Abstract
Vitamin D is involved in mineral and bone homeostasis, immune responses, anti-inflammation, anti-infection, and cancer prevention. Vitamin D receptor (VDR) is a nuclear receptor that mediates most biological functions of 1,25(OH)2D3 or vitamin D3, the active form of vitamin D. Recently, vitamin D3-induced autophagy has been reported. Autophagy is a lysosome-mediated catabolic pathway classified into three different types: macroautophagy, microautophagy, and chaperone-mediated autophagy. Autophagy contributes to anti-aging, antimicrobial defense, and tumor suppression. The functions of autophagy overlap remarkably with those of vitamin D/VDR signaling. This review focuses on vitamin D3, VDR, and macroautophagy in inflammation and infection. We place emphasis on the regulatory roles of vitamin D3 on autophagy at different steps, including induction, nucleation, elongation to maturation, and degradation. We summarize the known molecular mechanisms of vitamin D/VDR signaling on autophagy homeostasis. The potential application of the insights gleaned from these research findings to anti-inflammation and anti-infection is also discussed.
Introduction
Vitamin D deficiency is a critical factor in the pathology of at least 17 varieties of cancer, as well as autoimmune diseases, diabetes, osteoarthritis, periodontal disease, and more (Adorini and Penna, 2008; Blaney et al., 2009; Campbell et al., 2010; Cannell et al., 2008; Gocek and Studzinski, 2009; Grau et al., 2003; Heaney, 2008) (http://www.vitamindcouncil.org). Vitamin D receptor (VDR) is a nuclear receptor that mediates most biological functions of vitamin D3, the active form of vitamin D (Baeke et al., 2010). Activation of VDR signaling affects many processes, including calcium metabolism, apoptosis, immunity, and autophagy (Bikle, 2010; Hewison, 2008; Jo, 2010; Norman, 2008; White, 2008). Autophagy influences various aspects of disease progression, including stress adaptation, lifespan extension, development, immunity, and cancer (Brest et al., 2010; Levine and Kroemer, 2008; White et al., 2010; De Meyer et al., 2009). There is increasing concern regarding the use of vitamin D as a cheap and convenient supplement for disease prevention. In this article, we review the recent advances of the signaling pathways associated with vitamin D/VDR and autophagy. We discuss the molecular mechanisms and potential application of vitamin D3 signaling to autophagy homeostasis.
Vitamin D and VDR
There are two biologically relevant forms of vitamin D. One is ergocalciferol, or vitamin D2, and the other is cholecalciferol, or vitamin D3. The enzyme 25-hydroxyvitamin D-1alpha-hydroxylase, which catalyzes 25-hydroxyvitamin D3 into 1,25(OH)2D3, is critical to the production of the active form of vitamin D. After being taken up by target cells, vitamin D3 binds to its cognate receptor, VDR.
VDR is a member of the nuclear receptor superfamily (Adams et al., 2004). In mammals, VDR is highly expressed in metabolic tissues, such as intestine, kidney, skin, and thyroid gland, and moderately expressed in nearly all tissues (Bookout et al., 2006; Norman, 2008). Moreover, VDR is expressed in many malignant tissues (Sertznig et al., 2009; Silvagno et al., 2010). Active VDR binds to vitamin D response elements (VDREs) located in promoter regions of target genes, thereby controlling the transcription of these genes (Carlberg, 2003; Haussler et al., 2008). VDR affects the transcription of at least 913 genes in human SCC25 cells (head and neck squamous cell carcinoma cell line) (Wang et al., 2005). The impacted biological processes range from calcium metabolism to the expression of key antimicrobial peptides (Albert et al., 2009; Kamen and Tangpricha, 2010; Sun, 2010). Therefore, it is not surprising that vitamin D3/VDR signaling is involved in mineral and bone homeostasis, modulation of growth, cardiovascular processes, cancer prevention, and regulation of immune responses, including autophagy. Dysfunction of VDR and vitamin D3 deficiency can cause poor bone development and health, as well as increase the risk of many chronic diseases, including type 1 diabetes, rheumatoid arthritis, Crohn’s disease, infectious diseases, and cancer (Holick, 2010).
Autophagy
Autophagy is a lysosome-mediated catabolic pathway that occurs ubiquitously in all eukaryotic cells (Reggiori and Klionsky, 2002). Depending on the route of delivery to the lysosome, autophagy is classified into three different types: macroautophagy (delivery of cytosolic contents to the lysosome by autophagosomes), microautophagy (inward invagination of the lysosomal membrane), and chaperone-mediated autophagy (direct translocation across the lysosomal membrane) (Mizushima and Levine, 2010). We focus on macroautophagy, which is hereafter simply termed autophagy, in this review.
The process of mammalian autophagy is divided into six principal steps: initiation or induction, nucleation, elongation, closure, maturation, and degradation or extrusion (Kang et al., 2011). Nucleation is the formation of the isolation membrane/phagophore. The nascent membranes are fused at their edges to form double-membrane vesicles, called autophagosomes. Elongation and closure lead to completion of the mature autophagosome (Vellai, 2009). The autophagosome fuses with a lysosome to form an autolysosome, and then its content is degraded (Levine et al., 2011, 2008; Roy and Debnath, 2010). More than 30 autophagy-related genes (ATG) regulate autophagy at the molecular level (Huang and Klionsky, 2007).
The housekeeping function of autophagy is to maintain cellular energy levels and cell survival by recycling amino acids and fatty acids during periods of metabolic stress (Onodera and Ohsumi, 2005; Levine et al., 2008). Moreover, autophagy protects the cell by degrading damaged proteins and organelles as well as intracellular pathogens. The functions of autophagy include tumor suppression (Roy and Debnath, 2010), antimicrobial defense (Deretic, 2010), inhibition of cardiac hypertrophy (De Meyer and Martinet, 2009), anti-aging (Vellai, 2009), and others (Fleming, 2011). Remarkably, the functions of autophagy overlap with those of the vitamin D/VDR signaling.
Pathways Involved in Vitamin D3-associated Autophagy
Autophagy can be induced by cellular stress, including starvation, hypoxia, biologic agents, and chemicals. Some studies have reported autophagy induced by vitamin D3 and its analogs in human myeloid leukemia cells, macrophages, breast cancer cells, and head and neck squamous cancer cells (Table 1) (Demasters et al., 2006; Hoyer-Hansen et al., 2005). The signaling pathways regulated by vitamin D3 include Bcl-2, beclin-1, mammalian target of rapamycin (mTOR), the class III phosphatidylinositol 3-kinase complex (PI3KC3), cathelicidin, calcium metabolism, and cyclin-dependent kinase (Table 1). These pathways are critical in host defense and inflammatory responses. Hence, vitamin D3 and autophagy are associated with innate immunity (Fabri and Modlin, 2009; Jo, 2010; Liu and Modlin, 2008), inflammatory bowel diseases (Verway et al., 2010), infection, and cancer (Gewirtz et al., 2009; Hoyer-Hansen et al., 2010).
Table 1.
