Abstract
Transition metals such as iron, zinc, copper and manganese are essential for the growth and development of organisms ranging from bacteria to mammals. Numerous studies have focused on the impact of iron availability during bacterial and fungal infections, and increasing evidence suggests that copper is also involved in microbial pathogenesis. Not only is copper an essential co-factor for specific microbial enzymes, but several recent studies also strongly suggest that copper is used to restrict pathogen growth in vivo. Here, we review evidence that animals use copper as an anti-microbial weapon and, in turn, microbes have developed mechanisms to counteract the toxic effects of copper.
Introduction
The mammalian host provides an attractive niche for microbes, as it provides a rich supply of nutrients and biochemical co-factors including metals. Almost all fungi and bacteria require iron (Fe) for growth, and the acquisition of this metal has been extensively studied as an important step in microbial pathogenesis. Fe is the most abundant transition element in humans (3 to 5 g in an average person) but is mostly in a highly inaccessible, hemoglobin-bound form. Serum concentrations of Fe and zinc (Zn) can decrease upon the onset of acute bacterial and parasitic infections (Prentice et al., 2007), and Zn is also found in reduced amounts in tissue abscesses caused by Staphylococcus aureus (Corbin et al., 2008). Host deprivation of metals such as Fe, manganese, and Zn, or “nutritional immunity”, is a critical mechanism used by the host to control the growth of potentially pathogenic organisms (Weinberg, 1975). Perhaps not surprisingly, increased Fe availability is often correlated with aggravated infections (Doherty, 2007; Weinberg, 2009) and pathogens have developed numerous mechanisms to extract essential metals from its host (Kehl-Fie and Skaar, 2010; Schaible and Kaufmann, 2004).
Copper (Cu) is a critical component of proteins involved in a variety of cellular processes. As a redox-active metal ion, Cu exists in the reduced ["Cu(I)" or "Cu+"] or oxidized state ["Cu(II)" or "Cu2+"], thereby providing a rich chemical environment for diverse biological ligands that are partners for its many structural and catalytic roles. Enzymes and proteins such as Cu, Zn superoxide dismutase, cytochrome oxidase, methane mono-oxidase, dopamine β-hydroxylase and the ethylene receptor all bind Cu as an essential ligand for their activity. Computational genome analysis for proteins with potential Cu-binding domains estimates bacterial proteomes are ~0.3% cuproproteins (Andreini et al., 2008). Furthermore, analysis of 450 bacterial genomes found 72% encode at least one putative Cu-dependent protein (Ridge et al., 2008).
Despite the critical role of Cu in a wide array of biological processes, too much Cu is toxic. The antimicrobial benefits of Cu have been known for thousands of years and Cu has been used in healthcare and agriculture by many cultures. One of the earliest testimonies of Cu dates as far back as 2400 B.C. in an ancient Egyptian medical text known as the Smith Papyrus, where Cu was reported for its water and wound sterilization properties (Dollwet, 1985). The benefits of Cu to human health were also reported during the cholera epidemics in Paris in the 1800’s, when Cu workers were found to be less susceptible to the disease (Burq, 1867). Perhaps one of the most important developments in agricultural Cu history came in the 1880s with the creation of the Bordeaux mixture by Pierre-Marie-Alexis Millardet. Cu sulfate and lime mixtures were sprayed onto grape vines to keep them mildew-free. Thereafter, this mixture proved useful in preventing fungal infections in other plants, controlling the growth of algae in water reservoirs and on timber, as well as preserving fabric (Borkow and Gabbay, 2005).
Today, Cu continues to be used for its antimicrobial properties in plumbing (Borkow and Gabbay, 2005), and trials are underway to determine if Cu-containing surfaces can significantly reduce nosocomial infections (Casey et al., 2010; Marais et al., 2010; Mikolay et al., 2010). In 2008, the U.S. Environmental Protection Agency officially registered Cu alloys as antimicrobials (EPA, 2008).
Copper and infection
Cu is an essential cofactor for a wide variety of enzymes that are critical for cell growth, differentiation and survival in organisms from bacteria to plants to mammals. Moreover, the redox chemistry of Cu that provides biochemical catalytic power also underlies its potential as a cellular toxin. Consequently, the use by metazoan hosts of Cu as an antimicrobial agent may account, in part, for its essentiality to the host. As discussed earlier, although the antimicrobial benefits of Cu ex vivo are well established it has only recently become apparent that Cu may represent an important innate immune defense in mammals. The notion that Cu plays an important role in controlling infections may not be so surprising since dietary Cu deficiency in farm animals has been associated with increased susceptibility to bacterial infection and patients who have Menke's disease, a lethal Cu deficiency disorder, are highly prone to microbial infection (Agertt et al., 2007; Gunn et al., 1984; Kreuder et al., 1993; Uno and Arya, 1987).
Macrophages and the use of Cu as an anti-microbial weapon
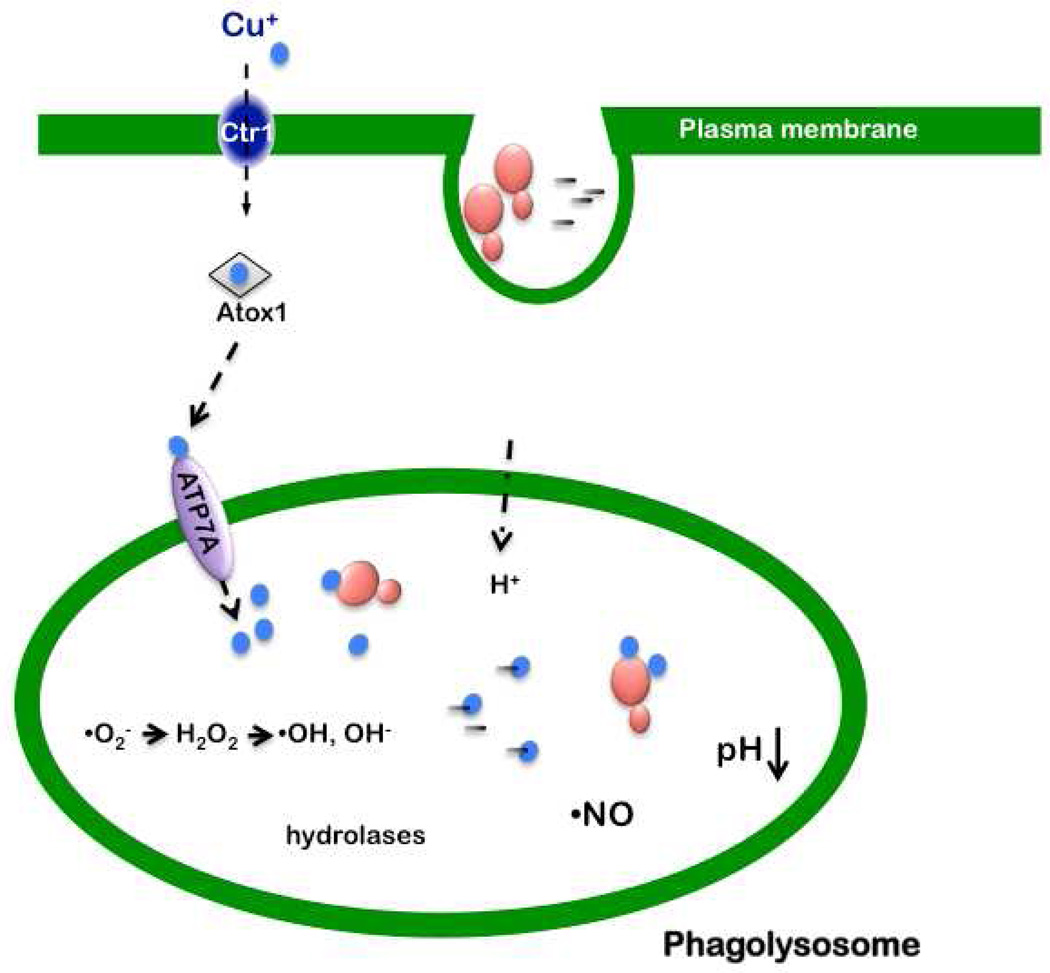
Phagocytes such as macrophages represent one of the first lines of defense against invading microbial pathogens. The uptake of microbes results in a cascade of events including the production of microbiocidal compounds and the secretion of pro-inflammatory mediators by the phagocyte. Within the lumen of the phagolysosome microbes are presented with a host of insults including an acidic pH, the generation of nitric oxide (•NO), a bevy of hydrolytic enzymes, antimicrobial peptides, and reactive oxygen species such as superoxide anion (•O2−) [reviewed in (Nathan and Shiloh, 2000; Shiloh and Nathan, 2000)] (Fig. 1).
