Abstract
Background
Black patients are commonly believed to have higher stroke mortality. However, several recent studies have reported better survival in black patients with stroke.
Objective
To examine racial differences in stroke mortality and explore potential reasons for these differences.
Design
Observational cohort study.
Setting
164 hospitals in New York.
Participants
5319 black and 18 340 white patients 18 years or older who were hospitalized with acute ischemic stroke between January 2005 and December 2006.
Measurements
Influence of race on mortality, examined by using propensity score analysis. Secondary outcomes were selected aspects of end-of-life treatment, use of tissue plasminogen activator, hospital spending, and length-of-stay. Patients were followed for mortality for 1 year after admission.
Results
Overall in-hospital mortality was lower for black than for white patients (5.0% vs. 7.4%; P < 0.001), as was 30-day (6.1% vs. 11.4%; P < 0.001) and 1-year (16.5% vs. 24.4%; <0.001) all-cause mortality. After propensity score adjustment, black race was independently associated with lower in-hospital mortality (odds ratio [OR], 0.77 [95% CI, 0.61 to 0.98]) and all-cause mortality up to 1-year (OR, 0.86 [CI, 0.77 to 0.96]). The adjusted hazard ratio was 0.87 (CI, 0.79 to 0.96). After adjusting for the probability of dying in the hospital, black patients with stroke were more likely to receive life-sustaining interventions (OR, 1.22 [CI, 1.09 to 1.38]) but less likely to be discharged to hospice (OR, 0.25 [CI, 0.14 to 0.46]).
Limitations
Study used hospital administrative data that lacked a stroke severity measure. Study design precluded determination of causality.
Conclusion
Among patients with acute ischemic stroke, black patients had lower mortality than white patients. This could be the result of differences in receipt of life-sustaining interventions and end-of-life care.
The racial disparity in stroke is an enormous public health concern. Compared with white patients, black patients have a greater prevalence of stroke risk factors (1, 2), experience more severe deficits at stroke onset (3, 4), are more likely to be hospitalized for stroke, and have less access to specialty care or newer technologies (5). Numerous appeals have been made for a better understanding of both the underlying reasons for these disparities and the resulting excess burden of disease (6).
Although vital statistics have documented excess stroke mortality among black persons for decades (7), several recent studies (8–11) have suggested a consistent pattern of lower short-term mortality for black patients who are hospitalized for stroke than for white patients, even after adjustment for age and clinical risk factors. This conflicts with the prevailing views that black patients have less access to evidence-based stroke treatments and worse overall quality of care (11).
Potential reasons offered for this paradox include differences in illness severity and stroke subtypes (11, 12), survivorship bias (9, 10), selection bias (13), different methods of risk adjustment (14), and different lengths of outcome assessment (15). However, most deaths from short-term ischemic stroke are due to the withholding or withdrawing of life-sustaining interventions (16). In addition, black and white patients are known to have different access to and preferences for treatments and services when they are seriously ill or at the end of life (17–20). We therefore examined racial differences in risk for death after being hospitalized with an acute ischemic stroke and explored the differential use of hyperacute and endof-life treatments as a possible explanation.
Methods
Study Population
Our primary data source was hospital administrative data from the New York Statewide Planning and Research Cooperative System (SPARCS), a comprehensive data reporting system that collects detailed information on every hospital and emergency department admission in New York. For this analysis, we included black or white patients not of Hispanic origin, 18 years or older, who were hospitalized for acute ischemic stroke between 1 January 2005 and 31 December 2006. An ischemic stroke diagnosis was defined as a principal diagnosis of acute ischemic stroke (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] diagnosis codes 433.×1 [occlusion and stenosis of precerebral arteries with cerebral infarction] or 434.×1 [occlusion of cerebral arteries with cerebral infarction]. Our sample was restricted to patients who lived in metropolitan statistical areas, because few black patients reside in the rural areas of New York. After patients who had missing data or resided outside of New York were excluded, our study population comprised 5319 black and 18 340 white patients from 164 hospitals in New York (total, 23 659 patients).
Patient Follow-up and Measurements
The SPARCS data were linked to the Social Security Administration Death Master File to follow patients' vital status for 1 year after the initial hospitalization. We examined in-hospital mortality (death occurring during the hospital stay) and all-cause mortality at 30, 90, and 180 days and 1 year. The ICD-9-CM procedure codes were condensed into procedure categories by using the Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project Clinical Classification Software.
We also examined the use of 6 potential life-sustaining interventions (respiratory intubation or mechanical ventilation, tracheostomy with mechanical ventilation for at least 96 hours, hemodialysis, cardiopulmonary resuscitation, gastrostomy, and enteral or parenteral nutrition) (21), as well as discharge to hospice rate, intensive care unit (ICU) admission rate, total length of stay, ICU length of stay, total hospital charges, and use of tissue plasminogen activator. These measures were chosen by using clinical judgment and data availability.
Statistical Analyses
We used the Wilcoxon rank-sum test or the chi-square test, as appropriate, to test for differences between black and white patients. A propensity score method (22) was used to balance baseline characteristics between black and white patients. The propensity score, defined as the probability of being black, was estimated by using a multivariate logistic regression model that included age, sex, health insurance status, family income, principal diagnosis, presence of atrial fibrillation on admission, a modified version of the Charlson Comorbidity Index tailored for ischemic stroke outcome studies (23), hospital teaching status, hospital bed size, and living distance from the admitting hospital. Comorbid conditions were differentiated from inpatient complications by using the present-on-admission indicator. A secondary diagnosis was identified as a comorbid condition if it was present when the patient was hospitalized, which allowed for cleaner adjustment for comorbid conditions and improved mortality risk adjustment (24). The propensity score model also included hospital indicator variables and hospital-by-characteristic interaction terms. This partial pooling approach incorporated the multilevel structure of the data, in which patients were clustered within hospitals (Appendix, available at www.annals.org) (25). The propensity scores were converted into deciles and included as a covariate in a Cox proportional hazards model to estimate the racial effect (black vs. white) on mortality. The robust sandwich estimator (26) was used to control for within-hospital clustering. Because age is a major determinant of stroke mortality, our analyses were stratified by age group (18 to 64, 65 to 74, 75 to 84, and ≥85 years), and an adjusted hazard ratio (HR) is reported for each age stratum. To evaluate the robustness of our findings, the propensity score model was first compared with a direct risk adjustment model that included all of the covariates. The Elixhauser index (27), an alternate and more comprehensive risk adjustment method that has been proposed for predicting mortality in stroke outcome studies (28), was then used to control for comorbid conditions.
