| Rectum | 59.0 ± 7.51 (n = 10) | 71.5 ± 10.4 1,2 (n = 11) | 28.5 ± 4.3 |
| Water content | |||
| Duodenum | 5.40 ± 0.14 (n = 9) | 6.05 ± 0.35 (n = 11) | 6.22 ± 0.57 |
| Jejunum | 5.32 ± 0.26 (n = 10) | 5.71 ± 0.41 (n = 11) | 4.86 ± 0.36 |
| Ileum | 4.47 ± 0.17 (n = 10) | 4.66 ± 0.27 (n = 11) | 4.51 ± 0.33 |
| Colon | 4.96 ± 0.10 (n = 10) | 4.70 ± 0.10 (n = 11) | 5.04 ± 0.20 |
| Caecum | 5.71 ± 0.10 (n = 10) | 5.48 ± 0.19 (n = 11) | 5.73 ± 0.33 |
| Rectum | 4.27 ± 0.06 (n = 10) | 4.43 ± 0.09 (n = 11) | 4.33 ± 0.08 |
P < 0.05
when compared to Non-expanded control with one-tailed Student's t test (one-tailed test used because one could only expect volumes to increase relative to Non-expanded control)
when compared to Non-expanded control with one-way ANOVA.
Figure 3. Normalized clearances in GI tissues.
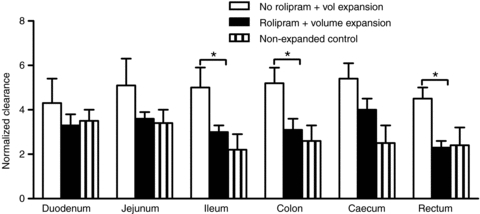
The normalized clearances in GI segments (30 min clearance divided by local vascular volume, Tables 2 and 3) indicate significant rolipram-dependent decreases in real vascular permeability in 3 of the 6 GI segments (*P < 0.05).
Table 4 summarizes 30 min clearances with and without rolipram pretreatment in heart, spleen, lung and kidney of male mice. There was no significant action of rolipram to reduce 30 min albumin clearance in volume-expanded animals in these tissues. Thus these tissues do not contribute significantly to selective volume retention with rolipram pretreatment. The lack of measurable change in these results does not rule out an action of ANP or rolipram to modify microvascular function in other tissues, such as coronary microvessels, under non-volume-expanded conditions (Huxley et al. 2007).
Table 4.
Thirty minute albumin clearances (μl (g dry wt)−1), local vascular volumes (μl (g dry wt)−1), normalized clearance and water content (ml (g dry wt)−1) in heart, spleen, lung and kidney
| No rolipram + volume expansion | Rolipram + volume expansion | |
|---|---|---|
| Clearance | ||
| Heart | 230.6 ± 26.4 (n = 10) | 214.0 ± 18.7 (n = 11) |
| Spleen | 478.8 ± 33.4 (n = 10) | 496.4 ± 38.3 (n = 11) |
| Lung | 448.3 ± 38.9 (n = 9) | 497.5 ± 65.2 (n = 9) |
| Kidney | 486.6 ± 34.2 (n = 10) | 485.2 ± 20.3 (n = 11) |
| Vascular volume | ||
| Heart | 189.0 ± 17.0 (n = 10) | 233.6 ± 22.9 (n = 11) |
| Spleen | 296.7 ± 29.5 (n = 10) | 350.8 ± 16.0 (n = 11) |
| Lung | 835.1 ± 89.4 (n = 9) | 1145.7 ± 133.0 (n = 9) |
| Kidney | 461.4 ± 32.0 (n = 10) | 451.0 ± 37.1 (n = 11) |
| Normalized clearance | ||
| Heart | 1.33 ± 0.21 (n = 10) | 0.98 ± 0.11 (n = 11) |
| Spleen | 1.79 ± 0.25 (n = 10) | 1.44 ± 0.13 (n = 11) |
| Lung | 0.61 ± 0.07 (n = 8) | 0.53 ± 0.13 (n = 9) |
| Kidney | 1.09 ± 0.09 (n = 10) | 1.13 ± 0.08 (n = 11) |
| Water content | ||
| Heart | 3.37 ± 0.10 (n = 9) | 3.36 ± 0.10 (n = 11) |
| Spleen | 4.75 ± 0.08 (n = 10) | 4.84 ± 0.06 (n = 11) |
| Lung | 5.03 ± 0.34 (n = 9) | 5.10 ± 0.22 (n = 9) |
| Kidney | 4.40 ± 0.11 (n = 10) | 4.36 ± 0.09 (n = 11) |
Table 1 also includes measurements of total tissue water content for all treatments in muscle and skin on a gram dry weight basis. In both hamstring and quadriceps muscle there was a small, but significant, increase in water content following volume expansion equal to close to 5% of total water content. We show in the Discussion that this increase in water content would account for infused fluid lost from the plasma volume during the initial volume expansion with or without rolipram. This increase was not modified by pretreatment with rolipram suggesting it occurred mainly due to increased surface area for exchange. We did not measure any consistent change in the water content of other tissues including skin (Table 1) and GI segments (Table 3). This does not rule out small fluid shifts of up to 2% of total water content during volume expansion or after rolipram treatment because the corresponding changes in wet/dry weight are beyond the resolution of our measurements. Finally we note that it has been suggested in some animal models that the spleen is an important site of ANP action to control plasma volume (Hamza & Kaufman, 2009). We measured a small increase in spleen weight with volume expansion (68.7 ± 0.4 mg with volume expansion vs. 67.3 ± 2.8 mg control), but there was no significant effect of rolipram on spleen weight showing that the spleen did not contribute to the action of rolipram to conserve the expanded plasma volume.