Fig. 1. Model for the trafficking of Cu to the macrophage phagolysosome for microbiocidal activity.
Shown are yeast (red) and bacterial cells (black) phagocytosed into a macrophage phagosome, where the hostile environment for microbial survival includes low Fe, low pH, the presence of hydrolases, and the generation of nitric oxide (•NO) and superoxide anion (•O2−). In activated macrophages there is a stimulation of Ctr1 and ATP7A expression and increased levels of the Ctr1 Cu+ importer on the plasma membrane and the ATP7A Cu+ pump on the phagolysosomal membrane. Atox1 is a cytosolic Cu chaperone that carries Cu+ (blue) to the cytosolic Cu binding domains of ATP7A. It is currently unclear whether lumenal Cu is microbiocidal due to its generation of hydroxyl radical via Fenton chemistry, its inherent toxicity to microorganisms via disruption of Fe-S clusters, or a combination of both mechanisms.
More recently, several reports provide compelling evidence that Cu also plays a role in antimicrobial activity. X-ray microprobe analysis demonstrated that, while some elements exhibit a decreased abundance in the phagosome of interferon-γ (IFN-γ) treated macrophages, Cu levels increase dramatically in the presence of mycobacteria (Wagner et al., 2005). The increased levels of phagosome-associated Cu may be due to the IFN-γ-dependent elevation of the steady state levels of the plasma membrane Ctr1 high affinity Cu+ importer and ATP7A, a P-type ATPase Cu+ pump (White et al., 2009). P-type ATPases are cytoplasmic membrane proteins found in both prokaryotes and eukaryotes and are frequently associated with metal ion transport [reviewed in (Palmgren and Nissen, 2011)]. ATP7A is normally localized to the trans-Golgi network where it delivers Cu+ to the lumen for loading onto Cu+-dependent secreted enzymes [reviewed in (Kim et al., 2008))] (Fig 1). Under conditions of either IFN-γ or LPS activation, a population of ATP7A, the protein associated with Menke’s disease, localizes to the phagosomal membrane. This localization can be abrogated with the potent intracellular Cu+ chelator tetrathiomolybdate, suggesting that ATP7A-phagosome co-localization occurs in response to the influx of Cu driven by elevated levels of Ctr1. RNAi depletion of ATP7A in RAW264.7 cells reduces microbiocidal activity against E. coli consistent with the notion that ATP7A pumps Cu+ into the phagosome to help control bacterial growth (White et al., 2009). Interestingly, another study showed that the introduction of bacteria into a sterile mouse intestine results in a three-fold elevation of mRNA encoding the Ctr1 Cu+ importer, with a concomitant five-fold reduction in metallothionein (MT) transcript levels in host intestinal epithelial cells (Hooper et al., 2001). MTs are low molecular weight, cysteine-rich proteins that bind metals, and protect against metal-mediated toxicity. Collectively, these data suggest that, among other functions, this host response could stimulate the accumulation of bio-available Cu to ward off invading microbes.
How does copper kill?
In phagocytic cells, hydrogen peroxide (H2O2) is generated by the disproportionation of •O2− produced by a membrane bound NADPH oxidase. As redox-active metals, Cu and Fe engage in chemical reactions with H2O2 that lead to the generation of the highly toxic hydroxyl radical (•OH) and hydroxyl anion (OH−):
These radicals can damage lipids, nucleic acids, and proteins, leading to cell death (Halliwell and Gutteridge, 1985). Consistent with this hypothesis, the bacteriocidal activity of Cu added to a macrophage-like cell line (RAW264.7) is enhanced by H2O2 (White et al., 2009). However, other studies suggest alternative mechanisms of Cu toxicity. Imlay and co-workers first observed that Cu toxicity in E. coli does not involve oxidative DNA damage, even in the presence of exogenously added H2O2 (Macomber et al., 2007). Curiously, they also showed that Cu could suppress Fe-mediated oxidative damage to DNA in E. coli for reasons that are unclear. A follow up study proposed an alternative mechanism of Cu toxicity to bacteria. Macomber and Imlay observed that Cu blocks branched-chain amino acids synthesis and hypothesized that dehydratases involved in branched-chain amino acids biosynthetic pathways are the primary targets of Cu, which could displace Fe atoms from dehydratase Fe-S clusters. Supporting this hypothesis, it was demonstrated that the addition of branched-chain amino acids to E. coli cultures restores bacterial growth impeded by Cu treatment (Macomber and Imlay, 2009). The observation that silver (Ag) has potent and clinically useful antimicrobial activity is also interesting in this regard. Ag is a redox inert metal that is electronically similar to Cu+, but not Cu2+, and its thiophilic nature would also predict an ability to displace Fe from Fe-S clusters. This suggests that Ag is antimicrobial due to its ability to inactivate Fe-S clusters, rather than via redox cycling.
McEwan and colleagues confirmed that the supplementation of Cu-treated E. coli cultures with branched-chain amino acids rescues bacterial survival; however, the addition of exogenous amino acids cannot rescue a Salmonella multi-copper oxidase mutant from Cu-mediated toxicity (Achard et al., 2010). Therefore the targets for Cu in this Salmonella strain are unclear. Taken together, the precise mechanism for how Cu (and other metals) kills microorganisms is likely to be multi-factorial and may vary depending on the microbe and is clearly in need of further investigation.