We examined the influence of race on end-of-life treatment on the basis of the probability of dying in the hospital (21, 29). An patient's probability of death was estimated from a multivariate logistic regression model with patient sociodemographic, disease, and comorbid condition characteristics; hospital characteristics; hospital indicator variables; and 2-way interactions between these variables as independent variables. A c-statistic of 0.86 indicated excellent discrimination of a patient's risk for death during hospitalization on the basis of the model. The estimated probability of dying in the hospital was then included with race as a variable in the logistic regression model to predict the probability of receiving end-of-life treatment. Inclusion of the probability of death as a covariate facilitated a parsimonious regression model and captured the racial influence on end-of-life treatment decisions for patients who may be dying. Generalized estimating equations were used to account for within-hospital clustering (30).
A 2-sided P value of less than 0.05 was considered to be statistically significant. All analyses were performed by using SAS, version 9.2 (SAS Institute, Cary, North Carolina). The University of Rochester Research Subject Review Board approved this study.
Role of the Funding Source
This study was partially funded by the American Heart Association Founders Affiliate; the National Center for Research Resources, a component of the National Institutes of Health; the National Institutes of Health Roadmap for Medical Research; and a Paul B. Beeson Career Development Award. The funding sources had no role in the design, data collection, analysis, or interpretation of the study, preparation of the manuscript, or the decision to submit the manuscript for publication.
Results
We identified 23 659 patients (5319 black and 18 340 white patients) hospitalized for acute ischemic stroke in 164 New York State hospitals between 1 January 2005 and 31 December 2006. Table 1 shows the differences in sociodemographic, disease, and hospital characteristics between black and white patients. On average, black patients were somewhat younger and poorer and had greater prevalence of diabetes and renal disease. Black patients also lived closer to the hospital and were more likely to be admitted to large teaching hospitals.
Table 1.
Selected Baseline Characteristics, by Race
| Characteristic | Black Patients (n = 5319) | White Patients (n = 18 340) | P Value | Adjusted P Value* |
|---|---|---|---|---|
| Mean age (SD), y | 66.9 (14.3) | 75.7 (13.2) | <0.001 | 0.46 |
| Men, n (%) | 2203 (41.4) | 8219 (44.8) | <0.001 | 0.86 |
| Socioeconomic status | ||||
| Medicare, n (%) | 3027 (56.9) | 14 095 (76.9) | <0.001 | 0.65 |
| Medicaid, n (%) | 848 (15.9) | 564 (3.1) | <0.001 | 0.58 |
| Private insurance, n (%) | 1142 (21.5) | 3172 (17.3) | <0.001 | 0.79 |
| Other insurance, n (%) | 302 (5.7) | 509 (2.8) | O.001 | 0.84 |
| Eligible for both Medicare and Medicaid, n (%) | 1122 (21.1) | 1704 (9.3) | <0.001 | 0.77 |
| Mean family income (SD), $ | 43 234 (19 438) | 62 631 (23,284) | <0.001 | 0.45 |
| ICD-9-CM code for primary diagnosis, n (%) | ||||
| 433.x1 | 226 (4.3) | 1181 (6.4) | <0.001 | 0.91 |
| 434.x1 | 5093 (95.8) | 17 159 (93.6) | <0.001 | 0.91 |
| Mean Charlson Comorbidity Index score (SD) | 1.2 (1.4) | 1.2 (1.5) | 0.39 | 0.80 |
| Comorbid conditions, n (%) † | ||||
| Myocardial infarction | 268 (5.0) | 1490 (8.1) | <0.001 | 0.89 |
| Congestive heart failure | 726 (13.7) | 2843 (15.5) | 0.001 | 0.95 |
| Peripheral vascular disease | 241 (4.5) | 1329 (7.3) | <0.001 | 0.97 |
| Dementia | 321 (6.0) | 1220 (6.7) | 0.108 | 0.83 |
| Chronic obstructive pulmonary disease | 556 (10.5) | 2826 (15.4) | <0.001 | 0.99 |
| Connective tissue disease | 97 (1.8) | 410 (2.2) | 0.068 | 0.79 |
| Peptic ulcer disease | 38 (0.71) | 157 (0.9) | 0.31 | 0.77 |
| Diabetes without complications | 2,004 (37.7) | 4460 (24.3) | <0.001 | 0.90 |
| Diabetes with complications | 227 (4.3) | 538 (2.9) | 0.001 | 0.90 |
| Renal disease | 500 (9.4) | 1,245 (6.8) | <0.001 | 0.80 |
| Cancer | 123 (2.3) | 759 (4.1) | <0.001 | 0.99 |
| Metastatic carcinoma | 52 (1.0) | 325 (1.8) | <0.001 | 0.90 |
| Liver disease | 39 (0.7) | 134 (0.7) | 0.98 | 0.80 |
| Atrial fibrillation | 533 (10.0) | 4731 (25.8) | <0.001 | 0.83 |
| Hospital | ||||
| Mean distance to admitting hospital (SD), miles | 3.1 (4.3) | 6.1 (11.1) | <0.001 | 0.79 |
| Teaching hospital, n (%) | 4145 (77.9) | 11 006 (60.0) | <0.001 | 0.93 |
| Mean hospital beds (SD), n | 463 (209) | 368 (203) | <0.001 | 0.82 |
Adjusted for propensity score deciles in the propensity score analysis.
From the Charlson Comorbidity Index.
Propensity score adjustment was used to balance the baseline characteristics between black and white patients. To achieve a reliable and stable study sample, we excluded patients with extreme propensity score values (0% or 100% probability of being either black or white) and further restricted our analysis to hospitals that had at least 10 black and 10 white patients. Thus, the propensity score study sample comprised 4238 black patients (80%) and 10 284 white patients (56%) among 71 hospitals (43%) that contributed 14 522 of the available patients (61%). The initial imbalance between black and white patients was not present after adjustment for propensity score deciles (Table 1; all adjusted P values > 0.050). The baseline characteristics of the black patients included in the propensity score analysis were similar to those of the entire sample of black patients. However, the entire sample of white patients was older and had more comorbid conditions than the subsample used in the propensity score analysis (Appendix).
