Abstract
Clostridium difficile spores play a pivotal role in the transmission of infectious diarrhoea, but in order to cause disease spores must complete germination and return to vegetative cell growth. While the mechanisms of spore germination are well understood in Bacillus, knowledge of C. difficile germination remains limited. Previous studies have shown that bile salts and amino acids play an important role in regulating the germination response of C. difficile spores. Taurocholate, in combination with glycine, can stimulate germination, whereas chenodeoxycholate has been shown to inhibit spore germination in a C. difficile clinical isolate. Our recent studies of C. difficile sporulation characteristics have since pointed to substantial diversity among different clinical isolates. Consequently, in this study we investigated how the germination characteristics of different C. difficile isolates vary in response to bile salts. By analysing 29 isolates, including 16 belonging to the BI/NAP1/027 type, we show that considerable diversity exists in both the rate and extent of C. difficile germination in response to rich medium containing both taurocholate and glycine. Strikingly, we also show that although a potent inhibitor of germination for some isolates, chenodeoxycholate does not inhibit the germination, or outgrowth, of all C. difficile strains. Finally, we provide evidence that components of rich media may induce the germination of C. difficile spores, even in the absence of taurocholate. Taken together, these data suggest that the mechanisms of C. difficile spore germination in response to bile salts are complex and require further study. Furthermore, we stress the importance of studying multiple isolates in the future when analysing the nutrients or chemicals that either stimulate or inhibit C. difficile spore germination.
Introduction
Clostridium difficile is a Gram-positive, anaerobic spore former and the major underlying cause of hospital-acquired diarrhoea. C. difficile infection (CDI) is estimated to affect more than 500,000 people per year in the USA alone and the spread of CDI has led to patient isolation, ward closures and even hospital closures [1]. Infection with C. difficile may manifest as asymptomatic colonisation, but can also lead to severe diarrhoea that may then progress into a potentially fatal pseudo-membranous colitis [2]. Endospores, formed during sporulation, play a pivotal role in the transmission of disease. Spores shed in the faeces are able to withstand a variety of cleaning agents and can reside on hospital surfaces for prolonged periods of time [3]. Therefore, spores are regarded as the infectious stage of C. difficile. However, following ingestion by susceptible individuals, the progression to disease relies first on a return to vegetative cell growth through germination and then on production of the characteristic toxins [4].
Germination is defined as the irreversible loss of spore-specific characteristics and ultimately leads to vegetative cell growth. The mechanisms of germination have been studied extensively in Bacillus spp. in which nutrients and chemicals, termed germinants, can bind to specific receptors at the inner spore membrane [5]. At this point, the spore becomes committed to germination and subsequent events include release of monovalent cations (H+, K+ and Na+) and the spores' large depot of calcium dipicolinic acid (CaDPA) [6]. This redistribution of ions and water in the spore core likely precedes the activation of specific lytic enzymes that degrade the spore cortex, a thick layer of peptidoglycan differing subtly from vegetative cell peptidoglycan [7], [8]. Following cortex degradation, the spore becomes fully rehydrated, which in turn allows for a return to enzyme activity, metabolism and, finally, vegetative cell growth. To date, germination has been studied in several spore formers, including some Clostridium spp., in which germinant receptors have been identified [9]–[11]. However, in C. difficile the absence of homologues to known germinant receptors has severely limited research into the mechanisms of germination in this species [12]–[14].
The mechanisms by which C. difficile spores sense a suitable environment for germination have not yet been investigated in great depth. However, recent work has revealed that bile salts play a pivotal role. It has been shown that C. difficile spores can germinate in response to the secondary bile salt taurocholate, which acts as a co-germinant with glycine [15], [16], and there is now evidence to suggest that, besides glycine, further amino acids may also act as co-germinants in combination with taurocholate [17], [18]. Furthermore, recent studies by Sorg and Sonenshein have described the role of the primary bile salt chenodeoxycholate in the inhibition of spore germination in a C. difficile clinical isolate [19], [20]. This adds an interesting element to the model of C. difficile colonisation in the gut. Increasing the concentration of chenodeoxycholate has been shown to reduce the efficacy of taurocholate as a germinant while, similarly, an increase in the concentration of taurocholate has been shown to reduce the inhibitory effect of chenodeoxycholate on germination [20]. However, spores of C. difficile are suggested to have a higher affinity for chenodeoxycholate than for taurocholate [20]. Therefore, in equal concentrations both cholate and chenodeoxycholate derivatives may compete for binding to putative C. difficile germinant receptors and germination may be inhibited. However, as the rate of absorption of chenodeoxycholate by the colon is ten times that of cholate [21], spores reaching the large intestine encounter a higher concentration of cholate derivatives, such as taurocholate. This suggests that germination may be inhibited until C. difficile spores reach the anaerobic environment of the large bowel, where conditions are suitable for vegetative cell growth.
The recent emergence of so-called ‘hypervirulent’ strains of C. difficile, such as those belonging to restriction endonuclease type BI, North American pulsed-field type 1, and PCR-ribotype 027 (BI/NAP1/027), presents a continuing challenge in healthcare settings worldwide. Strains belonging to this type are becoming increasingly common among clinical isolates, with some of these strains thought to be associated with more severe disease and an expanded repertoire of antibiotic resistance. As a result, understanding how these strains might differ from strains less frequently associated with ‘severe’ disease is of interest [22], [23]. Some strains of the BI/NAP1/027 type are believed to produce higher levels of toxin in vitro [24] and a number of studies have also concluded that this type is more prolific in terms of sporulation in vitro than other C. difficile types [25]–[30]. However, recent studies have now shown that BI/NAP1/027 strains do not have an increased sporulation rate compared with other types and these studies have also indicated that significant diversity exists in sporulation rates within the BI/NAP1/027 type [31], [32]. Interestingly, our previous studies of C. difficile sporulation rates have also indicated that the proportion of spores completing germination to form colonies may vary significantly among different isolates, suggesting that differences may exist in the germination response of different C. difficile strains to taurocholate [31], [32]. As germination is a prerequisite for colonisation, toxin production and subsequent disease, it is desirable to discover if germination characteristics vary among important clinical isolates. Furthermore, if variation exists in the germination response of different C. difficile isolates to taurocholate, it is conceivable that differences may also exist in the inhibitory effect of chenodeoxycholate on C. difficile spore germination.