Mechanisms for copper regulation in pathogenic bacteria
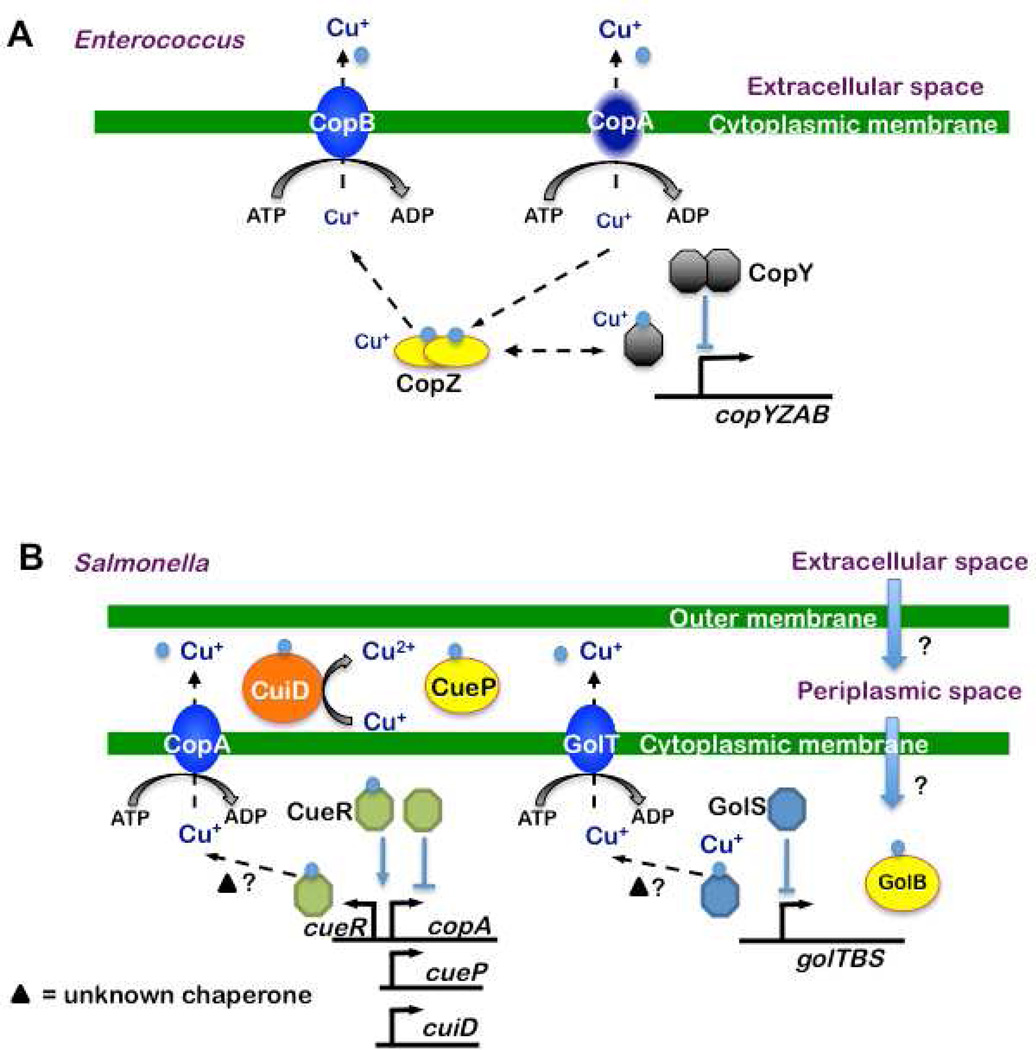
Bacteria tightly regulate cytoplasmic Cu concentrations in order to minimize toxicity while ensuring an adequate supply for cuproproteins. First, bacteria in general encode very few Cu-dependent enzymes. Second, bacterial cuproproteins tend to be periplasmic or extracellular, rather than cytoplasmic. In the event Cu levels become too high, bacteria have also developed mechanisms to alleviate Cu-induced stress. Some of the initial observations that suggested bacteria encode Cu resistance systems came from studies of the Gram-positive bacterium Enterococcus hirae (E. hirae). E. hirae is highly resistant to Cu due to the presence of a four gene operon, copYZAB (Odermatt et al., 1992) (Fig. 2A). CopA and CopB are P-type ATPases, similar to the ATP7A and ATP7B proteins in humans, which undergo conformational changes to drive Cu+ ion transport across membranes [reviewed in (Palmgren and Nissen, 2011)]. In E. hirae, CopA was believed to be a Cu importer, while CopB functions as a Cu exporter. The idea that CopA is a Cu importer was based on observations that copA mutants do not grow well in Cu-limiting media (Solioz and Stoyanov, 2003). However, recent data from Arguello and colleagues demonstrate that CopA is a Cu exporter (Raimunda et al., 2011). Thus it remains to be determined why a copA mutant grows poorly under Cu-limiting conditions.
Fig. 2. Cu homeostasis pathway models in Gram-positive and -negative bacterial pathogens.
(A) Gram-positive bacteria, based on E. hirae. It is believed Cu (blue) enters the cell via CopA. CopZ, a Cu chaperone, forms dimers that bind two Cu+ atoms and transfers them to CopY or other proteins. Consequently, a dimer of CopY bound to four Cu+ detaches from the copA promoter and derepresses expression of copYZAB operon, allowing resistance to Cu toxicity. (B) Gram-negative bacteria, based on Salmonella. Left: Cue system. Cu enters the periplasmic space, most likely through porins in the outer membrane, and crosses the inner membrane into the cytoplasm by an unknown mechanism. Within the cytoplasm, the Cue system responds to Cu: CueR changes its conformation upon Cu+ binding, resulting in the expression of cueP, copA, cuiD and cueR. CopA exports Cu+ to the periplasmic space and CuiD oxidizes Cu+ to Cu2+. Maximum induction of copA and cueO genes upon Cu exposure is detected within 2–3 minutes (Thieme et al., 2008). CueR is extremely sensitive to Cu+ (10−21 M) (Changela et al., 2003) and therefore appears to be the primary Cu sensor in these bacteria. Right: Gol system. When Cu is in the inner space, GolS is thought to bind Cu+, resulting in derepression of the gol operon. GolT is proposed to export Cu+ to the perisplasm. CueP and GolB are proposed to be perisplasmic and cytoplasmic, respectively, Cu chaperones.
In addition to the P-type Cu-transporting ATPases, the E. hirae cop operon encodes the Cu chaperone CopZ and the transcriptional regulator CopY (Fig. 2A). CopZ binds two Cu+ atoms in a solvent accessible manner, presumably to facilitate their transfer to CopY or other proteins. Under low Cu conditions, Zn2+ binds CopY, which represses expression from the copA promoter. In the presence of Cu+, Zn2+ is replaced with Cu and CopY releases from the copA operator allowing the expression of the copYZAB operon and resistance to Cu toxicity [reviewed in (Solioz and Stoyanov, 2003)]. Although E. hirae Cu resistance has not been the subject of pathogenesis studies, the identification of P-type ATPase transporters, a Cu+ chaperone and a transcription factor provided a model Cu-resistance paradigm for studies in other bacteria.
