Summary
Background
RNA interference (RNAi) is a powerful tool for suppressing gene function. The tetracycline (tet)-regulated expression system has recently been adapted to allow inducible RNAi in mice, however its efficiency in a particular cell type in vivo depends on a transgenic tet transactivator expression pattern and is often highly variable.
Objective
We aimed to establish a transgenic strategy that allows efficient and inducible gene knockdown in particular hematopoietic lineages in mice.
Methods and results
Using a tet-regulated reporter gene strategy, we found that transgenic mice expressing the rtTA (tet-on) transactivator under control of the cytomegalovirus (CMV) promoter (CMV-rtTA mice) display inducible reporter gene expression with unusual and near-complete efficiency in megakaryocytes and platelets. To test whether the CMV-rtTA transgene can drive inducible and efficient gene knockdown within this lineage, we generated a novel mouse strain harboring a tet-regulated short hairpin RNA (shRNA) targeting Bcl-xL, a pro-survival Bcl-2 family member known to be essential for maintaining platelet survival. Doxycycline treatment of adult mice carrying both transgenes induces shRNA expression, depletes Bcl-xL in megakaryocytes and triggers severe thrombocytopenia, whereas doxycycline withdrawal shuts off shRNA expression, normalizes Bcl-xL levels and restores platelet numbers. These effects are akin to those observed with drugs that target Bcl-xL, clearly demonstrating that this transgenic system allows efficient and inducible inhibition of genes in megakaryocytes and platelets.
Conclusions
We have established a novel transgenic strategy for inducible gene knockdown inmegakaryocytes and platelets that will be useful for characterizing genes involved in platelet production and function in adult mice.
Keywords: megakaryocyte, mouse, platelet, RNA interference, tetracycline
Introduction
Genetically modified mice are important tools for characterizing mammalian gene function in vivo, and conditional knockout strategies based on the Cre/lox system allow deletion of genes in specific cell types. Hundreds of transgenic mouse strains expressing Cre recombinase from various general or tissue-specific promoters are available, including a recently developed Pf4-Cre mouse strain that restricts gene knockout to the megakaryocyte/platelet lineage [1]. The tetracycline (tet)- regulated expression system is an alternative method for studying gene function inmice, providing the additional benefit of temporal regulation. Originally developed for inducible gene overexpression [2–4], the tet-regulated system has recently been adapted for inducible gene knockdown in cultured cells and mice via expression of microRNA-based short hairpin RNAs (shRNAs) [5,6]. Effective tet-regulated overexpression or knockdown in mice relies on mouse strains that express the rtTA (tet-on; activated by doxycycline) or tTA (tet-off; repressed by doxycycline) transactivators under the control of general or tissue-specific promoters. Many transactivator mouse strains are available, but none have been shown to effectively drive gene knockdown in the megakarocyte lineage. In the present study, we have established a genetic strategy for inducible gene knockdown in megakaryocytes and platelets in adult mice.
Materials and methods
Transgenic mice
The TRE-GFP-shBcl-xL transgene was targeted to the type I collagen (Col1a1) locus in embryonic stem (ES) cells using a highly efficient, FLP-mediated recombination strategy developed by the Jaenisch laboratory for gene overexpression [7] and adapted by the Lowe laboratory for shRNA expression (P.K. Premsrirut and S. W. Lowe, unpublished data). Briefly, a variant of the pBS31 targeting vector [7] was modified to express green fluorescent protein (GFP) and sequences encoding the Bcl-xL.231 shRNA. This vector and a vector encoding FLP recombinase were co-electroporated into KH2 ES cells, which harbor an frt ‘landing pad’ at the Col1a1 locus [7]. Hygromycin-resistant clones were isolated and targeting was verified by observing doxcycline-induced GFP expression driven by the ROSA26-M2rtTA expressed in KH2 cells. Targeted clones were injected into C57Bl/6 blastocysts and chimeras were crossed to C57Bl/6 female mice. TRE-GFP-shLuc mice were generated using the same approach (P. K. Premsrirut and S. W. Lowe, unpublished data). CMV-rtTA mice were obtained from Harold Varmus [8]. All mouse colonies were maintained by C57Bl/6 backcrossing. TRE-GFP-shRNA transgenes were detected by PCR using forward primers specific for each shRNA (Bcl-xL.231: TGTATTAGATCACTGAACGCTCTCCG; Luc.1309: GTATTAATCAGAGACTTCAGGCGG) and a common reverse primer (GAAAGAACAATCAAGGGTCC) yielding a 210-bp product. The CMV-rtTA transgene was detected using rtTA specific forward (GCTTGGTGTAGAGCAGCCTACAC) and reverse (CAGCGCTGAGTGCATATAACGCG) primers, yielding a 311-bp product. Doxycycline was administered in the diet at 600 mg kg−1 food (Specialty Feeds, Glen Forrest, WA, Australia). All mouse experiments were approved by theWalter and Eliza Hall Institute Animal Ethics Committee.