Here, we sought to investigate the germination and outgrowth characteristics of different C. difficile isolates (Table 1) in rich medium containing chenodeoxycholate. We then examined the differences in the germination response to a medium containing both taurocholate and glycine. Finally, we aimed to identify generic germination differences between BI/NAP1/027 and non-BI/NAP1/027 strains while also studying how the C. difficile germination response varies within the BI/NAP1/027 type. We observed substantial diversity in germination characteristics among our group of clinical isolates and, perhaps most importantly, noted that chenodeoxycholate does not inhibit the germination of all C. difficile isolates.
Table 1. C. difficile strains used in this study.
| Strain name | PCR-ribotype | Country | Source/Reference |
| 9001966 | 002 | The Netherlands | Ed Kuijper |
| G,08,0000780 | 002 | The Netherlands | Ed Kuijper |
| 630Δ erm | 012 | Laboratory strain | [37] |
| 8085054 | 014 | The Netherlands | Ed Kuijper |
| 8079089 | 017 | The Netherlands | Ed Kuijper |
| 675,1 | 017 | Romania | Ed Kuijper |
| 5108111 | 027 | The Netherlands | Ed Kuijper |
| CDC 38 | 027 | USA | [38] |
| M13042 | 027 | Canada | [38] |
| DH1432 | 027 | London, Barnet, UK | Val Hall |
| DH1834 | 027 | East of England, Ipswich, UK | Val Hall |
| DH1466 | 027 | East Midlands, Northampton, UK | Val Hall |
| DH1858 | 027 | NE England,Sunderland, UK | Val Hall |
| DH478 | 027 | SW England, Taunton, UK | Val Hall |
| DH361 | 027 | London, Lewisham, UK | Val Hall |
| DH1329 | 027 | West Midlands, UK | Val Hall |
| R20352 | 027 | Canada | Val Hall |
| R12087 | 027 | Historical EU strain (mid 80 s) | Val Hall |
| R20291 | 027 | Stoke Mandeville, UK | Jon Brazier |
| 26131 | 027 | Finland | Ed Kuijper |
| 26173 | 027 | Finland | Ed Kuijper |
| 05-1223-046 | 027 | Belgium | Ed Kuijper |
| 2016 | 078 | Ireland | Ed Kuijper |
| 7004578 | 078 | The Netherlands | Ed Kuijper |
| 7009825 | 078 | The Netherlands | Ed Kuijper |
| CD 2315 | 078 | Hungary | Ed Kuijper |
| R9764 | 081 | Cardiff, UK | Jon Brazier/Ed Kuijper |
| R12801 | 106 | Bristol, UK | Val Hall |
| R10459 | 106 | Cardiff, UK | Jon Brazier/Ed Kuijper |
Results
Chenodeoxycholate does not inhibit colony formation from spores of all C. difficile isolates
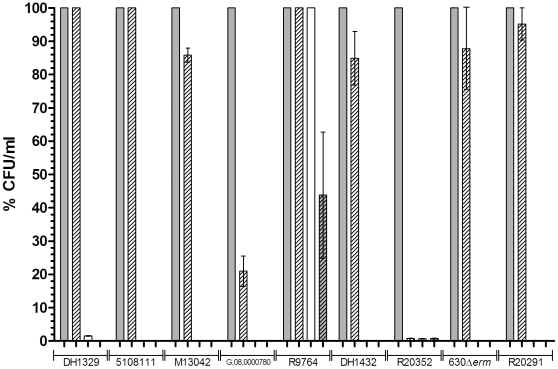
Chenodeoxycholate has previously been shown to inhibit spore germination in a C. difficile clinical isolate [19], [20]. As our previous work has indicated that differences may exist in the germination properties of different C. difficile isolates [31], [32], we elected to analyse if chenodeoxycholate inhibits the spore germination of nine isolates. Our initial investigations focused on the ability of dormant spores to return to vegetative cell growth following incubation with chenodeoxycholate. Accordingly, we measured the number of colonies recovered on BHIS agar following incubation of spores in BHIS medium supplemented with either 0.1% (w/v) taurocholate alone, or 0.1% (w/v) taurocholate further supplemented with 2 mM chenodeoxycholate (a concentration previously shown to inhibit germination of C. difficile spores and similar to the concentration of bile that enters the large intestine) [19], [33]. As negative controls, colony-forming units (CFU) were also enumerated on BHIS agar after incubation of spores in BHIS and dH2O, separately. We observed that chenodeoxycholate either completely or partially inhibited colony formation (i.e. the result of germination and outgrowth) from spores in six of the tested isolates (Fig. 1). When incubated with either i) BHIS supplemented with taurocholate and chenodeoxycholate; ii) BHIS alone; or iii) dH2O, R20352 was found to produce 100-fold fewer CFU/ml compared with taurocholate incubation. Five of the tested isolates (G,08,0000780, M13042, DH1432, 630Δerm, and R20291) were able to form colonies following incubation with chenodeoxycholate, although not necessarily to the same level as the CFU/ml observed after incubation with taurocholate alone (21%, 85%, 85%, 88%, and 95%, respectively compared with taurocholate alone). Additionally, three of the tested isolates (DH1329, 5108111, and R9764) were able to produce CFU comparable to the number observed following incubation with taurocholate alone (i.e. the positive control) (Fig. 1). Interestingly, we observed that R9764 (PCR-ribotype 081) was able to produce CFU when incubated with both BHIS alone and dH2O alone (100% and 44%, respectively compared to taurocholate only). The observation of colony formation following incubation in dH2O alone can likely be explained by the fact that the spore suspensions were subsequently spread onto BHIS agar, where germination could then have occurred. These data suggest that chenodeoxycholate does not inhibit spore germination in all C. difficile strains. Furthermore, they also suggest that spores of some isolates of C. difficile are able to germinate in the absence of bile salts, in BHIS alone.