For a general overview on bacterial Cu homeostasis we refer readers to Osman and Cavet (Osman and Cavet, 2008). We apologize to authors whose work could not be covered in this review due to space limitations. Here, we focus on the Cu resistance mechanisms that have been implicated in bacterial pathogenesis.
Cue (Cu efflux) systems
Salmonella and E. coli
The Gram-negative Enterobacteriaceae Eschericia coli (E. coli) and Salmonella enterica sv. Typhimurium (Stm) encode highly similar Cu responsive regulons known as the Cue system. Stm is a common cause of gastroenteritis after the consumption of contaminated food or water. The Cue regulon includes CopA, a P-type ATPase Cu+ efflux protein; a periplasmic multi-copper oxidase (CueO in E. coli; CuiD in Stm); and CueR, a DNA binding protein that activates transcription from the copA and cueO/cuiD promoters in the presence of Cu (Fig. 2B) (Achard et al., 2010; Espariz et al., 2007; Kim et al., 2002; Lim et al., 2002; Osman et al., 2010; Outten et al., 2000; Petersen and Moller, 2000; Rensing et al., 2000; Stoyanov et al., 2001). Unlike some of the other Cu responsive regulators to be discussed in this review, CueR, a MerR-family protein [reviewed in (Brown et al., 2003)], does not dissociate from DNA to allow gene expression but rather changes in conformation to either activate (with Cu+) or repress (no Cu+) transcription. In E. coli CueR binds one equivalent of Cu+ to activate gene expression (Chen et al., 2003).
Mutations in the Cu exporter gene copA sensitize Stm and E. coli to Cu (Espariz et al., 2007; Outten et al., 2001). However, an Stm copA mutant has a relatively mild Cu sensitive phenotype compared to an E. coli copA mutant, suggesting Stm has compensatory Cu detoxification systems (Osman et al., 2010). Cavet and colleagues noticed that Stm harbors a cluster of genes previously associated with gold resistance called golTBS that is not present in E. coli (Osman et al., 2010). GolT is a likely P-type ATPase, GolS is a distant CueR homologue, and GolB is a predicted cytoplasmic metal chaperone (Checa et al., 2007) (Fig. 2B). The gol operon is Cu-inducible, yet deletion of golT alone does not sensitize Stm to Cu under conditions tested so far (Checa et al., 2007). However, a highly Cu-sensitive phenotype is observed when both golT and copA (ΔgolTΔcopA) are deleted and the bacteria are grown in minimal media prior to Cu treatment (Osman et al., 2010). Thus, it appears CopA and GolT work together to extrude Cu from the bacterial cytoplasm under these conditions. Despite this encouraging result, the ΔgolTΔcopA strain has a subtle growth defect in RAW264.7 macrophages and no attenuation is detected in orally infected C57BL/6 mice (Osman et al., 2010).
In contrast to the ΔgolTΔcopA mutant, an Stm strain deficient in the Cu oxidase CuiD is not only sensitive to Cu in vitro (Espariz et al., 2007) but is also attenuated in a mouse model of infection (Achard et al., 2010). It is notable that the difference in results between the ΔgolTΔcopA and cuiD studies may be due to the bacterial doses used in these experiments [109 ΔgolTΔcopA versus 107 colony forming units of ΔcuiD] (Achard et al., 2010; Osman et al., 2010). It may be necessary to perform 50% lethal dose (LD50) or competition experiments to detect a phenotype associated with these mutants in vivo.
Unlike what is observed in Stm, disruption of cueO (cuiD) does not attenuate uropathogenic E. coli (UPEC) in a systemic infection model, despite increasing bacterial sensitivity to Cu in vitro (Tree et al., 2008). However, a systemic mouse model may not best represent a natural UPEC infection, which normally occurs in the urinary tracts of humans. Thus it remains possible that host factors such as Cu play an important role in urinary tract infections.
In Stm, CueR also regulates the expression of a gene encoding a second periplasmic Cu binding protein called CueP (STM3650), the function of which is unknown (Osman et al., 2010; Pontel and Soncini, 2009). Like CuiD/CueO, CueP is a periplasmic protein in Stm that confers Cu resistance under anaerobic conditions by an undetermined mechanism (Osman et al., 2010; Pontel and Soncini, 2009). Presumably CueP binds Cu to shield it from other proteins during anaerobiosis. Thus far, cueP is only found in bacteria that do not have the Cus (Cu sensitive) Cu-responsive system found in E. coli K-12 strains, an observation that led Pontel and Soncini to speculate that CueP functionally substitutes for the cus system for periplasmic Cu-resistance (Pontel and Soncini, 2009). Intriguingly, other enteropathogenic bacteria encode CueP-like proteins, perhaps suggesting that CueP is involved in combating gut-associated Cu (Hooper et al., 2001). It remains to be determined if cueP in any bacterial species is important in an animal infection model.
Pseudomonas
Pseudomonas aeruginosa (Pa) is an opportunistic animal and plant pathogen that flourishes in hospitals and is among the most common nosocomial infections in the United States and is particularly problematic in patients with the pulmonary disease cystic fibrosis (Driscoll et al., 2007). Pa is well known for its antibiotic resistance and is also highly resistant to Cu (Deredjian et al., 2011; Driscoll et al., 2007; Kerr and Snelling, 2009). Pa harbors cueAR, which encodes a P-type ATPase (known as CueA or CopA1 in Pa) and the CueR repressor (Thaden et al., 2010). As predicted, a cueA/copA1 mutant is hypersensitive to Cu in vitro (Schwan et al., 2005; Teitzel et al., 2006). Folger and colleagues speculated that perhaps this Cu-resistance system was important not only during environmental exposure to Cu, but also during infections. Indeed, a cueA mutant is highly attenuated in a systemic murine infection model (Schwan et al., 2005). The cueA/copA1 mutant has an LD50 50 times higher than that of wild type Pa. Furthermore, in vivo competition analysis between cueA/copA1 and wild type strains showed a 20-fold reduction of mutant bacteria in spleens after 24 hours (Schwan et al., 2005), supporting the hypothesis that Pa must resist Cu in a mammalian host.