Cell culture and western blotting
Primary mouse embryonic fibroblasts (MEFs) were isolated from e13.5 embryos and cultured in Dulbecco’s modified Eagle’s medium (DME)/10% fetal calf serum (FBS). Doxycycline (Sigma-Aldrich, St Louis, MO, USA) was added to cultures at 1 μg mL−1. Cell lysates were western blotted with anti-Bcl-xL antibody BD610212 (BD Biosciences, San Jose, CA, USA), anti-GFP antibody A6455 (Invitrogen, Carlsbad, CA, USA) and anti-α-tubulin antibody T5168 (Sigma- Aldrich). To generate megakaryocytes in culture, bone marrow cells were depleted for various lineage antigens (B220, CD2, CD3, CD4, CD5, CD8, CD19, Gr1 and Mac1) and cultured for 4 days in thrombopoietin based on previously described methods [9]. Cells were subjected to albumin gradient separation and megakaryocytes were isolated from the 3% albumin layer.
Flow cytometry and blood analysis
Blood was collected from the retro-orbital plexus and parameters were measured with an Advia 2120 hematological analyzer (Bayer, Leverkusen, Germany). Platelet-rich plasma was prepared by centrifuging blood in phosphate-buffered saline (PBS) at 125 × g for 7 min, and stained with PE-conjugated anti-CD41 antibody BD558040 (BD Biosciences). Single-cell suspensions were prepared from bone marrow, thymus and peripheral blood. After red blood cell lysis, cells were stained with allophycocyanin (APC)-conjugated anti-B220 (BD553092) or anti-CD8 (BD553035), or PE-conjugated anti-CD4 (BD553049) or anti-Mac1 (BD557397). Stained cells were analyzed on a fluorescence-activated cell sorter (BD FACSCalibur; BD Biosciences). Megakaryocyte progenitors (Lin–Sca1–cKit+CD150+CD41+) [10] were identified by first staining bone marrow cells with a cocktail of biotin-conjugated lineage antibodies (anti-B220, -CD4, -CD8, -Gr-1, - Mac-1, and -Ter119), followed by streptavidin-PE-TR (BD 551487), PCP-conjugated anti-cKit (Biolegend 105824), PE-Cy7- conjugated anti-Sca1 (BD 558162), APC-conjugated CD150 (Biolegend 115910) and PE-conjugated anti-CD41. Stained cells were analyzed on a fluorescence-activated cell sorter (BD LSRII).
Results and discussion
The cytomegalovirus (CMV) promoter has a broad expression pattern in transgenic mice [11] and a CMV-rtTA transgenic mouse strain recently generated by the Varmus laboratory has been used to inducibly overexpress genes in various tissues including the liver and lung [8,12]. To characterize rtTA function in the hematopoietic system of these mice, we utilized a reporter mouse strain (P. K. Premsrirut and S. W. Lowe, unpublished data) in whichGFP is under the control of the tet-responsive TRE promoter (Fig. 1A). This reporter cassette is targeted to the type I collagen (Col1a1) genomic locus, shown previously to facilitate tet-regulated GFP expression in a variety of tissues [7]. In addition to GFP, the TRE transcript of this reporter mouse encodes a microRNA-based short hairpin RNA (shRNA) targeting firefly luciferase (Luc.1309 or shLuc), a control shRNA with no known endogenous target in mammalian cells (Fig. 1A). We crossed CMV-rtTA and TRE-GFP-shLuc mice to produce bi-transgenic mice along with littermate single transgenic and wild-type controls.
