Abstract
A new tripeptide, pre-sclerotiotide F (3), was isolated from a marine sediment-derived fungus, Aspergillus insulicola, along with five known compounds, one of which was new at the time of isolation, scerotiotide F (4). The absolute configuration elucidation of the new compound was determined using a combination of NMR, HR-ESI-MS, and optical rotation analyses. Cytotoxicities were measured in vitro against selected cancer cells. The effects of pre-sclerotiotide F (3) and sclerotiotide F (4) on LPS-induced NF-κB and iNOS expression were also measured.
Keywords: Tripeptide, Aspergillus insulicola, Configuration elucidation, Cytotoxicity, Anti-inflammatory
1. Introduction
Strikingly, there are two fungal genera, Aspergillus and Penicillum that appear to be the most favored entities for metabolomics and chemical screening ventures. For example, a Scifinder bioorganic chemistry current awareness search for the year 2010 using these two key words revealed hundreds of hits for each organism, Aspergillus = 287, and Penicillum = 121. We have representatives of both genera, all marine-derived, in our repository and continue to place a greater interest on investigations involving the former. We are guardedly optimistic in the belief that the biosynthetic products of certain marine-derived Aspergillus strains will be different vs. those from the well-studied terrestrial counterparts. A few illustrative examples from our past research revealing unique biosynthetic products from marine-derived Aspergillus include: (a) an unusual diketopiperazine dimer, asperazine (Varoglu et al., 1997), (b) an unprecedented polyketide, asperic acid (Varoglu and Crews, 2000), and (c) halogenated polyketides, the chlorocarolides (Abrell et al., 1996). We also recognize that there are several examples known that counterbalance these phenomena. One extensively studied case pertains to the diketopiperazine mycotoxins, headed by gliotoxin, which show congruence in compound profiles in culture broths from terrestrial and marine derived strains (Ebel, 2010).