Related Pathways Involved Autophagy Induction by Vitamin D Compounds
| Related Pathway | Experimental System | Actions of Vitamin D and Its Analog |
|---|---|---|
| mTOR | human myeloid leukemia cells (HL60) | Decrease mTOR protein level to induce autophagy (Wang et al., 2008) |
| Calcium | Human breast carcinoma (MCF-7, MCF10A) | Increase free cytosolic calcium to inhibit mTOR and induce autophagy (Hoyer-Hansen et al., 2007) |
| Bcl-2 | MCF-7 | Decrease inhibition of Bcl-2 on Beclin 1 to induce autophagy; decrease endoplasmic reticulum Bcl-2 to increase calcium to induce autophagy (Hoyer-Hansen et al., 2007) |
| Beclin 1 | MCF-7, HL60, human primary monocytes/macrophages, human monocytes THP-1 | Increase Beclin 1 to induce autophagy (Hoyer-Hansen et al., 2005; Wang et al., 2008; Yuk et al., 2009) |
| PI3KC3 | HL60 | Increase PI3KC3 to induce autophagy (Wang et al., 2008) |
| Cathelicidin | THP-1 | Increase cathelicidin to increase Beclin 1, promote lysosome to fuse with autophagosome, increase autophagy (Yuk et al., 2009) |
| Cyclin-dependent kinase (CDK) inhibitor p19INK4D | Human head and neck squamous cell carcinoma (SCC25) | Decrease p19INK4D to increase autophagy (Tavera-Mendoza et al., 2006) |
| N/A | MCF-7 | Increase radiation-induced autophagy (Demasters et al., 2006) |
| N/A | Murine macrophages (Raw 264.7) | Increase autophagy (Yuk et al., 2009) |
Note: N/A, not discussed in this article.
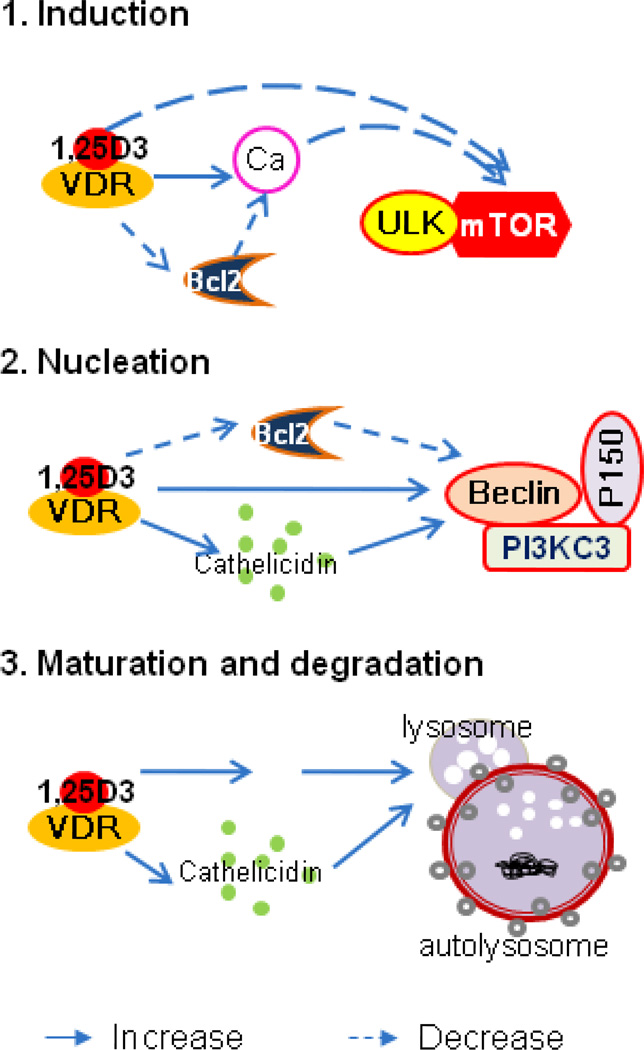
According to recent reports, vitamin D3 signaling is able to regulate autophagy at the following steps (Figure 1):
Figure 1.
Vitamin D3 regulation of autophagy at different levels. 1. Induction: Inhibits mTOR by increasing free calcium to induce autophagy. 2. Nucleation: Increases Beclin 1 and PI3K3 to induce autophagy. 3. Maturation and degradation: Increases lysosome to increase autophagy.
1. Vitamin D3 increases free cytosolic calcium and decreases mTOR induction in autophagy
Vitamin D3 is a major regulator of calcium metabolism (Fleet, 2006). Increased circulating vitamin D3 activates VDR, leading to increased intestinal calcium absorption (Song et al., 2003). In excitable cells such as neurons, calcium is released from the sarcoplasmic or endoplasmic reticulum (ER) to activate calcium-dependent kinases and phosphatases, thereby regulating numerous cellular processes, including autophagy. ER calcium induces autophagy when stimulated by vitamin D3 (Hoyer-Hansen et al., 2007). This process is inhibited by mTOR, a negative regulator of macroautophagy, and induces massive accumulation of autophagosomes in a beclin-1- and ATG7-dependent manner since they are not fused with lysosomes (Hoyer-Hansen et al., 2007). Vitamin D3 can down-regulate the expression of mTOR protein, thus inducing autophagy by inhibiting the mTORC1 complex (Loewith et al., 2002; Wang et al., 2008).
The Bcl-2 family also regulates autophagy (Chipuk et al., 2010). The published reports on the relationships between vitamin D3 and Bcl-2 are contradictory. Vitamin D3 and vitamin D analogs significantly induced the expression of Bcl-2 in psoriasis (Adisen et al., 2006). However, Xu et al. (1993) reported that vitamin D3 protected HL60 cells against apoptosis but down-regulated the expression of the Bcl-2 gene. Wagner et al. (2003) found that vitamin D3-induced apoptosis of Y79 cells was accompanied by a reduction of Bcl-2 and increase of Bax protein.
A recent study further determined that Bcl-2 inhibits autophagy by repressing calcium signals, depending on Bcl-2’s location. Bcl-2 inhibits autophagy only when Bcl-2 resides in the ER, where it has been suggested to regulate cellular Ca2+ homeostasis (Hoyer-Hansen et al., 2007). To fully understand the relationships between VDR and Bcl-2, all Bcl-2 family members need to be investigated.
2. Vitamin D3 regulates nucleation through beclin-1 and PI3KC3
Beclin-1 sits at the core of autophagy regulation (Sun et al., 2009). It is a key component of the PI3KC3 complex, which is important for the localization of autophagic proteins to a pre-autophagosomal structure (Kang et al., 2011; Kihara et al., 2001). Beclin-1 is regulated by many factors, such as Bcl-2 (Pattingre et al., 2005), NF-κB (Copetti et al., 2009b), vitamin D3, and vitamin D3 analogs (Hoyer-Hansen et al., 2005; Wang et al., 2008; Yuk et al., 2009). Inhibition of the VDR target gene cathelicidin significantly weakens vitamin D3-enhanced beclin-1 expression and vitamin D3-induced autophagy (Wang et al., 2008; Yuk et al., 2009). However, the mechanism by which vitamin D3 increases beclin-1 expression remains unclear.
Vitamin D3 can also increase beclin-1 expression through Bcl-2. Beclin-1 is a Bcl-2-homology-3 (BH3)-only protein (Sinha and Levine, 2008). Bcl-2 binds directly to a BH3 domain in beclin-1, inhibiting beclin-1 and consequently autophagy. Silencing of endogenous Bcl-2 increases the level of starvation-induced autophagy, possibly due to the release of beclin-1 from the Bcl-2-beclin-1 complex, allowing a sufficient amount of beclin-1 to be recruited to bind to PI3KC3 (Maiuri et al., 2007; Pattingre et al., 2005; Zhang et al., 2009).
Besides its effects on beclin-1, vitamin D3 signaling activates PI3K signaling pathway to induce autophagy. PI3K represents a family of kinases that phosphorylate the 3-hydroxyl group in phosphatidylinositol inositides (Saji and Ringel, 2010). Vitamin D3 activates the PI3K pathway in THP-1 cells (Sly et al., 2001), enhances the expression of beclin-1, and induces the expression of PI3KC3 in leukemia cells (Wang et al., 2008).
3. Vitamin D3 increases lysosome function to promote maturation and degradation
Cathelicidin, a VDR downstream gene, is essential in autophagosome formation. Cathelicidin is recruited into autophagosomes through the Ca2+/calmodulin-dependent kinase (kinase-beta) and AMP-activated protein kinase signaling pathways in human monocytes treated with vitamin D3 (Yuk et al., 2009).