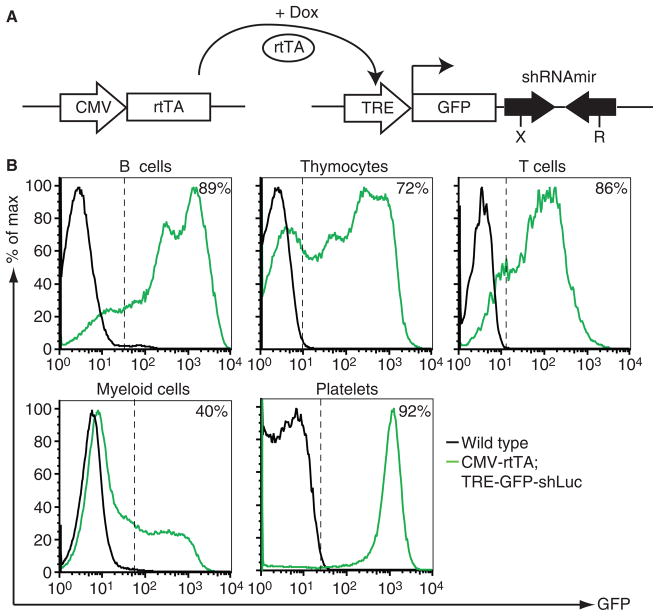
Fig. 1.
CMV-rtTA drives efficient tet-regulated green fluorescent protein (GFP) reporter expression in platelets. (A) Schematic of the CMV-rtTA and TRE-GFP-shRNA transgenes. The rtTA (tet-on) protein transactivates the TRE promoter upon addition of doxycycline (Dox). The TRE promoter transcript encodes GFP and contains microRNA-based shRNA sequences in the 3′ untranslated region. XhoI (X) and EcoRI (R) restriction sites facilitate cloning of sequences encoding unique shRNAs [21]. (B) Comparison of doxycycline-induced GFP expression measured by flow cytometry in different hematopoietic cell types isolated from representative CMV-rtTA; TRE-GFP-shLuc bi-transgenic mice. Percent GFP+ cells in bi-transgenic samples are indicated. B cells: B220+ bone marrow cells. Thymocytes: CD4+CD8+ thymocytes. T cells: CD8+peripheral blood cells. Myeloid cells: Mac1+ bone marrow cells. Platelets: CD41+ peripheral blood platelets.
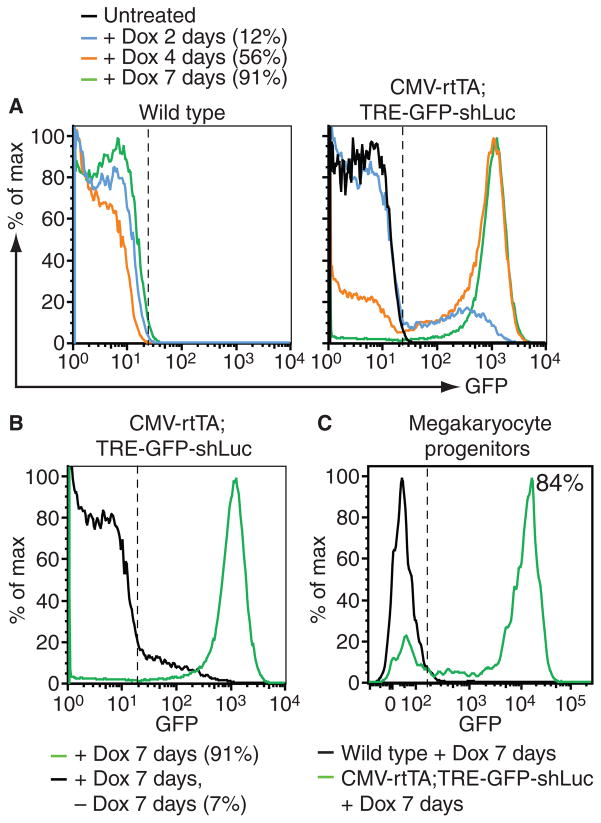
As anticipated, doxycycline treatment of adult CMV-rtTA; TRE-GFP-shLuc bi-transgenic mice induced GFP expression in all hematopoietic cell types examined by flow cytometry. Different lymphoid and myeloid cell sub-populations showed a range of GFP expression (Fig. 1B), suggesting variable TRE promoter activity within and between cell lineages. Interestingly, unlike the heterogeneous expression observed inmost cell types, over 90% of platelets displayed uniformly high GFP expression (Fig. 1B). Time course analysis revealed a low percentage of GFP+ platelets after 2 days of doxycycline treatment, rising to maximal (> 90%) levels after 7 days (Fig. 2A). As expected, GFP expression in platelets was reversed upon doxycycline withdrawal (Fig. 2B). Platelets are anucleate and transcriptionally incompetent, therefore GFP expression in plateletsmust derive from transcripts produced in megakaryocytes. As the lifespan of circulating platelets in mice is approximately 5 days [13], our results suggest that GFP expression in megakaryocytes is rapidly induced upon doxycycline treatment of CMV-rtTA; TRE-GFP-shLuc mice, resulting in production of GFP+ platelets that eventually predominate as older platelets are cleared. Consistent with this, we observed uniformly high GFP expression in over 80% of megakaryocyte progenitors [10] isolated from the bone marrow of doxycycline-treated CMV-rtTA; TRE-GFP-shLuc mice (Fig. 2C).
