Abstract
The mating system plays a key role during the process of plant invasion. Contemporary evolution of uniparental reproduction (selfing or asexuality) can relieve the challenges of mate limitation in colonizing populations by providing reproductive assurance. Here we examined aspects of the genetics of colonization in Ambrosia artemisiifolia, a North American native that is invasive in China. This species has been found to possess a strong self-incompatibility system and have high outcrossing rates in North America and we examined whether there has been an evolutionary shift towards the dependence on selfing in the introduced range. Specifically, we estimated outcrossing rates in one native and five invasive populations and compared levels of genetic diversity between North America and China. Based on six microsatellite loci we found that, like the native North American population, all five Chinese populations possessed a completely outcrossing mating system. The estimates of paternity correlations were low, ranging from 0.028–0.122, which suggests that populations possessed ∼8–36 pollen donor parents contributing to each maternal plant in the invasive populations. High levels of genetic diversity for both native and invasive populations were found with the unbiased estimate of gene diversity ranging from 0.262–0.289 for both geographic ranges based on AFLP markers. Our results demonstrate that there has been no evolutionary shift from outcrossing to selfing during A. artemisiifolia's invasion of China. Furthermore, high levels of genetic variation in North America and China indicate that there has been no erosion of genetic variance due to a bottleneck during the introduction process. We suggest that the successful invasion of A. artemisiifolia into Asia was facilitated by repeated introductions from multiple source populations in the native range creating a diverse gene pool within Chinese populations.
Introduction
The main challenge for populations of self-incompatible outcrossing plants is the mate acquisition phase of sexual reproduction. This part of the life cycle can be especially tenuous for invasive species because it depends on the number and distribution of genetically compatible genotypes. A biological invasion typically originates with the transport of individuals from the species' native to the introduced range. Since long-distance introduction involves a subsampling of the native range gene pool [1], [2], [3], [4] founding populations are usually initiated by small numbers of propagules that face genetic and demographic challenges that increase the probability of local extinction [5], [6].
Smaller and more isolated populations of self-incompatible wind-pollinated plants often experience increased pollen limitation [7]. Such negative effects of low abundance on individual performance and population growth known as Allee Effects can dramatically decrease rate of spread, even preventing invasion altogether [8], [9], [10]. Under such conditions, natural selection may favor the adaptive evolution of selfing [5], [11], [12], [13], [14], which will allow a rapid build-up of the population from a small number of colonists. This association between uniparental reproduction and colonizing ability is often referred to as Baker's Law [15]. Therefore, we may expect to see invasive species exhibiting breeding system transitions in the introduced range to avoid these constraints [11], [16], [17], [18], [19], [20]. By providing reproductive assurance, selfing and asexual reproduction can facilitate population establishment under conditions of low density [11], [21], [22], [23], [24]. However, efforts to explore the evolution of plant reproductive systems during biological invasion have only been made relatively recently [11], [25], [26]. Out of five case studies in which the breeding system has been estimated in both native and invasive ranges [27], [28], [29], [30], [31], only one exhibited a shift from self-incompatibility to self-compatibility following introduction to the novel range [31].
Founder events and genetic bottlenecks are generally thought to result in a reduction in the genetic diversity when compared to the total diversity present in the native range [32], [33]. Indeed, loss of genetic diversity has been reported for selectively neutral markers [34], [35], [36] and for loci under selection [37], [38]. A recent meta-analysis revealed the significant loss of both allelic richness and heterozygosity in invasive populations [39]. On the other hand, for several of successful invaders (e.g. Ambrosia artemisiifolia, Bromus tectorum, Clidemia hirta) the level of genetic diversity is actually greater in the introduced ranges [2], [18], [40], although the meta-analysis demonstrated that this pattern is rare [39]. One way to explain the preservation of genetic variation in the introduced range is that the colonization phase was associated with high levels of propagule pressure [16], [41], [42], thereby overcoming any Allee effects or affecting the capacity of invasive species to adapt to its new environment [43]. Under such conditions, selection for subsequent evolution of the mating system would be relaxed if reproductive success is not limited by the number of genetically compatible mates.