VDR also regulates autophagy through p19INK4D. p19INK4D is a cyclin-dependent kinase inhibitor. Vitamin D3 induces the expression of p19INK4D in SCC25 cells, thus protecting cells from autophagy-induced death (Tavera-Mendoza et al., 2006). It is clear that vitamin D3 signaling increases p19INK4D which in turn decreases autophagy and decreases VDR bound to the promoter of the p19INK4D gene (Tavera-Mendoza et al., 2006). However, the mechanism of p19INK4D function in autophagy still remains largely unclear.
Vitamin D analog EB1089 can increase the volume of the acidic compartment of lysosomes and the protease activity of lysosomes in a time-dependent manner starting before any apparent changes in cell morphology or DNA fragmentation are detectable (Hoyer-Hansen et al., 2005). Therefore, vitamin D3 signaling can enhance autolysosome maturation and degradation.
Vitamin D/VDR Regulation of Inflammatory Signaling in Autophagy
Inflammation (inflammare in Latin, to set on fire) is the body’s immediate response to damage to its tissues and cells by pathogens, noxious stimuli such as chemicals, or physical injury (Weiss, 2008; Medzhitov, 2008). Both autophagy and VDR signaling play critical roles in controlling inflammatory responses. Below we discuss in more depth the inflammatory signaling pathways associated with vitamin D and/or autophagy.
1. NF-κB affects nucleation through beclin-1
The nuclear factor-κB (NF-κB) family plays diverse roles in immunity, inflammation, and cancer (Karin, 2006). VDR down-regulates NF-κB activity (Wu et al., 2010a; 2010b). A NF-κB binding site is found in the promoter of the beclin-1 gene (Copetti et al., 2009b). Active NF-κB p65 up-regulates the expression of beclin-1 and stimulates autophagy in several cellular systems (Copetti et al., 2009a).
Constitutively active IκB kinase (IKK) subunits stimulate autophagy. Inhibition or ablation of NF-κB p65 fails to suppress IKK-induced autophagy (Criollo et al., 2010). At this point, it is clear that vitamin D3 signaling decreases autophagy through NF-κB. However, the effects of NF-κB on autophagy are inconsistent.
In contrast to the stimulatory role of NF-κB in the regulation of autophagy, NF-κB has emerged as a negative regulator of autophagy, as induced by tumor necrosis factor, reactive oxygen species (ROS), and starvation in some cell lines (Djavaheri-Mergny et al., 2006). NF-κB inhibits starvation-dependent autophagy in the acute myeloid leukemia (AML) cell line U937 (Fabre et al., 2007). Prolonged NF-κB activation prevents E. coli-induced autophagy in macrophages by down-regulating the expression of Atg5 and beclin-1 (Schlottmann et al., 2008). Further research in various systems will be required to fully clarify the roles of NF-κB in autophagy.
2. Vitamin D3 may inhibit tumor necrosis factor-alpha (TNF-α) in autophagy
TNF-α is a pleiotropic inflammatory cytokine produced by activated immune cells as well as stromal cells (Esposito and Cuzzocrea, 2009). TNF-α significantly increases the expression of beclin-1 through the JNK pathway in human atherosclerotic vascular smooth cells (Jia et al., 2006). Vitamin D3 inhibits TNF-α in mycobacteria-infected macrophages and peripheral blood mononuclear cells from pulmonary tuberculosis patients (Martineau et al., 2007; Prabhu Anand et al., 2009). The vitamin D analog cholecalciferol reduces the circulating level of TNF-α (Stubbs et al., 2010). In addition, TNF-α significantly increases the expression of MAP1LC3 (ATG8) to induce autophagy. MAP1LC3 expression is induced via both the Akt and JNK pathways in human atherosclerotic vascular smooth cells (Jia et al., 2006). Therefore, vitamin D3 signaling may decrease TNF-α-induced autophagy.
3. NOD2 recruits ATG16 to regulate elongation
NOD2 is an intracellular pattern recognition receptor that recognizes muramyldipeptide (MDP), an integral component of bacterial cell walls. NOD2 is expressed in myelomonocytic cells, dendritic cells, and intestinal epithelial cells (Lecat et al., 2010). NOD2 triggering by MDP induces autophagy in dendritic cells. This effect requires receptor-interacting serine-threonine kinase-2, ATG5, ATG7, and ATG16L1 (Cooney et al., 2010). Vitamin D3 robustly stimulates the expression of the NOD2 gene and protein in primary human monocytic and epithelial cells (Wang et al., 2010). Moreover, NOD2 is known to trigger autophagy and eliminate intracellular bacteria through the recruitment of ATG16L1 to the site of bacterial entry (Travassos et al., 2010). Therefore, vitamin D3 may increase vesicle elongation through the NOD2 pathway.
4. Autophagy via Interferon-gamma (IFN-γ)
IFN-γ is a cytokine produced by lymphocytes that has antiviral, immunoregulatory, and anti-tumor properties (Schroder et al., 2004). Vitamin D3 inhibits IFN-γ in naive CD62 ligand+CD4+ T cells (Staeva-Vieira and Freedman, 2002) and mycobacteria-infected peripheral blood mononuclear cells and macrophages (Martineau et al., 2007; Prabhu Anand et al., 2009). IFN-γ activates and increases lysosome activity in macrophages. It directly induces autophagy and the recruitment of autophagy proteins to the mycobacterial phagosome in macrophages (Al-Zeer et al., 2009; Gutierrez et al., 2004; Singh et al., 2006). Autophagy induced by IFN-γ depends on ATG5 (Chang et al., 2010). IFN-γ activation of macrophages also induces nitric oxide production, which in turn promotes autophagy through an autocrine positive-feedback loop (Ghadimi et al., 2010). IFN-γ level increases when cells are under certain stresses, such as Salmonella infection (Liu et al., 2010). However, there is no direct evidence to show that vitamin D3 signaling may decrease autophagy through IFN-γ.
Overall Functions of Vitamin D3 Signaling in Autophagy Homeostasis
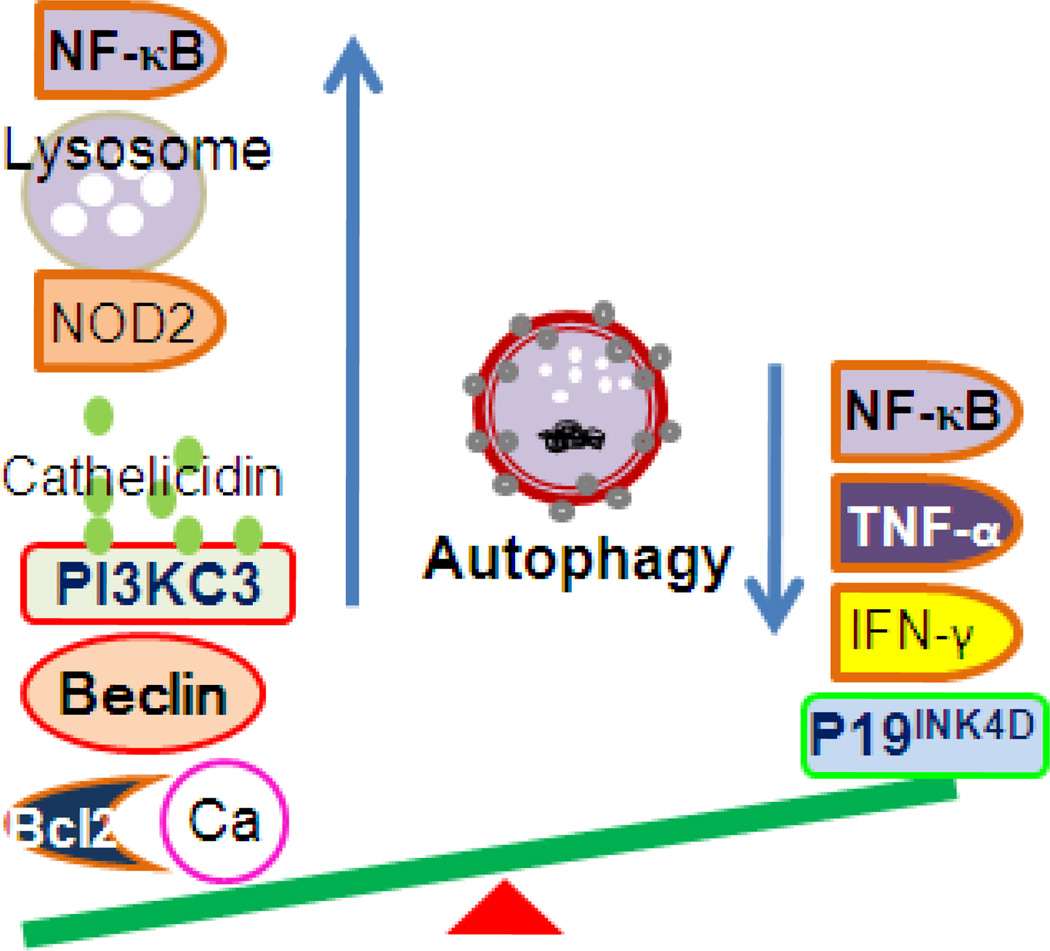
Vitamin D3 signaling affects autophagy at several levels, the outcome of which is two-sided (Figure 2). On one hand, vitamin D3 signaling increases the level of free cytosolic calcium and consequently decreases mTOR activity and induces autophagy; vitamin D3 signaling also increases beclin-1 through several pathways, decreases the inhibition of Bcl-2, increases cathelicidin, and down-regulates NF-κB, which may decrease beclin-1 level. Vitamin D3 signaling can increase PI3KC3 protein, enhancing nucleation. To promote elongation, vitamin D3 signaling increases NOD2 level to recruit ATG16, increases lysosomal protease activity, and induces autophagosomes to fuse with lysosomes through cathelicidin.