Figure 1. The CFU/ml formed on BHIS agar by nine C. difficile isolates after incubation with germinants.
Spores of C. difficile were heat treated at 60°C for 25 min. CFU/ml were then enumerated on BHIS agar after 2 h incubation of spores with either i) BHIS supplemented with 0.1% (w/v) taurocholate (filled bars); ii) BHIS supplemented with 0.1% (w/v) taurocholate and 2 mM chenodeoxycholate (empty bars, diagonal lines); iii) BHIS alone (empty bars); or iv) dH2O (filled bars, diagonal lines). Colony formation was expressed as the percentage of CFU/ml observed compared to the CFU/ml observed after incubation with the positive control, BHIS supplemented with taurocholate. The data represent the average of three independent experiments and error bars indicate the standard errors of the means.
Chenodeoxycholate does not inhibit the germination of all C. difficile isolates
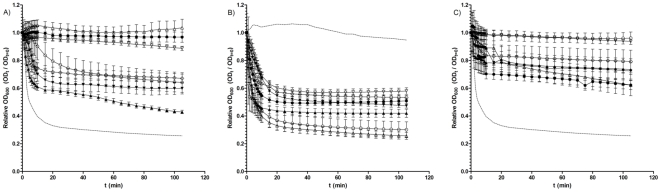
Following the observation that spores of some C. difficile isolates can complete germination and form colonies in the presence of chenodeoxycholate, we reasoned that the germination response of C. difficile spores to chenodeoxycholate may vary. Consequently, we analysed how the initiation of germination varied when spores of 15 different C. difficile isolates were exposed to chenodeoxycholate. We expressed germination as the loss of optical density at 600 nm (OD600), a known indicator of bacterial spore germination [20], [34]. Accordingly, we measured the loss of OD600 in response to BHIS supplemented with both 0.1% (w/v) taurocholate and 2 mM chenodeoxycholate. Interestingly, we observed that while the germination of most isolates appeared to be inhibited by chenodeoxycholate, this was not the case with all of the isolates tested (Fig. 2A and Table 2). Although no isolate germinated to the same degree as the germination positive control (05-1223-046, a strain found, in preliminary studies, to exhibit a 74% drop in OD600 in response to taurocholate), a number of isolates, DH1834 (57%), 5108111 (40%), 7004578 (36%) and CD 2315 (33%) were able to germinate in the presence of chenodeoxycholate. Interestingly, when analysing 11 BI/NAP1/027 isolates, two (DH1834, and 5108111) were able to germinate even in the presence of chenodeoxycholate, while germination appeared to be inhibited in spores of the other nine isolates (Fig. 3A). These data suggest that, despite being a potent inhibitor of spore germination in many C. difficile isolates (over two-thirds of our entire sample set) chenodeoxycholate does not universally inhibit spore germination in all C. difficile strains. Furthermore, variation appears to exist in the extent of chenodeoxycholate inhibition of germination within the BI/NAP1/027 type.
Figure 2. Loss of spore OD600 of C. difficile isolates during incubation in A) BHIS supplemented with taurocholate and chenodeoxycholate; B) BHIS supplemented with taurocholate; or C) BHIS alone.
C. difficile spores were heat treated at 60°C for 25 min and then incubated in BHIS with or without supplement. Germination was expressed as the loss of spore OD600. A) Loss of OD600 of spores of C. difficile isolates incubated in BHIS supplemented with 0.1% (w/v) taurocholate and 2 mM chenodeoxycholate. ▴, DH1834; ▾, 5108111; •, 9001966; ▵, 26131; ○, 7004578; ◊, CD 2315; and ▿, CDC38. The dotted line represents the loss of OD600 of the positive control strain (05-1223-046) in response to BHIS supplemented with 0.1% (w/v) taurocholate alone. B) Loss of OD600 of spores of C. difficile isolates incubated with BHIS supplemented with 0.1% (w/v) taurocholate. ▴, DH1834; ▾, 5108111; •, DH1432; ▵ 05-1223-046; □, CD 2315; ▿, CDC 38; and ○, DH1858. The dotted line represents the negative control strain (DH361) during incubation with both 0.1% (w/v) taurocholate and 2 mM chenodeoxycholate (a strain found, in preliminary studies, not to germinate when exposed to chenodeoxycholate). C) Loss of OD600 of spores of C. difficile isolates incubated with BHIS alone. ▴, DH1834; ▾, 5108111; •, 8085054; ▵, 05-1223-046; ○, 26173; and ▿, CDC38. The dotted line represents the loss of OD600 of the positive control strain (05-1223-046) in response to BHIS supplemented with 0.1% (w/v) taurocholate alone. All data represent the average of three independent experiments and error bars indicate the standard errors of the means.