The goal of the present study was to document the mating system and levels of genetic variation in Ambrosia artemisiifolia, a plant that has invaded China. This wind-pollinated, monoecious annual is reported to possess a strong self-incompatibility mechanism and exhibit high outcrossing rates in its native North American range [44]. However, molecular studies have revealed a deficit of heterozygotes in both native and invasive populations [45], [46], [47]. This pattern may be the result of either selfing and/or biparental inbreeding. Hence, a study to quantify the degree of selfing is critical to help us understand the factors contributing to this species' Chinese invasion. We wished to determine whether the spread of this species in China was associated with an evolutionary transition from outcrossing to selfing. We had two specific objectives. First, we estimated the selfing rates from North American and Chinese populations using microsatellite markers. Second, in order to evaluate the effects of propagule pressure, we quantified the levels of genetic diversity in four native North American populations and six invasive Chinese populations using AFLP (amplified fragment length polymorphism) markers.
Methods
Ethics Statement
No specific permits were required for the described field studies. The sampling location is not privately-owned or protected in any way, and the field studies did not involve endangered or protected species.
Study Species
The common ragweed, Ambrosia artemisiifolia L. (Asteraceae) is an aggressive annual weed of agricultural fields and disturbed sites that is native to North America and has become invasive in Europe and Eurasia [48]. Ambrosia artemisiifolia was introduced into China in 1930s and now occurs across a large range from Guangdong Province in the south to Heilongjiang Province in the north, and has invaded a variety of plant communities [49]. The species has a monoecious, wind-pollinated breeding system and previous work has found the mating system in its native North America to be primarily outcrossing due to a self-incompatibility system [44].
Estimation of the Mating System
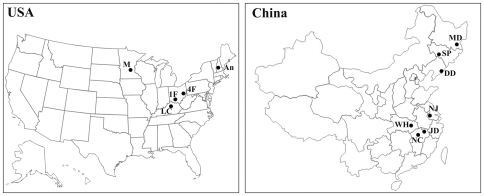
The mating system (selfing rate) was determined using microsatellite markers for one native North American population (LC) and five populations (MD, SP, DD, NJ, and NC) sampled over a large latitudinal gradient in eastern China (Figure 1). For each population, we detected the genotypes of all the seeds randomly sampled from 12 mother plants and 8 mature seeds per mother plants across six SSR loci (GenBank accession numbers: FJ595150, FJ595151, FJ595152, FJ595154, FJ595155, and FJ595156). Total genomic DNA was extracted using a plant genomic DNA extraction kit (Tiangen, China). All the SSR fragments were amplified via standard PCR in 25 µl volume containing 2.5 µl 10×PCR buffer, 2 µl 2.5 mM dNTPs, 1.5 µl 25 mM MgCl2, 0.5 µl 10 mM each fluorescence-labeled forward and reverse primers, 1 U Taq DNA polymerase (TaKaRa, Liaoning, China), 16.8 µl H2O and 1 µl (ca. 10–20 ng) genomic DNA. PCR amplifications were performed in a Bio-Rad thermal cycler as follows: an initial denaturation step at 94°C for 5 min followed by 30 cycles of 30 s at 94°C, 45 s at 50°C and 45 s at 72°C, with a final extension period of 8 min at 72°C. The fluorescence-labeled PCR products were denatured and analyzed on an ABI 3100 genetic analyzer, using the ROX 500 as an internal size standard. The genotypes were scored using GENEMAPPER software version 3.7 (Applied Biosystems).
Figure 1. Map of Ambrosia artemisiifolia populations used in this study from China and North America.
Population MD, SP, DD, NJ, NC, and LC were used for outcrossing rates estimation with microsatellite; MD, SP, DD, WH, NJ, NC, 1F, 4F, M, and An were for genetic diversity estimation with AFLP.
We estimated the single-locus outcrossing rate (ts), multilocus outcrossing rate (tm), outcrossing rate between related individuals (tm−ts), inbreeding coefficient of maternal parents (F), and the correlation of paternity or proportion of full sibs among outcrossed progeny (rp) for all six populations using the expectation-maximization method in the MLTRWIN program (version 3.4) [50]. Standard deviations were based on 1000 bootstrap values, with options of re-sampling families. The distribution of bootstrap values was analyzed according to Friedman and Barrett [44] and Eckert and Barrett [51] to determine whether outcrossing rates differed significantly from 1.0. Plants are characterized as obligate outcrossers when outcrossing rates do not deviate from one. When values are significantly less than one the species is self-compatible and exhibits a mixed mating system.