Fig. 2.
Reversible green fluorescent protein (GFP) reporter expression in the megakaryocyte/platelet lineage. (A) Flow cytometry analysis of GFP expression in CD41+ platelets in peripheral blood of representative wild type (left panel) and CMV-rtTA; TRE-GFP-shLuc bi-transgenic (right panel) mice after doxycycline treatment of indicated durations. Percent GFP+ cells in bi-transgenic samples are indicated. (B) Flow cytometry analysis of GFP expression in peripheral blood platelets of representative CMV-rtTA; TRE-GFP-shLuc mice after 1 week on doxycycline (green) followed by a week off doxycycline (black), showing reversible GFP expression in bi-transgenic platelets. PercentGFP+cells are indicated. (C) Flow cytometry analysis of doxycycline-induced GFP expression in Lin– Sca1–cKit+CD150+CD41+ megakaryocyte precursors isolated from bone marrow of wild type (black) and CMV-rtTA; TRE-GFP-shLuc bi-transgenic (green) mice. Percent GFP+ cells from bi-transgenic mice is indicated.
We have recently shown that microRNA-based shRNAs driven from the TRE promoter can inhibit the expression of endogenous genes in transgenic mice [6]. As GFP expression in CMV-rtTA; TRE-GFP-shLuc mice is transcriptionally linked to shRNA production, our observations suggested that this transgene combination may drive particularly efficient shRNA production in the megakaryocyte/platelet lineage. To test this hypothesis we focused on Bcl-xL (also known as Bcl2l1), a protein required for mouse development [14] and for maintaining platelet survival in adult mice [15].
After screening several microRNA-based Bcl-xL shRNAs by retroviral transduction of mouse fibroblasts [5], we found that a shRNA-designated Bcl-xL.231 (hereafter referred to as shBcl-xL; generously provided by H. Jiang and M. Hemann) effectively suppressed Bcl-xL expression. We cloned sequence encoding Bcl-xL.231 into the targeting vector used to generate TRE-GFP-shLuc mice, replacing shLuc (Fig. 1A). This vector was also targeted to the Col1a1 locus in ES cells, which were then used to generate TRE-GFP-shBcl-xL transgenic mice.