CsoR (copper sensitive operon) systems
Mycobacterium tuberculosis
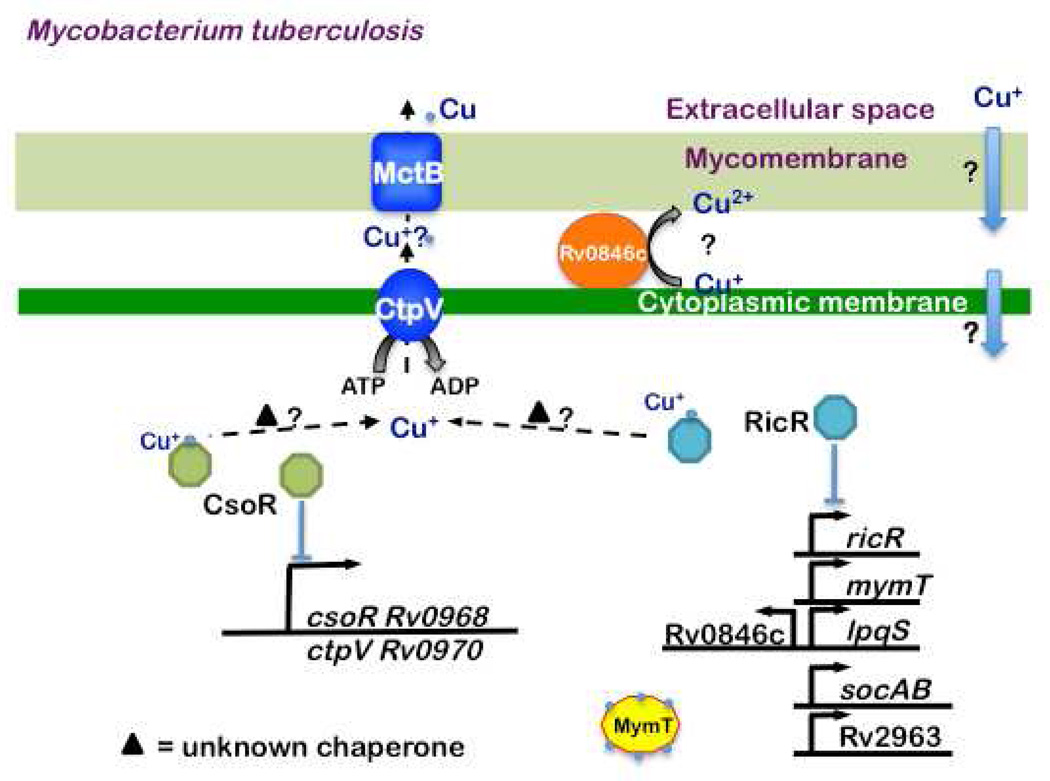
The causative agent of the pulmonary disease tuberculosis is Mycobacterium tuberculosis (Mtb), a bacterial pathogen that is only naturally found in humans. The first clue for the presence of Cu during tuberculosis infections was provided by the transcriptional analysis of the virulent Mtb strain H37Rv. The comparison of Mtb grown in BALB/c mice versus broth revealed a locus that was termed the in vivo expressed genomic island (iVEGI) (Talaat et al., 2004). Among the iVEGI genes are ctpV (cation transport protein V), which encodes a presumed P-type ATPase metal transporter, and Rv0967, a putative transcriptional regulator. Giedroc and co-workers determined that Rv0967 is a Cu sensing regulator that binds to DNA as a dimer of dimers in the absence of Cu+ (Liu et al., 2007). Binding of Cu+ results in the derepression of transcription of the Rv0967-ctpV operon (Fig. 3). Based on these results Rv0967 was named CsoR for Cu sensitive operon repressor. CsoR is the founding member of a large family of regulators found in numerous bacterial species.
Fig. 3. Cu homeostasis in M. tuberculosis.
There are currently three known Cu responsive pathways in Mtb. The RicR regulon is unique to pathogenic mycobacteria while MctB and CtpV are found in both pathogenic and non-pathogenic species. Interestingly, CsoR is less well conserved than RicR among bacterial species. Both CsoR and RicR dissociate from DNA upon Cu (light blue circles) binding. Rv0846c is a putative multi-copper oxidase. LpqS and Rv2963 are predicted to encode a lipoprotein and permease, respectively, but their roles, if any, in Cu homeostasis are unknown. MymT is a Cu+ MT that binds up to six Cu+ and protects against Cu toxicity. A Cu chaperone has not yet been identified in Mycobacteria.
Based on these studies, Talaat and colleagues hypothesized that Mtb encountered Cu in the host, an idea also supported by a study showing Cu concentrations transiently increase in peritoneal macrophages infected with Mtb (Wagner et al., 2005). A microarray analysis of Mtb exposed to various levels of Cu sulfate demonstrated that 30 genes are differentially expressed in response to Cu in vitro, including ctpV and csoR (Ward et al., 2008). Three other genes, annotated to encode putative metal responsive regulators (furA/Rv1909c, Rv1994, and Rv2642), were also identified. Because csoR was the most strongly Cu-induced regulator, it seemed likely that disruption of CsoR-regulated genes would be worth pursuing. Along these lines, the Talaat group characterized an Mtb mutant with a deletion-disruption mutation in ctpV. The ctpV mutant is sensitive to Cu, a phenotype that could be partially complemented. The ctpV mutant does not show any difference in the colonization of BALB/c mouse lungs and has a modestly reduced ability to colonize guinea pig lungs at early stages of infection (Ward et al., 2010). In a long-term survival assay in mice, animals infected with the ctpV mutant live longer than mice infected with wild type Mtb. Complementation of the ctpV mutation, however, does not restore full virulence of the mutant, perhaps because Cu resistance is also not fully complemented. Nonetheless, CtpV appears to have a role in Mtb pathogenesis in these models of infection.
In addition to CsoR, it was noted that additional putative metal-responsive regulators were present in Mtb (Liu et al., 2007; Ward et al., 2008). CsoR is highly similar to Rv0190, which was eventually determined to be a Cu responsive regulator named RicR (regulated in Cu repressor). Interestingly, RicR is more similar to CsoR homologues in several other bacterial species, including Bacillus subtilis (Smaldone and Helmann, 2007), than Mtb CsoR. Under low Cu concentrations RicR appears to directly repress the expression of five loci distributed throughout the Mtb chromosome, including ricR itself (Festa et al., 2011) (Fig. 3). Intriguingly, four of the RicR-regulated genes are unique to pathogenic mycobacteria, including a gene that encodes a Cu MT. MTs had previously only been characterized in eukaryotes and in the cyanobacterium Synechococcus (Olafson et al., 1988). Mycobacterium methallothionein, MymT, was found fortuitously in a screen for Mtb genes that conferred resistance to a compound that inhibited the growth of a distantly related, non-pathogenic Mycobacterium species, M. smegmatis (Msm) (Gold et al., 2008). Like other MTs, MymT is small, cysteine-rich and binds Cu+. Consistent with the hypothesis that MTs have a protective effect against Cu, deletion of mymT sensitizes Mtb to Cu sulfate, and this sensitivity can be reversed with the addition of a Cu+ chelating agent. Genetic deletion of mymT does not attenuate virulent Mtb in a mouse model of infection, thus the contribution of MymT to pathogenesis remains unclear.