Racial Differences in Mortality
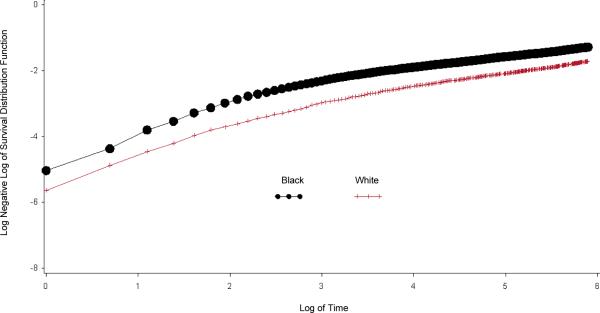
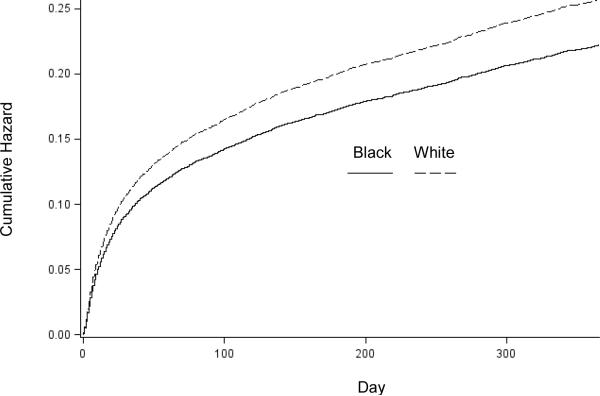
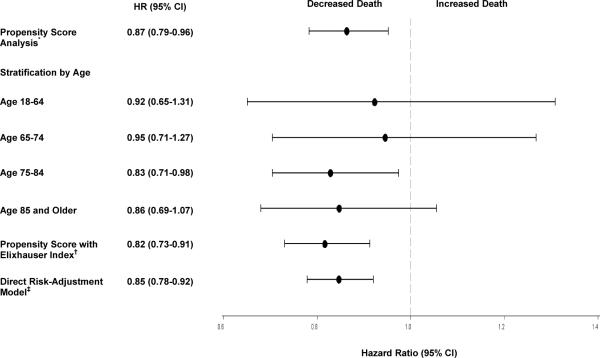
In unadjusted analyses (Table 2), black patients had lower in-hospital mortality (5.0% vs. 7.4%; unadjusted odds ratio [OR], 0.65 [95% CI, 0.56 to 0.77]). Black patients also had consistently lower 30-day to 1-year all-cause mortality than white patients (all P values < 0.001). The cumulative hazard plots showed a more favorable survival experience in black patients (Figure 1). After adjusting for propensity score deciles, black race was still independently associated with lower in-hospital mortality and 30-day to 1-year all-cause mortality. These mortality differences are reflected in the attenuated adjusted OR over time, from 0.69 at 30 days to 0.86 at 1 year. In the Cox proportional hazards model, the adjusted HR of death for black relative to white patients was 0.87 (CI, 0.79 to 0.96), which indicates lower mortality among black patients. No evidence indicated that the proportional hazards assumption was violated (Appendix). We observed a consistent pattern of lower risk for death for black patients in each age stratum, although the CIs were wide and not statistically significant (Figure 2).
Table 2.
Mortality, by Race, in Propensity Score Analysis
| Mortality Assessment | Black Patients (n = 4238), n (%) | White Patients (n = 10 284), n (%) | Unadjusted Odds Ratio (95% CI) | Adjusted Odds Ratio (95% CI)* |
|---|---|---|---|---|
| In-hospital | 211 (5.0) | 762 (7.4) | 0.65 (0.56–0.77) | 0.77 (0.61–0.98) |
| 30-d | 258 (6.1) | 1172 (11.4) | 0.50 (0.44–0.58) | 0.69 (0.57–0.84) |
| 90-d | 414 (9.8) | 1665 (16.2) | 0.56 (0.50–0.63) | 0.79 (0.68–0.93) |
| 180-d | 533 (12.6) | 2032 (19.8) | 0.58 (0.53–0.65) | 0.84 (0.74–0.95) |
| 1-y | 701 (16.5) | 2504 (24.4) | 0.62 (0.56–0.68) | 0.86 (0.77–0.96) |
Adjusted for propensity score deciles.
Appendix Figure 1.
Distribution of Propensity Score for Black and White Patients.
Appendix Figure 2.
Kaplan-Meier Curves to Assess the Proportional Hazards Assumption
The direct risk-adjusted Cox model, which included all of the covariates in the entire population, demonstrated similar mortality differences (adjusted HR, 0.85 [CI 0.78 to 0.92]). The survival advantage for black patients was even greater when the Elixhauser Index was used to adjust for comorbid conditions (adjusted HR, 0.82 [CI, 0.73 to 0.91]) (Figure 2).
Racial Differences in Hospital Spending, Length of Stay, and Thrombolytic Therapy
Black patients stayed 1 day longer on average (median stay, 6 days [interquartile range {IQR}, 4 to 9] for black patients vs. 5 days [IQR, 3 to 8] for white patients; adjusted P = 0.002) and had higher total hospital charges than white patients (median, $23 880 vs. $22 453; adjusted P = 0.050). Black and white patients had ICU stays of similar duration (median, 3 days [IQR, 2 to 6] vs. 3 days [IQR, 2 to 5]; adjusted P = 0.45). Only 126 black patients (3.0%) received thrombolytic therapy, compared with 468 white patients (4.6%) (adjusted OR, 0.76 [CI, 0.58 to 1.00]).
Racial Differences in End-Of-Life Care
In the unadjusted analysis, black patients were more likely to receive more aggressive life-sustaining interventions but less likely to be admitted to an ICU or hospice than white patients (Table 3). After adjustment for the probability of dying in the hospital, black race was independently associated with more aggressive end-of-life treatment, including tracheostomy with mechanical ventilation for at least 96 hours (adjusted OR, 1.53 [CI, 1.11 to 2.11]), hemodialysis (adjusted OR, 2.88 [CI, 2.19 to 3.78]), cardiopulmonary resuscitation (adjusted OR, 1.59 [CI, 1.05 to 2.43]), or any life-sustaining intervention (adjusted OR, 1.22 [CI, 1.09 to 1.38]). Black patients were also less likely to be discharged to hospice (adjusted OR, 0.25 [CI, 0.14 to 0.46]).
Table 3.
