Abstract
In this basic research study, Ganapathy-Kanniappan et al advance our understanding of how to block the glycolytic pathway to inhibit tumor progression by using image-guided procedures.
Summary:
In this basic research study, Ganapathy-Kanniappan et al advance our understanding of how to block the glycolytic pathway to inhibit tumor progression by using image-guided procedures (1). This was accomplished by demonstrating their ability to perform molecular targeting of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in human hepatocellular carcinoma (HCC) by using percutaneous injection of either inhibitor—3-bromopyruvate (3-BrPA) or short hairpin RNA (shRNA). They take the critical step of providing further rationale for potentially advancing this therapy into clinical trials by demonstrating that GAPDH expression strongly correlates with c-jun, a proto-oncogene involved in liver tumorigenesis in human HCC (2).
The Setting
During the past decade, we have seen such a dramatic increase in the number of image-guided options available to treat cancer to the point of developing a whole new subspecialty, interventional oncology. These techniques have included an expanding repertoire of agents to be directly injected either percutaneously into the tumor or delivered by using a transcatheter approach focally to the target site (3), as well as multiple energy sources to perform both thermal and nonthermal ablation (4). Injectable agents available now go far beyond a wide array of chemotherapeutic agents to include biologic agents (such as antiangiogenics), radioactive particles (such as yttrium 90), and gene therapies. Although this proliferation of parallel developments represents a wonderful opportunity for helping a greater number of patients, it also raises many questions as to how to best match and tailor the myriad of potential approaches to certain cancers and individuals.
Whereas in the past, in general, more empirical approaches were tried, the efforts of Ganapathy-Kanniappan et al fortunately continue the trend of transitioning to a mechanistic approach of molecular-based therapies. This article represents a key paradigm shift in our overall thinking—moving from an empirical approach of “try it because it might work, because the therapy has been shown to work in a few clinical series” to providing a clear rationale for the choice of a given intervention by identifying key mechanisms that can be exploited by administering specific agents to arrest the growth or eradicate a given tumor that shows over- or underexpression for gene X or compound Y. Here, the investigators continue their elegant work targeting the glycolytic pathway, specifically GAPDH, which although is regulated by a housekeeping gene expressed in all cells, it is an obligate enzyme for many tumors particularly those that live in a hypoxic environment (5).
The Science
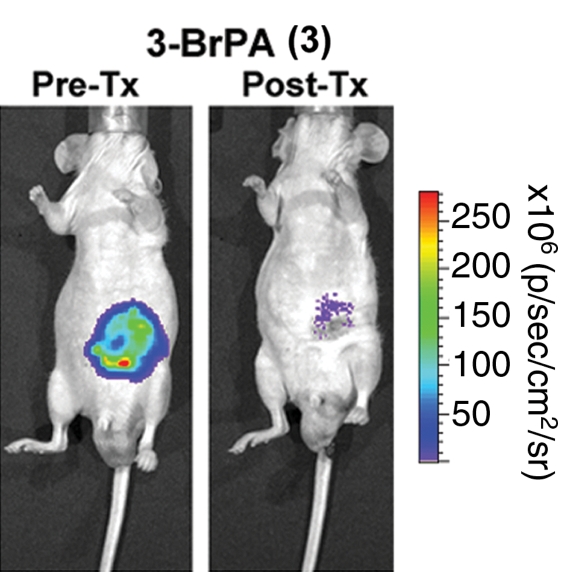
Ganapathy-Kanniappan et al use cutting-edge molecular biologic techniques to demonstrate that GAPDH antagonists can reduce GAPDH activity, which in turn influences tumor viability. They accomplish this by using an HCC cell line that was transfected with the luciferase (ie, firefly) gene, which enables the use of bioluminescence detection to allow straightforward quantification of the number of viable cells expressing this protein. Both chemical (3-BrPA) and genetic (shRNA that blocks production of GAPDH) agents were administered. First, they showed reduced viability in cell cultures to 3-BrPA in a dose-dependent manner. Next, they transitioned their studies into an in vivo mouse tumor model and showed at 7 days that 3-BrPA treatment primarily inhibited GAPDH activity (74.5%), with an associated decrease in mRNA expression to approximately 34.3% of baseline in these fluorescent HCC tumors (Figure) (3). Likewise, GAPDH shRNA inhibited both activity (60.6%) and expression (44.4%). Targeted inhibition of GAPDH by using 3-BrPA or shRNA also induced apoptosis as demonstrated by immunohistochemical and Western blotting techniques. Last, HCC samples from human patients demonstrated a strong correlation between GAPDH upregulation and the proto-oncogene c-jun expression, which was seen in 59% of the samples.
The Practice
Although we may still be a short distance from routine clinical use of 3-BrPA, this work in concert with the substantial research effort of the Johns Hopkins group has certainly progressed to point where future clinical trials can be envisioned. The investigators indeed note many future opportunities and some challenges, the first being characterization and optimization of dosing regimen to maximize the therapeutic benefit versus any potential short- or long-term negative effects to homeostasis or energy requirements of normal tissues where GAPDH is expressed, first in animals and then likely in early phase I and II clinical studies. The best methods for distribution (ie, percutaneous injection vs transcatheter delivery) will also need to be explored, again likely sequentially in animals and then humans. Once efficacy has been established in more long-term animal studies, controlled and ideally randomized trials comparing 3-BrPA with other interventional and more conventional therapies will undoubtedly be warranted. It is however most likely that a combination of strategies (ie, simultaneously targeting more than one oncogenic pathway) will prove most efficacious in clinical practice. This holds especially true for therapies that are not going to affect 100% of potentially mutative tumor cells and for tumors such as HCC that do not always invariably have a given mutation such as c-jun. Indeed, this variable genetic expression raises the issue of what is likely to become an ever more important function of the interventional radiologist in the era of personalized medicine, namely increased performance of tumor biopsy with the goal of determining specific biomarkers to enable targeting of tumors with specific agents on the basis of gene expression (6).