Figure 2.
Vitamin D3 signaling regulates autophagy homeostasis. Vitamin D3 signaling may increase autophagy through the following factors: elevated cytosolic calcium; Beclin 1, cathelicidin, and PI3KC3; and NOD2, lysosomal protease activity, and decreased NF-κB activity. Vitamin D3 signaling may decrease autophagy through the following regulators: P19INK4D, activation of NF-κB, TNF-α, and IFN-γ.
On the other hand, vitamin D3 signaling may decrease autophagy through different mechanisms, especially under certain stresses. Vitamin D3 may decrease the level of NF-κB, TNF-α, or IFN-γ, thus decreasing autophagy. In addition, vitamin D3 increases the level of p19INK4D, which protects cells from autophagy-induced death (Figure 2).
Vitamin D, VDR, and Macroautophagy in Inflammation and Infectious Disease
Acute inflammation is considered a host defense strategy to remove the injurious stimuli. Inflammation plays a critical role in wound healing and infection resolution. Inflammation is not a synonym for infection. Infection is caused by an exogenous pathogen, such as bacteria, viruses, and parasites, whereas inflammation is one of the host responses to the pathogen.
Although a successful inflammatory response is normally closely regulated by the body, inflammation could become pathologic and out of control. If the acute inflammation fails to eliminate the pathogen, the inflammatory process persists and acquires new characteristics (Medzhitov, 2008). Chronic inflammation is a prolonged, dysregulated, and maladaptive response that involves active inflammation, tissue destruction, and attempts at tissue repair (Weiss, 2008). Compelling evidence demonstrates that both vitamin D signaling and autophagy play a critical role in the pathogenesis of chronic inflammation and infection.
1. Vitamin D signaling and autophagy in inflammatory bowel diseases
Inflammatory bowel disease (IBD) is a dysregulated response of the immune system associated with intestinal tissues to the commensal microbiota in a genetically susceptible host (Kaser and Blumberg, 2008). The major types of IBD are Crohn’s disease (CD) and ulcerative colitis. The pathogenesis of IBD involves a complex interplay between genetic, microbial, immunological, and environmental factors (Bouma and Strober, 2003). More than 30 genetic loci associated with IBD have been identified in genome-wide association studies (Schreiber, 2009). Autophagy-associated genes ATG16L1 and IRGM are confirmed susceptibility loci for CD (Hampe et al., 2007; Parkes et al., 2007; Rioux et al., 2007). Variants in the NOD2 locus are associated with the strongest risk of developing CD (Hugot et al., 2001).
Mucosal inflammations in patients with IBD are accompanied by elevated levels of activated NF-κB, particularly p65 (Rogler et al., 1998; Schreiber et al., 1998). NOD2 and NF-κB play important roles in regulating autophagy. Paneth cells play an important role in intestinal innate immunity by means of secreting granule contents, including antimicrobial peptides and lysozyme (Ouellette, 2010). In experimental models, Paneth cells show notable abnormalities in the granule exocytosis pathway in ATG5- and ATG16L1-deficient mice (Cadwell et al., 2008). In human study, NOD2 mutations have been largely linked to ileal CD and have been associated with reduced expression of α-defensins HD-5 and HD-6 in isolates of ileal Paneth cells (Wehkamp et al., 2004). Taken together, the data strongly implicate autophagy in the pathogenesis of IBD (Kaser and Blumberg, 2008).
Deficiency of vitamin D3 has been suggested as an important environmental factor for IBD (Laverny et al., 2010). Vitamin D3 signaling regulates autophagy through several steps, which may affect the efficacy of treatments with vitamin D3 and its analogs on IBD. Vitamin D3 can increase NOD2 expression in human intestinal epithelial cells (Wang et al., 2010). In rabbits that were given a plant containing high levels of vitamin D3 for 15 or 30 days, time- and dose-dependent increases in the size and number of Paneth cells were found in the jejunum (Zanuzzi et al., 2008). In a pilot clinical study in IBD patients, Miheller et al. (2009) reported a short-term beneficial effect on Crohn’s disease activity after one-year administration of vitamin D3. However, there is no direct in vivo evidence of vitamin D3 signaling in the regulation of autophagy in IBD.
2. Vitamin D, autophagy, and infectious diseases
Some microorganisms have developed mechanisms to counteract or take control of the autophagic pathway as a survival strategy (Colombo et al., 2006; Kirkegaard et al., 2004). Coxiella burnetii resides in a phagosome that interacts with autophagic vacuoles and then with lysosomes to generate a large replicative niche. This bacterially driven interaction with autophagosomes and its transit through the autophagic pathway favor Coxiella replication in the host cell. We speculate that vitamin D3 signaling may inhibit autophagy and kill the bacteria through cathelicidin. However, we found no published reports on the effects of vitamin D3 signaling and Coxiella burnetii.
Cathelicidins are one of the major antimicrobial peptide families. In human, there is only one cathelicidin family member, human cationic antimicrobial protein (hCAP-18), which is cleaved to release LL37 (Durr et al., 2006). LL-37 has shown a broad spectrum of activity against both Gram-negative and Gram-positive bacteria, various viruses, and fungi (Tjabringa et al., 2006). In humans, cathelicidin contains activating VDREs in its promoter region, 507 bp upstream of its transcription initiation site (Wang et al., 2004). Activation of VDR results in the expression of cathelicidin at both the mRNA and protein levels in monocytes/macrophages (Liu et al., 2006; Peric et al., 2008; Wang et al., 2004).
There is a long history of using vitamin D to treat mycobacterial infections (Liu and Modlin, 2008; Hewison, 2010). Vitamin D3’s antagonism of M. tuberculosis involves antimicrobial peptides (Liu et al., 2006) and autophagy (Yuk et al., 2009). Vitamin D3-induced antimicrobial activity is completely inhibited in the presence of siRNA against cathelicidin (Liu et al., 2007; Yuk et al., 2009). Hence, cathelicidin is essential for the induction of autophagy by vitamin D3 in bacterial infection.