The recent results from our initial study of Aspergillus insulicola (UCSC marine-sediment derived strain No. 088708a, identified by molecular taxonomy) adds to the record of observing unique compounds from ocean-sourced fungi (Wu et al., 2010). The bioactive metabolite identified in this work proved be the known cytotoxic terpene nitrobenzoyl ester, insulicolide A (1) (Fig. 1) (Belofsky et al., 1998). It was first reported in 1997 from A. insulicola (marine-derived) (Rahbaek et al., 1997), but subsequently described, also as a new compound, in 1998 from A. versicolor (marine-derived). Significantly, experiments reported in the former study proved that: (a) 1 is a taxonomic marker for three Aspergillus species, insulicola, bridgeri and sclerotiorum, and (b) there was biosynthetic crossover between environments because the latter fungi were terrestrial. By contrast, the other compound we reported was the novel hexacyclic dipeptide, azonazine (2), which at this junction appears to be unique to the marine environment. The LC-MS scans of the HP-20 subfraction shown in Fig. 2 (coded as HP3, see Supporting Information Chart S1) containing 1 and 2, revealed the presence of at least five more compounds.
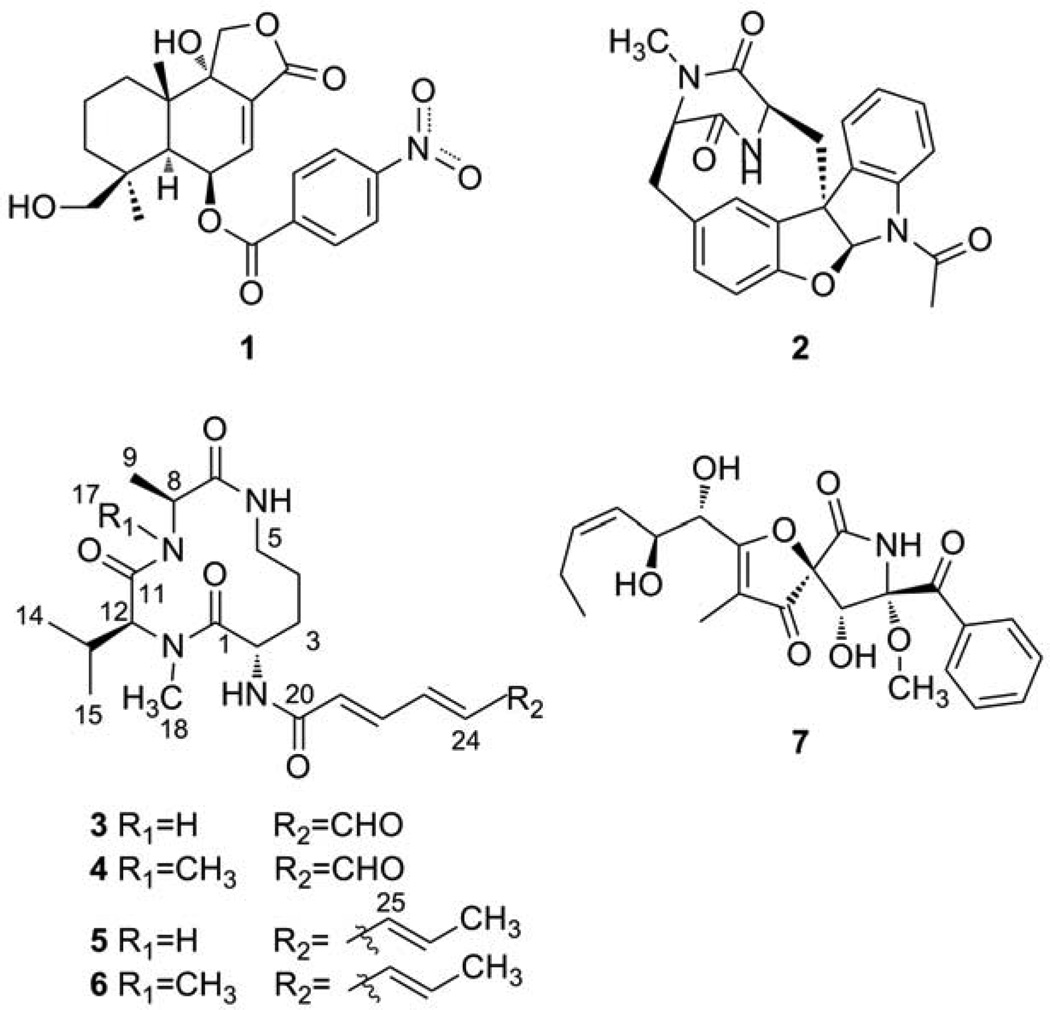
Fig. 1.
The structures of compounds 1–7.
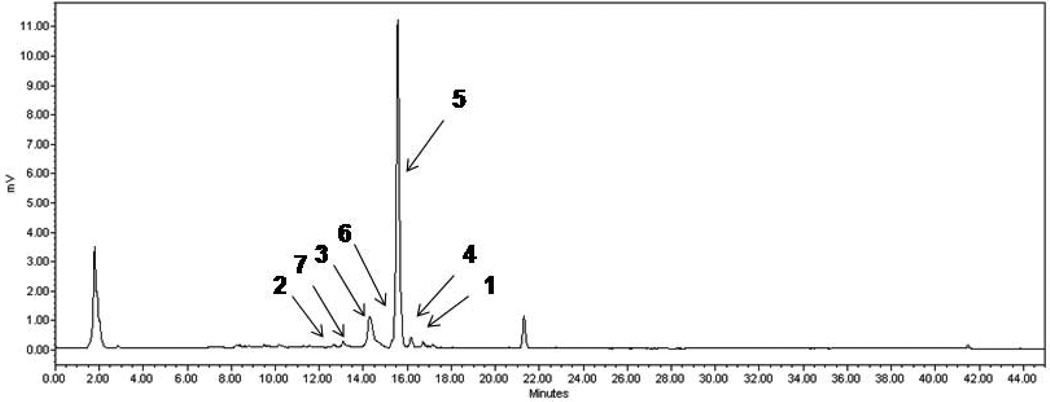
Fig. 2.
LC-ELSD trace of the semi-pure extract fraction pursued for the isolation work.
Compound Name Codes: insulicolide (1), (+) azonazine (2), pre-sclerotiotide F (3), sclerotiotide F (4), JBIR-15 (5), aspochracin (6), and pseurotin A (7).