Analysis of Genetic Diversity
To compare genetic variation between North American and Chinese populations of Ambrosia artemisiifolia, leaves were collected for AFLP analysis from the six Chinese populations in addition to four from North America (Figure 1). Leaves of 11–55 individuals were sampled at random in every population, dried using silica gel, and then preserved at room temperature. Total genomic DNA from all samples was isolated using a plant genomic DNA extraction kit (Tiangen, China).
AFLP fingerprinting was performed as described by Vos et al. [52] with some minor modifications. The digestion and ligation were carried out simultaneously. In brief, 100–200 ng genome DNA was digested with 8 U EcoRI and 2 U MseI for 3 h at 37°C and 10 min at 70°C, and simultaneously ligation adapters EcoRI and MseI were ligated to the sticky ends of the digested DNA fragments with 80 U T4 ligase. Selective pre-amplifications were performed with primers that match the adapter sequence and contained an additional selective base at the 3′ end. After a 20-fold dilution step, the resulting products were amplified in a second round with primers containing three selective bases and fluorescently labeled with 6-FAM (Sangon, China). The selective PCR amplifications were performed with a touchdown cycling process programmed with the following temperature profile: an initial denaturation step 94°C for 2 min, followed by 13 cycles of 94°C for 30 s, 65°C for 30 s, and 72°C for 1 min with a reduction of the annealing temperature at each cycle by 0.7°C; the annealing temperature was maintained at 56°C for the remaining 23 cycles. Four pairs of primers (E-AAC/M-CAG, E-ACT/M-CAC, E-ACT/M-CTG, and E-ATG/M-CTG) were used to amplify the DNA of 254 plants from four American populations and six Chinese populations.
The selective PCR products were denatured and separated on an ABI 3100 genetic analyzer (Applied Biosystems) with a ROX 500 as internal size standard. Fragment sizes were assessed using GENEMAPPER software version 3.7 (Applied Biosystems). Allele size determinations were performed twice manually to reduce scoring errors. The multilocus AFLP profiles were scored as present or absent to create binary matrices.
Based on the observed frequencies of AFLP fragments, allelic frequencies at each locus were estimated using Bayesian method with non-uniform prior distribution proposed by Zhivotovsky [53] in AFLP-SURV 1.0 [54]. These allelic frequencies were then used as the input for the analyses of genetic diversity following the method described in Lynch and Milligan [55]. Specifically, we calculated: 1) percentage of polymorphic loci (PLP, 5% level); 2) unbiased estimates of genetic diversity (Hj, analogous to He) to estimate the within-population diversity; and the degree of genetic differentiation (Fst) among populations within each of the continents.
Results
Mating System
Both Chinese and North American A. artemisiifolia populations displayed high outcrossing rates (Table 1). The multilocus outcrossing rates (mean ± S.D.) estimated from microsatellites for Chinese populations ranged from 0.928±0.042 to 1.000±0.000. These values are not significantly different from 1.0, indicating a completely outcrossing mating system for Chinese ragweed populations. The native North American population was also completely outcrossing, with a multilocus outcrossing rate of 1.000±0.000 (Table 1).
Table 1. Estimates of mating system parameters for the six populations of Ambrosia artemisiifolia based on six microsatellite loci.
| Population | tm | ts | tm−ts | F | rp |
| North America | |||||
| LC | 1.000 (0.000) | 0.937 (0.020) | 0.063 (0.020) | 0.008 (0.020) | 0.018 (0.012) |
| China | |||||
| MD | 0.979 (0.018) | 0.913 (0.033) | 0.065 (0.028) | 0.002 (0.009) | 0.034 (0.016) |
| SP | 0.942 (0.036) | 0.763 (0.040) | 0.179 (0.025) | 0.000 (0.000) | 0.122 (0.047) |
| DD | 0.928 (0.042) | 0.753 (0.051) | 0.175 (0.035) | 0.000 (0.000) | 0.081 (0.037) |
| NJ | 1.000 (0.000) | 0.923 (0.024) | 0.077 (0.024) | 0.000 (0.000) | 0.028 (0.017) |
| NC | 0.982 (0.013) | 0.865 (0.032) | 0.118 (0.027) | 0.001 (0.009) | 0.070 (0.032) |
The values in brackets are S.D. The tm, multilocus outcrossing rate; ts, single-locus outcrossing rate; tm−ts, outcrossing rate between related individuals; F, inbreeding coefficient of maternal parents; rp, the correlation of paternity or proportion of full sibs among outcrossed progeny.