Conclusion and Future Directions
Increasing evidence supports the idea that autophagy is regulated by vitamin D3 signaling at different levels, including induction, nucleation, elongation to maturation, and degradation. Vitamin D3 signaling plays essential roles in regulating autophagy to avoid autophagy-induced damage and to inhibit invasion of microorganisms.
To understand the effects of vitamin D3 and autophagy in inflammation and infectious diseases, we need to explore mechanisms by which vitamin D3 signaling regulates autophagy, especially in disease-associated experimental models in vivo. The unknown questions include: 1) the relationships between VDR and Bcl-2; 2) the mechanism by which vitamin D3 increases beclin-1 expression; 3) the mechanism by which p19INK4D decreases autophagy; 4) the effects of NF-κB on autophagy; 5) whether vitamin D3 signaling is directly involved in the regulation of autophagy in IBD; and 6) bacterial-host interactions by which bacteria use autophagy as a survival strategy. Studies on the above aspects will improve our understanding of the functions of vitamin D3 signaling and apply the insights to the development of effective therapy in anti-inflammation and anti-infection.
Acknowledgment
This work was supported by the National Institutes of Health (DK075386-0251, R03DK089010-01), the American Cancer Society (RSG-09-075-01-MBC), and the IDEAL award from New York State’s Empire State Stem Cell Board (N09G-279) to Jun Sun.
Footnotes
Disclosure
The authors report no conflicts of interest.
References
- Adams JS, Chen H, Chun R, Gacad MA, Encinas C, Ren S, Nguyen L, Wu S, Hewison M, Barsony J. Response element binding proteins and intracellular vitamin D binding proteins: novel regulators of vitamin D trafficking, action and metabolism. J Steroid Biochem Mol Biol. 2004;89–90(1–5):461–465. doi: 10.1016/j.jsbmb.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Adisen E, Gulekon A, Erdem O, Dursun A, Gurer MA. The effects of calcipotriol and methylprednisolone aseponate on bcl-2, p53 and ki-67 expression in psoriasis. J Eur Acad Dermatol Venereol. 2006;20(5):527–533. doi: 10.1111/j.1468-3083.2006.01508.x. [DOI] [PubMed] [Google Scholar]
- Adorini L, Penna G. Control of autoimmune diseases by the vitamin D endocrine system. Nat Clin Pract Rheumatol. 2008;4(8):404–412. doi: 10.1038/ncprheum0855. [DOI] [PubMed] [Google Scholar]
- Al-Zeer MA, Al-Younes HM, Braun PR, Zerrahn J, Meyer TF. IFN-gamma-inducible Irga6 mediates host resistance against Chlamydia trachomatis via autophagy. PLoS One. 2009;4(2):e4588. doi: 10.1371/journal.pone.0004588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert PJ, Proal AD, Marshall TG. Vitamin D: the alternative hypothesis. Autoimmun Rev. 2009;8(8):639–644. doi: 10.1016/j.autrev.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Baeke F, Gysemans C, Korf H, Mathieu C. Vitamin D insufficiency: implications for the immune system. Pediatr Nephrol. 2010;25(9):1597–1606. doi: 10.1007/s00467-010-1452-y. [DOI] [PubMed] [Google Scholar]
- Bikle DD. Vitamin D: newly discovered actions require reconsideration of physiologic requirements. Trends Endocrinol Metab. 2010;21(6):375–384. doi: 10.1016/j.tem.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaney GP, Albert PJ, Proal AD. Vitamin D metabolites as clinical markers in autoimmune and chronic disease. Ann N Y Acad Sci. 2009;1173:384–390. doi: 10.1111/j.1749-6632.2009.04875.x. [DOI] [PubMed] [Google Scholar]
- Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell. 2006;126(4):789–799. doi: 10.1016/j.cell.2006.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3(7):521–533. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- Brest P, Corcelle EA, Cesaro A, Chargui A, Belaid A, Klionsky DJ, Vouret-Craviari V, Hebuterne X, Hofman P, Mograbi B. Autophagy and Crohn’s disease: at the crossroads of infection, inflammation, immunity, and cancer. Curr Mol Med. 2010;10(5):486–502. doi: 10.2174/156652410791608252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadwell K, Liu JY, Brown SL, Miyoshi H, Loh J, Lennerz JK, Kishi C, Kc W, Carrero JA, Hunt S, Stone CD, Brunt EM, Xavier RJ, Sleckman BP, Li E, Mizushima N, Stappenbeck TS, Virgin HWT. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature. 2008;456(7219):259–263. doi: 10.1038/nature07416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell FC, Xu H, El-Tanani M, Crowe P, Bingham V. The yin and yang of vitamin D receptor (VDR) signaling in neoplastic progression: operational networks and tissue-specific growth control. Biochem Pharmacol. 2010;79(1):1–9. doi: 10.1016/j.bcp.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell JJ, Hollis BW, Zasloff M, Heaney RP. Diagnosis and treatment of vitamin D deficiency. Expert Opin Pharmacother. 2008;9(1):107–118. doi: 10.1517/14656566.9.1.107. [DOI] [PubMed] [Google Scholar]
- Carlberg C. Current understanding of the function of the nuclear vitamin D receptor in response to its natural and synthetic ligands. Recent Results Cancer Res. 2003;164:29–42. doi: 10.1007/978-3-642-55580-0_2. [DOI] [PubMed] [Google Scholar]
- Chang YP, Tsai CC, Huang WC, Wang CY, Chen CL, Lin YS, Kai JI, Hsieh CY, Cheng YL, Choi PC, Chen SH, Chang SP, Liu HS, Lin CF. Autophagy facilitates IFN-gamma-induced Jak2-STAT1 activation and cellular inflammation. J Biol Chem. 2010;285(37):28715–28722. doi: 10.1074/jbc.M110.133355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL-2 family reunion. Mol Cell. 2010;37(3):299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo MI, Gutierrez MG, Romano PS. The two faces of autophagy: Coxiella and Mycobacterium. Autophagy. 2006;2(3):162–164. doi: 10.4161/auto.2827. [DOI] [PubMed] [Google Scholar]
- Cooney R, Baker J, Brain O, Danis B, Pichulik T, Allan P, Ferguson DJ, Campbell BJ, Jewell D, Simmons A. NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat Med. 2010;16(1):90–97. doi: 10.1038/nm.2069. [DOI] [PubMed] [Google Scholar]
- Copetti T, Bertoli C, Dalla E, Demarchi F, Schneider C. p65/RelA modulates BECN1 transcription and autophagy. Mol Cell Biol. 2009a;29(10):2594–2608. doi: 10.1128/MCB.01396-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copetti T, Demarchi F, Schneider C. p65/RelA binds and activates the beclin 1 promoter. Autophagy. 2009b;5(6):858–859. doi: 10.4161/auto.8822. [DOI] [PubMed] [Google Scholar]
- Criollo A, Senovilla L, Authier H, Maiuri MC, Morselli E, Vitale I, Kepp O, Tasdemir E, Galluzzi L, Shen S, Tailler M, Delahaye N, Tesniere A, De Stefano D, Younes AB, Harper F, Pierron G, Lavandero S, Zitvogel L, Israel A, et al. The IKK complex contributes to the induction of autophagy. EMBO J. 2010;29(3):619–631. doi: 10.1038/emboj.2009.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meyer GR, Martinet W. Autophagy in the cardiovascular system. Biochim Biophys Acta. 2009;1793(9):1485–1495. doi: 10.1016/j.bbamcr.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Demasters G, Di X, Newsham I, Shiu R, Gewirtz DA. Potentiation of radiation sensitivity in breast tumor cells by the vitamin D3 analogue, EB 1089, through promotion of autophagy and interference with proliferative recovery. Mol Cancer Ther. 2006;5(11):2786–2797. doi: 10.1158/1535-7163.MCT-06-0316. [DOI] [PubMed] [Google Scholar]
