Abstract
Non-technical summary
A single bout of resistance exercise stimulates the synthesis of new muscle proteins. Chronic performance of resistance exercise (i.e. weight training) is what makes your muscles grow bigger; a process known as hypertrophy. However, it is unknown if increasing the time that muscle is under tension will lead to greater increases in muscle protein synthesis. We report that leg extension exercise at 30% of the best effort (which is a load that is comparatively light), with a slow lifting movement (6 s up and 6 s down) performed to fatigue produces greater increases in rates of muscle protein synthesis than the same movement performed rapidly (1 s up and 1 s down). These results suggest that the time the muscle is under tension during exercise may be important in optimizing muscle growth; this understanding enables us to better prescribe exercise to those wishing to build bigger muscles and/or to prevent muscle loss that occurs with ageing or disease.
Abstract
We aimed to determine if the time that muscle is under loaded tension during low intensity resistance exercise affects the synthesis of specific muscle protein fractions or phosphorylation of anabolic signalling proteins. Eight men (24 ± 1 years (sem), BMI = 26.5 ± 1.0 kg m−2) performed three sets of unilateral knee extension exercise at 30% of one-repetition maximum strength involving concentric and eccentric actions that were 6 s in duration to failure (SLOW) or a work-matched bout that consisted of concentric and eccentric actions that were 1 s in duration (CTL). Participants ingested 20 g of whey protein immediately after exercise and again at 24 h recovery. Needle biopsies (vastus lateralis) were obtained while fasted at rest and after 6, 24 and 30 h post-exercise in the fed-state following a primed, constant infusion of l-[ring-13C6]phenylalanine. Myofibrillar protein synthetic rate was higher in the SLOW condition versus CTL after 24–30 h recovery (P < 0.001) and correlated to p70S6K phosphorylation (r = 0.42, P = 0.02). Exercise-induced rates of mitochondrial and sarcoplasmic protein synthesis were elevated by 114% and 77%, respectively, above rest at 0–6 h post-exercise only in the SLOW condition (both P < 0.05). Mitochondrial protein synthesis rates were elevated above rest during 24–30 h recovery in the SLOW (175%) and CTL (126%) conditions (both P < 0.05). Lastly, muscle PGC-1α expression was increased at 6 h post-exercise compared to rest with no difference between conditions (main effect for time, P < 0.001). These data show that greater muscle time under tension increased the acute amplitude of mitochondrial and sarcoplasmic protein synthesis and also resulted in a robust, but delayed stimulation of myofibrillar protein synthesis 24–30 h after resistance exercise.
Introduction
High intensity resistance exercise is an effective stimulus to increase muscle protein synthesis rates (Chesley et al. 1992; Phillips et al. 1997; Kumar et al. 2009). We recently demonstrated that lifting relatively low loads (∼30% of maximum strength) to the point of fatigue was equally effective as high intensity resistance exercise for stimulating rates of myofibrillar protein synthesis (Burd et al. 2010b). In fact, performance of lower intensity contractions allows for higher total work by the time fatigue is achieved because the rate of fatigue increases exponentially with intensity (Fuglevand et al. 1993). Hence, the stimulus for myofibrillar protein synthesis, in its basic physiological nature, appears to be a matter of muscle fibre recruitment, with exercise performed to fatigue resulting in eventual maximal fibre recruitment (Burd et al. 2010b). This thesis is consistent with the work of Henneman et al. (1965) which demonstrated that motor units, and thus muscle fibres, are recruited in accordance with the size principle during voluntary contractions. Also, our recent work suggests that the eventual elicitation of full muscle fibre recruitment, during fatiguing acute resistance exercise, is integral to inducing an enhanced sensitivity of myofibrillar protein synthesis to protein feeding during longer-term (i.e. 24 h later) exercise recovery (Burd et al. 2011a).
Based on our previous findings (Burd et al. 2010b, 2011a) and as a further test of the thesis that complete muscle fibre recruitment is an important driver of myofibrillar protein synthesis rates, we manipulated the time that loaded muscle was under tension during low intensity resistance exercise using a slow cadence to achieve fatigue (i.e. maximal fibre activation) and compared post-exercise muscle protein synthetic responses, intramuscular signalling, and PGC1α mRNA responses with a work-matched condition that was performed using a faster lifting cadence that did not result in fatigue. We did not confine our conclusions to the mixed muscle protein synthetic response. Instead, we studied the responses in the myofibrillar, sarcoplasmic and mitochondrial enriched protein fractions. This approach allowed for the characterization of the muscle protein synthetic responses at the fraction-specific level to underpin the true acute phenotypic response during exercise recovery (Wilkinson et al. 2008; Burd et al. 2010b). We hypothesized that a longer time under muscle tension leading to fatigue, and thus ‘full’ muscle fibre recruitment, will result in greater increases in rates of muscle protein synthesis (i.e. myofibrillar, mitochondrial and sarcoplasmic), intramuscular signalling protein phosphorylation, and PGC-1α mRNA responses compared to a low-intensity external work-matched control condition. Certainly, to study the effects of contractile intensity and muscle fibre recruitment on muscle protein synthesis rates in vivo in humans has its inherent limitations. An assumption made with the methodology employed is that a small population of sampled muscle fibres reflects that of the entire vastus lateralis.
Methods
Participants
Eight recreationally resistance-trained men (23.5 ± 1 years (sem); 88.3 ± 5 kg; BMI = 26.5 ± 1.0 kg m−2) were recruited for the study. Participants were habitually active and engaged in lower body resistance exercise at least 2 times per week for ≥2 years at the time of the study. All participants were deemed healthy based on their response to a routine medical screening questionnaire. We chose to recruit resistance-trained subjects to increase the reliability of our strength measurements and to eliminate the potential for non-specific muscle protein synthetic responses due to the novelty of a resistance exercise stimulus (Wilkinson et al. 2008). Participants were informed of the purpose of the study, experimental procedures, and all its potential risks prior to providing written consent to participate. The study was approved by the local Research Ethics Board of McMaster University and Hamilton Health Sciences and conformed to standards for the use of human subjects in research as outlined in the fifth Declaration of Helsinki and with current Tri-Council Canadian Government funding agency guidelines for use of human subjects in research.