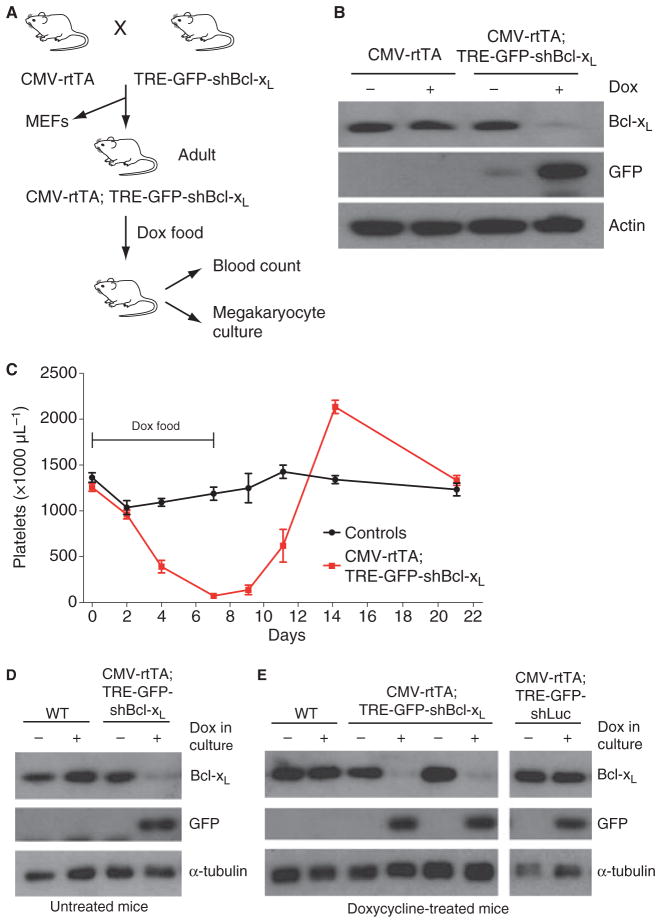
We initially characterized TRE-GFP-shBcl-xL transgene function in primary embryonic fibroblasts (MEFs) derived from a cross between TRE-GFP-shBcl-xL mice and CMV-rtTA transgenic mice (Fig. 3A). Short-term doxycycline treatment resulted in GFP expression and significant Bcl-xL knockdown in bi-transgenic MEFs but not untreated or single transgenic controls (Fig. 3B). Low levels of GFP in untreated bitransgenic MEFs suggests some leaky expression from the TRE promoter in this cell type, however, Bcl-xL levels were unaffected (Fig. 3B). As anticipated, untreated adult CMV-rtTA; TRE-GFP-shBcl-xL bi-transgenic mice were healthy, with a normal peripheral blood cell profile (Table 1). To induce shBcl-xL shRNA expression in bi-transgenics, litters were given doxycycline food for 1 week. This had no obvious effect on health, but caused a modest decrease in peripheral blood lymphocyte counts of bi-transgenic mice (Table 1). Although Bcl-xL promotes erythroid cell survival [16], we did not observe changes in hematocrit after 1 week (Table 1) or several weeks on doxycycline, potentially suggesting ineffective knockdown in this lineage. However, in keeping with the known role of Bcl-xL in maintaining platelet survival [15], 1 week of doxycycline treatment induced a severe thrombocytopenia in bi-transgenic mice, with platelet levels falling to< 10% of those in littermate controls or untreated mice (Fig. 3C, Table 1). Decreased platelet counts were observed after 4 days on doxycycline, and nadir after 7 days (Fig. 3C). Thrombocytopenia was stable over several weeks of doxycycline treatment, but was reversed by doxycycline withdrawal (Fig. 3C).
Fig. 3.
Inducible knockdown of Bcl-xL causes reversible thrombocytopenia. (A) Strategy for analysis of CMV-rtTA; TRE-GFP-shBcl-xL bi-transgenic mice and cells. (B) Western blot showing Bcl-xL expression in MEFs derived from CMV-rtTA; TRE-GFP-shBcl-xL bi-transgenic and CMV-rtTA littermate control embryos. MEFs were treated with 1 μg mL−1 doxycycline for 72 h as indicated, refreshed after 48 h. (C) Peripheral blood platelet counts of CMV-rtTA; TRE-GFP-shBcl-xL bi-transgenic mice (red squares) and littermate controls (pooled single transgenic and wild type; black circles) in response to 7 days of doxycycline administration in the food as indicated. Individual mice were bled between 6 and 14 weeks of age, a maximum of three times, and not more than once per week. n = at least four mice per data point. Error bars represent standard error of the mean. The transient thrombocytosis observed in bi-transgenic mice 1 week after doxycycline withdrawal is a common rebound response to thrombocytopenia [22]. (D, E) Western blot of Bcl-xL expression in megakaryocytes generated by culturing bone marrow from mice of indicated genotypes in thrombopoietin for 4 days, with or without doxycycline treatment in culture. Mice were either untreated (D) or doxycycline treated for one week (E) before bonemarrow harvest. GFP expression indicates TRE promoter activity and shLuc or shBcl-xL production. Samples from two independent CMV-rtTA; TRE-GFP-shBcl-xL bi-transgenic mice are shown in (E).
Table 1.
Peripheral blood counts
| Untreated
|
Doxycycline 7 days
|
|||
|---|---|---|---|---|
| Littermate controls (n = 12) | CMV-rtTA; TRE-GFP-shBclxL (n = 5) | Littermate controls (n = 24) | CMV-rtTA; TRE-GFP-shBclxL (n = 8) | |
| Erythrocytes (× 106 lL−1) | 11.2 ± 0.1 | 11.5 ± 0.2 | 11.1 ± 0.1 | 10.9 ± 0.2 |
| Hematocrit (%) | 51.9 ± 0.6 | 53.4 ± 0.4 | 52.3 ± 0.5 | 52.2 ± 0.8 |
| MCV (femtoliters) | 46.3 ± 0.5 | 46.5 ± 0.8 | 47.3 ± 0.3 | 48.0 ± 0.3 |
| Leukocytes (× 103 lL−1) | 9.8 ± 0.4 | 9.2 ± 0.8 | 9.7 ± 0.4 | 6.7 ± 0.4* |
| Neutrophils (× 103 lL−1) | 1.3 ± 0.2 | 1.0 ± 0.1 | 1.4 ± 0.2 | 0.9 ± 0.1 |
| Lymphocytes (× 103 lL−1) | 7.9 ± 0.3 | 7.6 ± 0.8 | 7.7 ± 0.3 | 5.2 ± 0.4† |
| Monocytes (× 103 lL−1) | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 |
| Platelets (× 103 lL−1) | 1364 ± 51 | 1258 ± 44 | 1273 ± 63 | 68 ± 16‡ |
| MPV (femtoliters) | 7.4 ± 0.1 | 7.4 ± 0.1 | 7.4 ± 0.1 | 11.3 ± 0.6¶ |
All mice were bled between 6 and 14 weeks of age. Littermate controls include wild type, CMV-rtTA single transgenic and TRE-GFP-shBclxL single transgenic mice.
MCV, mean corpuscular volume; MPV, mean platelet volume.
P < 0.001 compared with treated littermate controls (t-test).
P < 0.001 compared with treated littermate controls (t-test).
P < 10−11 compared with treated littermate controls (t-test).
P < 10−10 compared with treated littermate controls (t-test).
As platelets are anucleate and unable to produce shRNA, our results suggested that inducible Bcl-xL knockdown occurs in megakaryocytes, which then produce Bcl-xL-low platelets. To examine this possibility, we isolated bone marrow cells from adult mice and cultured them in conditions that promote megakaryocyte differentiation [9]. Just 4 days of culture in doxycycline was sufficient to induce Bcl-xL knockdown in CMV-rtTA; TRE-GFP-shBcl-xL megakaryocytes (Fig. 3D). Conversely, when bone marrow from doxycycline pre-treated mice was cultured without doxycycline, Bcl-xL expression was restored in the resulting megakaryocytes (Fig. 3E).
In summary, we have identified a novel transgenic approach for efficient, inducible and reversible knockdown of endogenous genes in megakaryocytes and platelets in vivo. Unlike previously described Pf4-Cre and Pf4-tTA mouse strains, which allow deletion of floxed alleles or overexpression of transgenes specifically in the megakaryocyte lineage [1,17], the transgenic knockdown system described here is not restricted exclusively to megakaryocytes. However, our results reveal particularly effective knockdown in this lineage, presumably at least partly as a result of optimal transactivator levels and TRE promoter accessibility. Indeed, other transactivator mouse strains we have tested show little TRE-GFP-shLuc reporter gene induction in this lineage. In addition, we have avoided the variability of traditional transgenic methods by employing a highly efficient TRE-shRNA targeting strategy, so in principle any gene can be perturbed in vivo using this approach.
Germline or megakaryocyte-specific deletion of genes involved in hemostasis and thrombosis can cause lethality as a result of perinatal bleeding [18,19]. In principle this can be circumvented by inducing gene knockdown in megakaryocytes and platelets in adult mice, a major advantage of the approach outlined here. We observed that inducing Bcl-xL knockdown caused severe thrombocytopenia within 1 week, which was completely reversible upon doxycycline withdrawal. Interestingly, the Bcl-xL/Bcl-2 inhibitor ABT-737 causes an acute, transient thrombocytopenia in mice by inducing platelet apoptosis [15,20]. Although thrombocytopenia developed more slowly in our transgenic model, which relies on shRNA-mediated Bcl-xL knockdown in megakaryocytes, our results demonstrate that transgenic gene knockdown can mimic the effects of targeted therapies on the megakaryocyte/platelet lineage.
Acknowledgments
We thank H. Varmus and F. Cong (Memorial Sloan Kettering Cancer Center) for CMV-rtTA mice; and H. Jiang and M. Hemann (Massachusetts Institute of Technology) for the shBcl-xL.231 shRNA sequence. We thank R. Lane, L. Nicolson, M. Salzone, D. Nguyen, K. Dennemoser, M. Dayton, J. Corbin and WEHI Bioservices staff for excellent technical assistance; and E. Major, W. Alexander and D. Hilton for ES cell and mouse resources. This work was supported by the National Health and Medical Research Council of Australia (Project grants 516725 and 575535; Program grant 461221; IRIIS grant 361646; and Fellowships to D. C. S. Huang, B. T. Kile, and R. A. Dickins), the Leukemia and Lymphoma Society (SCOR 7413-07 and Fellowship to E. C. Josefsson), the European Molecular Biology Organization (Fellowship to C. James), the Leukaemia Foundation of Australia (Fellowship to C. L. Carmichael), the Sylvia and Charles Viertel Charitable Foundation (Fellowship to B. T. Kile), Victorian State Government OIS grants, and the Victorian Endowment for Science, Knowledge and Innovation (VESKI; Fellowship to R. A. Dickins).
Footnotes
Addendum
C. James, E.C. Josefsson, C.L. Carmichael, P.K. Premsrirut, S.W. Lowe, J.R. Hamilton, D.C.S. Huang and B.T. Kile designed and researched and analyzed data. M. Takiguchi and R.A. Dickins designed and performed research, analyzed data and wrote the paper.
Disclosure of Conflicts of Interests
The authors state that they have no conflicts of interest.
References
- 1.Tiedt R, Schomber T, Hao-Shen H, Skoda RC. Pf4-Cre transgenic mice allow the generation of lineage-restricted gene knockouts for studying megakaryocyte and platelet function in vivo. Blood. 2007;109:1503–6. doi: 10.1182/blood-2006-04-020362. [DOI] [PubMed] [Google Scholar]
- 2.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci U S A. 1992;89:5547–51. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furth PA, St Onge L, Boger H, Gruss P, Gossen M, Kistner A, Bujard H, Hennighausen L. Temporal control of gene expression in transgenic mice by a tetracycline-responsive promoter. Proc Natl Acad Sci U S A. 1994;91:9302–6. doi: 10.1073/pnas.91.20.9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kistner A, Gossen M, Zimmermann F, Jerecic J, Ullmer C, Lubbert H, Bujard H. Doxycycline-mediated quantitative and tissue-specific control of gene expression in transgenic mice. Proc Natl Acad Sci U S A. 1996;93:10933–8. doi: 10.1073/pnas.93.20.10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dickins RA, Hemann MT, Zilfou JT, Simpson DR, Ibarra I, Hannon GJ, Lowe SW. Probing tumor phenotypes using stable and regulated synthetic microRNA precursors. Nat Genet. 2005;37:1289–95. doi: 10.1038/ng1651. [DOI] [PubMed] [Google Scholar]
- 6.Dickins RA, McJunkin K, Hernando E, Premsrirut PK, Krizhanovsky V, Burgess DJ, Kim SY, Cordon-Cardo C, Zender L, Hannon GJ, Lowe SW. Tissue-specific and reversible RNA interference in transgenic mice. Nat Genet. 2007;39:914–21. doi: 10.1038/ng2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beard C, Hochedlinger K, Plath K, Wutz A, Jaenisch R. Efficient method to generate single-copy transgenic mice by site-specific integration in embryonic stem cells. Genesis. 2006;44:23–8. doi: 10.1002/gene.20180. [DOI] [PubMed] [Google Scholar]
- 8.Sotillo R, Hernando E, Diaz-Rodriguez E, Teruya-Feldstein J, Cordon-Cardo C, Lowe SW, Benezra R. Mad2 overexpression promotes aneuploidy and tumorigenesis in mice. Cancer Cell. 2007;11:9–23. doi: 10.1016/j.ccr.2006.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baccini V, Roy L, Vitrat N, Chagraoui H, Sabri S, Le Couedic JP, Debili N, Wendling F, Vainchenker W. Role of p21(Cip1/Waf1) in cell-cycle exit of endomitotic megakaryocytes. Blood. 2001;98:3274–82. doi: 10.1182/blood.v98.12.3274. [DOI] [PubMed] [Google Scholar]