- Deretic V. Autophagy in infection. Curr Opin Cell Biol. 2010;22(2):252–262. doi: 10.1016/j.ceb.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djavaheri-Mergny M, Amelotti M, Mathieu J, Besancon F, Bauvy C, Souquere S, Pierron G, Codogno P. NF-kappaB activation represses tumor necrosis factor-alpha-induced autophagy. J Biol Chem. 2006;281(41):30373–30382. doi: 10.1074/jbc.M602097200. [DOI] [PubMed] [Google Scholar]
- Durr UH, Sudheendra US, Ramamoorthy A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim Biophys Acta. 2006;1758(9):1408–1425. doi: 10.1016/j.bbamem.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Esposito E, Cuzzocrea S. TNF-alpha as a therapeutic target in inflammatory diseases, ischemia-reperfusion injury and trauma. Curr Med Chem. 2009;16(24):3152–3167. doi: 10.2174/092986709788803024. [DOI] [PubMed] [Google Scholar]
- Fabre C, Carvalho G, Tasdemir E, Braun T, Ades L, Grosjean J, Boehrer S, Metivier D, Souquere S, Pierron G, Fenaux P, Kroemer G. NF-kappaB inhibition sensitizes to starvation-induced cell death in high-risk myelodysplastic syndrome and acute myeloid leukemia. Oncogene. 2007;26(28):4071–4083. doi: 10.1038/sj.onc.1210187. [DOI] [PubMed] [Google Scholar]
- Fabri M, Modlin RL. A vitamin for autophagy. Cell Host Microbe. 2009;6(3):201–203. doi: 10.1016/j.chom.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Fleet JC. Molecular regulation of calcium and bone metabolism through the vitamin D receptor. J Musculoskelet Neuronal Interact. 2006;6(4):336–337. [PubMed] [Google Scholar]
- Fleming A, Noda T, Yoshimori T, Rubinsztein DC. Chemical modulators of autophagy as biological probes and potential therapeutics. Nat Chem Biol. 2011;7:9–17. doi: 10.1038/nchembio.500. [DOI] [PubMed] [Google Scholar]
- Gewirtz DA, Hilliker ML, Wilson EN. Promotion of autophagy as a mechanism for radiation sensitization of breast tumor cells. Radiother Oncol. 2009;92(3):323–328. doi: 10.1016/j.radonc.2009.05.022. [DOI] [PubMed] [Google Scholar]
- Ghadimi D, De Vrese M, Heller KJ, Schrezenmeir J. Lactic acid bacteria enhance autophagic ability of mononuclear phagocytes by increasing Th1 autophagy-promoting cytokine (IFN-gamma) and nitric oxide (NO) levels and reducing Th2 autophagy-restraining cytokines (IL-4 and IL-13) in response to Mycobacterium tuberculosis antigen. Int Immunopharmacol. 2010;10(6):694–706. doi: 10.1016/j.intimp.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Gocek E, Studzinski GP. Vitamin D and differentiation in cancer. Crit Rev Clin Lab Sci. 2009;46(4):190–209. doi: 10.1080/10408360902982128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau MV, Baron JA, Sandler RS, Haile RW, Beach ML, Church TR, Heber D. Vitamin D, calcium supplementation, and colorectal adenomas: results of a randomized trial. J Natl Cancer Inst. 2003;95(23):1765–1771. doi: 10.1093/jnci/djg110. [DOI] [PubMed] [Google Scholar]
- Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119(6):753–766. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- Hampe J, Franke A, Rosenstiel P, Till A, Teuber M, Huse K, Albrecht M, Mayr G, De La Vega FM, Briggs J, Gunther S, Prescott NJ, Onnie CM, Hasler R, Sipos B, Folsch UR, Lengauer T, Platzer M, Mathew CG, Krawczak M, et al. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat Genet. 2007;39(2):207–211. doi: 10.1038/ng1954. [DOI] [PubMed] [Google Scholar]
- Haussler MR, Haussler CA, Bartik L, Whitfield GK, Hsieh JC, Slater S, Jurutka PW. Vitamin D receptor: molecular signaling and actions of nutritional ligands in disease prevention. Nutr Rev. 2008;66(10) Suppl 2:S98–S112. doi: 10.1111/j.1753-4887.2008.00093.x. [DOI] [PubMed] [Google Scholar]
- Heaney RP. Vitamin D in health and disease. Clin J Am Soc Nephrol. 2008;3(5):1535–1541. doi: 10.2215/CJN.01160308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewison M. Vitamin D and innate immunity. Curr Opin Investig Drugs. 2008;9(5):485–490. [PubMed] [Google Scholar]
- Hewison M. Antibacterial effects of vitamin D. Nat Rev Endocrinol. 2011 Jan. 25 doi: 10.1038/nrendo.2010.226. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D and health: evolution, biologic functions, and recommended dietary intakes for vitamin D. In: Holick MF, editor. Vitamin D. New York, New York, USA: Humana Press; 2010. pp. 3–33. [Google Scholar]
- Hoyer-Hansen M, Bastholm L, Mathiasen IS, Elling F, Jaattela M. Vitamin D analog EB1089 triggers dramatic lysosomal changes and Beclin 1-mediated autophagic cell death. Cell Death Differ. 2005;12(10):1297–1309. doi: 10.1038/sj.cdd.4401651. [DOI] [PubMed] [Google Scholar]
- Hoyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, Farkas T, Bianchi K, Fehrenbacher N, Elling F, Rizzuto R, Mathiasen IS, Jaattela M. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell. 2007;25(2):193–205. doi: 10.1016/j.molcel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Hoyer-Hansen M, Nordbrandt SP, Jaattela M. Autophagy as a basis for the health-promoting effects of vitamin D. Trends Mol Med. 2010;16(7):295–302. doi: 10.1016/j.molmed.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Huang J, Klionsky DJ. Autophagy and human disease. Cell Cycle. 2007;6(15):1837–1849. doi: 10.4161/cc.6.15.4511. [DOI] [PubMed] [Google Scholar]
- Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, Almer S, Tysk C, O’morain CA, Gassull M, Binder V, Finkel Y, Cortot A, Modigliani R, Laurent-Puig P, Gower-Rousseau C, Macry J, Colombel JF, Sahbatou M, Thomas G. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411(6837):599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- Jia G, Cheng G, Gangahar DM, Agrawal DK. Insulin-like growth factor-1 and TNF-alpha regulate autophagy through c-jun N-terminal kinase and Akt pathways in human atherosclerotic vascular smooth cells. Immunol Cell Biol. 2006;84(5):448–454. doi: 10.1111/j.1440-1711.2006.01454.x. [DOI] [PubMed] [Google Scholar]
- Jo EK. Innate immunity to mycobacteria: vitamin D and autophagy. Cell Microbiol. 2010;12(8):1026–1035. doi: 10.1111/j.1462-5822.2010.01491.x. [DOI] [PubMed] [Google Scholar]
- Kamen DL, Tangpricha V. Vitamin D and molecular actions on the immune system: modulation of innate and autoimmunity. J Mol Med. 2010;88(5):441–450. doi: 10.1007/s00109-010-0590-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011;18(4):571–580. doi: 10.1038/cdd.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441(7092):431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- Kaser A, Blumberg RS. Paneth cells and inflammation dance together in Crohn’s disease. Cell Res. 2008;18(12):1160–1162. doi: 10.1038/cr.2008.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara A, Kabeya Y, Ohsumi Y, Yoshimori T. Beclin-phosphatidylinositol 3-kinase complex functions at the trans-Golgi network. EMBO Rep. 2001;2(4):330–335. doi: 10.1093/embo-reports/kve061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkegaard K, Taylor MP, Jackson WT. Cellular autophagy: surrender, avoidance and subversion by microorganisms. Nat Rev Microbiol. 2004;2(4):301–314. doi: 10.1038/nrmicro865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laverny G, Penna G, Vetrano S, Correale C, Nebuloni M, Danese S, Adorini L. Efficacy of a potent and safe vitamin D receptor agonist for the treatment of inflammatory bowel disease. Immunol Lett. 2010;131(1):49–58. doi: 10.1016/j.imlet.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Lecat A, Piette J, Legrand-Poels S. The protein Nod2: an innate receptor more complex than previously assumed. Biochem Pharmacol. 2010;80(12):2021–2031. doi: 10.1016/j.bcp.2010.07.016. [DOI] [PubMed] [Google Scholar]