Experimental protocol
Two weeks prior to the infusion trials, all participants reported to the laboratory for familiarization with the exercise protocol and to establish their unilateral one repetition maximum (1RM) on each leg for knee extension exercise. Participants’ unilateral 1RM for the right and left legs were 105 ± 6 kg and 101 ± 6 kg, respectively (P = 0.7). Each participant recorded his dietary intake for 3 days prior to the resting and exercise infusion trial (trial 1). A unilateral model was used, whereby each individual served as his own comparison with the opposite condition, and including a rested fasted control measurement. This ensured that acute changes in muscle protein synthesis and protein phosphorylation after exercise and feeding were due to the imposed stimuli. On the morning of trial 1 (Fig. 1), participants reported to the laboratory at 07.00 h after an overnight fast and after refraining from any physical activity for the preceding 3 days. An 18-gauge catheter was inserted in an antecubital vein of one arm for blood sampling and kept patent with 0.9% saline drip for repeated blood sampling. After a baseline blood sample was drawn, a second catheter was inserted in the contralateral arm for the primed constant infusion (PHD 2000; Harvard Apparatus, Natick, MA, USA) of l-[ring-13C6]phenylalanine (prime: 2 μmol kg−1; 0.05 μmol kg−1 min−1; Fig. 1), which was passed through a 0.2 μm filter. Except for during the exercise bout, the participants rested comfortably on a bed throughout the infusions. At 3.5 ± 0.1 h after the start of the infusion, a single muscle biopsy was obtained from the dominant leg to measure fasting rates of muscle protein synthesis (Fast). After the biopsy, the participants’ legs were shaved with a hand razor and cleaned with isopropyl alcohol prior to electrode placement. Bipolar self-adhesive Ag/AgCl monitoring electrodes (Kendall Meditrace 133, Chicopee, MA, USA) were placed on the medial portion of the muscle bellies of the vastus lateralis, vastus medialis, and rectus femoris in line with the direction of muscle fibre orientation. The reference electrode was placed on the head of the fibula and electromyography (EMG) was measured during exercise.
Figure 1. Schematic diagram of the experimental infusion protocols.
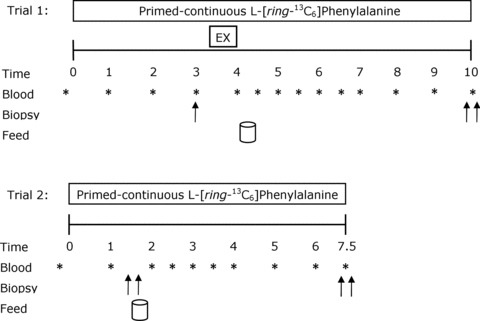
Double arrows indicate bilateral biopsies were obtained at corresponding time points. Subjects consumed 20 g of whey protein isolate at the feeding time points.
Participants subsequently performed bouts of unilateral leg extension exercise at 30% of their previously established concentric 1RM. Legs were randomized and balanced for dominance based on maximal strength to perform exercise at a slow lifting (SLOW) or an external work-matched control (CTL) conditions. The leg assigned to the SLOW condition performed exercise with a lifting/lowering cadence of 6 s concentric phase and a 6 s eccentric phase with no pauses until volitional fatigue (i.e. failure). Failure was defined as the point at which the participant could not lift through the full range, or his technique to lift the load included motions at joints other than the knee. The CTL condition was completed with the contralateral leg and was matched to the experimental condition for contraction volume such that the leg performed an identical number of repetitions at an equivalent load, but not to failure, and was performed with a lifting cadence of 1 s concentric phase and a 1 s eccentric phase. Participants performed three sets with 2 min rest between each set for each condition and were instructed on proper lifting cadence using verbal cues and a metronome. A goniometer was positioned on the leg extension machine to record knee joint angle. The time derivative of the flexion angle (angular velocity) was used to identify the concentric, isometric and eccentric phases of each repetition.
After completion of the exercise, participants returned to the resting position and a blood sample was collected and placed on ice. Subsequently, participants consumed a drink containing 20 g of whey protein isolate (Fonterra Alacen-895-I, Auckland, New Zealand). This amount of protein has been established to maximally stimulate rates of muscle protein synthesis after resistance exercise in young men (Moore et al. 2009a). To minimize disturbances in isotopic equilibrium, the drinks were enriched to 4% with tracer according to a measured phenylalanine content of 3.5% in the whey protein; this approach is explained in detail elsewhere (Burd et al. 2011b). Bilateral biopsies were taken at 6 h after completion of unilateral resistance exercise. After completion of trial 1, participants were fed a meal from the cafeteria that represented ∼2500–3000 kJ and instructed to eat a subsequent meal that was representative of the meals they previously recorded on the 3 day dietary log. This meal was to be consumed no later than 22.00 h to ensure a 10 h fast prior to the beginning of the 24 h post-exercise protein synthesis measurement (trial 2). Participants were instructed to refrain from physical activity for the evening.
In the morning participants returned to the laboratory for trial 2 and underwent the previously described infusion trial procedures. Bilateral biopsies were obtained at 1.5 h after the start of the infusion, followed by the consumption of a tracer-enriched protein drink containing 20 g of whey isolate. Infusion trial 2 was concluded by bilateral biopsies at 6.5 h. Muscle biopsies, all via separate incisions separated by ∼4 cm, were performed with a Bergström needle that was custom-modified for manual suction under local anaesthesia (2% xylocaine). All biopsies were obtained from the vastus lateralis. Biopsy samples were blotted and freed of any visible fat and connective tissue, immediately frozen in liquid nitrogen and stored at −80°C until further analysis. During trials 1 and 2, blood samples drawn every 0.5 or 1 h and were processed as previously described (Moore et al. 2009a) (Fig. 1).
Blood analyses
Plasma [ring-13C6] phenylalanine enrichments were determined as previously described (Glover et al. 2008). Blood amino acid concentrations were analysed by HPLC as previously described (Moore et al. 2005). Blood glucose concentrations were analysed using a blood glucose meter (OneTouch Ultra 2, Lifescan Inc., Milpitas, CA, USA) within 2 min of blood collection. Plasma insulin was measured using a commercially available immunoassay kit (ALPCO Diagnostics, Salem, NH, USA).
Electromyography analyses
The raw electromyographic (EMG) signals were sampled at 1024 Hz, using a custom-made bioamplifier, and were collected with acquisition software (LabVIEW v 8.2; National Instruments, Austin, TX, USA). All raw EMG signals were digitized and stored on an external hard drive and analysed as previously described (Burd et al. 2010a).
Muscle protein synthesis
A piece of wet muscle (∼100 mg) was homogenized with a Dounce glass homogenizer on ice in an ice-cold homogenizing buffer (10 μl mg−1; 1 m sucrose, 1 m Tris/HCl, 1 m KCl, 1 m EDTA) supplemented with a Complete Mini, protease inhibitor and phosphatase cocktail tablets (PhosSTOP, Roche Applied Science, Mannhein, Germany) per 10 ml of buffer. The homogenate was transferred to an Eppendorf tube and spun at 700 g for 15 min at 4°C to pellet a fraction enriched with myofibrillar and cytoskeleton proteins. The supernatant was transferred to another Eppendorf tube and spun at 12,000 g for 20 min at 4°C to pellet the mitochondrial enriched protein fraction. The supernatant was placed in two separate aliquots for intramuscular signalling and sarcoplasmic protein fraction. The sarcoplasmic proteins were precipated by the addition of 1 ml of 1 m perchloric acid (PCA). The mitochondrial enriched pellet was washed twice with 500 μl of an ice-cold homogenizing buffer (1 m sucrose, 1 m Tris/HCl, 1 m KCl, 1 m EGTA/Tris) and spun at 12,000 g for 5 min at 4°C. The supernatant was discarded and the pellet washed with 95% ethanol and spun at 12,000 g for 5 min. The supernatant was discarded and the pellet was lyophilized. Amino acids were liberated by adding 1.5 ml of 6 m HCl and heating to 110°C for 24 h. The myofibrillar and collagen pellets that remained from the initial 700 g spin were washed twice with the homogenization buffer and spun at 700 g for 5 min at 4°C. The supernatant was discarded. The myofibrillar enriched proteins were solubilised by adding 1.5 ml of 0.3 m NaOH and heating to 37°C for 30 min with vortex mixing every 10 min. Samples were centrifuged at 700 g for 5 min at 4°C and the supernatant containing the myofibrillar-enriched fractions was collected and the collagen pellets were discarded. Myofibrillar proteins were precipitated by the addition of 1 ml of 1 m PCA and spinning at 700 g for 10 min at 4°C. The myofibrillar and sarcoplasmic enriched fractions were washed twice with 70% ethanol and the latter was lyophilized. The amino acids were liberated from the myofibrillar, sarcoplasmic, and mitochondrial enriched fractions by adding 1.5 ml of 6 m HCl and heating to 110°C for 24 h.