End-of-Life Treatments, by Race
| Measure | Black Patients (n = 5319), n (%) | White Patients (n = 18 34(1), n (%) | Unadjusted Odds Ratio (95% CI) | Adjusted Odds Ratio (95% CI)* |
|---|---|---|---|---|
| Intubation or mechanical ventilation | 269 (5.1) | 835 (4.6) | 1.12 (0.97–1.29) | 1.15 (0.95–1.40) |
| Tracheostomy | 70 (1.3) | 144 (0.8) | 1.69 (1.26–2.25) | 1.53 (1.11–2.11) |
| Hemodialysis | 179 (3.4) | 238 (1.3) | 2.65 (2.18–3.22) | 2.88 (2.19–3.78) |
| Cardiopulmonary resuscitation | 27 (0.5) | 72 (0.4) | 1.29 (0.83–2.01) | 1.59 (1.05–2.43) |
| Gastrostomy | 305 (5.7) | 937 (5.1) | 1.13 (0.99–1.29) | 1.01 (0.84–1.20) |
| Enteral or parenteral nutrition | 507 (9.5) | 1445 (7.9) | 1.23 (1.11–1.37) | 0.97 (0.83–1.13) |
| Any life-sustaining intervention | 819 (15.4) | 2307 (12.6) | 1.26 (1.16–1.38) | 1.22 (1.09–1.38) |
| ICU admission | 766 (14.4) | 3320 (18.1) | 0.76 (0.70–0.83) | 0.91 (0.83–1.01) |
| Discharge to hospice | 26 (0.5) | 400 (2.2) | 0.22 (0.15–0.33) | 0.25 (0.14–0.46) |
Adjusted for the probability of dying in the hospital
Discussion
In this large cohort of patients with acute ischemic stroke, we found that black patients had lower in-hospital mortality and all-cause mortality for up to 1 year after stroke onset than white patients. The survival difference persisted after we controlled for the observed confounders in propensity score analyses and used a regular risk-adjustment model that included all of the covariates.
These data highlight the difference between stroke mortality and case-fatality rates. Compared with white persons, black persons have excess overall stroke-related mortality because of higher stroke incidence (31, 32). Conflicting data have been reported on stroke case-fatality by race (6, 32, 33), but an increasing body of evidence from hospital administrative data (9, 10) and stroke registries (8, 11) suggests that patients from racial or ethnic minority groups have a lower risk for all-cause mortality after being hospitalized for stroke than white patients.
We found that black patients with stroke received life-sustaining interventions more often and had longer lengths of stay, higher hospital spending, and a lower hospice admission rate. Our data are consistent with previous research (17–19, 34) that shows that black patients more often receive life-sustaining interventions, including gastrostomy, hemodialysis, and intubation and tracheostomy, than nonblack patients. In addition, black patients are less likely to access palliative care services or hospice (16, 18, 20). Previous research (35) has shown that early care limitations (such as do-not-resuscitate orders) independently predict death after intracerebral hemorrhage. Therefore, a philosophy of care that involves providing more or less intensive treatment and services may influence short- to intermediate-term survival.
Our data are also consistent with recent research (36) that shows a better survival rate for up to 180 days at acute-care hospitals in Pennsylvania with higher-intensity end-of-life treatment. Although the racial differences in mortality that we observed (30-day adjusted OR, 0.69 [CI, 0.57 to 0.84]) were greater than those seen between the high- versus average-intensity (30-day adjusted OR, 0.97 [CI, 0.95 to 0.99]) or the low- versus average-intensity Pennsylvania hospitals (30-day adjusted OR, 1.09 [CI, 1.07 to 1.11]), the differences attenuated over time in both studies. However, despite the narrowing mortality gap in our study, the differences persisted through 1 year, which is consistent with the facts that patients with stroke who opt for aggressive treatment may live months or years with continued supportive care and more intensive treatment may produce short or intermediate survival benefit. In a recent study (37), black Medicare patients with stroke had a short-term (30-day) mortality benefit (4.4%), after which the survival advantage decreased over time (to 0.6% at 1 year) and eventually reversed after 2 years of follow-up (to a 1.2% advantage for white patients).
The mortality differences are unlikely to be due to disparities in the delivery of effective care, because most research suggests that black patients have poorer access to and adherence to evidence-based treatment (11, 38). Although we had limited information relating to evidence-based process measures, we observed that fewer black patients received thrombolytic therapy, which is consistent with previous studies (11, 39). In addition, our data emphasize the limitations of using stroke mortality rates as a measure of quality; their indiscriminate use would imply that black patients had better quality of care than white patients (40, 41). This is a concern because stroke mortality is a common outcome measure used in clinical trials, and inpatient stroke mortality is the most common publicly reported measure used to judge the quality of stroke care. The Centers for Medicare & Medicaid Services is considering including 30-day ischemic stroke mortality as one of its publicly reported measures.
Although we have no information on advance directives or patient or family preferences, our results highlight the potential importance of value-laden end-of-life decisions on survival outcomes in stroke care. This involves the complex process of shared decision-making and deliberation to arrive at treatment decisions with end-of-life implications. More research is required to develop methods for measuring the quality of these preference-sensitive decisions for seriously ill stroke patients. Such methods would need to capture the degree of truly informed patient choice, adequacy of physician communication, attention to health literacy, and respect for individual spiritual beliefs. The quality of these decisions may differ by race, as suggested by a recent study (42) that found that white patients with cancer received end-of-life care consistent with their preferences more frequently than black patients. The policy push toward patient-centered care will also require us to confront the possibility that well-informed patients who decide to forgo life-prolonging treatments and allow a natural death might receive excellent-quality care but have higher mortality than poorly informed patients who decide to use life-prolonging treatments (43).
Because we used administrative data, we could not adjust for admission stroke severity, measure disability, or functional status. We do not believe that a lower severity of initial strokes in black patients (for example, as a result of more small vessel vs. large vessel or cardioembolic disease) explains our findings. First, we used several risk adjustment methods, including the Charlson Comorbidity Index, the Elixhauser Index, the presence of atrial fibrillation, and present-on-admission diagnoses, to improve mortality risk adjustment and provide a more accurate assessment of the relationship between race and mortality risk. Second, after adjusting for the probability of dying in the hospital, the ICU admission and mechanical ventilation rates were similar between black and white patients, which suggests that the groups had similar proportions of severe strokes and need for respiratory support. Third, previous research (3, 4) has shown that black patients have more severe deficits at presentation, a confounding variable that would increase rather than decrease mortality in black patients. Finally, research (9, 10) has also shown that black patients 65 years and older have lower adjusted 30-day mortality than white patients after being hospitalized for several conditions, including congestive heart failure, acute myocardial infarction, hip fracture, and gastrointestinal bleeding, which suggests something more systemic than disease-specific confounding. Further research is needed to confirm these findings.