Our previous account provided important background information pertaining to the possible biogenetic origin of 2, and a summary of the bioactivities observed for 1 and 2. The details of the scale-up culture (10L), processing via HP-20 resin, and the selection of a single wash fraction (100% methanol, coded as HP-3, Figure 1) for further chromatography have also been previously described. Additional LC separations provided fractions (Chart S1) containing milligram amounts of each of these. As noted above, the fastest and slowest retention time peaks consisting of 2 (1.1 mg) and 1 (2.2 mg) respectively were the first targets of our structure elucidation efforts. We now report that three compounds (Fig. 2) have been dereplicated and previously observed from terrestrial Aspergillus. These consisted of: (−)-pseurotin A (7) (1.7 mg) (Boot et al., 2007), (−)-aspochracin (6) (2.5 mg) (Myokei et al., 1969), and (−)-JBIR-15 (5) (1.0 mg) (Motohashi et al., 2009), the N-demethyl analog of 6. The remaining two compounds, which were undescribed at the point of our isolation-structure elucidation, consisted of a faster retention time peak (13 min, 3.2 mg) and a slower retention time major component (16 min, 11.7 mg). These compounds were eventually named as (−) pre-sclerotiotide F (3), C20H30N4O5 from the (+) HR-ESI-MS quasi-molecular ion peak at 429.21362 [M+Na]+ (calcd for C20H30N4O5Na, 429.21084), and (−) sclerotiotide F (4) C21H32N4O5 from the HR-ESI-MS quasi-molecular ion peak at m/z 421.24650 [M+H]+ (calcd for C21H33N4O5, 421.24455). Described next are the basis for structure assignments of this pair and the final biological activity assessments of all compounds.
2. Results and discussion
Parallel study of compounds 3 and 4, each isolated as an amorphous powder, was warranted as they possessed very similar properties. Both compounds contained 8 unsaturation equivalents and the carbon types and functionalities were assessed by NMR, gHMQC, broadband-decoupled 13C NMR, and DEPT spectra summarized in Table 1. These data revealed for 3, a sum of 4 quaternary, 9 methine, 3 methylene, and 4 methyl carbons of overall formula C20H27. The three remaining Hs at δ 6.44 (brd, J = 6.0 Hz), δ 7.04 (d, J = 7.2 Hz) and δ 7.20 (d, J = 9.0 Hz) were attributed to three NH groups (Amagata et al., 2006) and an additional NCH3 (δ 3.0/30.3) was evident. Further analysis of the 13C NMR data revealed four amide carbonyl carbons, an isopropyl group, two trans conjugated double bonds, further appended to an aldehyde group. Since these data accounted for all of the atoms in the molecular formula, it was then clear that a macrocyclic ring was needed to rationalize the remaining unsaturation.
Table 1.
NMR Data for Pre-sclerotiotide F (3) and Sclerotiotide F (4) a
| Pre-sclerotiotide (3) |
Sclerotiotide F (4) |
|||
|---|---|---|---|---|
| position | δH, mult. (J in Hz) | δC, type | δH, mult. (J in Hz) | δC, type |
| 1 | 173.1, C | 172.6, C | ||
| 2 | 5.05, ddd (7.2, 7.2, 0.6) | 49.7, CH | 5.00, ddd (7.2, 6.6, 1.2) | 49.8, CH |
| 3 | 2.37, m, 1.71, m | 28.2, CH2 | 2.39, m, 1.69, m | 28.3, CH2 |
| 4 | 1.76, m, 1.58, m | 21.8, CH2 | 1.70, m, 1.58, m | 21.7, CH2 |
| 5 | 3.48, m, 2.95, m | 39.9, CH2 | 3.34, m, 3.12, m | 39.7, CH2 |
| 6 | 6.44, brd (6.0) | 5.80, t (6.0) | ||
| 7 | 172.5, C | 171.3, C | ||
| 8 | 4.19, dq (9.0, 7.2) | 51.7, CH | 4.63, q (7.2) | 55.3, CH |
| 9 | 1.45, d (7.2) | 19.3, CH3 | 1.52, d (7.2) | 16.7, CH3 |
| 11 | 170.8, C | 169.1, C | ||
| 12 | 4.98, d (10.2) | 58.4, CH | 5.11, d (10.2) | 58.7, CH |
| 13 | 2.39, m | 26.3, CH | 2.43, m | 26.9, CH |
| 14 | 0.75, d (6.6) | 17.8, CH3 | 0.75, d (7.2) | 17.9, CH3 |
| 15 | 0.96, d (6.0) | 19.7, CH3 | 0.92, d (6.6) | 19.8, CH3 |
| 17 | 7.20, d (9.0) | 3.05, s | 29.8, CH3 | |
| 18 | 3.00, s | 30.3, CH3 | 2.98, s | 30.3, CH3 |
| 19 | 7.04, d (7.2) | 6.89, d (6.6) | ||
| 20 | 163.4, C | 163.2, C | ||
| 21 | 6.40, d (15.0) | 132.0, CH | 6.34, d (15.0) | 131.8, CH |
| 22 | 7.38, dd (15.0, 11.4) | 137.5, CH | 7.38, dd (15.0, 11.4) | 137.7, CH |
| 23 | 7.13, dd (15.0, 11.4) | 147.5, CH | 7.15, dd (15.0, 11.4) | 147.4, CH |
| 24 | 6.41, dd (15.0, 8.4) | 136.5, CH | 6.41, dd (15.0, 7.8) | 136.6, CH |
| 25 | 9.65, d (8.4) | 193.0, CH | 9.96, d (7.8) | 193.0, CH |
Spectra were recorded in CDCl3 at 600 and 150 MHz for 1H and 13C NMR, respectively (TMS as internal standard).