Free amino acids from myofibrillar, mitochondrial and sarcoplasmic enriched fractions were purified using cation-exchange chromatography (Dowex 50WX8-200 resin; Sigma-Aldrich Ltd) and converted to their N-acetyl-n-propyl ester derivatives for analysis by gas chromatography combustion-isotope ratio mass spectrometry (GC-C-IRMS: Hewlett Packard 6890; IRMS model Delta Plus XP, Thermo Finnagan, Waltham, MA, USA). Muscle intracellular amino acids were extracted from a separate piece of wet muscle (∼20 mg) with ice-cold 0.6 m PCA. Muscle was homogenized on ice with a Teflon-coated pestle and then centrifuged at 12,000 g for 10 min at 4°C. The supernatant was then collected and this process was repeated two more times. All three supernatants were combined and taken as the intracellular amino acids and purified by cation-exchange chromatography and converted to their heptafluorobutyrate (HFB) derivatives before analysis by GC-MS (models 6890 GC and 5973 MS; Hewlett-Packard, Palo Alto, CA, USA) as previously described (Moore et al. 2009b).
Intramuscular signalling
The methods for determination of the extent of phosphorylation of Akt on Ser473, mTOR on Ser2448, p70S6K on Thr389, rps6 on Ser240/244, 4EBP1 on Thr37/46, eIF2Bɛ on Ser539, p38 MAPK on Thr180/Try182, Erk1/2 on Thr202/Tyr204, p90RSK on Thr573, rps6 on Ser235/236 and total protein were performed as described in our previous work (Burd et al. 2010a). All data are expressed as the ratio between the phosphorylated to the total protein and analysed accordingly.
Real-time quantitative polymerase chain-reaction
Total RNA was isolated from wet muscle samples (∼20 mg) as described in previous work (Cochran et al. 2010). RNA was transcribed and quantitative RT-PCR reactions were conducted as described previously (Cochran et al. 2010). Fold change in PGC-1α expression were calculated using the ΔΔCt method (Livak & Schmittgen, 2001), normalized to the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH), which did not change across time (P = 0.75). GAPDH primers were as follows: forward 5′-CCTCCTGCACCACCAACTGCTT-3′ and reverse 5′-GAGGGGCCATTCACAGTCTTCT-3′.
Calculations
The fractional synthetic rates (FSR) of muscle proteins were calculated using the standard precursor–product equation as described (Moore et al. 2009b; West et al. 2009; Burd et al. 2010a,b). The recruitment of ‘tracer-naive’ participants allowed us to use the pre-infusion blood sample, which we have measured as being equivalent in enrichment to a pre-infusion biopsy (unpublished), as the pre-infusion baseline enrichment (Ep1) for the calculation of resting muscle protein synthesis (Fast). This single biopsy approach to determine basal muscle protein FSR has been validated within our laboratory (Burd et al. 2011b). Moreover, this approach has been shown to be valid by others, but instead the workers used a baseline (i.e. pre-infusion and thus non-enriched) muscle biopsy (Smith et al. 2010).
Statistics
A within-subject repeated measures design was used for the current study. Differences in muscle protein synthesis, mRNA responses, electromyography and intramuscular signalling were tested using a two-factor (condition × time) analysis of variance (ANOVA) with repeated measures on time. Muscle time under tension was analysed using a one-way ANOVA. Blood glucose, plasma insulin, and blood amino acid concentrations were analysed using one-factor (time) repeated measures ANOVA. Where significant interactions were identified in the ANOVA, Tukey's post hoc test was performed to determine differences between means for all significant main effects and interactions. Linear regression lines were fitted to plasma enrichments to assess the existence of any deviation in enrichment indicated by lines with a significant positive or negative slope. Pearson's r product–moment correlation was used to examine associations between different variables. For all analyses, differences were considered significant at P < 0.05. All results are presented as means ± standard error of the mean (SEM).
Results
Resistance exercise
There was no difference in the load lifted for SLOW (31 ± 2 kg) or CTL (30 ± 2 kg) conditions (P = 0.7). The repetitions performed during SLOW and CTL conditions were 12 ± 1, 7 ± 1 and 6 ± 1 for set 1, 2 and 3, respectively. Muscle time under muscle tension was greater for each exercise set (all, P < 0.05) in the SLOW condition (set 1, 198 ± 10 s; set 2, 119 ± 9 s; set 3, 90 ± 7 s) compared to the CTL condition (set 1, 25 ± 2 s; set 2, 14 ± 1 s; set 3, 11 ± 1 s) with a similar ∼8:1 ratio between contraction times for each set in the SLOW condition as compared to CTL. The total time the muscle was under tension was greater (P < 0.001) in the SLOW (407 ± 23 s) as compared to the CTL (50 ± 3 s) condition.
Blood glucose, plasma insulin and amino acid concentrations
Blood glucose remained stable during infusion trial 1 and 2 (Table 1). Plasma insulin concentration peaked (P < 0.05) at 1 h post-drink ingestion in trial 1 (4.7-fold increase above baseline) and in trial 2 (4.6-fold increase), but returned to basal concentrations by 2 h in both trials. Blood essential amino acid (EAA) concentrations peaked (P < 0.001) at 1 h post-drink ingestion and returned to basal by 2 h in both trials (Table 1). Similarly, blood leucine concentration peaked (P < 0.001) at 1 h for trial 1 and trial 2 and returned to basal levels by 2 h in both trials (Table 1).
Table 1.