The estimates for the outcrossing rate between related individuals (tm−ts) were low for Chinese populations (range: 0.065±0.028 to 0.179±0.025) and North American population (0.063±0.020). These values were significantly greater than zero, suggesting some degree of biparental inbreeding in both Chinese and North American A. artemisiifolia populations. The maternal inbreeding coefficients (F) were less than 0.008±0.020 and did not differ from zero for all populations, indicating obligate outcrossing. The correlations of paternity (rp) were 0.028–0.122 in Chinese populations, suggesting that the populations consisted of a large number of pollen donor parents. However, there was a higher number of pollen donor parents in the North American population (Table 1).
Genetic Diversity and Structure
The four pairs of FAM-EcoRI/MseI primer combinations in the ten populations yielded a total of 322 AFLP fragments, of which 321 (99.7%) were polymorphic at the 5% level. North American and Chinese populations shared 320 out of the 322 bands. Only two bands were private between continents: one band occurred in four North American populations but was absent in China while another occurred in three Chinese populations but was not found in North American populations.
Overall genetic diversity was similar in North America and China (Table 2). The proportion of polymorphic loci for North American populations and Chinese populations was 82.2% and 78.7% respectively. The unbiased estimation of gene diversity ranged from 0.262–0.289 for the four North American populations and from 0.263–0.283 for six Chinese populations. The Fst values indicate that there is a greater degree of genetic structure in the introduced Chinese range compared to North America. Approximately 94% of the genetic variation exists within North American populations compared to 87% in the invasive Chinese range (Table 2).
Table 2. Genetic diversity indices for Chinese and North American populations of Ambrosia artemisiifolia based on AFLP markers.
| Population | N | PLP (%) | Hj | Fst |
| North America | ||||
| 1F | 11 | 81.1 | 0.280 (0.009) | |
| 4F | 11 | 80.4 | 0.262 (0.010) | |
| M | 55 | 87.3 | 0.276 (0.008) | |
| An | 15 | 80.1 | 0.289 (0.009) | |
| Total North America | 0.295 | 0.060 | ||
| China | ||||
| MD | 28 | 80.4 | 0.280 (0.009) | |
| SP | 26 | 78.3 | 0.283 (0.009) | |
| DD | 20 | 75.8 | 0.263 (0.009) | |
| WH | 29 | 79.2 | 0.272 (0.009) | |
| NJ | 30 | 80.7 | 0.269 (0.009) | |
| NC | 29 | 77.6 | 0.265 (0.009) | |
| Total China | 0.311 | 0.127 |
N is the number of scored individuals; PLP is the proportion of polymorphic loci at the 5% level; Hj is Nei's genetic diversity; Fst is Wright's Fst.
Discussion
The plant mating system is subject to environmental and genetic influences that operate at both ecological and evolutionary time scales [56]. Limited mating often reduces the seed production of individuals in outcrossing populations [57], and the ability to self-fertilize can overcome this constraint on reproductive success [58], [59]. Invasions often begin with a small number of founders and subsequently there is the potential for compatible mates to be a limiting resource. Under these conditions, natural selection would be expected to favor uniparental reproduction in colonizing populations [11]. So far, only one study in the literature clearly discovered the shift from self-incompatibility to self-compatibility in the invasive range [31].Our study provides the first detailed evidence of the mating system of A. artemisiifolia in its invasive Asian range. The main result is that the outcrossing rates are very high and do not deviate from one in both native North American and invasive Chinese populations. In addition the indirect estimates of the paternity correlations (rp) were low suggesting that all of the populations possessed a high number of pollen donor parents contributing to each maternal plant. Moreover, the maternal inbreeding coefficients (F) were nearly equal to zero for all the populations, indicating the absence of inbreeding. Combined with the uniformly high outcrossing rates in North America reported by Friedman and Barrett [44], these results indicate that there has not been an evolutionary transition from the fully outcrossing sexual system that exists in North America during A. artemisiifolia's Asian invasion. Moreover, our results suggest that the positive fixation indices F IS reported in European populations of A. artemisiifolia [45], [46], [47] have likely not resulted from selfing, but from other factors such as the Wahlund effect, which refers to the reduction of heterozygosity due to subpopulation structure.