Disruption of the ricR repressor gene results in hyper-resistance to toxic levels of Cu. Hyper-resistance could be due solely to the elevated MymT levels found in the ricR mutant (Festa et al., 2011), but it remains to be determined if the other uncharacterized RicR-regulated genes also play a role. A putative multi-copper oxidase, Rv0846c, is also RicR regulated and may contribute to Cu resistance. It is predicted that Rv0846c is secreted beyond the cytoplasmic membrane by a twin arginine translocation (Tat) system (McDonough et al., 2005), perhaps to oxidize Cu+ to Cu2+ extracellularly. RicR does not regulate genes encoding P-type ATPases or homologues of other Cu responsive proteins. It is notable that unlike disruption of ricR, which affects at least five promoters, deletion of csoR only changes the expression of the cso operon (Festa et al., 2011) (Fig. 3). It is fascinating that RicR regulates genes unique to pathogenic Mycobacteria, suggesting host-adapted species of mycobacteria encounter Cu in animals. However, it remains to be determined if any of the RicR-regulated genes are required for Mtb pathogenesis.
It is notable that Mtb has two paralogous Cu-repressors that function to protect bacteria from Cu toxicity. It is possible that Mtb has evolved a graded response to Cu toxicity such that RicR and CsoR have different binding affinities for Cu. This may indicate that differing concentrations of Cu are encountered in the host.
Listeria monocytogenes (Lmo)
Lmo is a Gram-positive bacterium that typically causes gastroenteritis but can be fatal in immunocompromised individuals and pregnant women who have ingested contaminated food or water. A recent study examined the function of CsoR, CopA and CopZ encoded in a single Cu-inducible, CsoR-regulated operon in Lmo. Although deletion of the P-type ATPase transporter gene, copA, results in hyper-sensitivity to Cu, the ΔcopA::lacZ mutant is as virulent as wild type Lmo in a high dose oral mouse infection model (Corbett et al., 2011). Furthermore, although copZ over-expression protects against Cu toxicity in an Lmo strain that cannot respond to elevated Cu levels, a ΔcopZ mutant is as resistant to Cu as wild type bacteria (Corbett et al., 2011). This observation suggests that under very high Cu concentrations CopZ is not absolutely required to chaperone Cu to CopA for export in Lmo, or that alternative Cu chaperones could transfer Cu to CopA. Importantly, although CopZ is homologous to Cu chaperones, there are no data to support this function. CopZ may instead simply sequester Cu under elevated Cu conditions.
It is worth noting that early studies identified a plasmid-encoded P-type ATPase, CtpA, in Lmo strain DRDC8. A cognate regulator or chaperone was not reported in association with CtpA and CtpA appears to be needed for the full virulence of DRDC8 (Francis and Thomas, 1997). However, this locus is not present in other sequenced strains of Lmo (Corbett et al., 2011). Despite the lack of ctpA in other Lmo strains, the observation that increased Cu sensitivity could lead to virulence attenuation suggests that host Cu may play a role in controlling listeriosis under certain conditions.
Other Cu responsive pathways?
Up to now, bacterial Cu resistance has been largely defined by the CueR- or CsoR-like regulons. Recent work has shown however that other Cu-resistance pathways exist. For example, in addition to two parallel CsoR-type pathways in Mtb, a third pathway required for Cu resistance is present in Mycobacteria. Mycobacterial Cu transport protein B (MctB, Rv1698) was identified during the characterization of outer membrane protein mutants (Wolschendorf et al., 2011). MctB is conserved in both pathogenic and non-pathogenic mycobacterial species and regulates intracellular Cu concentrations. The authors of this study hypothesized that MctB acts as an outer membrane porin to remove excess Cu from the cell (Fig. 3). CtpV, which is predicted to be a cytoplasmic membrane efflux protein, may work in concert with MctB in this process. Niederweis and colleagues determined that the intracellular concentration of total Cu is more than 10,000 times higher in Msm than in Mtb (Wolschendorf et al., 2011). The authors of this work speculated that Mtb needs less Cu because it is a slow growing organism (Mtb doubling time ~20 hours versus 3–4 hours for Msm) or that Msm has more Cu binding proteins. Importantly, Mtb mctB mutant bacteria are far less capable of growing in mice and guinea pigs (Wolschendorf et al., 2011). Despite the strong association with Cu resistance, it does not yet appear that CsoR or RicR regulates mctB expression under conditions so far tested (Festa et al., 2011).
In addition to identifying a new Cu homeostasis protein in mycobacteria, Niederweis and colleagues made the important observation that Cu levels are elevated in granulomatous tissue isolated from Mtb infected guinea pig lungs (Wolschendorf et al., 2011), strongly suggesting that bacteria encounter Cu in the host. It is perhaps not surprising that Mtb, which is not found outside of humans, has evolved to have multiple Cu-responsive pathways. Furthermore, this may also explain why the disruption of a single gene such as ctpV or mymT is insufficient to produce a robust, attenuated in vivo phenotype.
Virulence roles for Cu in fungi
Cu homeostasis in the baker’s yeast S. cerevisiae
From a fungal pathogenesis point of view, and with respect to understanding the mechanisms of Cu homeostasis in general, the baker’s yeast Saccharomyces cerevisiae (S. cerevisiae) has been a powerful experimental system. While Cu homeostasis in yeast has been reviewed recently (Kim et al., 2008), a brief summary of Cu homeostasis in S. cerevisiae is useful here for understanding the potential role of Cu in fungal pathogenesis.
Under conditions of Cu deficiency, high affinity Cu uptake in S. cerevisiae is mediated by two functionally redundant and independent homo-trimeric Cu+ importers on the plasma membrane, Ctr1 and Ctr3. S. cerevisiae Ctr1 and Ctr3 are analogous in both their function in Cu+ transport and their general structures, possessing three transmembrane domains and methioninerich regions that may directly coordinate to Cu+ during the transport process. The expression of CTR1 and CTR3, as well as the FRE1 and FRE7-encoded metalloreductases, is activated by the Mac1 transcription factor, which reversibly binds to Cu Responsive Elements (CuREs) under Cu deficient conditions (Jungmann et al., 1993; Yamaguchi-Iwai et al., 1997).
In the presence of elevated and potentially toxic Cu levels, expression of the Ace1 Cu metalloregulatory transcription factor is activated. The Ace1 amino-terminal DNA binding domain forms a tetra-Cu+ cluster, activating its DNA binding function and delivering the potent transcription activation domain to target gene promoters. Ace1 targets include the CUP1 and CRS5 MT genes, and SOD1, which encodes a Cu,Zn superoxide dismutase (Ehrensberger and Bird, 2011; Rees and Thiele, 2004). Interestingly although MT gene transcription in mammals is induced in response to Cu, Cd, Zn and other metals, fungal MT genes appear to be activated only in response to elevated Cu.
Cryptococcus neoformans: Is Cu friend or foe?
With the dramatically increased incidence of life-threatening fungal infections on a global scale, studies of cellular homeostasis and regulatory mechanisms in general have recently expanded from S. cerevisiae to other fungal species of clinical importance. Certain fungal species are opportunistic pathogens that predominantly affect patients with immunodeficiency, either as a consequence of HIV/AIDS, cancer chemotherapies, diabetes or chronically administered immune suppressants. Here, we focus on Cryptococcus species, which have become among the most significant and aggressive fungal pathogens involved in many recent Cryptococcus outbreaks even in healthy populations, worldwide.