Blood amino acid concentrations, blood glucose, and plasma insulin concentrations in the fasted-state and after ingestion of 20 g of whey protein isolate during trial 1 and trial 2
| After drink | |||||||
|---|---|---|---|---|---|---|---|
| Fasted | 0 h | 0.5 h | 1.0 h | 1.5 h | 2.0 h | 3.0 h | |
| Trial 1: | |||||||
| Σ EAA (μmol l−1) | 554 ± 34 | 494 ± 44 | 1063 ± 66* | 1071 ± 95* | 689 ± 59 | 611 ± 42 | 503 ± 45 |
| Leucine (μmol l−1) | 89 ± 5 | 79 ± 7 | 233 ± 15* | 249 ± 23* | 145 ± 13* | 119 ± 8 | 103 ± 10 |
| Insulin (μU ml−1) | 4.4 ± 0.9 | 4.3 ± 0.7 | 18.9 ± 0.9* | 20.9 ± 3.6* | 6.6 ± 1.3 | 4.4 ± 1.3 | 4.03 ± 0.7 |
| Glucose (mm) | 5.0 ± 0.2 | 5.4 ± 0.3 | 5.1 ± 0.1 | 5.3 ± 0.1 | 5.2 ± 0.3 | 5.4 ± 0.1 | 4.9 ± 0.2 |
| Trial 2: | |||||||
| Σ EAA (μmol l−1) | 608 ± 26 | 562 ± 45 | 870 ± 49 | 1197 ± 159* | 970 ± 110* | 821 ± 128 | 726 ± 199 |
| Leucine (μmol l−1) | 99 ± 4 | 90 ± 7 | 190 ± 11* | 221 ± 24* | 163 ± 16* | 125 ± 19 | 102 ± 17 |
| Insulin (μU ml−1) | 4.1 ± 0.6 | 4.2 ± 1.0 | 12.2 ± 2.2* | 19.2 ± 1.3* | 6.1 ± 0.6 | 3.4 ± 0.2 | 3.7 ± 0.5 |
| Glucose (mm) | 5.3 ± 0.1 | — | 5.3 ± 0.2 | 5.1 ± 0.2 | 4.9 ± 0.2 | 4.9 ± 0.2 | 5.0 ± 0.2 |
Values are means ± S.E.M. (n = 8). Drink composed of 20 g of whey protein isolate. EAA are sum of His, Ile, Leu, Lys, Met, Phe, Thr, Val (note: neither Trp nor Cys was measured).
Significantly different from Fast, P < 0.05.
Electromyography
All muscles of the quadriceps that were measured (i.e. vastus lateralis, vastus medialis and rectus femoris) showed similar EMG results and therefore only the results for the vastus lateralis are reported. EMG amplitude for the concentric phase of exercise significantly increased from the start of the set (0% set completion) to 60–100% set completion for set 1 (all, P < 0.001), whereas set 2 and set 3 significantly increased at 50–100% set completion (all P < 0.001) in the SLOW condition. EMG amplitude in the CTL condition significantly increased from 0% set completion at 50–100% set completion for set 1 and 2 (all P < 0.001), whereas set 3 significantly increased from the start of the set to 60–100% set completion (all P < 0.05). EMG amplitude (Fig. 2A) for the SLOW condition was greater than the CTL condition at 90–100% set completion (both P < 0.05) for set 1 and at 80–100% for set 2 (all P < 0.05). EMG amplitude for the third set of the SLOW condition was greater than the CTL condition at 0–100% set completion (all P < 0.001). There was a significant decrease in isometric mean power frequency (MPF) from the first repetition to the last repetition of the last set completion only after performing the SLOW condition (P < 0.001), whereas the CTL condition did not show a significant reduction in MPF from beginning to the end of the contractile protocol (P = 0.09).
Figure 2. Percentage increase in vastus lateralis activation during the concentric phase of resistance exercise (A) and the change in mean power frequency (MPF) during the isometric phase of resistance exercise from the first repetition to the last repetition (B).
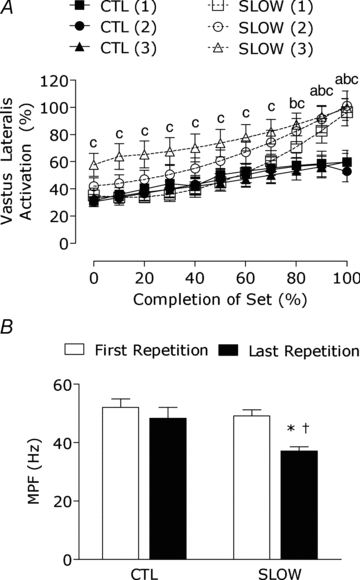
Numbers in parentheses following SLOW indicate set number. Lower case letter indicates significantly different from CTL for sets 1–3: a, SLOW(1); b, SLOW(2); c, SLOW(3); P < 0.05. *Significantly different from 0% set completion, P < 0.05. †Significantly different from CTL at that time point, P < 0.05.
Plasma and intracellular precursor enrichments
Intracellular precursor enrichments were similar across time during trial 1 in SLOW (0.051 ± 0.003 tracer/tracee and 0.052 ± 0.002 tracer/tracee) and CTL conditions (0.051 ± 0.003 tracer/tracee and 0.050 ± 0.002 tracer/tracee). Intracellular enrichments were also similar during trial 2 for SLOW (0.048 ± 0.002 and 0.049 ± 0.003 tracer/tracee at 1.5 and 6.5 h, respectively; P = 0.9) and CTL conditions (0.054 ± 0.3 and 0.053 ± 0.2 tracer/tracee at 1.5 and 6.5 h, respectively; P = 0.7). Furthermore, linear regression analysis indicated that the slopes of the plasma enrichments were not significantly different from zero during trial 1 or trial 2 (P = 0.7), indicating that isotopic plateau was achieved and that the use of the steady-state precursor product equation was appropriate.
Muscle protein synthesis
There was no detectable increase in rates of myofibrillar protein synthesis during 6 h of recovery in the SLOW or CTL conditions (both P > 0.05). The SLOW condition resulted in a stimulation of myofibrillar protein synthesis during 24–30 h recovery as indicated by a 2.3-fold increase above fasted rates (P < 0.001) and was greater than the 0–6 h response and the CTL condition at that time point (Fig. 3A). Mitochondrial protein synthesis rates were stimulated 2.1-fold above fast (P = 0.018) during 0–6 h recovery only in the SLOW condition; however, at 24–30 h post-exercise both SLOW (P < 0.001) and CTL (P = 0.002) were stimulated above fast by 2.8- and 2.3-fold, respectively (Fig. 3B). The mitochondrial protein synthetic responses at 24–30 h recovery were maintained in the SLOW condition and increased from 0–6 h in the CTL condition (P = 0.002). Sarcoplasmic protein synthesis rates were stimulated 1.8-fold during 0–6 h exercise recovery only in the SLOW condition (P < 0.001) and were greater than the CTL condition at this time point (P = 0.001).
Figure 3. Myofibrillar (A), mitochondrial (B), and sarcoplasmic (C) protein fractional synthetic rates (FSR) during protocols.