Discussion
The main new result is that the PDE4 inhibitor rolipram attenuated the fluid loss from the vascular space when the vascular volume was expanded by intravenous infusion of a balanced salt solution containing 4.5% albumin. The result demonstrates that, after intravenous fluid infusion, agents which stabilize endothelial barriers attenuate fluid loss from the vascular compartment in male mice. Although one of the mechanisms which shift fluid and protein from the vascular to the extravascular space during volume expansion is simply an increased surface area for exchange in the expanded vascular space, we conclude that the main mechanism to retain the fluid in the vascular space after rolipram pretreatment is attenuation of the physiological action of endogenously released ANP to increase vascular permeability. Although rolipram pretreatment is likely to attenuate increases in both water permeability (hydraulic conductivity) and albumin permeability, our results suggest that the reduction in albumin permeability is the modulator of fluid retention (see further discussion in section on ANP mechanisms below). One reason changes in the hydraulic conductivity do not appear to be the main mechanism is that the volume of fluid lost from the vascular space at the end of the vascular expansion is similar in mice with and without rolipram pretreatment. If the action of rolipram to reduce hydraulic conductivity induced significant fluid retention in the plasma volume, we would expect fluid loss from the plasma space at the end of intravenous infusion to be less in the rolipram-pretreated animals. The conclusion that the action of ANP is mainly to modulate albumin does not mean that other ANP-dependent mechanisms that may be modified by rolipram pretreatment are not active, but it does indicate that their contribution to the retention of extra infused fluid in the vascular compartment is relatively small. These mechanisms are considered first, before more detailed evaluation of the actions of ANP and rolipram to regulate vascular permeability and plasma volume.
Other actions of rolipram on ANP-dependent mechanisms after volume expansion
Phosphodiesterase inhibitors acting to modulate intracellular cAMP may modify ANP actions on renal water excretion (Smith & Scott, 2006; Stefan et al. 2007), vasodilatation (Somlyo et al. 2004; Curry et al. 2010; Zieba et al. 2011), and the release of ANP from atrial cells (Cui et al. 2002a; Wen et al. 2004). Here we found renal water excretion in volume expansion was reduced by rolipram pretreatment, but the measured water excretion after volume expansion was still significantly elevated above control with no volume expansion, and the reduction by rolipram pretreatment was too small to account for the measured vascular fluid retention. It is also known that increased cAMP in perfused heart atria can reduce ANP release (Cui et al. 2002b). Reduced ANP release would lower circulating ANP levels and thereby reduce the expected ANP-dependent increase in vascular permeability independent of actions of cAMP on endothelium (Tucker et al. 1992). However, it has been demonstrated that at least two different PDE4 inhibitors (one being rolipram) did not significantly change ANP release from heart atria even though cAMP efflux from the atria was increased (Cui et al. 2002a). Under the same conditions, phosphodiesterase 3 inhibitors did decrease atrial ANP release. These authors concluded that intracellular cAMP is compartmentalized in the regulation of atrial ANP release, and that the release is controlled by a phosphodiesterase subtype-specific mechanism that is independent of PDE4. Finally we note that the well-known action of ANP to induce vasodilatation is not likely to be inhibited by agents that increase cAMP which itself tends to relax vascular smooth muscle (Lugnier & Komas, 1993). On the basis of these observations we focus on the ANP-dependent mechanisms that increase vascular permeability and modify changes in vascular volume in this discussion.