Our study has additional limitations. First, it is descriptive in nature. Although we observed more aggressive end-of-life treatment and lower mortality in black patients, the causal relationship cannot be established with our study design. However, describing this pattern brings the literature closer to identifying the underlying sources of disparities in stroke outcomes. Second, the ICD-9-CM procedure codes might underestimate the prevalence of potential life-sustaining interventions and thrombolytic therapy. However, no data suggest that these procedures would be coded differently by race. Third, we used data from patients who live in urban areas of New York, and the generalizability of our findings, especially to rural areas and to other states, remains to be established. Fourth, because up to 50% of stroke deaths occur outside the hospital setting (44), a racially disproportionate number of out-of-hospital deaths could have affect mortality rates calculated from hospital records. Fifth, previous studies (5, 31, 45) have shown excess burden of stroke, particularly among young and middle-aged patients. Because most of our study patients were 65 years or older, survivor bias may exist if older black patients differed from older white patients. However, we stratified our analysis by age group and observed a consistent pattern of lower risk for death among black patients in each age stratum. Finally, propensity score methods can only adjust for observed confounders (22); unmeasured residual confounders, for which we could not adjust, could have been present.
In conclusion, black patients hospitalized with acute ischemic stroke had lower mortality rates than their white counterparts, an effect which persisted but attenuated over the year after the initial hospitalization. A racial effect, which may be explained by the differential use of intensive treatment in seriously ill patients with stroke, contributes to the mortality difference. Future research should focus on understanding what drives the racial variations in stroke care and developing optimal approaches for promoting patient-centered decision-making, including the ability to recognize, explore, and respect the cultural norms that each patient and family bring to every clinical encounter.
Supplementary Material
Figure 1.
Cumulative Hazards Plot of Death over Time
Figure 2. Hazard Ratio for Death by Race (Black vs. White).
*Age, gender, health insurance status, family income, principal diagnosis, 13 Charlson comorbidities, atrial fibrillation, hospital teaching status, hospital bed size, distance to the hospital, hospital site indicator and interactions were used to calculate the propensity score.
†Including all of the covariates for direct risk adjustment in the entire study population.
‡Elixhauser Index was used to calculate the propensity score.
Appendix Figure 3.
Schoenfeld Residual Plots to Assess the Proportional Hazards Assumption
Appendix Table 1.
Baseline Characteristics, by Race, in Patients Included in the Propensity Score Adjustment Model
| Variable | Black Patients (n = 4238), n (%) | White (n = 10 284), n (%) | P Value | Adjusted P Value* |
|---|---|---|---|---|
| Mean age (SD), y | 67.3 (14.2) | 74.8 (13.5) | <0.001 | 0.46 |
| Men, n (%) | 1725 (40.7) | 4691 (45.6) | <0.001 | 0.86 |
| Socioeconomic status | ||||
| Medicare, n (%) | 2466 (58.2) | 7758 (75.4) | <0.001 | 0.65 |
| Medicaid, n (%) | 607 (14.3) | 370 (3.6) | <0.001 | 0.58 |
| Private insurance, n (%) | 948 (22.4) | 1890 (18.4) | <0.001 | 0.79 |
| Other insurance, n (%) | 217 (5.1) | 266 (2.6) | <0.001 | 0.84 |
| Eligible for both Medicare and Medicaid, n (%) | 878 (20.7) | 1070 (10.4) | <0.001 | 0.77 |
| Mean family income (SD), $ | 44 441 (19 677) | 62 727 (22,927) | <0.001 | 0.45 |
| ICD-9-CM code for primary diagnosis, n (%) | ||||
| 433.x1 | 189 (4.5) | 562 (5.5) | 0.013 | 0.91 |
| 434.x1 | 4049 (95.5) | 9722 (94.5) | 0.013 | 0.91 |
| Mean Charlson Comorbidity Index score (SD) | 1.1 (1.3) | 1.0 (1.2) | <0.001 | 0.80 |
| Comorbid conditions, n (%) † | ||||
| Myocardial infarction | 219 (5.2) | 709 (6.9) | <0.001 | 0.89 |
| Congestive heart failure | 588 (13.9) | 1452 (14.1) | 0.70 | 0.95 |
| Peripheral vascular disease | 195 (4.6) | 631 (6.1) | <0.001 | 0.97 |
| Dementia | 245 (5.8) | 581 (5.7) | 0.76 | 0.83 |
| Chronic obstructive pulmonary disease | 454 (10.7) | 1385 (13.5) | <0.001 | 0.99 |
| Connective tissue disease | 68 (1.6) | 119 (1.2) | 0.030 | 0.79 |
| Peptic ulcer disease | 12 (0.3) | 15 (0.2) | 0.081 | 0.77 |
| Diabetes without complications | 1652 (39.0) | 2626 (25.5) | <0.001 | 0.90 |
| Diabetes with complications | 152 (3.6) | 262 (2.6) | <0.001 | 0.90 |
| Renal disease | 398 (9.4) | 629 (6.1) | <0.001 | 0.80 |
| Cancer | 85 (2.0) | 189 (1.8) | 0.50 | 0.99 |
| Metastatic carcinoma | 25 (0.6) | 53 (0.5) | 0.58 | 0.90 |
| Liver disease | 8 (0.2) | 11 (0.1) | 0.22 | 0.80 |
| Atrial fibrillation | 448 (10.6) | 2322 (22.6) | <0.001 | 0.83 |
| Hospital | ||||
| Mean distance to admitting hospital (SD), miles | 3.2 (4.0) | 5.4 (7.9) | <0.001 | 0.79 |
| Teaching hospital, n (%) | 3277 (77.3) | 7445 (72.4) | <0.001 | 0.93 |
| Mean hospital beds (SD), n | 479 (211) | 438 (206) | <0.001 | 0.82 |
Adjusted for propensity score deciles.
From the Charlson Comorbidity Index.
Appendix Table 2.