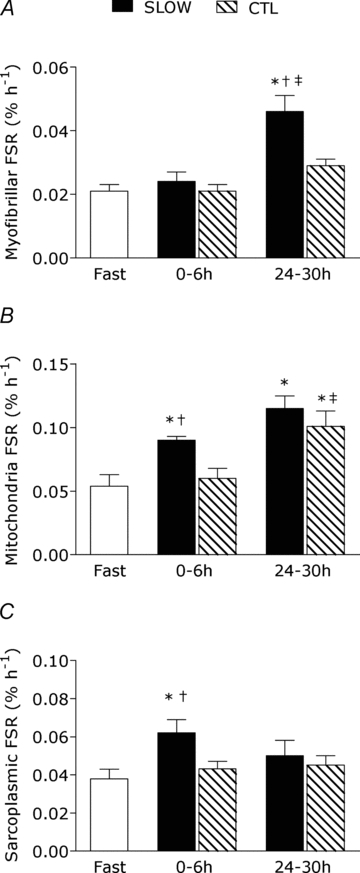
Note different scales on y-axes between graphs. Rates are from rested fasted and after resistance exercise with slow (SLOW) or external work match control (CTL) muscle time under tension. Values are means ± S.E.M. *Significantly different from fasting, P < 0.05. †Significantly different from CTL at that same time point, P < 0.05. ‡Significantly different from the 0–6 h response in the same condition, P < 0.05.
Intramuscular signalling
Phosphorylation of p70S6K (Fig. 4A) was increased above fast by 3.4-fold at 24 h post-exercise only in the SLOW condition (P = 0.002) and was phosphorylated more than CTL at this same time point (P = 0.004). There was a significant association between the extent of p70S6K phosphorylation at 24 h post-exercise and rates of myofibrillar protein synthesis during 24–30 h of recovery (r = 0.42, P = 0.02).
Figure 4. Ratio of phosphorylated to total of p70S6KThr389 (A), 4E-BP1Thr37/46 (B) and p90RSKThr573 (C) during the protocols.
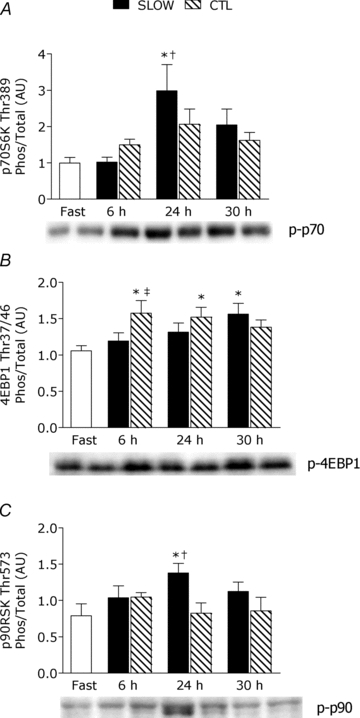
Ratios are from rested fasted and after resistance exercise with slow (SLOW) or external work match control (CTL) muscle time under tension. Values are means ± S.E.M. Data are expressed in arbitrary units (AU). *Significantly different from fast, P < 0.05. †Significantly different from CTL within that time point, P < 0.05. ‡Significantly different from SLOW within that time point, P < 0.05.
Phosphorylation of 4EBP1 was increased by 1.6- and 1.5-fold above fast at 6 h and 24 h post-exercise, respectively, only in the CTL condition (both P < 0.05); however, at 30 h post-exercise 4EBP1 was phosphorylated above fast (1.6-fold) only in the SLOW condition (P = 0.02). The phosphorylated-state of 4EBP1 in CTL was greater than SLOW condition at 6 h post-exercise (P = 0.004). Phosphorylation of p90RSK was significantly increased above fast by 2.5-fold only in the SLOW condition (P = 0.001). There was no change (P > 0.05) in phosphorylation of Erk1/2, p38 MAPK, Akt, mTOR, rps6 on Ser240/244 or 235/236, or eIF2Bɛ (supplemental figure).
PGC-1α mRNA
PGC-1α mRNA was increased by ∼3-fold above rest at 6 h post-exercise, with no difference between conditions (main effect for time, P = 0.001) and returned to baseline by 24 h (Fig. 5).
Figure 5. Peroxisome proliferator-activated receptor γ coactivator 1-α (PGC-1α) mRNA content at fast, 6 h and 24 h post-exercise.
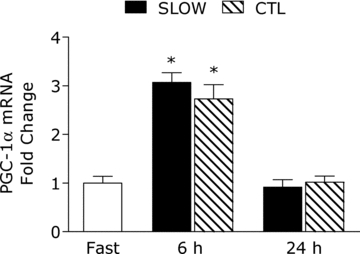
*Significantly different from fasting, P < 0.05.
Discussion
Our study is the first to demonstrate that a prolonged time under muscle tensi during resistance exercise did not stimulate an immediate rise in myofibrillar protein synthesis rates, but did result in a delayed stimulation that was significant at 24–30 h recovery with a subsequent feeding-induced increase of myofibrillar protein synthesis. We also report evidence that resistance exercise has a potent stimulatory effect on mitochondrial protein synthesis rates that were only partially dependent on muscle time under tension with a greater stimulation of the response early after a longer time under tension. However, there was a robust stimulation of mitochondrial protein synthesis rates in the SLOW and CTL conditions during 24–30 h recovery. Further, a longer time under muscle tension increased the acute (0–6 h) amplitude of sarcoplasmic protein synthesis rates; however, it had no influence on extending the duration of this response. Interestingly, acute low intensity resistance exercise increased muscle PGC-1 mRNA expression at 6 h post-exercise and this elevation was independent of muscle time under tension during the bout.
It is generally accepted that exercise-induced rates of muscle protein synthesis, in the fasted-state, are greatest immediately after an acute bout of resistance exercise and gradually decline in the hours and days that follow (Phillips et al. 1997). Thus, the effect of exercise per se, in the absence of feeding, is to stimulate a prolonged elevation in muscle protein synthesis rates. It is becoming clear that prior exercise affects nutrition-mediated myofibrillar protein synthesis rates at time points later (i.e. 24 h) in exercise recovery. What we observed here was a potentiated effect, from that seen in the fasted-state, of prior exercise in enhancing the feeding-induced myofibrillar protein synthetic rates. This effect appears to be dependent on maximal fibre activation during exercise, which is consistent with our previous observation (Burd et al. 2011a). The current study is noteworthy in that an enhanced effect of protein feeding during late exercise recovery was induced by a longer time under muscle tension rather than intensity-independent contraction volume, which we have previously examined (Burd et al. 2010a). These data, and our other observations (Burd et al. 2010b, 2011a), clearly show that contractile variables can be manipulated to affect responses of muscle protein synthesis. Thus, we speculate that maximal fibre activation, and not percentage of maximal strength, is fundamental to induce maximal rates of muscle protein synthesis and we would hypothesize other purportedly important variables that are thought to dictate hypertrophy (Ratamess et al. 2009) are largely redundant in their ability to elicit an anabolic response to exercise so long as high levels of muscle fibre recruitment are attained.