Since colonizing populations often establish with only a subset of the genotypes present in the native range, we expect less genetic variation to exist in introduced populations when compared to those in the native range [3]. However, AFLP markers revealed similarly high levels of polymorphism and genetic diversity in both Chinese and North American populations, although there was more structure present in Chinese populations. Ambrosia artemisiifolia has also invaded Europe where populations contain similar levels of allelic diversity and heterozygosity as in North America [45], [46], [47]. Perhaps the most parsimonious way to explain this departure from theoretical expectations is that the colonization phase experienced high levels of propagule pressure. If these initial populations were founded by repeated introductions from multiple native source populations, subsequent gene flow and admixture among historically divergent populations in the native range would result in high levels of genetic variation [16], [60], [61].
One of the goals of research on biological invasions is to understand the ecological and genetic factors that contribute to successful establishment and subsequent geographic spread. It is generally believed that owing to being limited to a single bout of reproduction, annual species should be primarily selfing and not possess a self-incompatibility system [2]. This association would be expected to be especially true for colonizing populations of invasive species owing to the dangers of reproductive failure resulting from low population densities that can occur along an invasion front [62]. Following this reasoning, it was assumed that A. artemisiifolia would be a selfer throughout its invasive range in Europe and Asia. However, it is apparent that the species has a highly outcrossed mating system in both its native North American range [44] and in Asian populations in China. Clearly this species has avoided the negative Allee Effects resulting from low abundance and self-incompatibility. The broad geographic distribution of ragweed indicates that while it is an outcrosser, the species exhibits some classic features of a successful invading colonizer [15] by being a rapid growing annual that produces prolific quantities of seeds [44]. Furthermore, not only are invasive populations outcrossing, but high levels of genetic variation are generated during reproduction from multiple paternity arising from the combination of copious quantities of pollen along with flowers that contain only a single ovule.
Acknowledgments
We thank Chuan Ni for his assistance in sample collection, Li-Hong Xu for help with the AFLP analysis, Jannice Friedman for providing helpful comments on a previous draft of the manuscript, and Dun-Yan Tan, Carol Baskin, and Jerry Baskin for helping with seeds collection.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was financially supported by the State Key Basic Research and Development Plan (2009CB119201), the National Natural Science Foundation of China (31121003), and Beijing Normal University. Dr. Wolfe's research is supported by the USA National Science Foundation (DEB 0349553). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Barrett SCH, Husband BC. The genetics of plant migration and colonization. In: Brown AHD, Clegg MT, Kahler AL, Weir BS, editors. Plant population genetics, breeding, and genetic resources. Sunderland, Massachusetts: Sinauer & Associates; 1990. pp. 254–277. [Google Scholar]
- 2.Brown AHD, Marshall DR. Evolutionary changes accompanying colonization in plants. In: Scudder GGE, Reveal JL, editors. Evolution today: Proceedings of the second international congress of systematic and evolutionary biology. Pittsburgh: Hunt Institute for Botanical Documentation, Carnegie-Mellon; 1981. pp. 351–363. [Google Scholar]
- 3.Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J, et al. The population biology of invasive species. Annual Review of Ecology and Systematics. 2001;32:305–332. [Google Scholar]