Patients Included in the Propensity Score Adjustment Model Versus the Entire Study Sample
| Variable | Black Patients | White Patients | ||||
|---|---|---|---|---|---|---|
| Propensity Score Analysis (n = 4238) | Entire Study Sample (n = 5319) | P Value | Propensity Score Analysis (n = 10 284) | Entire Study Sample (n = 18 340) | P Value | |
| Mean age (SD), y | 67.3 (14.2) | 66.9 (14.3) | 0.21 | 74.8 (13.5) | 75.7 (13.2) | <0.001 |
| Men, n (%) | 1725 (40.7) | 2203 (41.4) | 0.48 | 4691 (45.6) | 8219 (44.8) | 0.192 |
| Socioeconomic status | ||||||
| Medicare, n (%) | 2466 (58.2) | 3027 (56.9) | 0.21 | 7758 (75.4) | 14 095 (76.9) | 0.007 |
| Medicaid, n (%) | 607 (14.3) | 848 (15.9) | 0.029 | 370 (3.6) | 564 (3.1) | 0.017 |
| Private insurance, n (%) | 948 (22.4) | 1142 (21.5) | 0.29 | 1890 (18.4) | 3172 (17.3) | 0.021 |
| Other insurance, n (%) | 217 (5.1) | 302 (5.7) | 0.23 | 266 (2.6) | 509 (2.8) | 0.35 |
| Eligible for both Medicare and Medicaid, n (%) | 878 (20.7) | 1122 (21.1) | 0.65 | 1070 (10.4) | 1704 (9.3) | 0.002 |
| Mean family income (SD), $ | 44 441 (19 677) | 43 234 (19 438) | <0.001 | 62 727 (22 927) | 62 631 (23 284) | 0.024 |
| ICD-9-CM code for primary diagnosis | ||||||
| 433.x1 | 189 (4.5) | 226 (4.3) | 0.62 | 562 (5.5) | 1181 (6.4) | <0.001 |
| 434.x1 | 4049 (95.7) | 5093 (95.8) | 0.62 | 9722 (94.5) | 17 159 (93.6) | <0.001 |
| Mean Charlson Comorbidity Index score (SD) | 1.1 (1.3) | 1.2 (1.4) | 0.79 | 1.0 (1.2) | 1.2 (1.5) | <0.001 |
| Comorbid conditions, n (%)† | ||||||
| Myocardial infarction | 219 (5.2) | 268 (5.0) | 0.78 | 709 (6.9) | 1490 (8.1) | <0.001 |
| Congestive heart failure | 588 (13.9) | 726 (13.7) | 0.75 | 1452 (14.1) | 2843 (15.5) | 0.002 |
| Peripheral vascular disease | 195 (4.6) | 241 (4.5) | 0.87 | 631 (6.1) | 1329 (7.3) | <0.001 |
| Dementia | 245 (5.8) | 321 (6.0) | 0.60 | 581 (5.7) | 1220 (6.7) | <0.001 |
| Chronic obstructive pulmonary disease | 454 (10.7) | 556 (10.5) | 0.68 | 1385 (13.5) | 2826 (15.4) | <0.001 |
| Connective tissue disease | 68 (1.6) | 97 (1.8) | 0.41 | 119 (1.2) | 410 (2.2) | <0.001 |
| Peptic ulcer disease | 12 (0.3) | 38 (0.7) | 0.004 | 15 (0.2) | 157 (0.9) | <0.001 |
| Diabetes without complications | 1652 (39.0) | 2004 (37.7) | 0.192 | 2626 (25.5) | 4460 (24.3) | 0.022 |
| Diabetes with complications | 152 (3.6) | 227 (4.3) | 0.090 | 262 (2.6) | 538 (2.9) | 0.057 |
| Renal disease | 398 (9.4) | 500 (9.4) | 0.99 | 629 (6.1) | 1245 (6.8) | 0.027 |
| Cancer | 85 (2.0) | 123 (2.3) | 0.31 | 189 (1.8) | 759 (4.1) | <0.001 |
| Metastatic carcinoma | 25 (0.6) | 52 (1.0) | 0.035 | 53 (0.5) | 325 (1.8) | <0.001 |
| Liver disease | 8 (0.2) | 39 (0.7) | <0.001 | 11 (0.1) | 134 (0.7) | <0.001 |
| Atrial fibrillation | 448 (10.6) | 533 (10.0) | 0.38 | 2322 (22.6) | 4731 (25.8) | <0.001 |
| Hospital | ||||||
| Mean distance to admitting hospital (SD), miles | 3.2 (4.0) | 3.1 (4.3) | <0.001 | 5.4 (7.9) | 6.1 (11.1) | 0.003 |
| Teaching hospital, n (%) | 3277 (77.3) | 4145 (77.9) | 0.48 | 7445 (72.4) | 11 006 (60.0) | <0.001 |
| Mean hospital beds (SD), n | 479 (211) | 463 (210) | <0.001 | 438 (206) | 368 (203) | <0.001 |
| Mortality, n (%) | ||||||
| In-hospital | 211 (5.0) | 276 (5.2) | 0.64 | 762 (7.4) | 1488 (8.1) | 0.033 |
| 30-d | 258 (6.1) | 325 (6.1) | 0.96 | 1172 (11.4) | 2379 (13.0) | <0.001 |
| 90-d | 414 (9.8) | 531 (10.0) | 0.73 | 1665 (16.2) | 3379 (18.4) | <0.001 |
| 180-d | 533 (12.6) | 679 (12.8) | 0.78 | 2032 (19.8) | 4070 (22.2) | <0.001 |
| 1-y | 701 (16.5) | 908 (17.1) | 0.49 | 2504 (24.4) | 5006 (27.3) | <0.001 |
Acknowledgment
The authors thank Wenqin Pan, PhD, of Duke University School of Medicine, Durham, North Carolina, for data support and suggestions on statistical analyses, and Timothy E. Quill, MD; Curtis G. Benesch, MD, MPH; and Kevin Fiscella, MD, MPH, of University of Rochester School of Medicine and Dentistry, Rochester, New York, for their thoughtful comments.
Grant Support: By an American Heart Association Founders Affiliate Predoctoral Fellowship Award (0815772D) (Dr. Xian); the National Center for Research Resources, a component of the National Institutes of Health (NIH) and the NIH Roadmap for Medical Research (1 UL1 RR024160-01) (Drs. Holloway and Noyes); and a Paul B. Beeson Career Development Award (NIA 1K23AG028942) (Dr. Shah).
Footnotes
Current Author Addresses: Dr. Xian: Duke Clinical Research Institute, 2400 Pratt Street, Durham, NC 27701.
Dr. Holloway: Department of Neurology, University of Rochester, 601 Elmwood Avenue, Box 681, Rochester, NY 14642.
Drs. Noyes and Friedman: Department of Community and Preventive Medicine, University of Rochester, 601 Elmwood Avenue, Box 644, Rochester, NY 14642.
Dr. Shah: Department of Emergency Medicine, University of Rochester, 601 Elmwood Avenue, Box 655, Rochester, NY 14642.
Publisher's Disclaimer: This is the prepublication, author-produced version of a manuscript accepted for publication in Annals of Internal Medicine. This version does not include post-acceptance editing and formatting. The American College of Physicians, the publisher of Annals of Internal Medicine, is not responsible for the content or presentation of the author-produced accepted version of the manuscript or any version that a third party derives from it. Readers who wish to access the definitive published version of this manuscript and any ancillary material related to this manuscript (e.g., correspondence, corrections, editorials, linked articles) should go to www.annals.org or to the print issue in which the article appears. Those who cite this manuscript should cite the published version, as it is the official version of record.