An interesting question that arises from our current work is why did we not observe an increase in exercise-mediated rates of myofibrillar protein synthesis during 0–6 h recovery? This outcome was unexpected as our previous work demonstrated that low intensity exercise performed to failure, using a faster lifting cadence, stimulating robust increases in myofibrillar protein synthesis rates (Burd et al. 2010b). Indeed, this finding provided the basis for a thesis that achieving maximal muscle fibre activation during resistance exercise is fundamental to maximally stimulate rates of myofibrillar protein synthesis during acute exercise recovery. Certainly, our current protocol was successful in eliciting full muscle fibre recruitment using a prolonged time under tension (Fig. 2A); however, we did not find an immediate acute stimulation in myofibrillar protein synthesis rates. The explanation for this result is likely to relate to the timing of the muscle biopsies, training status of subjects, and the resistance exercise protocol. First of all, we specifically chose to study the muscle protein synthetic responses over 6 h to minimize an overriding feeding effect, which would peak at 3 h (Moore et al. 2009b), and thus capture a ‘true’ exercise effect, which normally is sustained for at least 5 h after high intensity resistance exercise (Moore et al. 2009b; West et al. 2011). Secondly, resistance training shortens the duration of the muscle protein synthetic response (i.e. we used trained subjects) (Tang et al. 2008) and may have further precluded our ability to detect a response. Finally, the resistance exercise protocol we employed is far from resembling any other resistance protocol used in other studies studying muscle protein metabolism in vivo in humans. Specifically, to minimize the repetitions performed during each exercise set and elicit fatigue with the low load, the time that the muscle was under tension in the SLOW condition was ∼2 min 16 s for each set, a duration that far exceeded the low intensity condition in our previous investigations (∼40 s each set) (Burd et al. 2010a,b). Thus, it seems that the hallmark response of loaded resistance exercise, as a stimulus for myofibrillar protein synthesis, was shifted instead toward increased synthesis of proteins in the mitochondrial and sarcoplasmic pools (Fig. 3). What facilitates the differences in synthesis of specific proteins within the muscle protein pools is still, at least at the muscle protein synthetic level, very much unclear. Importantly, such a finding would likely have been missed had we measured mixed muscle protein synthesis.
Despite the lack of an immediate stimulation of myofibrillar protein synthesis in the current study, our data do provide support that acute exercise until failure, likely through maximal fibre activation, results in a delayed sensitizing effect on myofibrillar protein synthesis with nutrition during late exercise recovery and provide further insight in the regulation of myofibrillar protein synthesis during 24 h of exercise recovery (Burd et al. 2011a). The increased sensitivity to protein feeding at 24 h post-exercise, reported previously (Burd et al. 2011a) and in the current study, are perhaps not overly surprising. However, since if basal fasting rates of muscle protein synthesis can be elevated for up to 48 h (Phillips et al. 1997) then the feeding-induced potentiation of myofibrillar protein synthesis over and above the fed-state response itself (Moore et al. 2009b) should be evident at 24 h and likely even at 48 h. Similar results have been seen in aged men who, while unable to mount a significant fed-state increase in mixed muscle protein synthesis in the absence of exercise, showed a significant stimulation at 18 h after 40 min of walking (Fujita et al. 2007b).
Our general understanding of the influence of resistance exercise on rates of mitochondrial protein synthesis in humans is very limited. Our laboratory has reported that an acute bout of resistance exercise has the capacity to increase rates of mitochondrial protein synthesis in the untrained state (Wilkinson et al. 2008), data which are consistent with the notion that resistance exercise can improve muscle oxidative potential (Tang et al. 2006). It appears the responsiveness of the mitochondrial protein pool, at least during acute recovery, to ‘conventional’ high intensity resistance exercise is attenuated after a training period when stimulated by the same absolute load as used prior to training (Wilkinson et al. 2008). However, our current data show that low intensity resistance exercise can stimulate mitochondrial protein synthesis rates during 0–6 h recovery in trained participants when muscle time under tension is increased during the exercise session. Indeed, it was unexpected that exercise induced mitochondrial protein synthesis rates were elevated to a similar extent at 24–30 h recovery between the SLOW and CTL conditions. Due to the exhaustive nature of the SLOW condition, we anticipated the mitochondrial synthetic response to be more robust. However, this finding may highlight just how sensitive the mitochondrial protein pool is to contraction, regardless of the exercise stimulus, during longer term recovery. In support, many genes involved in mitochondrial function are up-regulated at 48 h of recovery after endurance exercise, although we admit it is not fair to compare resistance exercise versus an endurance exercise stimulus (Rowlands et al. 2011). It is clear, however, that future investigations are needed, which would include a time course of the response, to underpin the physiological mechanism for up-regulation of mitochondrial protein synthesis rates during longer-term recovery. In a similar manner, we had hypothesized that muscle PGC-1α expression would be more robust after the SLOW condition (Egan et al. 2010). We found that muscle time under tension, and associated greater increase in metabolic work, had no influence on the PGC-1α mRNA response. Notable, the ∼3-fold increase in PGC-1α mRNA expression observed in the SLOW and CTL conditions are similar in amplitude (Fig. 5) to that observed after four 30 s ‘all out’ cycling sprints (Gibala et al. 2009). Also, it is worth highlighting that that our previous investigations which examined rates of sarcoplasmic protein synthesis (i.e. non-myofibrillar proteins) would also have contained the mitochondria protein pool (Moore et al. 2009b; Burd et al. 2010b). Here, we present rates of sarcoplasmic protein synthesis that are largely devoid of mitochondrial proteins and show that the increased time the muscle was under tension affected the acute amplitude of sarcoplasmic protein synthesis rates with no effect extending to the 24 h post-exercise period.
We studied candidate proteins within the Akt-mTOR and MAPK pathways to ascertain if the phosphorylation of intramuscular proteins involved in regulating mRNA translation and elongation were influenced by muscle time under tension. Indeed, it is difficult to fully understand which of these signalling pathways are involved in regulating the synthesis of specific muscle proteins (e.g. is p70S6K activation specific toward the stimulation of myofibrillar protein synthesis?), although there is likely to be interplay between protein kinases such that each coordinates the synthesis of more than one specific muscle protein pool. However, more work is necessary to address this question. Here, we found that 4E-BP1 phosphorylation was enhanced at 6 h post-exercise only in the CTL condition, a finding that is consistent with the notion that this signalling protein may be more responsive to feeding (Fujita et al. 2007a; Atherton et al. 2010; Moore et al. 2011) rather than contraction (Dreyer et al. 2006). We did not obtain muscle biopsies at 0.5–1.5 h after exercise, a time point when intramuscular signalling protein activation is typically higher (Camera et al. 2010), which may have precluded our ability to distinguish whether muscle time under tension affected the phosphorylation status of signalling proteins at a potentially more relevant time than 6 h post-exercise. There appears to be a substantial redundancy in the intramuscular signalling protein activation that may, in part, be mediating the delayed effect of resistance exercise on muscle protein synthesis rates at 24 h. Specifically, signalling proteins (i.e. p70S6K, 4EBP-1, and p90RSK) well known to be phosphorylated immediately after resistance exercise (Kumar et al. 2009; Camera et al. 2010; Terzis et al. 2010; Moore et al. 2011) were also phosphorylated at 24 and 30 h post-exercise. It remains to be clearly established, but the current data suggest that certain intramuscular signalling proteins may undergo relatively prolonged changes in their phosphorylated states during exercise recovery and may mediate rates of muscle protein synthesis during late exercise recovery. The present results also continue to add to the growing body of literature supporting the phosphorylated state of p70S6K as a proxy marker of myofibrillar protein synthesis rates after acute resistance exercise in humans (reviewed in West et al. 2010).