- 10.Pronk CJ, Rossi DJ, Mansson R, Attema JL, Norddahl GL, Chan CK, Sigvardsson M, Weissman IL, Bryder D. Elucidation of the phenotypic, functional, andmolecular topography of amyeloerythroid progenitor cell hierarchy. Cell Stem Cell. 2007;1:428–42. doi: 10.1016/j.stem.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt EV, Christoph G, Zeller R, Leder P. The cytomegalovirus enhancer: a pan-active control element in transgenic mice. Mol Cell Biol. 1990;10:4406–11. doi: 10.1128/mcb.10.8.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diaz-Rodriguez E, Sotillo R, Schvartzman JM, Benezra R. Hec1 overexpression hyperactivates the mitotic checkpoint and induces tumor formation in vivo. Proc Natl Acad Sci U S A. 2008;105:16719–24. doi: 10.1073/pnas.0803504105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berger G, Hartwell DW, Wagner DD. P-Selectin and platelet clearance. Blood. 1998;92:4446–52. [PubMed] [Google Scholar]
- 14.Motoyama N, Wang F, Roth KA, Sawa H, Nakayama K, Nakayama K, Negishi I, Senju S, Zhang Q, Fujii S, Loh DY. Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science. 1995;267:1506–10. doi: 10.1126/science.7878471. [DOI] [PubMed] [Google Scholar]
- 15.Mason KD, Carpinelli MR, Fletcher JI, Collinge JE, Hilton AA, Ellis S, Kelly PN, Ekert PG, Metcalf D, Roberts AW, Huang DC, Kile BT. Programmed anuclear cell death delimits platelet life span. Cell. 2007;128:1173–86. doi: 10.1016/j.cell.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 16.Wagner KU, Claudio E, Rucker EB, 3rd, Riedlinger G, Broussard C, Schwartzberg PL, Siebenlist U, Hennighausen L. Conditional deletion of the Bcl-x gene from erythroid cells results in hemolytic anemia and profound splenomegaly. Development. 2000;127:4949–58. doi: 10.1242/dev.127.22.4949. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen HG, Yu G, Makitalo M, Yang D, Xie HX, Jones MR, Ravid K. Conditional overexpression of transgenes in megakaryocytes and platelets in vivo. Blood. 2005;106:1559–64. doi: 10.1182/blood-2005-02-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clements JL, Lee JR, Gross B, Yang B, Olson JD, Sandra A, Watson SP, Lentz SR, Koretzky GA. Fetal hemorrhage and platelet dysfunction in SLP-76-deficient mice. J Clin Invest. 1999;103:19–25. doi: 10.1172/JCI5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petrich BG, Marchese P, Ruggeri ZM, Spiess S, Weichert RA, Ye F, Tiedt R, Skoda RC, Monkley SJ, Critchley DR, Ginsberg MH. Talin is required for integrin-mediated platelet function in hemostasis and thrombosis. J Exp Med. 2007;204:3103–11. doi: 10.1084/jem.20071800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, Joseph MK, Kitada S, Korsmeyer SJ, Kunzer AR, Letai A, Li C, Mitten MJ, Nettesheim DG, Ng S, Nimmer PM, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–81. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 21.Paddison PJ, Cleary M, Silva JM, Chang K, Sheth N, Sachidanandam R, Hannon GJ. Cloning of short hairpin RNAs for gene knockdown in mammalian cells. Nat Methods. 2004;1:163–7. doi: 10.1038/nmeth1104-163. [DOI] [PubMed] [Google Scholar]
- 22.Chenaille PJ, Steward SA, Ashmun RA, Jackson CW. Prolonged thrombocytosis in mice after 5-fluorouracil results from failure to down-regulate megakaryocyte concentration. An experimental model that dissociates regulation of megakaryocyte size and DNA content from megakaryocyte concentration. Blood. 1990;76:508–15. [PubMed] [Google Scholar]