- 4.Tsutsui ND, Suarez AV, Holway DA, Case TJ. Reduced genetic variation and the success of an invasive species. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:5948–5953. doi: 10.1073/pnas.100110397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Busch JW, Schoen DJ. The evolution of self-incompatibility when mates are limiting. Trends in Plant Science. 2008;13:128–136. doi: 10.1016/j.tplants.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Lande R, Engen S, Saether BE. Stochastic population dynamics in ecology and conservation: an introduction. Oxford: Oxford University Press; 2003. [Google Scholar]
- 7.Davis HG, Taylor CM, Lambrinos JG, Strong DR. Pollen limitation causes an Allee effect in a wind-pollinated invasive grass (Spartina alterniflora). Proceedings of the National Academy of Sciences of the United States of America. 2004;101:13804–13807. doi: 10.1073/pnas.0405230101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groom MJ. Allee effects limit population viability of an annual plant. The American Naturalist. 1998;151:487–496. doi: 10.1086/286135. [DOI] [PubMed] [Google Scholar]
- 9.Leung B, Drake JM, Lodge DM. Predicting invasions: Propagule pressure and the gravity of Allee effects. Ecology. 2004;85:1651–1660. [Google Scholar]
- 10.Taylor CM, Davis HG, Civille JC, Grevstad FS, Hastings A. Consequences of an Allee effect in the invasion of a pacific estuary by Spartina alterniflora. Ecology. 2004;85:3254–3266. [Google Scholar]
- 11.Barrett SCH, Colautti RI, Eckert CG. Plant reproductive systems and evolution during biological invasion. Molecular Ecology. 2008;17:373–383. doi: 10.1111/j.1365-294X.2007.03503.x. [DOI] [PubMed] [Google Scholar]
- 12.Cheptou PO. Allee effect and self-fertilization in hermaphrodites: reproductive assurance in demographically stable populations. Evolution. 2004;58:2613–2621. doi: 10.1111/j.0014-3820.2004.tb01615.x. [DOI] [PubMed] [Google Scholar]
- 13.Colautti RI, White NA, Barrett SCH. Variation of self-incompatibility within invasive populations of purple loosestrife (Lythrum salicaria L.) from eastern North America. International Journal of Plant Sciences. 2010;171:158–166. [Google Scholar]
- 14.Morgan MT, Wilson WG, Knight TM. Plant population dynamics, pollinator foraging, and the selection of self-fertilization. The American Naturalist. 2005;166:169–183. doi: 10.1086/431317. [DOI] [PubMed] [Google Scholar]
- 15.Baker HG. Self compatibility and establishment after long distance dispersal. Evolution. 1955;9:347–349. [Google Scholar]
- 16.Colautti RI, Grigorovich IA, MacIsaac HJ. Propagule pressure: A null model for biological invasions. Biological Invasions. 2006;8:1023–1037. [Google Scholar]
- 17.Davis MB, Shaw RG. Range shifts and adaptive responses to Quaternary climate change. Science. 2001;292:673–679. doi: 10.1126/science.292.5517.673. [DOI] [PubMed] [Google Scholar]
- 18.Maron JL, Vila M, Bommarco R, Elmendorf S, Beardsley P. Rapid evolution of an invasive plant. Ecological Monographs. 2004;74:261–280. [Google Scholar]
- 19.Reinartz JA. Life history variation of common mullein (Verbascum thapsus): I. Latitudinal differences in population dynamics and timing of reproduction. Journal of Ecology. 1984;72:897–912. [Google Scholar]
- 20.Stockwell CA, Hendry AP, Kinnison MT. Contemporary evolution meets conservation biology. Trends in Ecology & Evolution. 2003;18:94–101. [Google Scholar]
- 21.Baker HG. Support for Baker's law-as a rule. Evolution. 1967;21:853–856. doi: 10.1111/j.1558-5646.1967.tb03440.x. [DOI] [PubMed] [Google Scholar]
- 22.Pannell JR, Barrett SCH. Baker's law revisited: reproductive assurance in a metapopulation. Evolution. 1998;52:657–668. doi: 10.1111/j.1558-5646.1998.tb03691.x. [DOI] [PubMed] [Google Scholar]
- 23.Price SC, Jain SK. Are inbreeders better colonizers? Oecologia. 1981;49:283–286. doi: 10.1007/BF00349202. [DOI] [PubMed] [Google Scholar]
- 24.Rambuda TD, Johnson SD. Breeding systems of invasive alien plants in South Africa: does Baker's rule apply? Diversity and Distributions. 2004;10:409–416. [Google Scholar]
- 25.Barrett SCH, Husband BC. Ecology and genetics of ephemeral plant populations: Eichhornia paniculata (Pontederiaceae) in northeast Brazil. Journal of Heredity. 1997;88:277–284. [Google Scholar]