Admittedly, the methods to study muscle protein synthesis in vivo in humans only requires that a small population of muscle fibres are sampled. Thus, it is assumed that this small population of fibres is representative of the entire thigh muscle. Indeed, all the motor units, and the associated type I or II fibres, in a muscle do not fire at the same time (Sale, 1987). Thus, there is selective recruitment of the fast-twitch and slow-twitch motor units to produce enough force to overcome the load. In the current study, we employed a model that allowed us to test how various levels of recruitment affect specific protein pools within muscle. We are assuming that type II muscle fibres were eventually activated, which is supported by the EMG results, in the SLOW condition that led to some of the superior responses (Figs 2–4). Certainly, studying the response at the single fibre level would yield valuable insight into how specific fibre types are affected during low intensity resistance exercise. However, this approach also takes into account a small population of fibres and the feasibility of this methodology is difficult to employ on a large-scale basis (examining multiple time points post-exercise).
In summary, a prolonged muscle time under tension, only when fatigue leads to full motor unit recruitment (Fig. 2A), affects the acute amplitude of muscle protein sub-fractional synthesis (i.e. mitochondria and sarcoplasmic protein pools) and mediates a delayed effect on rates of myofibrillar synthesis during 24–30 h recovery. This delayed effect on myofibrillar protein synthesis rates during longer-term recovery, when accompanied by protein feeding, after fatiguing exercise highlights that separate, yet undefined, mechanisms are facilitating a nutrient enhancing effect on longer-term myofibrillar protein synthetic responses as compared to immediately after resistance exercise. Notable is that our current data highlight, and substantiate our previous findings (Burd et al. 2010b), that maximal fibre activation cannot be viewed as the exclusive driver of myofibrillar protein synthesis rates. It appears exercise volume is yet another fundamental variable that promotes p70S6K phosphorylation (Terzis et al. 2010) and a prolonged elevation of myofibrillar protein synthesis rates (Burd et al. 2010a,b). We are the first to provide a further time course (i.e. 24 h later) of mitochondrial protein synthesis rates after acute resistance exercise and report that low intensity resistance exercise has a potent stimulatory effect on the response at 24–30 h recovery. Additionally, low intensity resistance exercise has the capacity to increase muscle PGC-1α mRNA responses at 6 h post-exercise recovery. Our data provide further evidence of the value of studying muscle protein synthetic responses at the muscle fraction specific level in order to gain a clear understanding of the phenotypic response to an exercise stimulus.
Acknowledgments
The authors wish to thank Todd Prior, Tracy Rerecich, and Mike Percival for analytical assistance. We also thank Andrew Holwerda, Nathan Cain and Keegan Selby for their help in data collection and the participants for their time and effort. We wish to thank Colin De France of Inbalance Nutrition (Burlington, ON) for his generous gift of whey protein isolate used in the study. Lastly, we thank Justin Crane and Mark Tarnopolsky for confirming the purity of our enriched protein fractions. This research was supported by a research grant from Natural Sciences and Engineering Research Council of Canada to S.M.P.
Glossary
Abbreviations
- Akt
protein kinase B
- 4E-BP1
eukaryotic initiation factor 4E binding protein 1
- eIF2Bɛ
eukaryotic translation initiation factor 2B epsilon
- FSR
fractional synthetic rate
- MAPK
mitogen-activated protein kinase
- mTOR
mammalian target of rapamycin
- p38 MAPK
p38 mitogen-activated protein kinase
- p70S6K
70 kDa S6 protein kinase
- p90RSK
90 kDa ribosomal S6 protein kinase
- PGC-1α
peroxisome proliferator-activated receptor-γ coactivator-1α
- rpS6
ribosomal protein S6
Author contributions
N.A.B. and S.M.P. contributed to the conception and the design of the experiment. All authors contributed to collection, analysis, and interpretation of data. All authors contributed to drafting or revising intellectual content of the manuscript. All authors approved the final version of this manuscript.
Supplementary material
Supplemental Figure
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors
References
- Atherton PJ, Etheridge T, Watt PW, Wilkinson D, Selby A, Rankin D, Smith K, Rennie MJ. Muscle full effect after oral protein: time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am J Clin Nutr. 2010;92:1080–1088. doi: 10.3945/ajcn.2010.29819. [DOI] [PubMed] [Google Scholar]
- Burd NA, Holwerda AM, Selby KC, West DW, Staples AW, Cain NE, Cashaback JG, Potvin JR, Baker SK, Phillips SM. Resistance exercise volume affects myofibrillar protein synthesis and anabolic signalling molecule phosphorylation in young men. J Physiol. 2010a;588:3119–3130. doi: 10.1113/jphysiol.2010.192856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd NA, West DW, Moore DR, Atherton PJ, Staples AW, Prior T, Tang JE, Rennie MJ, Baker SK, Phillips SM. Enhanced amino acid sensitivity of myofibrillar protein synthesis persists for up to 24 h after resistance exercise in young men. J Nutr. 2011a;141:568–573. doi: 10.3945/jn.110.135038. [DOI] [PubMed] [Google Scholar]
- Burd NA, West DW, Rerecich T, Prior T, Baker SK, Phillips SM. Validation of a single biopsy approach and bolus protein feeding to determine myofibrillar protein synthesis in stable isotope tracer studies in humans. Nutr Metab (Lond) 2011b;8:15. doi: 10.1186/1743-7075-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd NA, West DW, Staples AW, Atherton PJ, Baker JM, Moore DR, Holwerda AM, Parise G, Rennie MJ, Baker SK, Phillips SM. Low-load high volume resistance exercise stimulates muscle protein synthesis more than high-load low volume resistance exercise in young men. PLoS One. 2010b;5:e12033. doi: 10.1371/journal.pone.0012033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camera DM, Edge J, Short MJ, Hawley JA, Coffey VG. Early time course of Akt phosphorylation after endurance and resistance exercise. Med Sci Sports Exerc. 2010;42:1843–1852. doi: 10.1249/MSS.0b013e3181d964e4. [DOI] [PubMed] [Google Scholar]
- Chesley A, MacDougall JD, Tarnopolsky MA, Atkinson SA, Smith K. Changes in human muscle protein synthesis after resistance exercise. J Appl Physiol. 1992;73:1383–1388. doi: 10.1152/jappl.1992.73.4.1383. [DOI] [PubMed] [Google Scholar]
- Cochran AJ, Little JP, Tarnopolsky MA, Gibala MJ. Carbohydrate feeding during recovery alters the skeletal muscle metabolic response to repeated sessions of high-intensity interval exercise in humans. J Appl Physiol. 2010;108:628–636. doi: 10.1152/japplphysiol.00659.2009. [DOI] [PubMed] [Google Scholar]
- Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol. 2006;576:613–624. doi: 10.1113/jphysiol.2006.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan B, Carson BP, Garcia-Roves PM, Chibalin AV, Sarsfield FM, Barron N, McCaffrey N, Moyna NM, Zierath JR, O’Gorman DJ. Exercise intensity-dependent regulation of peroxisome proliferator-activated receptor coactivator-1 mRNA abundance is associated with differential activation of upstream signalling kinases in human skeletal muscle. J Physiol. 2010;588:1779–1790. doi: 10.1113/jphysiol.2010.188011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuglevand AJ, Zackowski KM, Huey KA, Enoka RM. Impairment of neuromuscular propagation during human fatiguing contractions at submaximal forces. J Physiol. 1993;460:549–572. doi: 10.1113/jphysiol.1993.sp019486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita S, Dreyer HC, Drummond MJ, Glynn EL, Cadenas JG, Yoshizawa F, Volpi E, Rasmussen BB. Nutrient signalling in the regulation of human muscle protein synthesis. J Physiol. 2007a;582:813–823. doi: 10.1113/jphysiol.2007.134593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita S, Rasmussen BB, Cadenas JG, Drummond MJ, Glynn EL, Sattler FR, Volpi E. Aerobic exercise overcomes the age-related insulin resistance of muscle protein metabolism by improving endothelial function and Akt/mammalian target of rapamycin signaling. Diabetes. 2007b;56:1615–1622. doi: 10.2337/db06-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibala MJ, McGee SL, Garnham AP, Howlett KF, Snow RJ, Hargreaves M. Brief intense interval exercise activates AMPK and p38 MAPK signaling and increases the expression of PGC-1α in human skeletal muscle. J Appl Physiol. 2009;106:929–934. doi: 10.1152/japplphysiol.90880.2008. [DOI] [PubMed] [Google Scholar]
- Glover EI, Phillips SM, Oates BR, Tang JE, Tarnopolsky MA, Selby A, Smith K, Rennie MJ. Immobilization induces anabolic resistance in human myofibrillar protein synthesis with low and high dose amino acid infusion. J Physiol. 2008;586:6049–6061. doi: 10.1113/jphysiol.2008.160333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motoneurons. J Neurophysiol. 1965;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, Williams J, Smith K, Seynnes O, Hiscock N, Rennie MJ. Age-related differences in the dose–response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol. 2009;587:211–217. doi: 10.1113/jphysiol.2008.164483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(–ΔΔCT) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Moore DR, Atherton PJ, Rennie MJ, Tarnopolsky MA, Phillips SM. Resistance exercise enhances mTOR and MAPK signalling in human muscle over that seen at rest after bolus protein ingestion. Acta Physiol (Oxf) 2011;201:365–372. doi: 10.1111/j.1748-1716.2010.02187.x. [DOI] [PubMed] [Google Scholar]
- Moore DR, Phillips SM, Babraj JA, Smith K, Rennie MJ. Myofibrillar and collagen protein synthesis in human skeletal muscle in young men after maximal shortening and lengthening contractions. Am J Physiol Endocrinol Metab. 2005;288:E1153–E1159. doi: 10.1152/ajpendo.00387.2004. [DOI] [PubMed] [Google Scholar]
- Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, Prior T, Tarnopolsky MA, Phillips SM. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr. 2009a;89:161–168. doi: 10.3945/ajcn.2008.26401. [DOI] [PubMed] [Google Scholar]
- Moore DR, Tang JE, Burd NA, Rerecich T, Tarnopolsky MA, Phillips SM. Differential stimulation of myofibrillar and sarcoplasmic protein synthesis with protein ingestion at rest and after resistance exercise. J Physiol. 2009b;587:897–904. doi: 10.1113/jphysiol.2008.164087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol Endocrinol Metab. 1997;273:E99–E107. doi: 10.1152/ajpendo.1997.273.1.E99. [DOI] [PubMed] [Google Scholar]
- Ratamess NA, Alvar BA, Evetoch TK, Housh TJ, Kibler WB, Kraemer WJ, Triplett NT. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2009;41:687–708. doi: 10.1249/MSS.0b013e3181915670. [DOI] [PubMed] [Google Scholar]
- Rowlands DS, Thomson JS, Timmons BW, Raymond F, Fuerholz A, Mansourian R, Zwahlen MC, Metairon S, Glover E, Stellingwerff T, Kussmann M, Tarnopolsky MA. Transcriptome and translational signaling following endurance exercise in trained skeletal muscle: impact of dietary protein. Physiol Genomics. 2011;43:1004–1020. doi: 10.1152/physiolgenomics.00073.2011. [DOI] [PubMed] [Google Scholar]
- Sale DG. Influence of exercise and training on motor unit activation. Exerc Sport Sci Rev. 1987;15:95–151. [PubMed] [Google Scholar]
- Smith GI, Villareal DT, Lambert CP, Reeds DN, Mohammed BS, Mittendorfer B. Timing of the initial muscle biopsy does not affect the measured muscle protein fractional synthesis rate during basal, postabsorptive conditions. J Appl Physiol. 2010;108:363–368. doi: 10.1152/japplphysiol.00957.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang JE, Hartman JW, Phillips SM. Increased muscle oxidative potential following resistance training induced fibre hypertrophy in young men. Appl Physiol Nutr Metab. 2006;31:495–501. doi: 10.1139/h06-026. [DOI] [PubMed] [Google Scholar]
- Tang JE, Perco JG, Moore DR, Wilkinson SB, Phillips SM. Resistance training alters the response of fed state mixed muscle protein synthesis in young men. Am J Physiol Regul Integr Comp Physiol. 2008;294:R172–E178. doi: 10.1152/ajpregu.00636.2007. [DOI] [PubMed] [Google Scholar]
- Terzis G, Spengos K, Mascher H, Georgiadis G, Manta P, Blomstrand E. The degree of p70 S6k and S6 phosphorylation in human skeletal muscle in response to resistance exercise depends on the training volume. Eur J Appl Physiol. 2010;110:835–843. doi: 10.1007/s00421-010-1527-2. [DOI] [PubMed] [Google Scholar]
- West DW, Burd NA, Coffey VG, Baker SK, Burke LM, Hawley JA, Moore DR, Stellingwerff T, Phillips SM. Rapid aminoacidemia enhances myofibrillar protein synthesis and anabolic intramuscular signaling responses after resistance exercise. Am J Clin Nutr. 2011;94:795–803. doi: 10.3945/ajcn.111.013722. [DOI] [PubMed] [Google Scholar]
- West DW, Burd NA, Staples AW, Phillips SM. Human exercise-mediated skeletal muscle hypertrophy is an intrinsic process. Int J Biochem Cell Biol. 2010;42:1371–1375. doi: 10.1016/j.biocel.2010.05.012. [DOI] [PubMed] [Google Scholar]
- West DW, Kujbida GW, Moore DR, Atherton P, Burd NA, Padzik JP, De Lisio M, Tang JE, Parise G, Rennie MJ, Baker SK, Phillips SM. Resistance exercise-induced increases in putative anabolic hormones do not enhance muscle protein synthesis or intracellular signalling in young men. J Physiol. 2009;587:5239–5247. doi: 10.1113/jphysiol.2009.177220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson SB, Phillips SM, Atherton PJ, Patel R, Yarasheski KE, Tarnopolsky MA, Rennie MJ. Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J Physiol. 2008;586:3701–3717. doi: 10.1113/jphysiol.2008.153916. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