Table 2. Percentage loss of spore optical density after incubation of C. difficile spores with bile salts for 2 h.
| Strain name | PCR-ribotype | 0.1% taurocholate | 2 mM chenodeoxycholate | BHIS |
| 9001966 | 002 | 47% (0.529) | 3% (0.968) | 11% (0.894) |
| 8085054 | 014 | 72% (0.376) | 13% (0.868) | 38% (0.623) |
| 5108111 | 027 | 53% (0.473) | 40% (0.599) | 27% (0.725) |
| CDC 38 | 027 | 42% (0.580) | 12% (0.888) | 6% (0.938) |
| DH1432 | 027 | 49% (0.508) | 0% (1.031) | 15% (0.846) |
| DH1834 | 027 | 58% (0.420) | 57% (0.429) | 27% (0.734) |
| DH1466 | 027 | 64% (0.355) | 0% (1.079) | 15% (0.852) |
| DH1858 | 027 | 70% (0.301) | 9% (0.910) | 23% (0.768) |
| DH478 | 027 | 52% (0.479) | 0% (1.113) | 5% (0.947) |
| DH361 | 027 | 58% (0.422) | 5% (0.947) | 9% (0.911) |
| 26131 | 027 | 50% (0.500) | 0% (1.036) | 7% (0.934) |
| 26173 | 027 | 46% (0.537) | 5% (0.946) | 4% (0.961) |
| 05-1223-046 | 027 | 74% (0.257) | 0% (1.009) | 38% (0.623) |
| 7004578 | 078 | 46% (0.536) | 36% (0.644) | 7% (0.926) |
| CD 2315 | 078 | 47% (0.534) | 33% (0.671) | 21% (0.792) |
C. difficile spores were heat treated at 60°C for 25 min and then incubated with either i) BHIS supplemented with 0.1% (w/v) taurocholate; ii) BHIS supplemented with 0.1% (w/v) taurocholate and 2 mM chenodeoxycholate; or iii) BHIS alone. The data represent the average of three independent experiment and numbers in parentheses represent the mean of the final OD600 value.
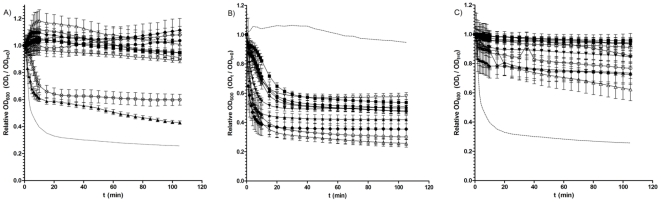
Figure 3. Loss of spore OD600 of 11 C. difficile BI/NAP1/027 isolates during incubation in A) BHIS supplemented with taurocholate and chenodeoxycholate; B) BHIS supplemented with taurocholate; or C) BHIS alone.
A), B), and C) ▴, DH1834; ▾, DH1432; ▪, 26173; •, 26131; ♦, DH478; ▵, 05-1223-046; □, DH361; ○, DH1858; ◊, DH1466; ▿, CDC38; and <$>\raster="rg4"<$>, 5108111. A) The dotted line represents the loss of OD600 of the positive control strain (05-1223-046) in response to BHIS supplemented with 0.1% (w/v) taurocholate alone. B) The dotted line represents the negative control strain (DH361) during incubation with both 0.1% (w/v) taurocholate and 2 mM chenodeoxycholate (a strain found, in preliminary studies, not to germinate when exposed to chenodeoxycholate). C) The dotted line represents the loss of OD600 of the positive control strain (05-1223-046) in response to BHIS supplemented with 0.1% (w/v) taurocholate alone. All data represent the average of three independent experiments and error bars indicate the standard errors of the means.
Diversity in the germination of C. difficile spores in response to taurocholate
We have previously reasoned that the proportion of spores that form colonies may vary from strain to strain, possibly due to differences in heat resistance and/or germination characteristics among different C. difficile isolates [31], [32]. When measuring the loss in OD600 of spores of 15 C. difficile isolates (of varying PCR-ribotypes) during incubation with BHIS supplemented with 0.1% (w/v) taurocholate (Fig. 2B and Table 2), we observed significant variation in the germination response among different C. difficile isolates (p<0.00001). Even though a maximal loss of OD600 was observed within the first 20 min for most isolates, the final percentage drop in spore OD600 was considerably different among our sample of isolates. Starting from a baseline adjusted OD600 of 1, the largest drop in OD600 was found to be 74% (05-1223-046), while the smallest drop was 42% (CDC 38). This indicates that different isolates of C. difficile may vary in both their rate and extent of germination in response to taurocholate.
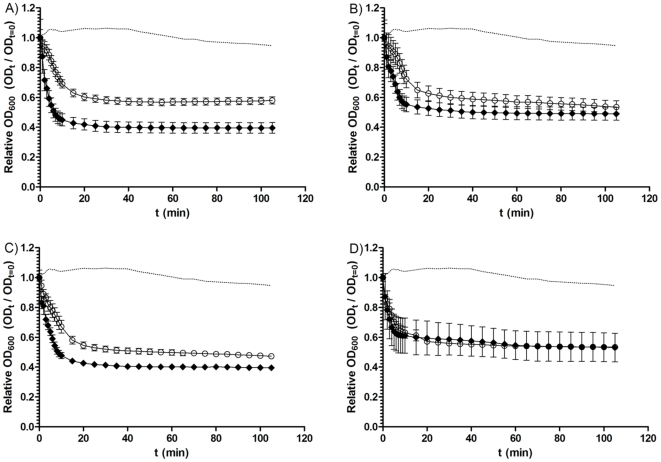
To evaluate if different concentrations of taurocholate alter the germination response of C. difficile spores, we incubated spores of four isolates with BHIS supplemented with either 0.1% or 1% (w/v) taurocholate (Fig. 4). Interestingly, we again observed differences among these isolates. For example, CD 2315 was found to germinate similarly in response to both concentrations of taurocholate. Conversely, CDC 38 appeared to germinate at a faster rate when incubated with 1% (w/v) taurocholate than when incubated with 0.1% (w/v) taurocholate and CDC 38 also appeared to show a larger drop in OD600 when exposed to 1% (w/v) taurocholate. This suggests that different isolates of C. difficile may vary in their response to different concentrations of germinants, which could be of interest for future studies.
Figure 4. Loss of spore OD600 of four C. difficile isolates during incubation with different concentrations of taurocholate.
C. difficile spores were heat treated at 60°C for 25 min and then incubated in BHIS supplemented with either 0.1% (w/v) taurocholate (○) or 1% (w/v) taurocholate (•) for 2 h. A) CDC38; B) 7004578; C) 5108111; D) CD2315. The data represent the average of three independent experiments and error bars indicate the standard errors of the means. The dotted line represents the negative control strain (DH361) during incubation with both 0.1% (w/v) taurocholate and 2 mM chenodeoxycholate (a strain found, in preliminary studies, not to germinate when exposed to chenodeoxycholate).
In keeping with our previous work [31], [32], we also sought to investigate the germination characteristics of 16 BI/NAP1/027 isolates, independent of other PCR-ribotypes. Interestingly, when examining the germination properties of 11 of these BI/NAP1/027 isolates spectrophotometrically, we observed significant variation in both the rate and extent of germination within the BI/NAP1/027 group in response to taurocholate (p<0.0001), with the drop in OD600 ranging from 74% in strain 05-1223-046 to 42% in strain CDC 38 (Fig. 3B). These data support our hypothesis that strains grouped according to a particular typing method do not necessarily exhibit similar in vitro characteristics.
Rich medium stimulates germination and outgrowth of C. difficile spores
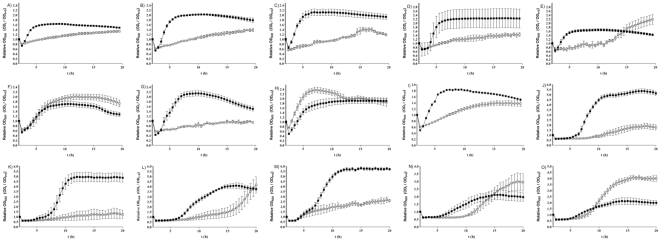
We observed above that spores of some C. difficile strains were able to form colonies on BHIS agar, even in the absence of known germinants such as taurocholate. To assess whether components of BHIS can act as germinants, spores of 15 C. difficile isolates were incubated with BHIS alone and the loss of OD600 was measured. Six isolates (8085054, 05-1223-046, DH1834, DH1858, 5108111 and CD 2315) of C. difficile showed a slow but significant (p<0.001) loss of OD600 (Fig. 2C and Table 2), suggesting that these isolates are able to germinate in response to BHIS alone. To investigate whether this germination would lead to vegetative cell growth, we next measured the change in OD600 of C. difficile cultures over a 20 h time period, during incubation of spores with either BHIS only or BHIS supplemented with 0.1% (w/v) taurocholate (Fig. 5). Once more, we observed considerable differences in the growth properties among strains. The isolates R12087, R12801, R10459, R20352, 9001966, 5108111, 8079089 and 675,1 were not able to grow during incubation with BHIS alone, while the isolates 7009825, 7045389, DH1329, 2016, 7004578, R9764 and G,08,0000780 were able to demonstrate cell growth without taurocholate supplement (Fig. 5). This suggests that components of rich media such as BHIS can induce germination of C. difficile spores, which can subsequently lead to vegetative cell growth.
Figure 5. Cell growth from spores of 15 C. difficile isolates.
Spores were heat treated at 60°C for 25 min and then incubated with either BHIS alone (□) or BHIS supplemented with 0.1% (w/v) taurocholate (▪). The change in OD600 was measured over 24 h. A) 9001966; B) R12087; C) R12801; D) 675,1; E) G,08,0000780; F) 7004578; G) 5108111; H) R9764; I) DH1329; J) 8079089; K) R10459; L) 7045389; M) R20352; N) 7009825; O) 2016. The data represent the average of three independent experiments and error bars indicate the standard errors of the means.
Discussion
Spores of C. difficile are pivotal for transmission of infection, but in order to cause disease spores must return to vegetative cell growth through germination. Bile salts likely play an important role in C. difficile germination in vivo and it has been shown previously that chenodeoxycholate can act as an inhibitor of germination in vitro [16], [19], [35]. Here, we examined how the germination response to bile salts varies among spores of 29 different C. difficile isolates. We found that, despite being a potent inhibitor of spore germination in many isolates, chenodeoxycholate did not inhibit the initiation of germination, or the subsequent vegetative cell outgrowth, in all isolates. We also observed substantial variation in both the rate and extent of germination in response to taurocholate among different C. difficile isolates, in addition to a possible concentration-dependent response to taurocholate. Furthermore, we provide evidence that components of a rich medium such as BHIS may be sufficient to induce germination of C. difficile spores, even in the absence of taurocholate.
Due to the proposed inhibitory effect on C. difficile spore germination, chenodeoxycholate has been suggested as a potential prophylactic drug for the prevention of C. difficile colonisation [20]. However, we have shown in this study that chenodeoxycholate does not appear to inhibit the spore germination of all C. difficile strains. Therefore, a drug based on chenodeoxycholate may not be sufficient for the control of CDI, as its inhibitory action on spore germination is unlikely to be specific to all strains of C. difficile. Given our evidence, we suggest that more studies regarding the germination mechanisms of C. difficile are needed in order to explore the true potential of germination-based therapeutic drugs. Although it has been suggested that there is kinetic evidence for the presence of germinant receptors [16], it is still unknown if germinants such as taurocholate bind to a receptor or simply diffuse through the spore and trigger the germination cascade. Consequently, it is difficult for us to speculate on what the molecular basis may be for the variation in germination characteristics that we have observed in this study.
We included 16 BI/NAP1/027 isolates in our study and observed significant diversity in the germination response to taurocholate among this group. We have previously found substantial variation in sporulation characteristics among C. difficile BI/NAP1/027 isolates [31], [32], [36]. As a result, the current study supports our hypothesis that isolates grouped according to a particular molecular typing technique cannot be assumed to have identical characteristics. To the contrary, this study indicates that general conclusions should not be made when discussing the BI/NAP1/027 type. The use of the term ‘hypervirulent’ should, therefore, perhaps be restricted to the description of individual isolates and should only be used when clearly defined and supported by appropriate clinical data.
In this study we also observed differences in the ease of preparing pure spore suspensions from different C. difficile isolates. Repeatedly washing spore suspensions in dH2O was sufficient for the preparation of pure spores from many C. difficile isolates. However, we noted that for some isolates it was not possible to obtain spore suspensions sufficiently free of vegetative cells and cell debris using this method. Furthermore, even when pure spore suspensions were prepared, excessive spore clumping in some isolates prevented the analysis of germination by the loss of spore OD600, as spore clumping results in a rapid loss of OD600 that may be misinterpreted as the onset of germination. For that reason, six of the isolates analysed for colony formation (M13042, G.08,0000780, R20352, R9764, 630Δerm, R20291) were unable to be studied for loss of OD600. This is an interesting finding and perhaps reinforces our hypothesis that different C. difficile isolates exhibit varying in vitro characteristics. Studying these differences in more detail is a clear area for future studies.
We have uncovered differences in the in vitro germination response of different C. difficile clinical isolates, but it is still unclear how these different germination properties relate to in vivo germination characteristics. Given the likely importance of germination for C. difficile colonisation, it is interesting that different isolates appear to germinate at different rates in response to bile salts. One could speculate that, ideally, a balance of spores and vegetative cells may need to be maintained in order for C. difficile to effectively colonise the gut. Therefore, a specific germination frequency may not only result in a sufficient number of vegetative cells required for colonisation, but also in a number of spores that could resist therapeutically administered antibiotics, such that they could then cause a relapse in disease at a later stage. However, further investigation is warranted in order to test this hypothesis.
Materials and Methods
C. difficile isolates and growth conditions
A total of 29 isolates were chosen for analysis (Table 1), including 16 BI/NAP1/027 isolates and 13 non-BI/NAP1/027 isolates. The non-BI/NAP1/027 group included isolates of PCR-ribotypes 002, 003, 012, 014, 017 (n = 2), 078 (n = 4), 081, and 106 (n = 2). The chosen isolates included isolates from the UK (n = 11), the USA, Canada (n = 2), Hungary, Finland (n = 2), Belgium, Ireland, Romania, and the Netherlands (n = 7), in addition to an historical EU isolate. C. difficile 630Δerm was derived previously through continuous subculturing of the 630 wild-type strain [37]. Unless otherwise stated, all C. difficile isolates were cultivated at 37°C, in an anaerobic workstation (Don Whitley, United Kingdom), in BHIS (brain heart infusion supplemented with yeast extract [5 mg/ml, Oxoid] and L-cysteine [0.1% (w/v), Sigma; United Kingdom]) broth or agar.
Preparation of C. difficile spores
C. difficile isolates were first cultivated on BHIS agar plates supplemented with cycloserine (250 µg/ml) and cefoxitin (8 µg/ml). Overnight cultures of C. difficile were prepared and 100 µl of these cultures was spread onto BHIS agar. These plates were then incubated at 37°C under anaerobic conditions for 5 days, to allow for efficient sporulation [12], [16]. After 5 days, all spores and vegetative cells were harvested from the plates using cell scrapers (Costar®, Corning Incorporated, NY, USA) and suspended in dH2O before incubation overnight at 4°C to encourage the release of the spore from the mother cell [20]. Spore suspensions were then washed at least ten times in dH2O (16 000× g, 4 min). Following each centrifugation step the supernatant and top layer of the pellet, containing vegetative cells and cell debris, was carefully removed. After at least ten washes, spores were re-suspended in dH2O and checked for purity using phase-contrast microscopy. Spore suspensions, free of vegetative cells and cell debris, were stored at −20°C before use.
Measurement of spore germination and outgrowth by colony formation
To measure the ability of C. difficile spores to complete germination and form colonies, spore suspensions were heat treated at 60°C for 25 min to kill any vegetative cells and then incubated in either i) BHIS; ii) BHIS supplemented with 0.1% (w/v) taurocholate; iii) BHIS supplemented with both 0.1% (w/v) taurocholate and 2 mM chenodeoxycholate; or iv) dH2O for 2 h. Following this incubation, the samples were washed and re-suspended in phosphate-buffered saline. Samples were then serially diluted and plated onto unsupplemented BHIS plates. CFU/ml were enumerated after 24 h incubation. A spo0A mutant, in which the master regulator of sporulation has been inactivated [16], was used as a negative control for colony formation after heat treatment.
Measurement of the germination of C. difficile spores by loss of optical density
Prior to any spectrophotometric experiments, spore suspensions were checked, using phase-contrast microscopy, for impurity and clumping which could interfere with the subsequent OD600 measurements. Spore suspensions which showed extensive impurity and/or clumping were excluded from analyses. Purified spores were heat treated at 60°C for 25 min to kill any vegetative cells. Spore suspensions were then adjusted to an OD600 of 1 and 450 µl of each suspension was used per measurement. Spores were centrifuged and re-suspended in either BHIS or dH2O, and then split into three wells of a 96-well plate (for triplicate measurements). To each well, an equivalent volume of the respective germinant solution was added and germination was measured over 2 h. Heat-treated spore suspensions were germinated in either i) BHIS; ii) BHIS supplemented with either 0.1% or 1% (w/v) taurocholate (dissolved in dH2O); or iii) BHIS supplemented with both 0.1% (w/v) taurocholate and 2 mM chenodeoxycholate (dissolved in Tris-buffer by pH adjustment with NaOH to pH 8.0). Germination was expressed as the loss of spore OD600 over time using an iMark™ Microplate Absorbance Reader, with 5 sec of shaking prior to each measurement to avoid adherence of spores to the wells of the microtitre plate. After measuring OD600 over 2 h at room temperature, spore suspensions were again examined using phase-contrast microscopy to identify clumping of spores during the incubation period. Spore suspensions that showed evidence of clumping were excluded from the analyses, as spore clumping can cause a rapid drop in optical density that may be misinterpreted as the initiation of germination.
Measurement of the return of C. difficile spores to vegetative cell growth
The return of dormant C. difficile spores to vegetative cell growth was evaluated by measuring the change in OD600 over a 20 h period following a 2 h incubation of spores with germinants. First, spore suspensions were heat treated at 60°C for 25 min to kill any vegetative cells and then incubated at room temperature for 2 h in either BHIS alone, or BHIS supplemented with 0.1% (w/v) taurocholate. Following this incubation, the suspensions were centrifuged at 8000× g for 4 min and re-suspended in BHIS. The samples were then incubated at 37°C under anaerobic conditions and cell growth was measured by the change in OD600 over 20 h using a microplate reader (TECAN GENiosPro 96/384).
Biological replicates and statistical analyses
All data presented in this manuscript represent the results of three independent experiments. Statistical analyses were carried out in GraphPad Prism using one-way analysis of variance with Tukey's post hoc compensation for multiple comparisons of individual isolates.
Acknowledgments
We thank Jon Brazier, Val Hall and Ed Kuijper for providing C. difficile strains and Sara Jabbari for advice on statistical analyses.
Footnotes
Competing Interests: The authors have read the journal's policy and have the following conflicts: Dr. David Burns is currently employed by Pharmacom Media. All work carried out by Dr. Burns and described in this manuscript has been carried out prior to this employment. Pharmacom Media has had no interest in the work described in this manuscript.
Funding: The research leading to these results received funding from the UK Medical Research Council (Grant G0601176) and the European Community's Seventh Framework Programmes ‘CLOSTNET’ (PEOPLE-ITN-2008-237942) and ‘HYPERDIFF’ (HEALTH-F3-2008-223585). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nature Review Microbiology. 2009;7:526–536. doi: 10.1038/nrmicro2164. [DOI] [PubMed] [Google Scholar]
- 2.Poxton IR, McCoubrey J, Blair G. The pathogenicity of Clostridium difficile. Clinical Microbiology & Infection. 2001;7:421–427. doi: 10.1046/j.1198-743x.2001.00287.x. [DOI] [PubMed] [Google Scholar]
- 3.Riggs MM, Sethi AK, Zabarsky TF, Eckstein EC, Jump RLP, et al. Asymptomatic carriers are a potential source for transmission of epidemic and nonepidemic Clostridium difficile strains among long-term care facility residents. Clinical Infectious Diseases. 2007;45:992–998. doi: 10.1086/521854. [DOI] [PubMed] [Google Scholar]
- 4.Kuehne SA, Cartman ST, Heap JT, Kelly ML, Cockayne A, et al. The role of toxin A and toxin B in Clostridium difficile infection. Nature. 2010;467:711–713. doi: 10.1038/nature09397. [DOI] [PubMed] [Google Scholar]
- 5.Paidhungat M, Setlow P. Role of Ger proteins in nutrient and nonnutrient triggering of spore germination in Bacillus subtilis. Journal of Bacteriology. 2000;182:2513–2519. doi: 10.1128/jb.182.9.2513-2519.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Setlow P. Spore germination. Current Opinion in Microbiology. 2003;6:550–556. doi: 10.1016/j.mib.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Sekiguchi J, Akeo K, Yamamoto H, Khasanov FK, Alonso JC, et al. Nucleotide sequence and regulation of a new putative cell wall hydrolase gene, cwlD, which affects germination in Bacillus subtilis. Journal of Bacteriology. 1995;177:5582–5589. doi: 10.1128/jb.177.19.5582-5589.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Popham DL, Helin J, Costello CE, Setlow P. Analysis of the peptidoglycan structure of Bacillus subtilis endospores. Journal of Bacteriology. 1996;178:6451–6458. doi: 10.1128/jb.178.22.6451-6458.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hudson KD, Corfe BM, Kemp EH, Feavers IM, Coote PJ, et al. Localization of GerAA and GerAC germination proteins in the Bacillus subtilis spore. Journal of Bacteriology. 2001;183:4317–4322. doi: 10.1128/JB.183.14.4317-4322.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moir A, Corfe B, Behravan J. Spore Germination. CMLS Cellular and Molecular Life Sciences. 2002;59:403–409. doi: 10.1007/s00018-002-8432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Broussolle V, Alberto F, Shearman CA, Mason DR, Botella L, et al. Molecular and physiological characterisation of spore germination in Clostridium botulinum and C. sporogenes. Anaerobe. 2002;8:89–100. [Google Scholar]
- 12.Burns DA, Heap JT, Minton NP. SleC is essential for germination of Clostridium difficile spores in nutrient-rich medium supplemented with the bile salt taurocholate. Journal of Bacteriology. 2010;192:657–664. doi: 10.1128/JB.01209-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burns DA, Heap JT, Minton NP. Clostridium difficile spore germination: an update. Research in Microbiology. 2010;161:730–734. doi: 10.1016/j.resmic.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Xiao Y, Francke C, Abee T, Wells-Bennik MHJ. Clostridial spore germination versus bacilli: Genome mining and current insights. Food Microbiology. 2011;28:266–274. doi: 10.1016/j.fm.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Wilson KH, Kennedy MJ, Fekety FR. Use of sodium taurocholate to enhance spore recovery on a medium selective for Clostridium difficile. Journal of Clinical Microbiology. 1982;15:443–446. doi: 10.1128/jcm.15.3.443-446.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sorg JA, Sonenshein AL. Bile salts and glycine as cogerminants for Clostridium difficile spores. Journal of Bacteriology. 2008;190:2505–2512. doi: 10.1128/JB.01765-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howerton A, Ramirez N, Abel-Santos E. Mapping interactions between germinants and Clostridium difficile spores. Journal of Bacteriology. 2011;193:274–282. doi: 10.1128/JB.00980-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wheeldon LJ, Worthington T, Hilton AC, Elliott TSJ, Lambert PA. Physical and chemical factors influencing the germination of Clostridium difficile spores. Journal of Applied Microbiology. 2008;105:2223–2230. doi: 10.1111/j.1365-2672.2008.03965.x. [DOI] [PubMed] [Google Scholar]