- 26.Olsson K, Ågren J. Latitudinal population differentiation in phenology, life history and flower morphology in the perennial herb Lythrum salicaria. Journal of Evolutionary Biology. 2002;15:983–996. [Google Scholar]
- 27.Lafuma L, Maurice S. Increase in mate availability without loss of self-incompatibility in the invasive species Senecio inaequidens (Asteraceae). Oikos. 2007;116:201–208. [Google Scholar]
- 28.Amsellem L, Noyer JL, Hossaert-McKey M. Evidence for a switch in the reproductive biology of Rubus alceifolius (Rosaceae) towards apomixis, between its native range and its area of introduction. American Journal of Botany. 2001;88:2243–2251. [PubMed] [Google Scholar]
- 29.Davis HG. r-Selected traits in an invasive population. Evolutionary Ecology. 2005;19:255–274. [Google Scholar]
- 30.Cano L, Escarre J, Blanco-Moreno JM, Sans FX. Assessing the effect of inbreeding and long-distance gene flow on the invasive potential of Senecio pterophorus (Asteraceae). Australian Journal of Botany. 2008;56:539–549. [Google Scholar]
- 31.Petanidou T, Godfree RC, Song DS, Kantsa A, Dupont YL, et al. Self-compatibility and plant invasiveness: Comparing species in native and invasive ranges. Perspectives in Plant Ecology, Evolution and Systematics. 2012 in press. [Google Scholar]
- 32.Barrett SCH. Genetics of weed invasions. In: Jain SK, Botsford L, editors. Applied population biology. Dordrecht: Kluwer Academic Publishers; 1992. pp. 91–119. [Google Scholar]
- 33.Novak SJ, Mack RN. Genetic bottlenecks in alien plant species: influence of mating systems and introduction dynamics. In: Sax D, Stachowicz J, Gaines S, editors. Species invasions: insights into ecology, evolution, and biogeography. Sunderland: Sinauer & Associates; 2005. pp. 201–228. [Google Scholar]
- 34.Amsellem L, Noyer JL, Le Bourgeois T, Hossaert-McKey M. Comparison of genetic diversity of the invasive weed Rubus alceifolius Poir. (Rosaceae) in its native range and in areas of introduction, using amplified fragment length polymorphism (AFLP) markers. Molecular Ecology. 2000;9:443–455. doi: 10.1046/j.1365-294x.2000.00876.x. [DOI] [PubMed] [Google Scholar]
- 35.Bossdorf O, Auge H, Lafuma L, Rogers WE, Siemann E, et al. Phenotypic and genetic differentiation between native and introduced plant populations. Oecologia. 2005;144:1–11. doi: 10.1007/s00442-005-0070-z. [DOI] [PubMed] [Google Scholar]
- 36.Estoup A, Wilson IJ, Sullivan C, Cornuet JM, Moritz C. Inferring population history from microsatellite and enzyme data in serially introduced cane toads, Bufo marinus. Genetics. 2001;159:1671–1687. doi: 10.1093/genetics/159.4.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eckert CG, Manicacci D, Barrett SCH. Genetic drift and founder effect in native versus introduced populations of an invading plant, Lythrum salicaria (Lythraceae). Evolution. 1996;50:1512–1519. doi: 10.1111/j.1558-5646.1996.tb03924.x. [DOI] [PubMed] [Google Scholar]
- 38.Giraud T, Pedersen JS, Keller L. Evolution of supercolonies: The Argentine ants of southern Europe. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:6075–6079. doi: 10.1073/pnas.092694199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dlugosch KM, Parker IM. Founding events in species invasions: genetic variation, adaptive evolution, and the role of multiple introductions. Molecular Ecology. 2008;17:431–449. doi: 10.1111/j.1365-294X.2007.03538.x. [DOI] [PubMed] [Google Scholar]
- 40.DeWalt SJ, Hamrick JL. Genetic variation of introduced Hawaiian and native Costa Rican populations of an invasive tropical shrub, Clidemia hirta (Melastomataceae). American Journal of Botany. 2004;91:1155–1163. doi: 10.3732/ajb.91.8.1155. [DOI] [PubMed] [Google Scholar]
- 41.Foxcroft LC, Pickett STA, Cadenasso ML. Expanding the conceptual frameworks of plant invasion ecology. Perspectives in Plant Ecology Evolution and Systematics. 2011;13:89–100. [Google Scholar]
- 42.Simberloff D. The Role of Propagule Pressure in Biological Invasions. 2009. pp. 81–102. Annual Review of Ecology Evolution and Systematics.