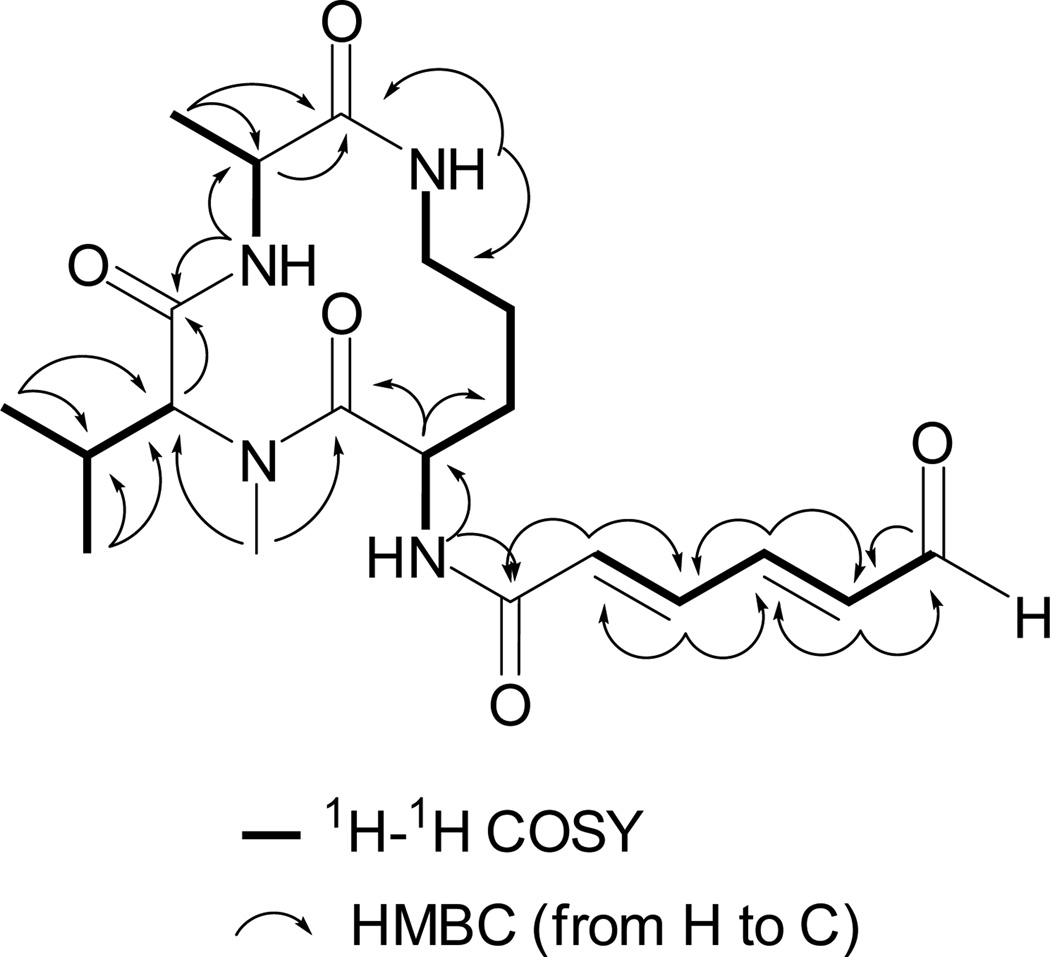
The 1H–1H COSY and gHMBC correlations shown for 3 in Figure 2 were used to assemble four substructures. These consisted of (a) (2E,4E)-6-oxohexa-2,4-dienamide, (b) ornithine, (c) NMe-valine, and (d) alanine. The data in Fig. 3 also allowed the connectivity sequence between these moieties to be established as shown in the final structure of (−) 3. Similar logic was used to establish the gross structure of (−) 4, which was the NMe-alanine analog of 3. Further evident was that this pair was closely related to (−) 5 and (−) 6 by replacement of their terminal carbon propenyl group by an aldehyde moiety. Both 5 and 6 have been shown to possess all L-amino acid subgroups, thus it was tempting to assign the amino acid residues in 3 and 4 as also all L-containing. This conclusion was buttressed by the following observations. On completion of our work we discovered that (−) 4 had been isolated and characterized from Aspergillus sclerotiorum and assigned the same overall structure including double bond geometries and absolute configurations we deduced (Zheng et al., 2010). Further consistent were NMR data J value comparisons, indicating the same 2α, 8β, and 12β relative configurations for 3 based on J’s of H-2/H-3a (7.2 Hz), H-2/H3b (0.6 Hz), H-2/H-19 (7.2 Hz), H-8/H-9 (7.2 Hz), and H-12/H-13 (10.2 Hz) versus those in 4 of (H-2/H-3a (7.2 Hz), H-2/H3b (1.2 Hz), H-2/H-19 (6.6 Hz), H-8/H-9 (7.2 Hz), and H-12/H-13 (10.2 Hz)). Also consistent were the results from NOE analysis of 3, irradiating the proton H-8, significant NOE enhancements were obtained at H-6, H-12, H-17 and H-18. When irradiating the proton H-12, significant NOE enhancement was also obtained at H-8, but no enhancement was observed at H-2. Hence, H-8 and H-12 were on the same plane, which was opposite to that of H-2. Finally the absolute configurations of C-2, C-8, and C-12 of 3 were probed using experimental