While it is likely overly optimistic to expect every radiologist to be continuously up to date with the fine details of the ever-expanding plethora of cutting-edge molecular biologic techniques, it is important for all concerned to be aware and appreciative of the potentially tremendous effect that these can have on our field. These techniques can and should be applied to answer many open but basic questions about what we as radiologists can and do accomplish with our treatments. Indeed, on the basis of the wealth of extant techniques, it is well beyond the scope and possibility of any Science to Practice article to fully review the spectrum of molecular biologic techniques that are now available. Accordingly, the interested reader is directed to the following reviews that more fully address the implementation and effect of key techniques used in this article including shRNA (7) and apoptosis detection (8), as well as to recommended animation at http://www.nature.com/nrg/multimedia/rnai/index.html. Equally important is awareness of an outstanding Web site www.protocol-online.org that explains the rationale and methods of these techniques (including bioluminescence imaging, mRNA expression, and other methods described) in an easy-to-grasp, straightforward format.
We hope that 10 years from now we will look back at this article and acknowledge it as one of the pioneering group that started the trend toward incorporating cutting-edge scientific techniques into interventional radiology. Indeed, we have much to gain by incorporating molecular biologic tools and strategies into interventional oncology research. This undoubtedly could be facilitated by allying with dedicated research scientists who use these techniques on a daily basis to advance medical science in other disciplines, as called for 7 years ago in “Society of Interventional Radiology Interventional Oncology Task Force: Interventional Oncology Research Vision Statement and Critical Assessment of the State of Research Affairs” (9) and as is in common practice for magnetic resonance imaging where incorporation of physicists into the research and clinical team is virtually mandated. Such building of bridges will assist us both in terms of better optimizing our extant platforms and, as this article shows, open new vistas for image-guided treatment. By the same token, it is also imperative that we go to the greatest lengths to educate our colleagues in cutting-edge scientific fields as to what image-guided techniques have to offer, not only for focal high-concentration percutaneous or catheter drug delivery but also for substantially improving other paradigms such as gene and stem cell therapy where the substantial barriers that exist currently to delivery and retention may very well be obviated by using interventional oncology techniques (10).
Disclosures of Potential Conflicts of Interest: S.N.G. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: author is consultant for Angiodynamics; institution receives grants from Angiodynamics and Hospital Services. Other relationships: none to disclose.
Footnotes
Funding: This work was supported by the National Institutes of Health (grants R01 CA133114, R01 CA100045, 2R01 HL55519, 1U54CA151881-01).
Supported by the Israel Science Foundation and the Israel Ministry of Health.
See also the article by Ganapathy-Kanniappan et al.
References
- 1.Ganapathy-Kanniappan S, Kunjithapatham R, Torbenson MS, et al. Human hepatocellular carcinoma in a mouse model: assessment of tumor response to percutaneous ablation by using glyceraldehyde-3-phosphate dehydrogenase antagonists. Radiology 2012;262(3)834–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feitelson MA, Pan J, Lian Z. Early molecular and genetic determinants of primary liver malignancy. Surg Clin North Am 2004;84(2):339–354 [DOI] [PubMed] [Google Scholar]
- 3.Lewandowski RJ, Geschwind JF, Liapi E, Salem R. Transcatheter intraarterial therapies: rationale and overview. Radiology 2011;259(3):641–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed M, Brace CL, Lee FT, Jr, Goldberg SN. Principles of and advances in percutaneous ablation. Radiology 2011;258(2):351–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganapathy-Kanniappan S, Vali M, Kunjithapatham R, et al. 3-bromopyruvate: a new targeted antiglycolytic agent and a promise for cancer therapy. Curr Pharm Biotechnol 2010;11(5):510–517 [DOI] [PubMed] [Google Scholar]
- 6.Prat A, Ellis MJ, Perou CM. Practical implications of gene-expression-based assays for breast oncologists. Nat Rev Clin Oncol 2011;9(1):48–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell 2009;136(4):642–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hale AJ, Smith CA, Sutherland LC, et al. Apoptosis: molecular regulation of cell death. Eur J Biochem 1996;237(3):884. [PubMed] [Google Scholar]
- 9.Goldberg SN, Bonn J, Dodd G, et al. Society of Interventional Radiology Interventional Oncology Task Force: interventional oncology research vision statement and critical assessment of the state of research affairs. J Vasc Interv Radiol 2005;16(10):1287–1294 [DOI] [PubMed] [Google Scholar]
- 10.Sawyer GJ, Rela M, Davenport M, Whitehorne M, Zhang X, Fabre JW. Hydrodynamic gene delivery to the liver: theoretical and practical issues for clinical application. Curr Gene Ther 2009;9(2):128–135 [DOI] [PubMed] [Google Scholar]