- 43.Lockwood JL, Cassey P, Blackburn TM. The more you introduce the more you get: the role of colonization pressure and propagule pressure in invasion ecology. Diversity and Distributions. 2009;15:904–910. [Google Scholar]
- 44.Friedman J, Barrett SCH. High outcrossing in the annual colonizing species Ambrosia artemisiifolia (Asteraceae). Annals of Botany. 2008;101:1303–1309. doi: 10.1093/aob/mcn039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaudeul M, Giraud T, Kiss L, Shykoff JA. Nuclear and chloroplast microsatellites show multiple introductions in the worldwide invasion history of common ragweed, Ambrosia artemisiifolia. PLoS ONE. 2011;6:15. doi: 10.1371/journal.pone.0017658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Genton BJ, Shykoff JA, Giraud T. High genetic diversity in French invasive populations of common ragweed, Ambrosia artemisiifolia, as a result of multiple sources of introduction. Molecular Ecology. 2005;14:4275–4285. doi: 10.1111/j.1365-294X.2005.02750.x. [DOI] [PubMed] [Google Scholar]
- 47.Chun YJ, Fumanal B, Laitung B, Bretagnolle F. Gene flow and population admixture as the primary post-invasion processes in common ragweed (Ambrosia artemisiifolia) populations in France. New Phytologist. 2010;185:1100–1107. doi: 10.1111/j.1469-8137.2009.03129.x. [DOI] [PubMed] [Google Scholar]
- 48.Bassett IJ, Crompton CW. The biology of Canadian weeds: 2. Ambrosia artemisiifolia L. and A. psilostachya D.C. Canadian Journal of Plant Science. 1975;55:463–476. [Google Scholar]
- 49.Wan FH, Guo JY, Zhang F. Research on biological invasions in China. Beijing: Science Press; 2009. [Google Scholar]
- 50.Ritland K. Extensions of models for the estimation of mating systems using n independent loci. Heredity. 2002;88:221–228. doi: 10.1038/sj.hdy.6800029. [DOI] [PubMed] [Google Scholar]
- 51.Eckert CG, Barrett SCH. Post-pollination mechanisms and the maintenance of outcrossing in self-compatible, tristylous, Decodon verticillatus (Lythraceae). Heredity. 1994;72:396–411. [Google Scholar]
- 52.Vos P, Hogers R, Bleeker M, Reijans M, Lee T, et al. AFLP: a new technique for DNA fingerprinting. Nucleic acids research. 1995;23:4407–4414. doi: 10.1093/nar/23.21.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhivotovsky LA. Estimating population structure in diploids with multilocus dominant DNA markers. Molecular Ecology. 1999;8:907–913. doi: 10.1046/j.1365-294x.1999.00620.x. [DOI] [PubMed] [Google Scholar]
- 54.Vekemans X, Beauwens T, Lemaire M, Roldan-Ruiz I. Data from amplified fragment length polymorphism (AFLP) markers show indication of size homoplasy and of a relationship between degree of homoplasy and fragment size. Molecular Ecology. 2002;11:139–151. doi: 10.1046/j.0962-1083.2001.01415.x. [DOI] [PubMed] [Google Scholar]
- 55.Lynch M, Milligan BG. Analysis of population genetic structure with RAPD markers. Molecular Ecology. 1994;3:91–99. doi: 10.1111/j.1365-294x.1994.tb00109.x. [DOI] [PubMed] [Google Scholar]
- 56.Barrett SCH, Husband BC, Cole WW. Variation in outcrossing rates in Eichhornia paniculata: Temporal changes in populations of contrasting style morph structure. Plant Species Biology. 1993;8:141–148. [Google Scholar]
- 57.Burd M. Bateman's principle and plant reproduction: the role of pollen limitation in fruit and seed set. The Botanical Review. 1994;60:83–139. [Google Scholar]
- 58.Eckert CG, Samis KE, Dart S. Reproductive assurance and the evolution of uniparental reproduction in flowering plants. In: Harder LD, Barrett SCH, editors. Ecology and evolution of flowers. Oxford: Oxford University Press; 2006. pp. 183–203. [Google Scholar]
- 59.Larson BMH, Barrett SCH. A comparative analysis of pollen limitation in flowering plants. Biological Journal of the Linnean Society. 2000;69:503–520. [Google Scholar]
- 60.Catford JA, Jansson R, Nilsson C. Reducing redundancy in invasion ecology by integrating hypotheses into a single theoretical framework. Diversity and Distributions. 2009;15:22–40. [Google Scholar]
- 61.Taylor DR, Keller SR. Historical range expansion determines the phylogenetic diversity introduced during contemporary species invasion. Evolution. 2007;61:334–345. doi: 10.1111/j.1558-5646.2007.00037.x. [DOI] [PubMed] [Google Scholar]
- 62.Barrett SCH. Why reproductive systems matter for the invasion biology of plants. In: Richardson DM, editor. Fifty years of invasion ecology: The legacy of Charles Elton. Oxford: Oxford University Press; 2011. pp. 195–210. [Google Scholar]