vs. calculated electronic optical rotation (OR) data. The B3LYP/6-311++G(b.p) method was used to confirm the absolute configuration (Frisch et al., 1998) as this approach is emerging as a powerful tool in the absolute configuration analysis of natural products (McCann and Stephens, 2006).The [α] values for the two enantiomeric configurations of 3 were calculated as follows: 2S, 8S, 12S = − 31.22 vs. that for 2R, 8R, 12R = + 0.76. The former orientation (all L-configuration amino acids) was in agreement with the experimental .
Fig. 3.
Key 1H-1H COSY and HMBC correlations observed for 3.
Unfortunately, no new useful insights were obtained about the bioactivity properties of the compounds isolated here. It is now clear that the previously reported selective and potent cytotoxicity of the EtOAc crude extract of the initial small-scale culture (125 mL) is entirely due to insulicolide A (1) (see SI Table S1). Compounds 2–7 were each inactive at 1 mg/mL against murine lymphocytic (L1210), Colon 38, human colon adenocarcinoma (HCT-116), and human lung adenocarcinoma (H125) (Amagata et al., 2006; Subramanian et al., 2006). Additionally, 3 and 4 were in active at 100 µM for in vitro cytotoxicity against human prostate (PC3), human breast adenocarcinoma (MCF-7) and murine macrophage (RAW) cancer cell lines using an MTT method (Wu et al., 2005). Finally, this pair of compounds exhibited no inhibition of NF-kB and iNOS when tested in the presence of LPS (Gilmore, 2006; Naylor, 1999).
A final issue pertains to 3 and 4 as being authentic natural products or artifacts of isolation. A recent publication showed that 6 could be converted into 4 by prolonged exposure to oxygen (Zheng et al., 2010). Clearly shown in Fig. 2, is that 3 and 4 were both observed early in the isolation process. This intimates that both compounds are bonafide constituents produced in the culture broths rather than arising from the post-isolation work-up.