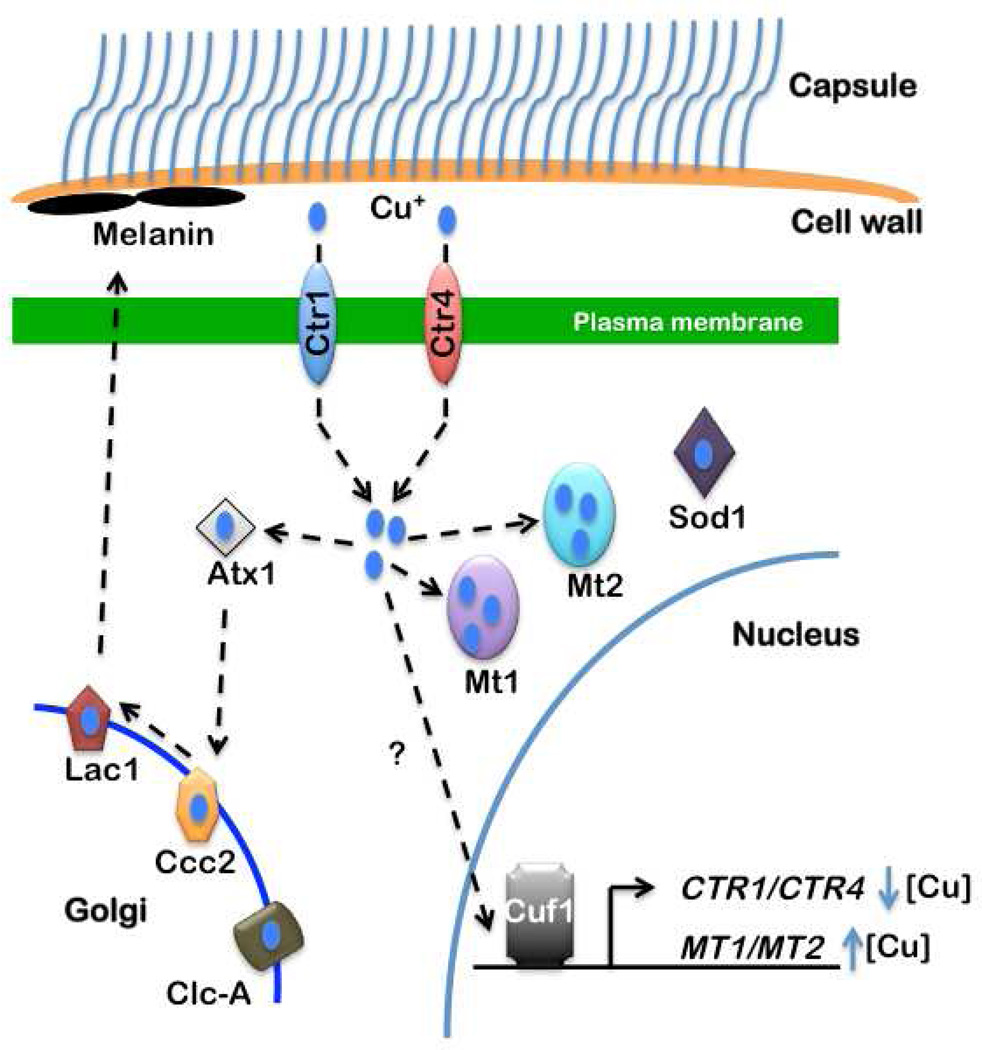
Cryptococcus species such as C. neoformans, are predominantly found in the environment on plants and in pigeon guano, and are transmitted in either the yeast or spore form. The spores are ~2 µm in diameter and enter their mammalian hosts through the respiratory route. Once in the bloodstream, C. neoformans crosses the blood brain barrier through an unknown mechanism, progresses to the encapsulated yeast phase and causes lethal meningitis. Recent work has identified conserved Cu homeostasis gene products in C. neoformans (Fig. 4). Two high affinity Cu+ transporters, Ctr1 and Ctr4, are localized to the plasma membrane and are partially functionally redundant for growth under Cu-deficient conditions. While both the CTR1 and CTR4 genes are transcriptionally activated in response to Cu deficiency, CTR1 has a high basal mRNA level whereas CTR4 is only readily detected under Cu deficient conditions. The Ccc2 P-Type Cu+ transporting ATPase and the CLC-A chloride channel facilitate Cu loading onto laccase (Lac1), a cell wall-associated, Cu-dependent oxidase required for melanin formation (Walton et al., 2005; Zhu and Williamson, 2003). Melanin formation is a critical virulence factor in protecting C. neoformans from killing by macrophages and in evading the host immune response during systemic infection (Garcia-Rivera et al., 2005; Liu et al., 1999). Melanin is thought to bind •O2− and provide other protective functions including cell wall maintenance and resistance to high temperature and UV light (Zhu and Williamson, 2004). Recent studies suggest that LAC1 expression is Cu-inducible, linking Cu status to melanin production and virulence in C. neoformans. Consistent with this hypothesis, mutations in genes encoding proteins involved in Cu delivery to the fungal secretory compartment (Atx1 and Ccc2), or Cu loading onto Lac1 dramatically decrease Lac1 activity and reduce C. neoformans virulence (Walton et al., 2005). Moreover, LAC1 is highly expressed early after infection, in keeping with its role in protection from killing by pulmonary macrophages (Garcia-Rivera et al., 2005; Liu et al., 1999).
Fig. 4. C. neoformans Cu acquisition and detoxification pathways.
The fungal pathogen C. neoformans has conserved many of the Cu homeostasis proteins found in other fungi and humans, but exhibits unusual regulation of gene expression in response to Cu. Melanin is synthesized by Lac1, which receives Cu in the secretory pathway through the action of the cytosolic Atx1 Cu chaperone and the Ccc2 Cu+ transporting ATPase. The Clc-A chloride channel facilitates Cu loading in the secretory compartment of S. cerevisiae. Ctr1 and Ctr4 are two partially redundant, high affinity Cu+ transporters largely localized to the plasma membrane. MT1 and MT2 are predicted to bind Cu+ and which have been shown to be essential for Cu detoxification. Sod1 mutants are sensitive to oxidative stress, have reduced melanin production and attenuated virulence in mice. The Cuf1 transcription factor is required for the activation of Ctr1 and Ctr4 expression in response to Cu deficiency and for MT1 and MT2 expression during Cu excess.
Interestingly, quantitative trait loci analysis in C. neoformans identified Cuf1, an orthologue of Mac1 in S. cerevisiae, as a gene that contributes to many virulence related biological processes including hyphal formation and melanin production (Lin et al., 2006). A subsequent report showed that C. neoformans cuf1Δ mutants exhibit low Lac1 activity and growth defects on Cu-deficient medium (Waterman et al., 2007). Moreover, Ctr4 expression is elevated when C. neoformans is in a macrophage-like cell line or in mouse brains (Waterman et al., 2007). The observation that cuf1Δ mutants are attenuated in mouse infection models suggested to these authors that Cu acquisition, rather than detoxification, plays a central role in C. neoformans pathogenesis. However, the precise relationship between C. neoformans Cuf1 and virulence is further complicated by recent studies that unexpectedly show a dependence on Cuf1 for MT expression in response to Cu excess (Ding et al., 2011). Correspondingly, cuf1Δ strains exhibit growth defects on both high Cu and Cu deficient medium. Additional investigations are merited to ascertain the relative contributions of the Cu acquisition and detoxification pathways in C. neoformans virulence.
The mating process in C. neoformans is another critical factor in C. neoformans virulence. C. neoformans mating leads to the generation of airborne spores, facilitating the infection process via inhalation. Interestingly, Cu has been reported to play a role in the filamentation of C. neoformans, a morphological switch that is required for mating. Heitman and colleagues observed that wild type C. neoformans filaments less well during Cu deficiency, whereas filamentation is greatly induced upon the addition of exogenous Cu to growth medium (Lin et al., 2006). In agreement with this observation, a ccc2Δ mutant dramatically impairs filamentation, suggesting that key components of the mating process that traverse the secretory pathway require Cu, which is loaded into the secretory lumen by Ccc2.
Questions going forward
The increased awareness of Cu homeostasis systems in microbial pathogens has raised numerous questions. How many pathogens are affected by Cu in vivo? Has the use of Cu in the environment affected the virulence of microbes? What regulates host Cu transport during infection? Does the host suffer “collateral damage” by Cu during an infection? Perhaps the compartmentalization of Cu+ in the phagolysosome would, at least in principle, solve the problem of Cu toxicity to host cells. In addition to Cu in macrophages, some microbial pathogens encounter Cu in the cerebral spinal fluid, where Cu concentrations have been estimated to reach over 100 micromolar under some conditions (Waggoner et al., 2000).
In addition to the research summarized here, it is worth noting that numerous studies have recently reported the identification of Cu responsive regulators in other important pathogens, including the Gram-positive bacteria Staphylococcus aureus (Baker et al., 2011; Grossoehme et al., 2011) and Streptococcus pneumoniae (Shafeeq et al., 2011). In S. pneumoniae, Cu-resistance is important for virulence in a mouse infection model, just as it is for several other pathogens discussed here. Taken together, it is likely that future studies will support the hypothesis that Cu is a component of our innate immune arsenal used to battle invading pathogens, and future research may help develop therapies that enhance microbiocidal Cu-toxicity delivered by the host.
Acknowledgements
We thank Ricky Festa for critical review of a draft version of this manuscript. Research on Cu metabolism in the Thiele laboratory is supported by NIH grants GM41840 and DK074192. The Darwin lab is supported by NIH grant HL92774. C.D. is a Duke Scholar in Infectious Disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achard ME, Tree JJ, Holden JA, Simpfendorfer KR, Wijburg OL, Strugnell RA, Schembri MA, Sweet MJ, Jennings MP, McEwan AG. The multi-copper-ion oxidase CueO of Salmonella enterica serovar Typhimurium is required for systemic virulence. Infect Immun. 2010;78:2312–2319. doi: 10.1128/IAI.01208-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agertt F, Crippa AC, Lorenzoni PJ, Scola RH, Bruck I, Paola L, Silvado CE, Werneck LC. Menkes' disease: case report. Arq Neuropsiquiatr. 2007;65:157–160. doi: 10.1590/s0004-282x2007000100032. [DOI] [PubMed] [Google Scholar]
- Andreini C, Banci L, Bertini I, Rosato A. Occurrence of copper proteins through the three domains of life: a bioinformatic approach. J Proteome Res. 2008;7:209–216. doi: 10.1021/pr070480u. [DOI] [PubMed] [Google Scholar]
- Baker J, Sengupta M, Jayaswal RK, Morrissey JA. The Staphylococcus aureus CsoR regulates both chromosomal and plasmid-encoded copper resistance mechanisms. Environ Microbiol. 2011;13:2495–2507. doi: 10.1111/j.1462-2920.2011.02522.x. [DOI] [PubMed] [Google Scholar]
- Borkow G, Gabbay J. Copper as a biocidal tool. Curr Med Chem. 2005;12:2163–2175. doi: 10.2174/0929867054637617. [DOI] [PubMed] [Google Scholar]
- Brown NL, Stoyanov JV, Kidd SP, Hobman JL. The MerR family of transcriptional regulators. FEMS Microbiol Rev. 2003;27:145–163. doi: 10.1016/S0168-6445(03)00051-2. [DOI] [PubMed] [Google Scholar]
- Burq V. Du cuivre contre le choléra: de l'immunité acquise par les ouvriers en cuivre par rapport au choléra. Paris: G. Baillière; 1867. [Google Scholar]
- Casey AL, Adams D, Karpanen TJ, Lambert PA, Cookson BD, Nightingale P, Miruszenko L, Shillam R, Christian P, Elliott TS. Role of copper in reducing hospital environment contamination. J Hosp Infect. 2010;74:72–77. doi: 10.1016/j.jhin.2009.08.018. [DOI] [PubMed] [Google Scholar]
- Changela A, Chen K, Xue Y, Holschen J, Outten CE, O'Halloran TV, Mondragon A. Molecular basis of metal-ion selectivity and zeptomolar sensitivity by CueR. Science. 2003;301:1383–1387. doi: 10.1126/science.1085950. [DOI] [PubMed] [Google Scholar]
- Checa SK, Espariz M, Audero ME, Botta PE, Spinelli SV, Soncini FC. Bacterial sensing of and resistance to gold salts. Mol Microbiol. 2007;63:1307–1318. doi: 10.1111/j.1365-2958.2007.05590.x. [DOI] [PubMed] [Google Scholar]
- Chen K, Yuldasheva S, Penner-Hahn JE, O'Halloran TV. An atypical linear Cu(I)-S2 center constitutes the high-affinity metal-sensing site in the CueR metalloregulatory protein. J Am Chem Soc. 2003;125:12088–12089. doi: 10.1021/ja036070y. [DOI] [PubMed] [Google Scholar]
- Corbett D, Schuler S, Glenn S, Andrew PW, Cavet JS, Roberts IS. The combined actions of the copper-responsive repressor CsoR and copper-metallochaperone CopZ modulate CopA-mediated copper efflux in the intracellular pathogen Listeria monocytogenes. Mol Microbiol. 2011;81:457–472. doi: 10.1111/j.1365-2958.2011.07705.x. [DOI] [PubMed] [Google Scholar]
- Corbin BD, Seeley EH, Raab A, Feldmann J, Miller MR, Torres VJ, Anderson KL, Dattilo BM, Dunman PM, Gerads R, et al. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science. 2008;319:962–965. doi: 10.1126/science.1152449. [DOI] [PubMed] [Google Scholar]
- Deredjian A, Colinon C, Brothier E, Favre-Bonte S, Cournoyer B, Nazaret S. Antibiotic and metal resistance among hospital and outdoor strains of Pseudomonas aeruginosa. Res Microbiol. 2011;162:689–700. doi: 10.1016/j.resmic.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Ding C, Yin J, Tovar EM, