Abstract
BACKGROUND
Data are needed on how life expectancy affects treatment decisions among women ≥80 years with early stage breast cancer.
METHODS
We used the linked Surveillance Epidemiology and End Results-Medicare claims dataset from 1992–2005 to identify women aged ≥80 newly diagnosed with lymph node negative, estrogen receptor positive tumors, ≤5 centimeters. To estimate life expectancy, we matched these women to women of similar age, region, and insurance, not diagnosed with breast cancer. We examined 5-year mortality of matched controls by illness burden (measured with the Charlson Comorbidity Index [CCI]) using Kaplan-Meier statistics. We examined treatments received by estimated life expectancy within CCI levels. We further examined factors associated with receipt of radiotherapy after breast conserving surgery (BCS).
RESULTS
Of 9,932 women, 39.6% underwent mastectomy, 30.4% received BCS plus radiotherapy, and 30.0% received BCS alone. Estimated 5-year mortality was 72% for women with CCIs of 3+, yet 38.0% of these women underwent mastectomy and 22.9% received radiotherapy after BCS. Conversely, estimated 5-year mortality was 36% for women with CCIs of 0 and 26.6% received BCS alone. Age 80–84, urban residence, higher grade, recent diagnosis, mammography use, and low comorbidity, were factors associated with receiving radiotherapy after BCS. Among women with CCIs of 3+ treated with BCS, 36.9% underwent radiotherapy.
CONCLUSIONS
Many women aged ≥80 with limited life expectancies receive radiotherapy after BCS for treatment of early stage breast cancers while many in excellent health do not. More consideration needs to be given to patient life expectancy when considering breast cancer treatments. KEY WORDS: Breast cancer, older women, treatment, life expectancy, radiation
Introduction
The benefits of radiotherapy after breast conserving surgery (BCS) among women aged ≥80 with early stage breast cancer are not well understood even though radiotherapy after BCS is standard treatment for most younger women.1 No studies have demonstrated a mortality benefit of radiotherapy after BCS among women aged ≥80, although some studies suggest a small reduction in local recurrence.1–4 Most experts agree that older women in excellent health without comorbid disease limiting their life expectancy would be the most likely to benefit from radiotherapy after BCS.2–5
A pooled analysis of data from several trials including women of all ages, (but few ≥70 years) found that radiotherapy after BCS resulted in 7% of women experiencing a local recurrence after 5 years compared to 26% of women who received BCS alone. 6 This study also showed a 5.4% reduction in breast cancer mortality and a 5.3% reduction in overall mortality after 15 years among women who received radiotherapy after BCS. Breast cancer recurrence was less common among women aged ≥70 (3% among women who received radiotherapy after BCS compared to 13% among those who did not).6 Data were not presented on whether the mortality benefit of radiotherapy after BCS persisted among women ≥70 years. A randomized control trial (RCT) comparing mastectomy, BCS alone, and BCS plus radiotherapy including women of all ages after 20 years follow-up found that radiotherapy after BCS reduced rates of ipsilateral recurrence (39.2% to 14.3%).7 There were no significant differences in distant-disease free or overall survival in the three groups; however, radiotherapy after BCS was associated with a nearly significant increase in breast cancer survival.8 These studies suggest that radiotherapy after BCS for early stage breast cancer significantly reduces local recurrence and possibly reduces breast cancer mortality after 15–20 years follow-up, which is beyond the life expectancy of most women ≥80 years.8
Few studies examining radiotherapy after BCS have focused on older women. One RCT included 636 women aged ≥70 with stage I, estrogen receptor positive (ER+) breast cancer treated with BCS, and randomized women to treatment with tamoxifen plus radiotherapy or tamoxifen alone. After 5 years, the group that received radiotherapy experienced fewer local and regional recurrences (1% versus 4%, p<0.001) but there were no significant differences in distant recurrence or survival.1 An observational study that included women with similar characteristics found that radiotherapy after BCS reduced breast cancer recurrence from 5.1% to 1.1%. 2 Another RCT that included 769 women aged ≥50 (~40% ≥70 years) with node negative breast tumors, 5 centimeters (cm) or less, found that radiotherapy after BCS reduced local recurrence (7.7% to 0.6%, p<0.001) but did not show significant differences in rates of distant relapse or overall survival after 5 years.3 A recent review recommended radiotherapy for all patients after BCS, except possibly women aged ≥70 with lymph node negative (LN-) tumors, 5 cm or less, being treated with hormonal therapy. 9 Although not all investigators agree that elderly women with favorable tumor characteristics can forgo radiotherapy after BCS, nearly all concur that those with limited life expectancies are unlikely to benefit.2,6,10,11
In this study, we examined initial treatment of women aged ≥80 with early stage breast cancers by life expectancy. We then examined characteristics associated with the use of radiotherapy after BCS among these women.
Methods
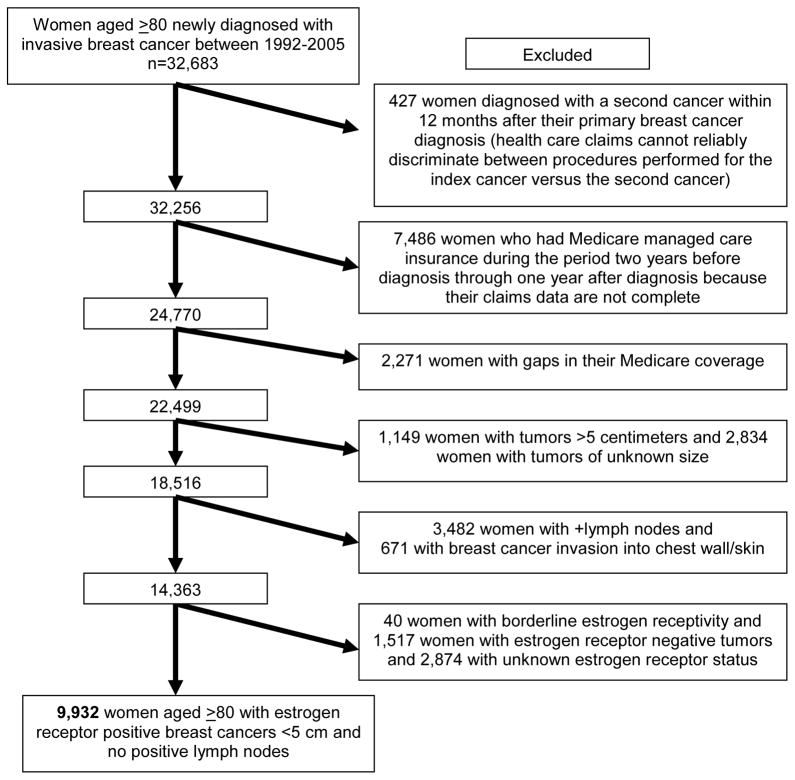
We used data from the National Cancer Institute’s linked SEER-Medicare dataset. Since 1992, SEER has included 11 population-based tumor registries in the metropolitan areas of San Francisco/Oakland, Detroit, Atlanta, and Seattle; Los Angeles county; the San Jose-Monterey area; and the states of Connecticut, Iowa, New Mexico, Utah, and Hawaii.12 These areas cover approximately 14% of the US population.13 For this study, we included 9,932 women aged ≥80 with continuous fee-for-service Medicare insurance newly diagnosed with a unilateral, invasive breast cancer with tumors that were 5 cm or less, no known positive lymph nodes [LN-], and ER+. Figure 1 illustrates selection of our sample population.
Figure 1.
Study Population
Characteristics
We obtained data on tumor characteristics at diagnosis from SEER including: tumor grade, size, stage, and histology. Patient characteristics available in SEER include: race/ethnicity, metropolitan vs. non-metropolitan residence, marital status, and year of diagnosis. Because SEER-Medicare data do not provide individual level data on income and/or education, we used census tract data and substituted ZIP code level data when not available.14 We grouped median household income and percentage of adults with less than a high school education into quintiles within registry. We determined mammography use in the previous 27 months since we wanted to examine two full years of utilization data, and we hypothesized that mammograms within 3 months of diagnosis might relate to diagnosis and not screening behaviors. We categorized previous mammography use sequentially as 1) non-users, 2) regular users (at least 2 mammograms 10 months apart or 1 mammogram coded as screening), 3) only diagnostic mammograms within 3 months of diagnosis, and 4) other.
Treatments
We used data from both SEER and Medicare claims to classify initial treatment with surgery or radiotherapy (RT). We considered women to have received surgical and/or radiation treatment if reported in SEER or if there were Medicare claims for these treatments within 12 months following diagnosis. We categorized initial treatment as mastectomy, BCS with radiotherapy, BCS alone, or no initial surgery.
Comorbidities
We considered individual comorbidities from the Charlson Comorbidity Index and the weighted index (CCI 0,1, 2, 3+).15 We used Klabunde’s modification of the Charlson Comorbidity Index derived from both inpatient and outpatient claims to define these conditions.15–17 We also considered several additional diagnoses from the Clinical Classifications Software (CCS).18 The CCS is a collection of 13,600 diagnoses codes and 3,700 procedure codes collapsed into 262 clinically meaningful categories. We considered CCS conditions that have a significant impact on the health and function of older adults, not already captured by the Charlson index, and were prevalent in ≥1% of the study population. The 10 CCS conditions we considered were Parkinson’s disease, hypertension, mood disorders, thyroid disorder, blindness, pneumonia, nutritional deficiencies, gastrointestinal hemorrhage, hip fracture, and/or falls in the past two years. As we did for the Charlson, we only considered a CCS diagnosis from outpatient claims if it appeared twice separated by greater than one month to avoid “rule-out” diagnoses. Life expectancy
To estimate life expectancy unconfounded by the diagnosis and treatment of breast cancer, we matched women in our sample (cases) with women in the 5% random sample of Medicare beneficiaries who reside in SEER areas but who do not have breast cancer (controls). We used 5-year mortality of the matched controls as the estimated probability of 5-year mortality of the cases at the time of diagnosis. Our methods for matching have been described previously.19 Briefly, we used the greedy matching algorithm (SAS Macro GMATCH) to match women by birth year, registry location, and year of registry. The date of diagnosis of the cases was assigned as the date to begin follow-up of the matched controls. We required that the controls, like cases, had to have continuous fee-for-service Medicare the two years before follow-up. We successfully matched 98.2% of our cases with a non-breast cancer control. Controls were similar to our cases in terms of comorbidity, race/ethnicity, and income and education of residence.
Survival
Survival time was measured from date of diagnosis (cases) or assigned date of diagnosis (controls) until death or December 31, 2006, whichever came first. We censored observations of women who were alive at the end of the follow-up period. For women diagnosed with breast cancer, we also examined breast cancer specific survival using data on cause of death from SEER through December 31, 2005.
Statistical Analyses
We used the Kaplan-Meier method to estimate 5-year mortality for the controls and to estimate 5-year overall and breast cancer mortality for the cases. We additionally estimated 5-year mortality for women with each individual Charlson and CCS condition and by the weighted Charlson Comorbidity Index (CCI) for both the cases and controls. We examined initial treatments received for women with each individual condition and by CCI (0, 1, 2, 3+). Since few women (1.8%, n=177) did not receive any initial surgery, we do not present data on these women. We then limited our sample to women treated with BCS (n=5,889) and we used multivariable logistic regression to identify sociodemographics, tumor characteristics and comorbidities (both individually and using the Charlson Comorbidity Index) associated with receiving radiotherapy after BCS. We converted adjusted odds ratios to relative risks (RR) using SAS PROC GENMOD’s Poisson regression capability.20 All statistical analyses used SAS version 9.1 (SAS Institute, Cary, NC). Beth Israel Deaconess Medical Center’s Institutional Review Board approved this study.
Results
Of the 9,932 women aged ≥80 diagnosed with favorable breast cancers, 58.2% were aged 80-84, 29.7% were aged 85–89, and 12.1% were aged ≥90; 43% were regular users of mammography. The majority were diagnosed with stage I disease (61.8%, Table 1). Approximately half (55.4%) had a CCI of 0 and 8.7% had a CCI ≥3 (Table 2). Based on data from controls, women with a CCI ≥3 had a substantially higher probability of 5-year mortality (72.4%) than women with a CCI of 0 (36.1%). Despite this large difference in overall mortality risk, women with a CCI ≥3 were nearly as likely to receive mastectomy (38.0% versus 40.5%) but were somewhat less likely to receive BCS+RT (22.9% versus 32.9%) than women with a CCI of 0 (Table 2). Five- year breast cancer mortality was low for all women and ranged from 5.6% among women with a CCI of 0 to 9.8% among women with a CCI ≥3.