3. Experimental
3.1. General experimental procedures
The optical rotations were determined on a Jasco DIP 370 digital polarimeter and UV data were obtained on an Agilent 8453 UV/Vis spectrophotometer. All NMR spectra were recorded in CDCl3 or CD3OD with 5mm resonance (HCN) probe. Chemical shifts are reported in ppm relative to CDCl3 (δH 7.27) and CD3OD (δH 3.31). A Mariner ESI mass spectrometer was used for low- and high-resolution mass measurements. Both ELSD and UV (254 nm) were used for peak detection. Semipreparative reverse-phase (RP) HPLC used a C-18 4 µm column, 10 × 250 mm and UV peak detection 254 nm. Compound purity (> 95%) was confirmed using both 1H and 13C NMR and LCMS (UV and ELSD detection) experiments.
3.2. Fungal material
Strain 088708aZA, from shallow water marine sediment collected in Hawaii, was cultured as previously described5 via our standard methods.18 It was identified as Aspergillus insulicola via molecular methods as previously described 5 and the isolation of insulicolide A (1), considered as a taxonomic marker for this genus,6 was consistent with this. The fungus is maintained as a cryopreserved glycerol stock at UCSC.
3.3. Fermentation, extraction, and isolation
The large-scale culture (10 L) was grown in Czapek-Dox media made with artificial seawater-based media adjusted to pH 7.0 with shaking (150 rpm) for 21 days at room temperature (23 °C). The broth and mycelia were separated through vacuum filtration and the broth was extracted with Hp20 resin. The Hp20 resin extraction was washed with water (Hp1) followed by 50% methanol/water (Hp2), methanol (Hp3), and isopropanol (Hp4) and the solvent was concentrated in vacuo. After LCMS analysis, Hp3 (443.2 mg) was subjected to RP-HPLC (30 – 50% acetonitrile / 0.1% formic acid-water, 50mins), and 42 fractions (F1~F42) were collected. Further purification of F11 yielded 2 (1.1 mg), F13 yielded 7 (1.7 mg), F17 via RP-HPLC yielded 3 (3.2 mg), F19 yielded 5 (1.0 mg) and 6 (2.5 mg), F24 yielded 4 (11.7 mg), and F34 yielded 1 (2.2 mg).
3.3.1. Pre-sclerotiotide F (3)
White amorphous solid, (c 0.1, MeOH), UV (MeOH) λmax (log ε) 201.0 (4.06) and 272.0 (3.98) nm, HR-ESI-MS [M+Na]+ m/z 429.21362 (calcd for C20H30N4O5Na, 429.21084), 1H and 13C NMR data (CDCl3) see Table 1.
3.3.2. Sclerotiotide F (4)
White amorphous solid, (c 0.1, MeOH), UV (MeOH) λmax (log ε): 201.0 (4.19) and 272.0 (4.29) nm, HR-ESI-MS [M+H]+ m/z 421.24650 (calcd for C21H33N4O5, 421.24455), 1H and 13C NMR data (CDCl3) see Table 1.
Highlights.
► Seven compounds were isolated from a marine sediment-derived fungus, Aspergillus insulicola. ► Pre-sclerotiotide F (3) was a new tripeptide. ► Compounds 3 and 5 were the N-demethyl analogs of 4 and 6. ► Cytotoxicities of compounds 1–7 were measured in vitro against selected cancer cells.