- 19.Sorg JA, Sonenshein AL. Chenodeoxycholate is an inhibitor of Clostridium difficile spore germination. Journal of Bacteriology. 2009;191:1115–1117. doi: 10.1128/JB.01260-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sorg JA, Sonenshein AL. Inhibiting the initiation of Clostridium difficile spore germination using analogs of chenodeoxycholic acid, a bile acid. Journal of Bacteriology. 2010;192:4983–4990. doi: 10.1128/JB.00610-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mekhjian H, Phillips S, Hofmann A. Colonic absorption of unconjugated bile acids: perfusion studies in man. Digestive Diseases and Sciences. 1979;24:545–550. doi: 10.1007/BF01489324. [DOI] [PubMed] [Google Scholar]
- 22.Kuijper E, van Dissel J, Wilcox M. Clostridium difficile: changing epidemiology and new treatment options. Current Opinion in Infectious Diseases. 2007;20:376–383. doi: 10.1097/QCO.0b013e32818be71d. [DOI] [PubMed] [Google Scholar]
- 23.Pépin J, Valiquette L, Alary ME, Villemure P, Pelletier A, et al. Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. Canadian Medical Association Journal. 2004;171:466–472. doi: 10.1503/cmaj.1041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warny M, Pepin J, Fang A, Killgore G, Thompson A, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366:1079–1084. doi: 10.1016/S0140-6736(05)67420-X. [DOI] [PubMed] [Google Scholar]
- 25.Cheng VCC, Yam W, Lam OTC, Tsang JLY, Tse EYF, et al. Clostridium difficile isolates with increased sporulation: emergence of PCR ribotype 002 in Hong Kong. European Journal of Clinical Microbiology & Infectious Diseases. 2011:1–11. doi: 10.1007/s10096-011-1231-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akerlund T, Persson I, Unemo M, Noren T, Svenungsson B, et al. Increased sporulation rate of epidemic Clostridium difficile type 027/NAP1. Journal of Clinical Microbiology. 2008;46:1530–1533. doi: 10.1128/JCM.01964-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fawley WN, Underwood S, Freeman J, Baines SD, Saxton K, et al. Efficacy of hospital cleaning agents and germicides against epidemic Clostridium difficile strains. Infection Control and Hospital Epidemiology. 2007;28:920–925. doi: 10.1086/519201. [DOI] [PubMed] [Google Scholar]
- 28.Merrigan M, Venugopal A, Mallozzi M, Roxas B, Viswanathan V, et al. Human hypervirulent Clostridium difficile strains exhibit increased sporulation as well as robust toxin production. Journal of Bacteriology. 2010;192:4904–4911. doi: 10.1128/JB.00445-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vohra P, Poxton I. Comparison of toxin and spore production in clinically relevant strains of Clostridium difficile. Microbiology. 2011;157:1343–1353. doi: 10.1099/mic.0.046243-0. [DOI] [PubMed] [Google Scholar]
- 30.Wilcox MH, Fawley WN. Hospital disinfectants and spore formation by Clostridium difficile. Lancet. 2000;356:1324. doi: 10.1016/S0140-6736(00)02819-1. [DOI] [PubMed] [Google Scholar]
- 31.Burns DA, Heeg D, Cartman ST, Minton NP. Reconsidering the Sporulation Characteristics of Hypervirulent Clostridium difficile BI/NAP1/027. PLoS ONE. 2011;6:e24894. doi: 10.1371/journal.pone.0024894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burns DA, Heap JT, Minton NP. The diverse sporulation characteristics of Clostridium difficile clinical isolates are not associated with type. Anaerobe. 2010;16:618–622. doi: 10.1016/j.anaerobe.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Northfield T, McColl I. Postprandial concentrations of free and conjugated bile acids down the length of the normal human small intestine. Gut. 1973;14:513–518. doi: 10.1136/gut.14.7.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paredes-Sabja D, Setlow P, Sarker MR. SleC is essential for cortex peptidoglycan hydrolysis during germination of spores of the pathogenic bacterium Clostridium perfringens. Journal of Bacteriology. 2009;191:2711–2720. doi: 10.1128/JB.01832-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson KH. Efficiency of various bile salt preparations for stimulation of Clostridium difficile spore germination. Journal of Clinical Microbiology. 1983;18:1017–1019. doi: 10.1128/jcm.18.4.1017-1019.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burns DA, Minton NP. Sporulation studies in Clostridium difficile. Journal of Microbiological Methods. 2011;87:133–138. doi: 10.1016/j.mimet.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 37.Hussain HA, Roberts AP, Mullany P. Generation of an erythromycin-sensitive derivative of Clostridium difficile strain 630 (630Δerm) and demonstration that the conjugative transposon Tn916ΔE enters the genome of this strain at multiple sites. Journal of Medical Microbiology. 2005;54:137–141. doi: 10.1099/jmm.0.45790-0. [DOI] [PubMed] [Google Scholar]
- 38.Killgore G, Thompson A, Johnson S, Brazier J, Kuijper E, et al. Comparison of seven techniques for typing international epidemic strains of Clostridium difficile: restriction endonuclease analysis, pulsed-field gel electrophoresis, PCR-ribotyping, multilocus sequence typing, multilocus variable-number tandem-repeat analysis, amplified fragment length polymorphism, and surface layer protein A gene sequence typing. Journal of Clinical Microbiology. 2008;46:431–437. doi: 10.1128/JCM.01484-07. [DOI] [PMC free article] [PubMed] [Google Scholar]