Table 1.
Sample Characteristics of 9,932 Women aged 80 and Older with Estrogen Receptor Positive Tumors, 5 Centimeters or Less, with No Known Positive Lymph Nodes.
| n | % | |
|---|---|---|
| Age | ||
| 80–84 | 5,779 | 58.2 |
| 85–89 | 2,951 | 29.7 |
| 90+ | 1,202 | 12.1 |
| Race/Ethnicity | ||
| Non-Hispanic White | 9,152 | 92.2 |
| Non-Hispanic Black | 362 | 3.6 |
| Hispanic | 79 | 0.8 |
| Asian | 169 | 1.7 |
| Other | 107 | 1.1 |
| Unknown | 63 | 0.6 |
| Year of diagnosis | ||
| 92–95 | 2,608 | 26.3 |
| 96–00 | 3,574 | 36.0 |
| 01–05 | 3,750 | 37.8 |
| Metropolitan status of residence | ||
| Metropolitan | 8,902 | 89.6 |
| Non-metropolitan | 1,030 | 10.4 |
| Marital status | ||
| Not married | 7,228 | 72.8 |
| Married | 2,371 | 23.9 |
| Unknown | 333 | 3.4 |
| Median income for area of residence | ||
| Lowest Quintile | 1,900 | 19.1 |
| 2nd Quintile | 1,997 | 20.1 |
| 3rd Quintile | 2,082 | 21.0 |
| 4th Quintile | 1,971 | 19.8 |
| 5th Quintile | 1,937 | 19.5 |
| Unknown | 45 | 0.5 |
| Quintiles of education (<12 years) for adults ≥25 in area of residence | ||
| Lowest Quintile | 1,832 | 18.5 |
| 2nd Quintile | 1,922 | 19.4 |
| 3rd Quintile | 2,011 | 20.3 |
| 4th Quintile | 2,077 | 20.9 |
| 5th Quintile | 2,045 | 20.6 |
| Unknown | 45 | 0.5 |
| Mammography use in the past 2 years | ||
| Non-users | 3,810 | 38.4 |
| Screeners | 4,237 | 42.7 |
| Peri-diagnosis mammogram | 1,411 | 14.2 |
| Other | 474 | 4.8 |
| SEER registry | ||
| Connecticut | 1,505 | 15.2 |
| San Francisco | 829 | 8.4 |
| Detroit | 1,204 | 12.1 |
| Hawaii | 153 | 1.5 |
| Iowa | 1,870 | 18.8 |
| New Mexico | 310 | 3.1 |
| Seattle | 1,302 | 13.1 |
| Utah | 485 | 4.9 |
| Atlanta | 547 | 5.5 |
| San Jose | 395 | 4.0 |
| Los Angeles | 1,332 | 13.4 |
| Stage | ||
| Stage I | 6,141 | 61.8 |
| Stage II | 2,093 | 21.1 |
| Unknown | 1,698 | 17.1 |
| Tumor Size | ||
| ≤1 cm | 3,605 | 36.3 |
| 1– ≤2 cm | 4,037 | 40.7 |
| 2– ≤5 cm | 2,290 | 23.1 |
| Histology | ||
| Ductal | 7,689 | 77.4 |
| Lobular | 811 | 8.2 |
| Mucinous | 699 | 7.0 |
| Other | 684 | 6.9 |
| Unknown | 49 | 0.5 |
| Tumor Grade | ||
| Well differentiated, 1 | 2,561 | 25.8 |
| Moderately differentiated, 2 | 4,354 | 43.8 |
| Poorly differentiated, 3 | 1,933 | 19.5 |
| Undifferentiated, 4 | 125 | 1.3 |
| Unknown | 959 | 9.6 |
| Lymph Nodes | ||
| None positive | 8,278 | 83.4 |
| None examined |
Table 2.
Five Year Breast and Overall Morbidity for 9,932 Women Aged 80 and Older with Favorable Breast Tumor Characteristics by Comorbidity and for 9,749 Non-Breast Cancer Controls.
| MATCHED CONTROLS | BREAST CANCER CASES | TREATMENT RECEIVED | ||||||
|---|---|---|---|---|---|---|---|---|
| % (n) | 5-year overall mortality% | % (n) | 5-year overall mortality % | 5-year breast cancer mortality % | Mastectomy | BCS+ Radiotherapy | BCS alone | |
| OVERALL | 100 (9,749) | 47.3 | 100 (9,932) | 41.1 | 6.7 | 39.6 | 30.4 | 30.0 |
| Charlson Index | ||||||||
| 0 | 51.3 (5,004) | 36.1 | 55.4 (5,498) | 31.6 | 5.6 | 40.5 | 32.9 | 26.6 |
| 1 | 24.5 (2,391) | 52.2 | 23.9 (2,374) | 46.6 | 7.6 | 39.8 | 27.8 | 32.4 |
| 2 | 13.1 (1,278) | 64.0 | 12.0 (1,196) | 55.2 | 8.8 | 36.4 | 28.8 | 34.7 |
| 3+ | 11.0 (1,076) | 72.4 | 8.7 (864) | 69.1 | 9.8 | 38.0 | 22.9 | 39.2 |
| Individual Illnesses: | ||||||||
| Dementia | 6.9 (676) | 80.4 | 3.0 (300) | 77.9 | 19.0 | 40.8 | 5.8 | 53.4 |
| Parkinson’s Disease | 2.0 (190) | 74.7 | 1.3 (129) | 70.0 | 8.1 | 39.5 | 20.2 | 40.3 |
| Pneumonia | 6.3 (609) | 72.7 | 4.3 (431) | 66.9 | 9.9 | 39.7 | 19.7 | 40.6 |
| Nutritional Deficiencies | 2.8 (274) | 70.2 | 1.6 (154) | 61.3 | 10.1 | 40.1 | 15.7 | 44.2 |
| Congestive Heart Failure | 14.7 (1,435) | 69.0 | 13.5 (1,340) | 65.5 | 10.8 | 37.8 | 20.8 | 41.5 |
| Chronic Renal Failure | 1.9 (181) | 68.5 | 1.4 (140) | 75.3 | 13.3 | 33.8 | 27.9 | 38.2 |
| Falls | 2.8 (273) | 68.7 | 2.1 (213) | 59.8 | 9.0 | 38.0 | 23.1 | 38.9 |
| Hip fracture | 3.7 (362) | 68.4 | 2.5 (243) | 61.0 | 11.9 | 43.9 | 15.6 | 40.5 |
| Myocardial infarction | 3.9 (378) | 63.8 | 3.6 (356) | 58.8 | 9.5 | 37.6 | 29.0 | 33.3 |
| Cerebrovascular Disease | 10.8 (1,055) | 64.5 | 9.0 (895) | 55.8 | 7.8 | 37.3 | 24.9 | 37.8 |
| Mood Disorder | 7.7 (752) | 64.4 | 6.1 (601) | 52.7 | 8.8 | 34.9 | 25.0 | 40.1 |
| COPD† | 12.8 (1,248) | 63.4 | 11.4 (1,134) | 55.7 | 8.0 | 39.6 | 27.7 | 32.7 |
| Peripheral Vascular Disease | 5.7 (554) | 63.4 | 5.2 (515) | 56.6 | 8.0 | 41.6 | 23.0 | 35.4 |
| Cancer | 3.9 (382) | 63.2 | 4.0 (395) | 54.6 | 8.3 | 31.5 | 30.5 | 38.0 |
| Diabetes | 14.1 (1,371) | 58.9 | 14.0 (1,394) | 55.5 | 7.7 | 39.8 | 30.5 | 29.7 |
| GI hemorrhage | 3.8 (371) | 57.1 | 3.8 (377) | 54.7 | 11.3 | 36.4 | 27.5 | 36.1 |
| Peptic Ulcer Disease | 2.9 (279) | 56.8 | 1.9 (189) | 54.8 | 12.9 | 39.3 | 22.4 | 38.3 |
| Rheumatologic Disease | 3.2 (315) | 51.0 | 2.5 (249) | 46.1 | 7.7 | 34.2 | 36.6 | 29.2 |
| Thyroid Disorders | 13.2 (1,289) | 50.4 | 14.1 (1,396) | 45.4 | 7.3 | 33.1 | 34.5 | 32.4 |
| Hypertension | 54.3 (5,297) | 47.7 | 56.7 (5,635) | 41.5 | 5.8 | 37.3 | 32.9 | 29.8 |
| Blindness | 3.9 (379) | 44.5 | 4.7 (468) | 39.5 | 4.8 | 37.6 | 33.3 | 29.2 |
We do not present data for women with liver disease and paraplegia since less than 1% of women received these diagnoses. However, women with these conditions were included in the Charlson Comorbidity Index.
COPD=Chronic Obstructive Pulmonary Disease
Examining individual comorbidities, we found that women with dementia (80.4%), Parkinson’s disease (74.7%), pneumonia (72.7%), or nutritional deficiencies (70.2%) in the past 2 years had the highest risk of 5-year mortality, based on data from controls (Table 2). However, nearly all women with a significant illness had greater than 50% risk of mortality in 5 years. Few women with dementia received BCS+RT (5.8%) while 40.8% received mastectomies and 53.4% were treated with BCS alone. Many women with other significant illnesses limiting their life expectancies were treated with BCS+RT. For example, although women with chronic renal failure have a 69.0% risk of 5-year mortality, 27.9% received BCS+RT. Women with dementia had the highest rate of 5-year breast cancer mortality at 19.0% and were the least likely to be treated with BCS+RT. However, women with chronic renal failure had the next highest rate of 5-year breast cancer mortality (13.3%) even though many were treated with BCS+RT. Women with a history of hip fractures were the most likely to undergo mastectomy (43.9%) and were less likely to undergo BCS+RT (15.6%).
In the subset of women who received BCS (n=5,889) for their surgical treatment, 50.3% also received radiotherapy (Table 3). Although women with a CCI ≥3 were less likely to receive radiotherapy after BCS (aRR 0.7 95% CI [0.7–0.8]) than women without comorbidity, 36.9% with a CCI ≥3 still received radiotherapy after BCS. Advancing age was strongly associated with decreased likelihood of receiving radiotherapy after BCS; only 12.7% of women aged ≥90 received radiotherapy after BCS compared to 65.1% of women aged 80–84 and 38.1% of women aged 85–89. Women residing in Metropolitan areas, recently diagnosed, regular users of mammography, and those with high grade tumors were more likely to receive radiotherapy after BCS.
Table 3.