Disclaimer: This study used the linked SPARCS-SSADMF database. The interpretation and reporting of these data are the sole responsibility of the authors.
Potential Conflicts of Interest: Disclosures can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M10-0622.
References
- 1.Gillum RF. The epidemiology of cardiovascular disease in black Americans [Editorial] N Engl J Med. 1996;335:1597–9. doi: 10.1056/NEJM199611213352110. PMID: 8900095. [DOI] [PubMed] [Google Scholar]
- 2.Sacco RL, Boden-Albala B, Abel G, Lin IF, Elkind M, Hauser WA, et al. Race-ethnic disparities in the impact of stroke risk factors: the northern Manhattan stroke study. Stroke. 2001;32:1725–31. doi: 10.1161/01.str.32.8.1725. PMID: 11486097. [DOI] [PubMed] [Google Scholar]
- 3.Kuhlemeier KV, Stiens SA. Racial disparities in severity of cerebrovascular events. Stroke. 1994;25:2126–31. doi: 10.1161/01.str.25.11.2126. PMID: 7974532. [DOI] [PubMed] [Google Scholar]
- 4.Jones MR, Horner RD, Edwards LJ, Hoff J, Armstrong SB, Smith-Hammond CA, et al. Racial variation in initial stroke severity. Stroke. 2000;31:563–7. doi: 10.1161/01.str.31.3.563. PMID: 10700486. [DOI] [PubMed] [Google Scholar]
- 5.Pathak EB, Sloan MA. Recent racial/ethnic disparities in stroke hospitalizations and outcomes for young adults in Florida, 2001–2006. Neuroepidemiology. 2009;32:302–11. doi: 10.1159/000208795. PMID: 19287184. [DOI] [PubMed] [Google Scholar]
- 6.Gorelick PB. Cerebrovascular disease in African Americans. Stroke. 1998;29:2656–64. doi: 10.1161/01.str.29.12.2656. PMID: 9836782. [DOI] [PubMed] [Google Scholar]
- 7.Gillum RF. Stroke mortality in blacks. Disturbing trends. Stroke. 1999;30:1711–5. doi: 10.1161/01.str.30.8.1711. PMID: 10436126. [DOI] [PubMed] [Google Scholar]
- 8.Wolfe CD, Smeeton NC, Coshall C, Tilling K, Rudd AG. Survival differences after stroke in a multiethnic population: follow-up study with the South London stroke register. BMJ. 2005;331:431. doi: 10.1136/bmj.38510.458218.8F. PMID: 16055452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Volpp KG, Stone R, Lave JR, Jha AK, Pauly M, Klusaritz H, et al. Is thirty-day hospital mortality really lower for black veterans compared with white veterans? Health Serv Res. 2007;42:1613–31. doi: 10.1111/j.1475-6773.2006.00688.x. PMID: 17610440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polsky D, Lave J, Klusaritz H, Jha A, Pauly MV, Cen L, et al. Is lower 30-day mortality posthospital admission among blacks unique to the Veterans Affairs health care system? Med Care. 2007;45:1083–9. doi: 10.1097/MLR.0b013e3180ca960e. PMID: 18049349. [DOI] [PubMed] [Google Scholar]
- 11.Schwamm LH, Reeves MJ, Pan W, Smith EE, Frankel MR, Olson D, et al. Race/ethnicity, quality of care, and outcomes in ischemic stroke. Circulation. 2010;121:1492–501. doi: 10.1161/CIRCULATIONAHA.109.881490. PMID: 20308617. [DOI] [PubMed] [Google Scholar]
- 12.Petty GW, Brown RD, Jr, Whisnant JP, Sicks JD, O'Fallon WM, Wiebers DO. Ischemic stroke subtypes : a population-based study of functional outcome, survival, and recurrence. Stroke. 2000;31:1062–8. doi: 10.1161/01.str.31.5.1062. PMID: 10797166. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg AL, Hofer TP, Strachan C, Watts CM, Hayward RA. Accepting critically ill transfer patients: adverse effect on a referral center's outcome and benchmark measures. Ann Intern Med. 2003;138:882–90. doi: 10.7326/0003-4819-138-11-200306030-00009. PMID: 12779298. [DOI] [PubMed] [Google Scholar]
- 14.Iezzoni LI, Shwartz M, Ash AS, Mackiernan YD. Predicting in-hospital mortality for stroke patients: results differ across severity-measurement methods. Med Decis Making. 1996;16:348–56. doi: 10.1177/0272989X9601600405. PMID: 8912296. [DOI] [PubMed] [Google Scholar]
- 15.Jencks SF, Williams DK, Kay TL. Assessing hospital-associated deaths from discharge data. The role of length of stay and comorbidities. JAMA. 1988;260:2240–6. PMID: 3050163. [PubMed] [Google Scholar]
- 16.Holloway RG, Ladwig S, Robb J, Kelly A, Nielsen E, Quill TE. Palliative care consultations in hospitalized stroke patients. J Palliat Med. 2010;13:407–12. doi: 10.1089/jpm.2009.0278. PMID: 20384501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Brien LA, Grisso JA, Maislin G, LaPann K, Krotki KP, Greco PJ, et al. Nursing home residents' preferences for life-sustaining treatments. JAMA. 1995;274:1775–9. PMID: 7500508. [PubMed] [Google Scholar]
- 18.Crawley L, Payne R, Bolden J, Payne T, Washington P, Williams S, et al. Initiative to Improve Palliative and End-of-Life Care in the African American Community Palliative and end-of-life care in the African American community. JAMA. 2000;284:2518–21. doi: 10.1001/jama.284.19.2518. PMID: 11074786. [DOI] [PubMed] [Google Scholar]
- 19.Diringer MN, Edwards DF, Aiyagari V, Hollingsworth H. Factors associated with withdrawal of mechanical ventilation in a neurology/neurosurgery intensive care unit. Crit Care Med. 2001;29:1792–7. doi: 10.1097/00003246-200109000-00023. PMID: 11546988. [DOI] [PubMed] [Google Scholar]
- 20.duPreez AE, Smith MA, Liou JI, Frytak JR, Finch MD, Cleary JF, et al. Predictors of hospice utilization among acute stroke patients who died within thirty days. J Palliat Med. 2008;11:1249–57. doi: 10.1089/jpm.2008.0124. PMID: 19021489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnato AE, Farrell MH, Chang CC, Lave JR, Roberts MS, Angus DC. Development and validation of hospital “end-of-life” treatment intensity measures. Med Care. 2009;47:1098–105. doi: 10.1097/MLR.0b013e3181993191. PMID: 19820614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127:757–63. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. PMID: 9382394. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein LB, Samsa GP, Matchar DB, Horner RD. Charlson Index comorbidity adjustment for ischemic stroke outcome studies. Stroke. 2004;35:1941–5. doi: 10.1161/01.STR.0000135225.80898.1c. PMID: 15232123. [DOI] [PubMed] [Google Scholar]