Comparison with previous studies
The action of rolipram to attenuate increased vascular permeability during volume expansion when ANP is released is consistent with our previous observation that the phosphodiesterase 4 inhibitor rolipram inhibited the action of exogenous ANP to increase vascular permeability in multiple organs of the mouse (Lin et al. 2011). In the same experiments, rolipram pretreatment completely blocked the ANP-induced reduction of plasma volume in mice exposed to ANP with no volume expansion. In these previous experiments, the rate of infusion of exogenous ANP was set to achieve circulating levels of exogenous ANP close to those measured in mice using the same intravenous infusion as used in the current experiments (Schreier et al. 2008). Our new results demonstrate that rolipram acts to attenuate the action of endogenously released ANP. Furthermore, our results showing retention of fluid in the vascular compartment are consistent with the observations of Schreier et al. (2008) that volume expansion causes more sustained elevation of central venous pressure in mice lacking ANP receptors in endothelial cells (EC GC-A KO mice). Similar to the rolipram-treated mice in the present experiments, the EC GC-A KO mice fail to increase vascular permeability when ANP levels are elevated. Experiments in both wild-type and genetically modified mice build systematically on the extensive previous investigations in other animal models (including dogs and rats) demonstrating ANP-dependent increases in vascular permeability with volume expansion (Tucker et al. 1992; Renkin & Tucker, 1998) and that infusion of exogenous ANP to achieve similar plasma concentrations causes similar vascular permeability increases (Kishimoto et al. 1996). The novel result in the present experiments is the demonstration that the physiological action of ANP to restore plasma volume after an acute increase in plasma volume is largely prevented by attenuating the action of ANP to increase permeability using a pharmacological agent to stabilize the endothelial barrier. Rolipram is one agent that increases cAMP and stabilizes the barrier.
Relative contributions of different organs to volume expansion-dependent whole body albumin clearance, vascular fluid retention, and role of renal water excretion
Table 5 summarizes the calculations of whole body albumin clearance taking into account individual tissue weights to obtain representative values in different organs during volume expansion and rolipram-modulated changes in albumin distribution. In the baseline state total albumin clearance from muscle, skin, kidney, heart and GI tissues was 134 μl in 30 min. Volume expansion increased the albumin clearance to 208 μl in 30 min. Rolipram pretreatment reduced the albumin clearance in the presence of expanded plasma volume to 151 μl. Therefore, the attenuation of ANP-induced albumin clearance by rolipram is 57 μl. The tissues that contribute most significantly to the reduction in total clearance are muscle and skin which together account for 70% of the total reduction (39.7/57) while the GI tissues contribute 29% (16.4/57). We conclude that although tissues such as GI have a high albumin clearance on a per gram basis, muscle and skin are the main sites determining blood-to-tissue redistribution of albumin because they make up 60% of the total body weight. This is consistent with our previous data comparing albumin clearances in EC GC-A KO mice and wild-type mice in response to exogenous ANP (Curry et al. 2010).
Table 5.
Contribution of tissues to total albumin clearance (all values are μl (30 min)−1 (30 g BW)−1)
| Tissue weight (g) | Non-volume expanded | No rolipram + volume expansion | Rolipram + volume expansion | |
|---|---|---|---|---|
| Muscle | 12 | 27.6 | 58.4 | 40.5 |
| Skin | 6 | 38.0 | 46.8 | 25.0 |
| Kidney | 0.3 | 40.5 | 36.5 | 36.4 |
| Heart | 0.1 | 4.5 | 5.8 | 5.4 |
| GI | 0.5 | 23.7 | 60.3 | 43.9 |
| Total | 134.3 | 207.8 | 151.2 | |
| Spleen | 0.07 | 8.4 | 8.7 | |
| Lung | 0.1 | 11.2 | 12.4 |
To calculate total tissue clearance by adding the contributions from each tissue take the mean 30 min clearance on a dry weight basis (Tables 1, 2 or 4), divide by wet/dry ratio (2.5 for skin and 4 for all other tissues) to give clearance on a per gram wet basis, then multiply by average tissue weight.
We demonstrated that the reduction in urine water excretion was insufficient to account for fluid retention in the vascular space. However, our measurements do show that urine flow was increased in response to plasma volume expansion. Control urine flow was close to 1.0 μl min−1. To estimate a minimum urine flow rate, we assumed the increase in urine flow above control occurred over the period of volume expansion and subsequent fluid retention. For example if we assume the increase of urine volume in mice with volume expansion and no rolipram pretreatment measured at the end of a period of 120 min relative to control (184–121 = 63 μl) occurred over the 90 min of fluid infusion and subsequent volume retention as in Fig. 1 (i.e. an increase of 73/90 = 0.8 μl min−1) the average urine flow during and after volume expansion was 1.7 μl min−1, nearly twice that in non-volume-expanded animals. The corresponding average urine flow with rolipram pretreatment over 90 min was 1.4 μl min−1. In preliminary experiments, we also measured urine excretion at the end of the shorter period of volume retention and estimated average urine flows of 3 μl min−1 over the 35 min (15 min of infusion plus 20 min of retention; see Fig. 1). This 3-fold increase in urine flow relative to control is the same as the 3-fold increase measured during previous investigations using exogenous ANP infusion.
It is important to emphasize that plasma volume was maintained in animals with rolipram pretreatment even though renal water excretion remained above normal values. Specifically, in the presence of rolipram 158 μl of fluid were excreted over 120 min and ultimately this volume must have been reabsorbed from the interstitial space. We and others (Renkin & Tucker, 1996; Curry et al. 2010) have argued that this reabsorption occurs when excretion of protein-free urine concentrates the plasma proteins thereby favouring an increase in plasma colloid osmotic pressure, a Starling force favouring transient fluid movement from interstitium to blood. ANP normally acts to offset this increase in plasma protein concentration by increasing exchange of plasma protein into the interstitial space and fluid is then preferentially lost from the plasma volume (Tucker et al. 1992; Sabrane et al. 2005; Curry et al. 2010; Lin et al. 2011). Our experiments show that, by attenuating ANP-dependent albumin clearance, rolipram blocked preferential plasma fluid loss in the presence of elevated ANP. Specifically the increase in blood-to-tissue albumin clearance above control (27 μl over 120 min) is less than the increase in renal water excretion (37 μl) over 120 min. In other words rolipram pretreatment favours conditions where the plasma osmotic pressure increases. This increase would be offset by blood-to-tissue exchange in the presence of ANP.
Mechanism of actions of ANP and rolipram after volume expansion: changes in permeability vs. change in area for exchange
There are several mechanisms where ANP can increase permeability. First, ANP increases cGMP levels by activating the guanylyl cyclase-A (GC-A) receptor, which can cause cAMP hydrolysis by stimulating cGMP-dependent PDE2 (Lugnier, 2006; Surapisitchat et al. 2007). Second, ANP clearance receptor (NPR-C) may reduce cAMP level by inhibiting adenylyl cyclase (Hempel et al. 1998). Rolipram will counter the fall in ANP-stimulated cAMP levels by inhibiting the PDE4-dependent hydrolysis of cAMP (Suttorp et al. 1993; Netherton & Maurice, 2005). Because cAMP regulates the stability of intercellular junctions via the Epac/Rap1/Rac pathway (Bos, 2005; Cullere et al. 2005; Adamson et al. 2008), the tendency to increase permeability of intercellular junctions as local levels of cAMP fall is offset by the reduced cAMP hydrolysis due to inhibition of PDE 4 activity. On the other hand, in tissues such as GI, the increase in albumin clearance with plasma volume expansion could be due to the predominant effect of ANP to relax vascular smooth muscle, causing vasodilatation in these tissues, and increase clearance resulting from increasing surface area for exchange with no significant increase in vascular permeability (Renkin & Tucker, 1998). The failure of rolipram to significantly attenuate ANP action in some GI tissue is understandable where this action predominates because rolipram itself would cause vasodilatation if it raised cAMP levels in vascular smooth muscle (β2-adrenergic effect). An increase in the area for exchange would also account for some of the initial loss of infused fluid which was similar in mice with volume expansion with and without rolipram pretreatment (i.e. at the end of the volume expansion, the plasma volumes were expanded by similar amounts, 560 μl untreated and 532 μl treated).
Other mechanisms not considered above
Finally we briefly comment on observations not emphasized above. As noted above, up to 300–320 μl of infused fluid left the vascular space (855 μl infused, 530–560 μl retained) during the initial volume expansion with and without rolipram pretreatment. There was a significant increase in muscle water content corresponding to an average of about 0.2 ± 0.1 ml (g dry wt)−1 (Table 1). This is enough to account for 300–320 μl lost during the first 15 min of volume expansion if the water content increased in half the total muscle mass in the body to a similar amount. This increased water content was not reduced by rolipram pretreatment suggesting that it was not due to increased vascular permeability. One likely mechanism was a passive increase in the surface area for exchange as more microvessels were perfused during the initial volume expansion. Another possible mechanism is an increase in capillary pressure. A similar mechanism may result in passive fluid loss in other tissues. We have argued above that increased area for exchange is not likely to be modified by rolipram which would tend to relax vascular smooth muscle. The same argument applies to increases in capillary pressure. Thus it is unlikely that changes in solvent drag (albumin flux coupled to water flow) contribute significantly to rolipram action.
In skin and other tissues we measured no significant changes in tissue water content. This is understandable if most of the water accumulates in muscle as suggested above, and other fluid volumes (of the order of 100–200 μl) are distributed into at least 10 g of tissue (wet weight of non-muscle tissue). This would result in an increase of, at most, 2% of water content, and measurement of such a small increase is beyond the resolution of our methods. Also we did not discuss albumin clearance in lung, kidney, liver, heart or spleen as part of the rolipram-modulated mechanisms. We have previously noted that changes in liver uptake probably represent scavenging of modified albumin unrelated to volume control. We carefully checked changes in albumin clearance and water content of mouse spleen and associated lymphatics and found no significant changes with rolipram pretreatment. We also previously noted that while it is possible that ANP and rolipram modulate changes in microvascular pressures in the renal medulla (Tenstad et al. 2001; Lin et al. 2011), their net effect to move albumin out of the plasma space over time periods comparable to our experiments is expected to be small. Finally, the albumin clearance, vascular volume, and water content in the lung with rolipram all tended to increase rather than decrease, suggesting that there was no significant contribution of the lung to fluid retention and that change in surface area rather than change in permeability was the predominant mechanism.
Application to management of vascular volume
Taken together with our previous experiments showing that rolipram attenuated the loss of plasma volume when exogenous ANP was infused, the present results suggest that further investigations of the action of agents that stabilize the endothelial barrier when ANP levels are elevated may lead to better control of plasma volume in some clinical situations. For example, plasma volume is often expanded prior to and during surgery to help maintain plasma volume, but this can result in significant fluid shift into the extravascular space and unwanted tissue fluid accumulation. In this situation the use of phosphodiesterase inhibitors which act preferentially to reduce cAMP hydrolysis in vascular endothelium may be appropriate. We are not aware of this approach but we note that β-adrenergic agents that are known to raise cAMP in endothelium have been shown to preserve plasma fluid volume after the use of intravenous fluids that do not contain colloid (Vane et al. 2004). It is not clear that a similar strategy would be appropriate in more chronic conditions such as heart failure.
Acknowledgments
This work was supported by a grant from the NIH National Heart, Lung, and Blood Institute (HL28607), and by pilot funds from the Clinical and Translational Science Centre, UC Davis, made possible by a grant from the National Centre for Research Resources (UL1 RR024146 and HL 28607).
Glossary
Abbreviations
- ANP
atrial natriuretic peptide3
- BSA
bovine serum albumin
- BW
body weight
- EC
endothelial cell
- GC-A
guanylyl cyclase-A
- GI
gastrointestinal
- KO
knock-out
- PBS
phosphate-buffered saline
- PDE4
phosphodiesterase 4
Author contributions
Y.-C.L., F.E.C., R.K.R. and R.H.A. contributed to the conception and design of experiments. J.F.C., Y.-C.L., R.H.A., F.E.C. and R.K.R. contributed to the collection, analysis and interpretation of experiments. Y.-C.L. wrote the initial draft of the manuscript and F.E.C. and R.H.A. contributed to writing and revising the manuscript. The experiments were carried out at University of California, Davis. All authors approved the final version of the manuscript.
References
- Adamson RH, Huxley VH, Curry FE. Single capillary permeability to proteins having similar size but different charge. Am J Physiol Heart Circ Physiol. 1988;254:H304–H312. doi: 10.1152/ajpheart.1988.254.2.H304. [DOI] [PubMed] [Google Scholar]
- Adamson RH, Ly JC, Sarai RK, Lenz JF, Altangerel A, Drenckhahn D, Curry FE. Epac/Rap1 pathway regulates microvascular hyperpermeability induced by PAF in rat mesentery. Am J Physiol Heart Circ Physiol. 2008;294:H1188–H1196. doi: 10.1152/ajpheart.00937.2007. [DOI] [PubMed] [Google Scholar]
- Almeida FA, Suzuki M, Maack T. Atrial natriuretic factor increases hematocrit and decreases plasma volume in nephrectomized rats. Life Sci. 1986;39:1193–1199. doi: 10.1016/0024-3205(86)90351-6. [DOI] [PubMed] [Google Scholar]
- Alvarez A, Piqueras L, Blazquez MA, Sanz MJ. Cyclic AMP elevating agents and nitric oxide modulate angiotensin II-induced leukocyte-endothelial cell interactions in vivo. Br J Pharmacol. 2001;133:485–494. doi: 10.1038/sj.bjp.0704096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter GF. The natriuretic peptides. Basic Res Cardiol. 2004;99:71–75. doi: 10.1007/s00395-004-0457-8. [DOI] [PubMed] [Google Scholar]
- Bingaman S, Huxley VH, Rumbaut RE. Fluorescent dyes modify properties of proteins used in microvascular research. Microcirculation. 2003;10:221–231. doi: 10.1038/sj.mn.7800186. [DOI] [PubMed] [Google Scholar]
- Bos JL. Linking Rap to cell adhesion. Curr Opin Cell Biol. 2005;17:123–128. doi: 10.1016/j.ceb.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Brenner BM, Ballermann BJ, Gunning ME, Zeidel ML. Diverse biological actions of atrial natriuretic peptide. Physiol Rev. 1990;70:665–699. doi: 10.1152/physrev.1990.70.3.665. [DOI] [PubMed] [Google Scholar]
- Cui X, Wen JF, Jin H, Li D, Jin JY, Kim SH, Kim SZ, Lee HS, Cho KW. Subtype-specific roles of cAMP phosphodiesterases in regulation of atrial natriuretic peptide release. Eur J Pharmacol. 2002a;451:295–302. doi: 10.1016/s0014-2999(02)02294-x. [DOI] [PubMed] [Google Scholar]
- Cui X, Wen JF, Jin JY, Xu WX, Kim SZ, Kim SH, Lee HS, Cho KW. Protein kinase-dependent and Ca2+-independent cAMP inhibition of ANP release in beating rabbit atria. Am J Physiol Regul Integr Comp Physiol. 2002b;282:R1477–R1489. doi: 10.1152/ajpregu.00316.2001. [DOI] [PubMed] [Google Scholar]
- Cullere X, Shaw SK, Andersson L, Hirahashi J, Luscinskas FW, Mayadas TN. Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood. 2005;105:1950–1955. doi: 10.1182/blood-2004-05-1987. [DOI] [PubMed] [Google Scholar]
- Curry FR, Rygh CB, Karlsen T, Wiig H, Adamson RH, Clark JF, Lin YC, Gassner B, Thorsen F, Moen I, Tenstad O, Kuhn M, Reed RK. Atrial natriuretic peptide modulation of albumin clearance and contrast agent permeability in mouse skeletal muscle and skin: role in regulation of plasma volume. J Physiol. 2010;588:325–339. doi: 10.1113/jphysiol.2009.180463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981;28:89–94. doi: 10.1016/0024-3205(81)90370-2. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwood W, Sun J, Barnes PJ, Giembycz MA, Chung KF. Inhibition of allergen-induced lung eosinophilia by type-III and combined type III- and IV-selective phosphodiesterase inhibitors in brown-Norway rats. Inflamm Res. 1995;44:83–86. doi: 10.1007/BF01793218. [DOI] [PubMed] [Google Scholar]
- Fluckiger JP, Waeber B, Matsueda G, Delaloye B, Nussberger J, Brunner HR. Effect of atriopeptin III on hematocrit and volemia of nephrectomized rats. Am J Physiol Heart Circ Physiol. 1986;251:H880–H883. doi: 10.1152/ajpheart.1986.251.4.H880. [DOI] [PubMed] [Google Scholar]
- Hamza SM, Kaufman S. Role of spleen in integrated control of splanchnic vascular tone: physiology and pathophysiology. Can J Physiol Pharmacol. 2009;87:1–7. doi: 10.1139/Y08-103. [DOI] [PubMed] [Google Scholar]
- Hempel A, Noll T, Bach C, Piper HM, Willenbrock R, Hohnel K, Haller H, Luft FC. Atrial natriuretic peptide clearance receptor participates in modulating endothelial permeability. Am J Physiol Heart Circ Physiol. 1998;275:H1818–H1825. doi: 10.1152/ajpheart.1998.275.5.H1818. [DOI] [PubMed] [Google Scholar]
- Huxley VH, Tucker VL, Verburg KM, Freeman RH. Increased capillary hydraulic conductivity induced by atrial natriuretic peptide. Circ Res. 1987;60:304–307. doi: 10.1161/01.res.60.2.304. [DOI] [PubMed] [Google Scholar]
- Huxley VH, Wang JJ, Sarelius IH. Adaptation of coronary microvascular exchange in arterioles and venules to exercise training and a role for sex in determining permeability responses. Am J Physiol Heart Circ Physiol. 2007;293:H1196–H1205. doi: 10.1152/ajpheart.00069.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsky MP, Vaid SU, Vane LA, Prough DS, Kramer GC. Effect of esmolol on fluid therapy in normovolemia and hypovolemia. Shock. 2008;30:55–63. doi: 10.1097/SHK.0b013e31815d1a85. [DOI] [PubMed] [Google Scholar]
- Kishimoto I, Dubois SK, Garbers DL. The heart communicates with the kidney exclusively through the guanylyl cyclase-A receptor: acute handling of sodium and water in response to volume expansion. Proc Natl Acad Sci U S A. 1996;93:6215–6219. doi: 10.1073/pnas.93.12.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YC, Samardzic H, Adamson RH, Renkin EM, Clark JF, Reed RK, Curry FR. Phosphodiesterase 4 inhibition attenuates atrial natriuretic peptide-induced vascular hyperpermeability and loss of plasma volume. J Physiol. 2011;589:341–353. doi: 10.1113/jphysiol.2010.199588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugnier C. Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutic agents. Pharmacol Ther. 2006;109:366–398. doi: 10.1016/j.pharmthera.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Lugnier C, Komas N. Modulation of vascular cyclic nucleotide phosphodiesterases by cyclic GMP: role in vasodilatation. Eur Heart J. 1993;14(Suppl. I):141–148. [PubMed] [Google Scholar]
- Nedrebo T, Reed RK. Different serotypes of endotoxin (lipopolysaccharide) cause different increases in albumin extravasation in rats. Shock. 2002;18:138–141. doi: 10.1097/00024382-200208000-00008. [DOI] [PubMed] [Google Scholar]
- Nedrebo T, Reed RK, Berg A. Effect of α trinositol on interstitial fluid pressure, edema generation, and albumin extravasation after ischemia-reperfusion injury in rat hind limb. Shock. 2003;20:149–153. doi: 10.1097/01.shk.0000072128.33223.15. [DOI] [PubMed] [Google Scholar]
- Netherton SJ, Maurice DH. Vascular endothelial cell cyclic nucleotide phosphodiesterases and regulated cell migration: implications in angiogenesis. Mol Pharmacol. 2005;67:263–272. doi: 10.1124/mol.104.004853. [DOI] [PubMed] [Google Scholar]
- Pearse RM, Rhodes A, Grounds RM. Clinical review: how to optimize management of high-risk surgical patients. Crit Care. 2004;8:503–507. doi: 10.1186/cc2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renkin EM, Tucker VL. Atrial natriuretic peptide as a regulator of transvascular balance. News Physiol Sci. 1996;11:138–143. [Google Scholar]
- Renkin EM, Tucker VL. Measurement of microvascular transport parameters of macromolecules in tissues and organs of intact animals. Microcirculation. 1998;5:139–152. [PubMed] [Google Scholar]
- Rumbaut RE, Harris NR, Sial AJ, Huxley VH, Granger DN. Leakage responses to l NAME differ with the fluorescent dye used to label albumin. Am J Physiol Heart Circ Physiol. 1999;276:H333–H339. doi: 10.1152/ajpheart.1999.276.1.H333. [DOI] [PubMed] [Google Scholar]
- Sabrane K, Kruse MN, Fabritz L, Zetsche B, Mitko D, Skryabin BV, Zwiener M, Baba HA, Yanagisawa M, Kuhn M. Vascular endothelium is critically involved in the hypotensive and hypovolemic actions of atrial natriuretic peptide. J Clin Invest. 2005;115:1666–1674. doi: 10.1172/JCI23360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier B, Borner S, Volker K, Gambaryan S, Schafer SC, Kuhlencordt P, Gassner B, Kuhn M. The heart communicates with the endothelium through the guanylyl cyclase-A receptor: acute handling of intravascular volume in response to volume expansion. Endocrinology. 2008;149:4193–4199. doi: 10.1210/en.2008-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith FD, Scott JD. Anchored cAMP signaling: onward and upward – a short history of compartmentalized cAMP signal transduction. Eur J Cell Biol. 2006;85:585–592. doi: 10.1016/j.ejcb.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Somlyo AV, Khromov AS, Webb MR, Ferenczi MA, Trentham DR, He ZH, Sheng S, Shao Z, Somlyo AP. Smooth muscle myosin: regulation and properties. Philos Trans R Soc Lond B Biol Sci. 2004;359:1921–1930. doi: 10.1098/rstb.2004.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan E, Wiesner B, Baillie GS, Mollajew R, Henn V, Lorenz D, Furkert J, Santamaria K, Nedvetsky P, Hundsrucker C, Beyermann M, Krause E, Pohl P, Gall I, MacIntyre AN, Bachmann S, Houslay MD, Rosenthal W, Klussmann E. Compartmentalization of cAMP-dependent signaling by phosphodiesterase-4D is involved in the regulation of vasopressin-mediated water reabsorption in renal principal cells. J Am Soc Nephrol. 2007;18:199–212. doi: 10.1681/ASN.2006020132. [DOI] [PubMed] [Google Scholar]
- Surapisitchat J, Jeon KI, Yan C, Beavo JA. Differential regulation of endothelial cell permeability by cGMP via phosphodiesterases 2 and 3. Circ Res. 2007;101:811–818. doi: 10.1161/CIRCRESAHA.107.154229. [DOI] [PubMed] [Google Scholar]
- Suttorp N, Weber U, Welsch T, Schudt C. Role of phosphodiesterases in the regulation of endothelial permeability in vitro. J Clin Invest. 1993;91:1421–1428. doi: 10.1172/JCI116346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira MM, Rossi AG, Williams TJ, Hellewell PG. Effects of phosphodiesterase isoenzyme inhibitors on cutaneous inflammation in the guinea-pig. Br J Pharmacol. 1994;112:332–340. doi: 10.1111/j.1476-5381.1994.tb13073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenstad O, Heyeraas KJ, Wiig H, Aukland K. Drainage of plasma proteins from the renal medullary interstitium in rats. J Physiol. 2001;536:533–539. doi: 10.1111/j.1469-7793.2001.0533c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker VL, Simanonok KE, Renkin EM. Tissue-specific effects of physiological ANP infusion on blood-tissue albumin transport. Am J Physiol Regul Integr Comp Physiol. 1992;263:R945–R953. doi: 10.1152/ajpregu.1992.263.4.R945. [DOI] [PubMed] [Google Scholar]
- Vane LA, Prough DS, Kinsky MA, Williams CA, Grady JJ, Kramer GC. Effects of different catecholamines on the dynamics of volume expansion of crystalloid infusion. Anesthesiology. 2004;101:1136–1144. doi: 10.1097/00000542-200411000-00013. [DOI] [PubMed] [Google Scholar]
- Wen JF, Cui X, Jin JY, Kim SM, Kim SZ, Kim SH, Lee HS, Cho KW. High and low gain switches for regulation of cAMP efflux concentration: distinct roles for particulate GC- and soluble GC-cGMP-PDE3 signaling in rabbit atria. Circ Res. 2004;94:936–943. doi: 10.1161/01.RES.0000123826.70125.4D. [DOI] [PubMed] [Google Scholar]
- Zieba BJ, Artamonov MV, Jin L, Momotani K, Ho R, Franke AS, Neppl RL, Stevenson AS, Khromov AS, Chrzanowska-Wodnicka M, Somlyo AV. The cAMP-responsive Rap1 guanine nucleotide exchange factor, Epac, induces smooth muscle relaxation by down-regulation of RhoA activity. J Biol Chem. 2011;286:16681–16692. doi: 10.1074/jbc.M110.205062. [DOI] [PMC free article] [PubMed] [Google Scholar]