Factors Associated with Receipt of Radiotherapy after BCS among 5,889 Women Aged 80 and Older who Underwent BCS.*
| n | % Received Radiotherapy after BCS | Adjusted Relative Risk of Radiotherapy after BCS† | |
|---|---|---|---|
| Overall | 5,889 | 50.3 | |
| Characteristics: | |||
| Age | |||
| 80–84 | 3,377 | 65.1 | 1.0 |
| 85–89 | 1,754 | 38.1 | 0.7 (0.6–0.7) |
| 90+ | 758 | 12.7 | 0.3 (0.2–0.3) |
| Metropolitan status of residence | |||
| Metropolitan | 5,444 | 52.0 | 1.0 |
| Non-Metropolitan | 445 | 29.3 | 0.8 (0.7–0.9) |
| Race/Ethnicity | |||
| Non-Hispanic White | 5,439 | 50.2 | 1.0 |
| Non-Hispanic Black | 218 | 48.2 | 1.0 (0.9–1.2) |
| Hispanic | 47 | 44.7 | 0.8 (0.6–1.1) |
| Asian | 90 | 68.9 | 1.1 (0.9–1.3) |
| Other | 61 | 52.5 | 0.9 (0.7–1.2) |
| Unknown | 34 | 35.3 | 0.8 (0.6–1.1) |
| Marital status | |||
| Not currently married | 4,240 | 46.8 | 1.0 |
| Currently married | 1,444 | 62.6 | 1.1 (1.0–1.1) |
| Unknown | 203 | 36.0 | 0.8 (0.7–0.9) |
| Year of diagnosis | |||
| 92–95 | 1,310 | 38.5 | 1.0 |
| 96–00 | 2,141 | 50.7 | 1.2 (1.1–1.3) |
| 01–05 | 2,438 | 56.2 | 1.2 (1.1–1.3) |
| Median income for area of residence | |||
| Lowest Quintile | 1,020 | 42.6 | 1.0 |
| 2nd Quintile | 1,156 | 47.7 | 1.0 (0.9–1.1) |
| 3rd Quintile | 1,205 | 50.0 | 1.0 (0.9–1.1) |
| 4th Quintile | 1,228 | 52.9 | 1.0 (0.9–1.2) |
| 5th Quintile | 1,254 | 57.1 | 1.1 (1.0–1.3) |
| Quintiles of education (<12 years) for those Age ≥ 25 in area of residence | |||
| Lowest Quintile | 976 | 45.2 | 1.0 |
| 2nd Quintile | 1,070 | 49.7 | 1.1 (1.0–1.1) |
| 3rd Quintile | 1,220 | 48.9 | 1.1 (1.0–1.2) |
| 4th Quintile | 1,274 | 52.9 | 1.1 (1.0–1.2) |
| 5th Quintile | 1,323 | 53.7 | 1.1 (1.0–1.2) |
| Mammography Use | |||
| Screeners | 2,846 | 63.0 | 1.0 |
| Non-users | 1,955 | 37.8 | 0.8 (0.8–0.9) |
| Peri-diagnosis | 814 | 35.9 | 0.8 (0.7–0.8) |
| Other | 274 | 50.0 | 0.9 (0.8–1.0) |
| Tumor stage | |||
| Stage I | 3,733 | 59.5 | 1.0 |
| Stage II | 842 | 46.2 | 1.0 (0.9–1.2) |
| Unknown | 1,314 | 26.8 | 0.8 (0.6–1.2) |
| Tumor Grade | |||
| Grade I | 1,740 | 50.6 | 1.0 |
| Grade II | 2,582 | 52.0 | 1.1 (1.0–1.1) |
| Grade III | 1,030 | 50.5 | 1.1 (1.0–1.2) |
| Grade IV | 56 | 57.1 | 1.4 (1.1–1.7) |
| Unknown | 481 | 38.5 | 1.0 (0.9–1.1) |
| Tumor size | |||
| ≤1cm | 2,477 | 55.6 | 1.0 |
| 1–≤2cm | 2,387 | 50.6 | 1.0 (1.0–1.1) |
| 2-≤5cm | 1,025 | 36.8 | 0.9 (0.8–1.1) |
| Tumor histology | |||
| Ductal | 4,568 | 50.8 | 1.0 |
| Lobular | 446 | 55.8 | 1.0 (0.9–1.1) |
| Mucinous | 417 | 47.5 | 1.0 (0.9–1.1) |
| Other | 436 | 41.7 | 0.8 (0.8–0.9) |
| Lymph node | |||
| None positive | 4,604 | 57.0 | 1.0 |
| None examined | 1,285 | 26.4 | 0.7 (0.5–1.0) |
| SEER registry | |||
| Connecticut | 1,031 | 52.4 | 1.0 |
| San Francisco | 548 | 51.1 | 1.0 (0.9–1.0) |
| Detroit | 740 | 52.2 | 1.0 (1.0–1.1) |
| Hawaii | 91 | 69.2 | 1.1 (0.9–1.3) |
| Iowa | 847 | 33.3 | 0.8 (0.7–0.9) |
| New Mexico | 166 | 50.0 | 1.0 (0.9–1.2) |
| Seattle | 791 | 56.5 | 1.1 (1.0–1.2) |
| Utah | 246 | 56.9 | 1.2 (1.0–1.3) |
| Atlanta | 294 | 41.5 | 0.8 (0.7–0.9) |
| San Jose | 235 | 52.3 | 1.0 (0.9–1.1) |
| Los Angeles | 900 | 55.0 | 1.0 (0.9–1.1) |
| Comorbidities | |||
| Dementia | 173 | 9.8 | 0.3 (0.2–0.4) |
| Nutrition Deficiencies | 88 | 26.1 | 0.7 (0.5–1.0) |
| Heart Failure | 812 | 33.4 | 0.8 (0.7–0.9) |
| Mood Disorder | 378 | 38.4 | 0.8 (0.7–0.9) |
| Other Cancer | 267 | 44.6 | 0.8 (0.7–0.9) |
| Peptic Ulcer Disease | 111 | 36.9 | 0.8 (0.7–1.1) |
| Hip fracture | 133 | 27.8 | 0.8 (0.6–1.0) |
| Parkinson’s Disease | 75 | 33.3 | 0.9 (0.6–1.2) |
| COPD | 667 | 45.9 | 0.9 (0.8–1.0) |
| Cerebral Vascular Disease | 547 | 39.7 | 0.9 (0.8–1.0) |
| Pneumonia | 248 | 32.7 | 0.9 (0.8–1.1) |
| Peripheral Vascular Disease | 292 | 39.4 | 0.9 (0.8–1.1) |
| Chronic Renal Failure | 90 | 42.2 | 0.9 (0.7–1.1) |
| Gastrointestinal Hemorrhage | 234 | 43.2 | 1.0 (0.8–1.1) |
| Thyroid Disorder (not cancer) | 916 | 51.5 | 1.0 (0.9–1.1) |
| Falls | 129 | 37.2 | 1.0 (0.8–1.3) |
| Diabetes | 823 | 50.7 | 1.0 (1.0–1.1) |
| Rheumatologic Disorders | 160 | 55.6 | 1.1 (0.9–1.2) |
| Blindness | 289 | 53.3 | 1.1 (1.0–1.2) |
| Hypertension | 3,471 | 52.4 | 1.0 (1.0–1.1) |
| Myocardial infarction | 217 | 46.5 | 1.1 (1.0–1.3) |
| Charlson Comorbidity Index ‡ | |||
| 0 | 3,223 | 55.4 | 1.0 |
| 1 | 1,402 | 46.2 | 0.9 (0.8–0.9) |
| 2 | 743 | 45.4 | 0.9 (0.8–0.9) |
| 3+ | 521 | 36.9 | 0.7 (0.7–0.8) |
Each model was adjusted for tumor characteristics (grade, tumor size, histology, stage), sociodemographics at diagnosis (age, marital status, race/ethnicity, educational attainment of census tract, ZIP code, median household income of census tract/income, metropolitan status of residence), year of diagnosis, lymph node status, mammography use, and tumor registry.
Odds ratios were converted to Relative Risks using SAS PROC GENMOD’s Poisson regression capability.
In a separate model, we categorized women by their Charlson Comorbidity Index (0,1,2,3+) rather than their individual comorbidities; however, we did include conditions from the Clinical Classifications Software that were not included in the Charlson.
Women with dementia, congestive heart failure, mood disorders, and other cancers, were significantly less likely to receive radiotherapy after BCS than women without comorbidity. However, other conditions (e.g. chronic renal failure, myocardial infarction, falls) were not associated with a decreased likelihood of treatment with radiotherapy after BCS. Only 9.8% of women with dementia were treated with radiotherapy after BCS, while 45.9% of women with chronic obstructive pulmonary disease received radiotherapy after BCS (Table 3). Conversely, 44.5% of women without any of the conditions associated with >50% mortality in 5-years (all conditions in Table 2 except hypertension and blindness) were not treated with radiotherapy after BCS.
Discussion
We found that half (50.3%) of women aged ≥80 with ER+, LN- breast tumors ≤5 cm received radiotherapy after breast conserving surgery. We also found that treatment with radiotherapy after BCS was not limited to the oldest women in good health; 37% of women with a 72% probability (CCI ≥3) of mortality in five years and very little chance of benefit received radiotherapy after BCS. Increasing age and the presence of dementia, which are two factors strongly associated with decreased life expectancy, were two of the strongest factors associated with not being treated with radiotherapy after BCS. However, women with many other conditions associated with high risk of 5-year mortality (e.g., chronic renal failure) commonly (42%) received radiotherapy after BCS. Conversely, 45% of women without significant comorbidities limiting their life expectancy did not receive radiotherapy after BCS. Some of these women may benefit from a reduction in 5-year local recurrence.
We found that many women aged ≥80 newly diagnosed with an early stage, ER+, LN- tumor but who also have a life threatening comorbidity are at high risk of non-breast cancer mortality in 5 years. Experts encourage clinicians to consider patient life expectancy when deciding on breast cancer treatments for the oldest women.4 To our knowledge, this is the first study to examine receipt of breast cancer treatments by estimated life expectancy among older women with ER+, LN-breast cancers ≤5 cm. Beyond age and dementia, clinicians may need to factor in older women’s other health conditions (e.g., chronic renal failure) when deciding which treatments to recommend for early stage breast cancer. A Comprehensive Geriatric Assessment (CGA), which consists of measurement of patient’s function, cognition, mood, mobility, nutritional status, medications, and social situation, can aid treatment decision making.21 Assessment-based treatment has been shown to improve the quality of life of elderly cancer patients.21,22
Although a local recurrence can be a significant event in an older woman’s life, few older women develop a recurrence.1,3,6 Meanwhile, nearly all experience fatigue during treatment,9,23 and radiotherapy can cause breast pain, skin fibrosis or retraction, and edema as well as arm and shoulder pain even months following treatment.9,24 Serious complications of radiotherapy are rare but include radiation pneumonitis (experienced by <5% of women).25 The potential benefits of radiotherapy after BCS must be weighed against the potential risks for each woman within the context of her life expectancy. Moreover, it can be difficult for older women to arrange transportation to a radiation center for treatments.9 Transportation needs can additionally burden patients’ family members and affect family members socially or financially.
We found that a substantial proportion of women without significant comorbidities, approximately 45%, did not receive treatment with radiotherapy after BCS. Although there are no data suggesting a mortality benefit of radiotherapy after BCS among women ≥80 years, some of the oldest women in excellent health may benefit due to a reduction in local recurrence.2 Furthermore, women aged 80 in excellent health have a life expectancy of more than 13 years.26 Based on data from younger women suggesting a small mortality benefit of radiation after BCS after 15–20 years, some of the women aged ≥80 in excellent health may not only benefit from a reduction in breast cancer recurrence but possibly even a reduction in breast cancer mortality. Studies have found that adults aged ≥80 in good health tolerate radiotherapy well. Increasingly, these women are also being offered shorter courses of radiotherapy which may be associated with fewer side effects and/or less transportation burden.24 Thoughtful consideration of patients’ comorbidities, life expectancy, and preferences, must occur when deciding on the optimal treatment of breast cancer among women aged ≥80.
Women with dementia had the greatest risk of 5-year mortality and were the least likely to receive radiotherapy after BCS, but these women were also at greater risk of death from breast cancer. These findings could suggest that women with dementia would suffer less breast cancer mortality if they were more commonly treated with radiotherapy after BCS. However, dementia is also known to be under-reported on death certificates,27 while other conditions like coronary heart disease have been shown to be overreported,28 and breast cancer may be more accurately reported.29 Therefore, among women with dementia and breast cancer, breast cancer may be more likely to be documented on the death certificate than in the case of women with breast cancer and other conditions. However, in post-hoc analyses, we found that women with dementia were more likely to have larger tumors and not have their lymph nodes assessed than other women in our study so it is also possible that women with dementia had less favorable tumors. There are no specific guidelines for treating breast cancer among women with dementia; however, studies have found that most caregivers prefer comfort care for women with severe dementia while there is great variation in preferences for women with mild to moderate dementia.30
Although this study focused on use of radiotherapy after BCS, we also found that 40% of the women aged ≥80 received a mastectomy for favorable breast cancers. Although mastectomies may have been necessary for women with the largest tumors, many others may have done well with BCS. Some women and their clinicians may have chosen mastectomy over BCS so as not to be faced with the decision of whether or not to undergo radiotherapy. Others may have received a mastectomy to avoid a second surgery in the case of a local recurrence. Such decision-making may have occurred in women that sustained hip fractures since few on average were treated with BCS+RT while more on average were treated with mastectomy. Identifying which of the oldest women may benefit from radiotherapy after BCS and which will not, may allow clinicians to feel more comfortable recommending BCS without radiotherapy rather than mastectomy to the oldest women with significant comorbidity, exposing fewer of these women to more invasive breast surgery.3
Women who were regular users of mammography screening were more likely to receive radiotherapy after BCS. This finding may reflect that older women who undergo screening are more likely to accept medical interventions in general, more likely to have access to transportation, and/or more likely to be in better health than those who are not screened. However, comorbidity scores were similar for regular users and non-users of mammography (60.7% of non-users had a Charlson score of 0 compared to 60.3% of regular users). Our findings may indicate that older women who are screened and diagnosed with breast cancer may be at greater risk of receiving treatments from which they have little chance to benefit such as radiotherapy after BCS than those who are not screened.
There are important limitations to our study. Although we do not have data on use of endocrine therapy, other studies have found that more than 80% of women ≥ 80 years with ER+ early stage tumors receive tamoxifen.31 Therefore, we limited our sample to women with known ER+ tumors. However, it is possible that some women chose radiotherapy after BCS rather than treatment with endocrine therapy. In addition, adjusting for endocrine therapy use in previous studies did not alter the relationship between recurrence and radiotherapy after BCS in previous studies of older women.4 Since our data are from administrative claims we do not have data on patient preferences or treatment discussions with physicians. Furthermore, administrative data may underestimate the prevalence of many chronic conditions. However, claims data likely captures individuals with more severe disease, suggesting that we found high rates of radiotherapy after BCS among women with more severe forms of disease.32,33 Some of the women categorized as having no significant diseases may be misclassified and this may partly explain why many of these women did not receive radiotherapy after BCS. However, we additionally excluded women with a history of falls and other geriatric conditions associated with mortality. We also lacked individual-level data on socioeconomic status; however, studies have found a moderate association between individual and aggregate socioeconomic characteristics. 14
In summary, breast cancer treatment choices for women aged ≥80 with ER+, LN- breast cancers ≤5 cm are associated with life expectancy. However, many elderly women with short life expectancies receive radiotherapy after BCS while others in excellent health do not. Clinicians should consider using available prognostic tools to determine which patients are more likely to benefit from radiotherapy after BCS based on life expectancy.34 Future studies are needed to better understand how to improve decision-making around breast cancer treatment for the oldest women.
Acknowledgments
Research Support: Dr. Schonberg was supported by a Paul B. Beeson Career Development Award in Aging supported by the National Institute on Aging K23 [K23AG028584], The John A. Hartford Foundation, The Atlantic Philanthropies, The Starr Foundation, and The American Federation for Aging Research. Drs. McCarthy, Marcantonio, and Silliman were supported by the American Cancer Society [RSGT-10-080-CPHSPS]. There are no financial disclosures.
Footnotes
Disclosures: The authors have no conflicts of interest to report.
Author Contributions: Concept and design: Schonberg, Marcantonio, Silliman, McCarthy Data collection: Schonberg, McCarthy Analysis and interpretation of data: Schonberg, Marcantonio, Ngo, Silliman, McCarthy Manuscript writing and approval: Schonberg, Marcantonio, Ngo, Silliman, McCarthy
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Mara A. Schonberg, Division of General Medicine and Primary Care, Department of Medicine, Harvard Medical School, Beth Israel Deaconess Medical Center, Boston, MA.
Edward R. Marcantonio, Email: emarcant@bidmc.harvard.edu, Division of General Medicine and Primary Care and Gerontology, Department of Medicine, Harvard Medical School, Beth Israel Deaconess Medical Center, Boston, MA.
Long Ngo, Email: lngo@bidmc.harvard.edu, Division of General Medicine and Primary Care, Department of Medicine, Harvard Medical School, Beth Israel Deaconess Medical Center, Boston, MA.
Rebecca A. Silliman, Email: rsillima@bu.edu, Geriatrics Section, Boston University Schools of Medicine and Public Health, Boston University Medical Center, Boston, MA.
Ellen P. McCarthy, Email: emccarth@bidmc.harvard.edu, Division of General Medicine and Primary Care, Department of Medicine, Harvard Medical School, Beth Israel Deaconess Medical Center, Boston, MA.
References
- 1.Hughes KS, Schnaper LA, Berry D, Cirrincione C, McCormick B, Shank B, et al. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med. 2004;351:971–977. doi: 10.1056/NEJMoa040587. [DOI] [PubMed] [Google Scholar]
- 2.Smith BD, Gross CP, Smith GL, Galusha DH, Bekelman JE, Haffty BG. Effectiveness of radiation therapy for older women with early breast cancer. J Natl Cancer Inst. 2006;98:681–690. doi: 10.1093/jnci/djj186. [DOI] [PubMed] [Google Scholar]
- 3.Fyles AW, McCready DR, Manchul LA, Trudeau ME, Merante P, Pintilie M, et al. Tamoxifen with or without breast irradiation in women 50 years of age or older with early breast cancer. N Engl J Med. 2004;351:963–970. doi: 10.1056/NEJMoa040595. [DOI] [PubMed] [Google Scholar]
- 4.Geiger AM, Thwin SS, Lash TL, Buist DS, Prout MN, Wei F, et al. Recurrences and second primary breast cancers in older women with initial early-stage disease. Cancer. 2007;109:966–974. doi: 10.1002/cncr.22472. [DOI] [PubMed] [Google Scholar]
- 5.Vinh-Hung V, Verschraegen C. Breast-conserving surgery with or without radiotherapy: pooled-analysis for risks of ipsilateral breast tumor recurrence and mortality. J Natl Cancer Inst. 2004;96:115–121. doi: 10.1093/jnci/djh013. [DOI] [PubMed] [Google Scholar]
- 6.Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 7.Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 8.Arias E. National Vital Statistics Reports. Vol. 56. 2007. United States Life Tables, 2004; p. 13. [PubMed] [Google Scholar]
- 9.Buchholz TA. Radiation therapy for early-stage breast cancer after breast-conserving surgery. N Engl J Med. 2009;360:63–70. doi: 10.1056/NEJMct0803525. [DOI] [PubMed] [Google Scholar]
- 10.Martelli G, Miceli R, Costa A, Coradini D, Zurrida S, Piromalli D, et al. Elderly breast cancer patients treated by conservative surgery alone plus adjuvant tamoxifen: fifteen-year results of a prospective study. Cancer. 2008;112:481–488. doi: 10.1002/cncr.23213. [DOI] [PubMed] [Google Scholar]
- 11.Hamaker ME, Schreurs WH, Uppelschoten JM, Smorenburg CH. Breast cancer in the elderly: retrospective study on diagnosis and treatment according to national guidelines. Breast J. 2009;15:26–33. doi: 10.1111/j.1524-4741.2008.00667.x. [DOI] [PubMed] [Google Scholar]
- 12.Procedure Codes for Seer-Medicare Analyses. 2009 October 29; Available at: http://healthservices.cancer.gov/seermedicare/considerations/procedure_codes.html.
- 13.Ries LAG, Kosary CL, Hankey BF, Miller BA, Edwards BK, editors. NIH Publication Number 97-2789. National Cancer Institute; Bethesda, MD: 1997. SEER Cancer Statistics Review, 1973–1994. [Google Scholar]
- 14.Bach PB, Guadagnoli E, Schrag D, Schussler N, Warren JL. Patient demographic and socioeconomic characteristics in the SEER-Medicare database applications and limitations. Med Care. 2002;40:19–25. doi: 10.1097/00005650-200208001-00003. [DOI] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 17.National Cancer Institute. SEER-Medicare: Calculation of Comorbidity Weights. 2011 July 25; http://healthservices.cancer.gov/seermedicare/program/comorbidity.html.
- 18.Clinical Classifications Software for Services and Procedures. 2011 July 25; http://www.hcup-us.ahrq.gov/toolssoftware/ccs/AppendixASingleDX.txt.
- 19.Schonberg MA, Marcantonio ER, Ngo L, Li D, Silliman RA, McCarthy EP. Causes of Death and Relative Survival of Older Women after a Breast Cancer Diagnosis. J Clin Oncol. 2011;29:1570–1577. doi: 10.1200/JCO.2010.33.0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 21.Wedding U, Pientka L, Hoffken K. Quality-of-life in elderly patients with cancer: a short review. Eur J Cancer. 2007;43:2203–2210. doi: 10.1016/j.ejca.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol. 2007;25:1824–1831. doi: 10.1200/JCO.2007.10.6559. [DOI] [PubMed] [Google Scholar]
- 23.Lopez E, Nunez MI, Guerrero MR, del Moral R, de Dios Luna J, del Mar Rodriguez M, et al. Breast cancer acute radiotherapy morbidity evaluated by different scoring systems. Breast Cancer Res Treat. 2002;73:127–134. doi: 10.1023/a:1015296607061. [DOI] [PubMed] [Google Scholar]
- 24.Zachariah B, Balducci L, Venkattaramanabalaji GV, Casey L, Greenberg HM, DelRegato JA. Radiotherapy for cancer patients aged 80 and older: a study of effectiveness and side effects. Int J Radiat Oncol Biol Phys. 1997;39:1125–1129. doi: 10.1016/s0360-3016(97)00552-x. [DOI] [PubMed] [Google Scholar]
- 25.Lind PA, Marks LB, Hardenbergh PH, Clough R, Fan M, Hollis D, et al. Technical factors associated with radiation pneumonitis after local +/- regional radiation therapy for breast cancer. Int J Radiat Oncol Biol Phys. 2002;52:137–143. doi: 10.1016/s0360-3016(01)01715-1. [DOI] [PubMed] [Google Scholar]
- 26.Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285:2750–2756. doi: 10.1001/jama.285.21.2750. [DOI] [PubMed] [Google Scholar]
- 27.Wachterman M, Kiely DK, Mitchell SL. Reporting dementia on the death certificates of nursing home residents dying with end-stage dementia. JAMA. 2008;300:2608–2610. doi: 10.1001/jama.2008.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lloyd-Jones DM, Martin DO, Larson MG, Levy D. Accuracy of death certificates for coding coronary heart disease as the cause of death. Ann Intern Med. 1998;129:1020–1026. doi: 10.7326/0003-4819-129-12-199812150-00005. [DOI] [PubMed] [Google Scholar]
- 29.Kircher T, Nelson J, Burdo H. The autopsy as a measure of accuracy of the death certificate. N Engl J Med. 1985;313:1263–1269. doi: 10.1056/NEJM198511143132005. [DOI] [PubMed] [Google Scholar]
- 30.Smyth KA. Current practices and perspectives on breast cancer screening and treatment in older women with dementia. J Am Geriatr Soc. 2009;57 (Suppl 2):S272–274. doi: 10.1111/j.1532-5415.2009.02510.x. [DOI] [PubMed] [Google Scholar]
- 31.Silliman RA, Guadagnoli E, Rakowski W, Landrum MB, Lash TL, Wolf R, et al. Adjuvant tamoxifen prescription in women 65 years and older with primary breast cancer. J Clin Oncol. 2002;20:2680–2688. doi: 10.1200/JCO.2002.08.137. [DOI] [PubMed] [Google Scholar]
- 32.Quan H, Parsons GA, Ghali WA. Validity of information on comorbidity derived rom ICD-9-CCM administrative data. Med Care. 2002;40:675–685. doi: 10.1097/00005650-200208000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Wilchesky M, Tamblyn RM, Huang A. Validation of diagnostic codes within medical services claims. J Clin Epidemiol. 2004;57:131–141. doi: 10.1016/S0895-4356(03)00246-4. [DOI] [PubMed] [Google Scholar]
- 34.Schonberg MA, Davis RB, McCarthy EP, Marcantonio ER. An Index to Predict 5-Year Mortality of Community-Dwelling Adults Aged 65 and Older. J Gen Intern Med. 2009;24(10):1115–1121. doi: 10.1007/s11606-009-1073-y. [DOI] [PMC free article] [PubMed] [Google Scholar]