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132(1):27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469(7330):323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PT, Modlin RL. Human macrophage host defense against Mycobacterium tuberculosis. Curr Opin Immunol. 2008;20(4):371–376. doi: 10.1016/j.coi.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol. 2007;179(4):2060–2063. doi: 10.4049/jimmunol.179.4.2060. [DOI] [PubMed] [Google Scholar]
- Liu X, Lu R, Xia Y, Sun J. Global analysis of the eukaryotic pathways and networks regulated by Salmonella typhimurium in mouse intestinal infection in vivo. BMC Genomics. 2010;11:722. doi: 10.1186/1471-2164-11-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, Oppliger W, Jenoe P, Hall MN. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell. 2002;10(3):457–468. doi: 10.1016/s1097-2765(02)00636-6. [DOI] [PubMed] [Google Scholar]
- Maiuri MC, Le Toumelin G, Criollo A, Rain JC, Gautier F, Juin P, Tasdemir E, Pierron G, Troulinaki K, Tavernarakis N, Hickman JA, Geneste O, Kroemer G. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J. 2007;26(10):2527–2539. doi: 10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau AR, Wilkinson KA, Newton SM, Floto RA, Norman AW, Skolimowska K, Davidson RN, Sorensen OE, Kampmann B, Griffiths CJ, Wilkinson RJ. IFN-gamma- and TNF-independent vitamin D-inducible human suppression of mycobacteria: the role of cathelicidin LL-37. J Immunol. 2007;178(11):7190–7198. doi: 10.4049/jimmunol.178.11.7190. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454(7203):428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Miheller P, Muzes G, Hritz I, Lakatos G, Pregun I, Lakatos PL, Herszenyi L, Tulassay Z. Comparison of the effects of 1,25 dihydroxyvitamin D and 25 hydroxyvitamin D on bone pathology and disease activity in Crohn’s disease patients. Inflamm Bowel Dis. 2009;15(11):1656–1662. doi: 10.1002/ibd.20947. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Levine B. Autophagy in mammalian development and differentiation. Nat Cell Biol. 2010;12(9):823–830. doi: 10.1038/ncb0910-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman AW. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr. 2008;88(2):491S–499S. doi: 10.1093/ajcn/88.2.491S. [DOI] [PubMed] [Google Scholar]
- Onodera J, Ohsumi Y. Autophagy is required for maintenance of amino acid levels and protein synthesis under nitrogen starvation. J Biol Chem. 2005;280(36):31582–31586. doi: 10.1074/jbc.M506736200. [DOI] [PubMed] [Google Scholar]
- Ouellette AJ. Paneth cells and innate mucosal immunity. Curr Opin Gastroenterol. 2010;26(6):547–553. doi: 10.1097/MOG.0b013e32833dccde. [DOI] [PubMed] [Google Scholar]
- Parkes M, Barrett JC, Prescott NJ, Tremelling M, Anderson CA, Fisher SA, Roberts RG, Nimmo ER, Cummings FR, Soars D, Drummond H, Lees CW, Khawaja SA, Bagnall R, Burke DA, Todhunter CE, Ahmad T, Onnie CM, Mcardle W, Strachan D, et al. Sequence variants in the autophagy gene IRGM and multiple other replicating loci contribute to Crohn’s disease susceptibility. Nat Genet. 2007;39(7):830–832. doi: 10.1038/ng2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122(6):927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Peric M, Koglin S, Kim SM, Morizane S, Besch R, Prinz JC, Ruzicka T, Gallo RL, Schauber J. IL-17A enhances vitamin D3-induced expression of cathelicidin antimicrobial peptide in human keratinocytes. J Immunol. 2008;181(12):8504–8512. doi: 10.4049/jimmunol.181.12.8504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhu Anand S, Selvaraj P, Narayanan PR. Effect of 1,25 dihydroxyvitamin D3 on intracellular IFN-gamma and TNF-alpha positive T cell subsets in pulmonary tuberculosis. Cytokine. 2009;45(2):105–110. doi: 10.1016/j.cyto.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Reggiori F, Klionsky DJ. Autophagy in the eukaryotic cell. Eukaryot Cell. 2002;1(1):11–21. doi: 10.1128/EC.01.1.11-21.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioux JD, Xavier RJ, Taylor KD, Silverberg MS, Goyette P, Huett A, Green T, Kuballa P, Barmada MM, Datta LW, Shugart YY, Griffiths AM, Targan SR, Ippoliti AF, Bernard EJ, Mei L, Nicolae DL, Regueiro M, Schumm LP, Steinhart AH, et al. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat Genet. 2007;39(5):596–604. doi: 10.1038/ng2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogler G, Brand K, Vogl D, Page S, Hofmeister R, Andus T, Knuechel R, Baeuerle PA, Scholmerich J, Gross V. Nuclear factor kappaB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology. 1998;115(2):357–369. doi: 10.1016/s0016-5085(98)70202-1. [DOI] [PubMed] [Google Scholar]
- Roy S, Debnath J. Autophagy and tumorigenesis. Semin Immunopathol. 2010;32(4):383–396. doi: 10.1007/s00281-010-0213-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saji M, Ringel MD. The PI3K-Akt-mTOR pathway in initiation and progression of thyroid tumors. Mol Cell Endocrinol. 2010;321(1):20–28. doi: 10.1016/j.mce.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlottmann S, Buback F, Stahl B, Meierhenrich R, Walter P, Georgieff M, Senftleben U. Prolonged classical NF-kappaB activation prevents autophagy upon E. coli stimulation in vitro: a potential resolving mechanism of inflammation. Mediators Inflamm. 2008;2008 doi: 10.1155/2008/725854. 725854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S. The genetic universe of inflammatory bowel disease. Gastroenterology. 2009;136(7):2402–2403. doi: 10.1053/j.gastro.2009.04.051. [DOI] [PubMed] [Google Scholar]
- Schreiber S, Nikolaus S, Hampe J. Activation of nuclear factor kappa B inflammatory bowel disease. Gut. 1998;42(4):477–484. doi: 10.1136/gut.42.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75(2):163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- Sertznig P, Dunlop T, Seifert M, Tilgen W, Reichrath J. Cross-talk between vitamin D receptor (VDR)- and peroxisome proliferator-activated receptor (PPAR)-signaling in melanoma cells. Anticancer Res. 2009;29(9):3647–3658. [PubMed] [Google Scholar]
- Silvagno F, Poma CB, Realmuto C, Ravarino N, Ramella A, Santoro N, D’Amelio P, Fuso L, Pescarmona G, Zola P. Analysis of vitamin D receptor expression and clinical correlations in patients with ovarian cancer. Gynecol Oncol. 2010;119(1):121–124. doi: 10.1016/j.ygyno.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Singh SB, Davis AS, Taylor GA, Deretic V. Human IRGM induces autophagy to eliminate intracellular mycobacteria. Science. 2006;313(5792):1438–1441. doi: 10.1126/science.1129577. [DOI] [PubMed] [Google Scholar]