- 24.Stukenborg GJ, Wagner DP, Harrell FE, Jr, Oliver MN, Heim SW, Price AL, et al. Which hospitals have significantly better or worse than expected mortality rates for acute myocardial infarction patients? Improved risk adjustment with present-at-admission diagnoses. Circulation. 2007;116:2960–8. doi: 10.1161/CIRCULATIONAHA.107.712323. PMID: 18071076. [DOI] [PubMed] [Google Scholar]
- 25.Griswold ME, Localio AR, Mulrow C. Propensity score adjustment with multilevel data: setting your sites on decreasing selection bias [Editorial] Ann Intern Med. 2010;152:393–5. doi: 10.7326/0003-4819-152-6-201003160-00010. PMID: 20231571. [DOI] [PubMed] [Google Scholar]
- 26.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84:1074–8. [Google Scholar]
- 27.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. PMID: 9431328. [DOI] [PubMed] [Google Scholar]
- 28.Zhu H, Hill MD. Stroke: the Elixhauser Index for comorbidity adjustment of in-hospital case fatality. Neurology. 2008;71:283–7. doi: 10.1212/01.wnl.0000318278.41347.94. PMID: 18645167. [DOI] [PubMed] [Google Scholar]
- 29.Bach PB, Schrag D, Begg CB. Resurrecting treatment histories of dead patients: a study design that should be laid to rest. JAMA. 2004;292:2765–70. doi: 10.1001/jama.292.22.2765. PMID: 15585737. [DOI] [PubMed] [Google Scholar]
- 30.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 31.Kissela B, Schneider A, Kleindorfer D, Khoury J, Miller R, Alwell K, et al. Stroke in a biracial population: the excess burden of stroke among blacks. Stroke. 2004;35:426–31. doi: 10.1161/01.STR.0000110982.74967.39. PMID: 14757893. [DOI] [PubMed] [Google Scholar]
- 32.Kleindorfer D, Broderick J, Khoury J, Flaherty M, Woo D, Alwell K, et al. The unchanging incidence and case-fatality of stroke in the 1990s: a population-based study. Stroke. 2006;37:2473–8. doi: 10.1161/01.STR.0000242766.65550.92. PMID: 16946146. [DOI] [PubMed] [Google Scholar]
- 33.Ellis C, Zhao Y, Egede LE. Racial/ethnic differences in stroke mortality in veterans. Ethn Dis. 2009;19:161–5. PMID: 19537227. [PubMed] [Google Scholar]
- 34.Barnato AE, Chang CC, Saynina O, Garber AM. Influence of race on inpatient treatment intensity at the end of life. J Gen Intern Med. 2007;22:338–45. doi: 10.1007/s11606-006-0088-x. PMID: 17356965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zahuranec DB, Brown DL, Lisabeth LD, Gonzales NR, Longwell PJ, Smith MA, et al. Early care limitations independently predict mortality after intracerebral hemorrhage. Neurology. 2007;68:1651–7. doi: 10.1212/01.wnl.0000261906.93238.72. PMID: 17502545. [DOI] [PubMed] [Google Scholar]
- 36.Barnato AE, Chang CC, Farrell MH, Lave JR, Roberts MS, Angus DC. Is survival better at hospitals with higher “end-of-life” treatment intensity? Med Care. 2010;48:125–32. doi: 10.1097/MLR.0b013e3181c161e4. PMID: 20057328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Polsky D, Jha AK, Lave J, Pauly MV, Cen L, Klusaritz H, et al. Short- and long-term mortality after an acute illness for elderly whites and blacks. Health Serv Res. 2008;43:1388–402. doi: 10.1111/j.1475-6773.2008.00837.x. PMID: 18355259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tuhrim S, Cooperman A, Rojas M, Brust JC, Koppel B, Martin K, et al. The association of race and sex with the underuse of stroke prevention measures. J Stroke Cerebrovasc Dis. 2008;17:226–34. doi: 10.1016/j.jstrokecerebrovasdis.2008.02.003. PMID: 18589344. [DOI] [PubMed] [Google Scholar]
- 39.Johnston SC, Fung LH, Gillum LA, Smith WS, Brass LM, Lichtman JH, et al. Utilization of intravenous tissue-type plasminogen activator for ischemic stroke at academic medical centers: the influence of ethnicity. Stroke. 2001;32:1061–8. doi: 10.1161/01.str.32.5.1061. PMID: 11340210. [DOI] [PubMed] [Google Scholar]
- 40.Holloway RG, Quill TE. Mortality as a measure of quality: implications for palliative and end-of-life care. JAMA. 2007;298:802–4. doi: 10.1001/jama.298.7.802. PMID: 17699014. [DOI] [PubMed] [Google Scholar]
- 41.Kelly A, Thompson JP, Tuttle D, Benesch C, Holloway RG. Public reporting of quality data for stroke: is it measuring quality? Stroke. 2008;39:3367–71. doi: 10.1161/STROKEAHA.108.518738. PMID: 18772446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loggers ET, Maciejewski PK, Paulk E, DeSanto-Madeya S, Nilsson M, Viswanath K, et al. Racial differences in predictors of intensive end-of-life care in patients with advanced cancer. J Clin Oncol. 2009;27:5559–64. doi: 10.1200/JCO.2009.22.4733. PMID: 19805675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Epstein RM, Fiscella K, Lesser CS, Stange KC. Why the nation needs a policy push on patient-centered health care. Health Aff (Millwood) 2010;29:1489–95. doi: 10.1377/hlthaff.2009.0888. PMID: 20679652. [DOI] [PubMed] [Google Scholar]
- 44.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics---2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. PMID: 19075105. [DOI] [PubMed] [Google Scholar]
- 45.Feng W, Nietert PJ, Adams RJ. Influence of age on racial disparities in stroke admission rates, hospital charges, and outcomes in South Carolina. Stroke. 2009;40:3096–101. doi: 10.1161/STROKEAHA.109.554535. PMID: 19542054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.