Acknowledgements
This work was supported by NIH grants CA 47135 (PC) and CA 052955 (PC), US Civilian Research and Development Foundation IDBI-21003-JA-08 of USA and National Basic Research Program (2007CB108903), the 111 Project, the Fundamental Research Funds for the Central Universities (LZUJBKY-2011-75), and Interdisciplinary Youth Innovation Fund of Lanzhou University (LZUJC2007005) of China. We thank Dr. Rinaldi & Dr. Thompson at UT San Antonio for molecular and phenotypic taxonomic identification, Mr. Mejia for technical assistance, Dr. Johnson, Dr. Sohn, and Prof. Bjeldanes in the Department of Nutritional Sciences and Toxicology at University of California, Berkeley for bioassays.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supporting Information. Extraction and separation scheme of Aspergillus insulicola, cytotoxicities of crude extracts and pure compounds, NMR and UV spectra for compounds 3 and 4. Those material are available free of charge via the internet at doi:
References
- Abrell L, Borgeson B, Crews P. Chloro polyketides from the cultured fungus (Aspergillus) separated from a marine sponge. Tetrahedron Lett. 1996;37:2331–2334. [Google Scholar]
- Amagata T, Morinaka BI, Amagata A, et al. A chemical study of cyclic depsipeptides produced by a sponge-derived fungus. J. Nat. Prod. 2006;69:1560–1565. doi: 10.1021/np060178k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belofsky GN, Jensen PR, Renner MK, et al. New cytotoxic sesquiterpenoid nitrobenzoyl esters from a marine isolate of the fungus Aspergillus versicolor. Tetrahedron. 1998;54:1715–1724. [Google Scholar]
- Boot CL, Gassner NC, Compton JE, et al. Pinpointing Pseurotins from a Marine-Derived Aspergillus as Tools for Chemical Genetics Using a Synthetic Lethality Yeast Screen. J. Nat. Prod. 2007;70:1672–1675. doi: 10.1021/np070307c. [DOI] [PubMed] [Google Scholar]
- Ebel R. In: Comp. Nat. Prod. II Chem. Biol. CONAP. Moore BS, Crews P, editors. Vol. 2. Elsevier; 2010. pp. 226–227. [Google Scholar]
- Frisch MJ, Trucks GW, Schlegel HB, et al. Gaussian 98, Revision A.9. Pittsburgh.: Gaussian, Inc.; 1998. [Google Scholar]
- Gilmore TD. Introduction to NF-.kappa.B: players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- McCann DM, Stephens PJ. Determination of absolute configuration using density functional theory calculations of optical rotation and electronic circular dichroism: chiral alkenes. J.Org. Chem. 2006;71:6074–6098. doi: 10.1021/jo060755+. [DOI] [PubMed] [Google Scholar]
- Motohashi K, Inaba S, Takagi M, et al. JBIR-15, a new aspochracin derivative, isolated from a sponge-derive fungus, Aspergillus sclerotiorum Huber SP080903f04. Biosci. Biotechnol. Biochem. 2009;73:1898–1900. doi: 10.1271/bbb.90228. [DOI] [PubMed] [Google Scholar]
- Myokei R, Sakurai A, Chang C-F, et al. Structure of aspochracin, an insecticidal metabolite of Aspergillus ochraceus. Tetraderon Lett. 1969;9:695–698. doi: 10.1016/s0040-4039(01)87785-6. [DOI] [PubMed] [Google Scholar]
- Naylor LH. Reporter gene technology: the future looks bright. Biochem. Pharm. 1999;58:749–757. doi: 10.1016/s0006-2952(99)00096-9. [DOI] [PubMed] [Google Scholar]
- Rahbaek L, Christopherson C, Frisvad J, et al. Insulicolide A: a new nitrobenzoyloxy-substituted sesquiterpene from the marine fungus Aspergillus insulicola. J. Nat. Prod. 1997;60:811–813. [Google Scholar]
- Subramanian B, Nakeff A, Tenney K, et al. A new paradigm for the development of anticancer agents from natural products. J. Exp. Ther. Oncol. 2006;5:195–204. [PMC free article] [PubMed] [Google Scholar]
- Varoglu M, Corbett TH, Valeriote FA, et al. Asperazine, a selective cytotoxic alkaloid from a sponge-derived culture of Aspergillus niger. J. Org. Chem. 1997;62:7078–7079. doi: 10.1021/jo970568z. [DOI] [PubMed] [Google Scholar]
- Varoglu M, Crews P. Biosynthetically diverse compounds from a saltwater culture of sponge-derived Aspergillus niger. J. Nat. Prod. 2000;63:41–43. doi: 10.1021/np9902892. [DOI] [PubMed] [Google Scholar]
- Wu QX, Crews MS, Draskovic M, et al. Azonazine, a novel dipeptide from a Hawaiian marine sediment-derived fungus, Aspergillus insulicola. Org. Lett. 2010;12:4458–4461. doi: 10.1021/ol101396n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu QX, Yang AM, Shi YP. Sesquiterpenoids from Ligularia virgaurea spp. oligocephala. Tetrahedron. 2005;61:10529–10535. [Google Scholar]
- Zheng J, Xu Z, Wang Y, et al. Cyclic tripeptides from the Halotolerant fungus Aspergillus sclerotiorum PT06-1. J. Nat. Prod. 2010;73:1133–1137. doi: 10.1021/np100198h. [DOI] [PubMed] [Google Scholar]