- Sinha S, Levine B. The autophagy effector Beclin 1: a novel BH3-only protein. Oncogene. 2008;27 Suppl 1:S137–S148. doi: 10.1038/onc.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sly LM, Lopez M, Nauseef WM, Reiner NE. 1alpha,25-Dihydroxyvitamin D3-induced monocyte antimycobacterial activity is regulated by phosphatidylinositol 3-kinase and mediated by the NADPH-dependent phagocyte oxidase. J Biol Chem. 2001;276(38):35482–35493. doi: 10.1074/jbc.M102876200. [DOI] [PubMed] [Google Scholar]
- Song Y, Peng X, Porta A, Takanaga H, Peng JB, Hediger MA, Fleet JC, Christakos S. Calcium transporter 1 and epithelial calcium channel messenger ribonucleic acid are differentially regulated by 1,25 dihydroxyvitamin D3 in the intestine and kidney of mice. Endocrinology. 2003;144(9):3885–3894. doi: 10.1210/en.2003-0314. [DOI] [PubMed] [Google Scholar]
- Staeva-Vieira TP, Freedman LP. 1,25-dihydroxyvitamin D3 inhibits IFN-gamma and IL-4 levels during in vitro polarization of primary murine CD4+ T cells. J Immunol. 2002;168(3):1181–1189. doi: 10.4049/jimmunol.168.3.1181. [DOI] [PubMed] [Google Scholar]
- Stubbs JR, Idiculla A, Slusser J, Menard R, Quarles LD. Cholecalciferol supplementation alters calcitriol-responsive monocyte proteins and decreases inflammatory cytokines in ESRD. J Am Soc Nephrol. 2010;21(2):353–361. doi: 10.1681/ASN.2009040451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J. Vitamin D and mucosal immune function. Curr Opin Gastroenterol. 2010;26(6):591–595. doi: 10.1097/MOG.0b013e32833d4b9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Fan W, Zhong Q. Regulation of Beclin 1 in autophagy. Autophagy. 2009;5(5):713–716. doi: 10.4161/auto.5.5.8524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavera-Mendoza L, Wang TT, Lallemant B, Zhang R, Nagai Y, Bourdeau V, Ramirez-Calderon M, Desbarats J, Mader S, White JH. Convergence of vitamin D and retinoic acid signalling at a common hormone response element. EMBO Rep. 2006;7(2):180–185. doi: 10.1038/sj.embor.7400594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjabringa GS, Ninaber DK, Drijfhout JW, Rabe KF, Hiemstra PS. Human cathelicidin LL-37 is a chemoattractant for eosinophils and neutrophils that acts via formyl-peptide receptors. Int Arch Allergy Immunol. 2006;140:103–112. doi: 10.1159/000092305. [DOI] [PubMed] [Google Scholar]
- Travassos LH, Carneiro LA, Ramjeet M, Hussey S, Kim YG, Magalhaes JG, Yuan L, Soares F, Chea E, Le Bourhis L, Boneca IG, Allaoui A, Jones NL, Nunez G, Girardin SE, Philpott DJ. Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat Immunol. 2010;11(1):55–62. doi: 10.1038/ni.1823. [DOI] [PubMed] [Google Scholar]
- Vellai T. Autophagy genes and ageing. Cell Death Differ. 2009;16(1):94–102. doi: 10.1038/cdd.2008.126. [DOI] [PubMed] [Google Scholar]
- Verway M, Behr MA, White JH. Vitamin D, NOD2, autophagy and Crohn’s disease. Expert Rev Clin Immunol. 2010;6(4):505–508. doi: 10.1586/eci.10.31. [DOI] [PubMed] [Google Scholar]
- Wagner N, Wagner KD, Schley G, Badiali L, Theres H, Scholz H. 1,25-dihydroxyvitamin D3-induced apoptosis of retinoblastoma cells is associated with reciprocal changes of Bcl-2 and bax. Exp Eye Res. 2003;77(1):1–9. doi: 10.1016/s0014-4835(03)00108-8. [DOI] [PubMed] [Google Scholar]
- Wang J, Lian H, Zhao Y, Kauss MA, Spindel S. Vitamin D3 induces autophagy of human myeloid leukemia cells. J Biol Chem. 2008;283(37):25596–25605. doi: 10.1074/jbc.M801716200. [DOI] [PubMed] [Google Scholar]
- Wang TT, Dabbas B, Laperriere D, Bitton AJ, Soualhine H, Tavera-Mendoza LE, Dionne S, Servant MJ, Bitton A, Seidman EG, Mader S, Behr MA, White JH. Direct and indirect induction by 1,25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin beta2 innate immune pathway defective in Crohn disease. J Biol Chem. 2010;285(4):2227–2231. doi: 10.1074/jbc.C109.071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, Tavera-Mendoza L, Lin R, Hanrahan JW, Mader S, White JH. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173(5):2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- Wang TT, Tavera-Mendoza LE, Laperriere D, Libby E, Macleod NB, Nagai Y, Bourdeau V, Konstorum A, Lallemant B, Zhang R, Mader S, White JH. Large-scale in silico and microarray-based identification of direct 1,25-dihydroxyvitamin D3 target genes. Mol Endocrinol. 2005;19(11):2685–2695. doi: 10.1210/me.2005-0106. [DOI] [PubMed] [Google Scholar]
- Wehkamp J, Harder J, Weichenthal M, Schwab M, Schaffeler E, Schlee M, Herrlinger KR, Stallmach A, Noack F, Fritz P, Schroder JM, Bevins CL, Fellermann K, Stange EF. NOD2 (CARD15) mutations in Crohn’s disease are associated with diminished mucosal alpha-defensin expression. Gut. 2004;53(11):1658–1664. doi: 10.1136/gut.2003.032805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss U. Inflammation. Nature. 2008;454(7203):427. doi: 10.1038/454427a. [DOI] [PubMed] [Google Scholar]
- White E, Karp C, Strohecker AM, Guo Y, Mathew R. Role of autophagy in suppression of inflammation and cancer. Curr Opin Cell Biol. 2010;22(2):212–217. doi: 10.1016/j.ceb.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JH. Vitamin D signaling, infectious diseases, and regulation of innate immunity. Infect Immun. 2008;76(9):3837–3843. doi: 10.1128/IAI.00353-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Liao AP, Xia Y, Li YC, Li JD, Sartor RB, Sun J. Vitamin D receptor negatively regulates bacterial-stimulated NF-kappaB activity in intestine. Am J Pathol. 2010a;177(2):686–697. doi: 10.2353/ajpath.2010.090998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Xia Y, Liu X, Sun J. Vitamin D receptor deletion leads to reduced level of IkappaBalpha protein through protein translation, protein-protein interaction, and post-translational modification. Int J Biochem Cell Biol. 2010b;42(2):329–336. doi: 10.1016/j.biocel.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HM, Tepper CG, Jones JB, Fernandez CE, Studzinski GP. 1,25-Dihydroxyvitamin D3 protects HL60 cells against apoptosis but down-regulates the expression of the bcl-2 gene. Exp Cell Res. 1993;209(2):367–374. doi: 10.1006/excr.1993.1322. [DOI] [PubMed] [Google Scholar]
- Yuk JM, Shin DM, Lee HM, Yang CS, Jin HS, Kim KK, Lee ZW, Lee SH, Kim JM, Jo EK. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe. 2009;6(3):231–243. doi: 10.1016/j.chom.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Zanuzzi CN, Fontana PA, Barbeito CG, Portiansky EL, Gimeno EJ. Paneth cells: histochemical and morphometric study in control and Solanum glaucophyllum intoxicated rabbits. Eur J Histochem. 2008;52(2):93–100. doi: 10.4081/1193. [DOI] [PubMed] [Google Scholar]
- Zhang XD, Wang Y, Wu JC, Lin F, Han R, Han F, Fukunaga K, Qin ZH. Down-regulation of Bcl-2 enhances autophagy activation and cell death induced by mitochondrial dysfunction in rat striatum. J Neurosci Res. 2009;87(16):3600–3610. doi: 10.1002/jnr.22152. [DOI] [PubMed] [Google Scholar]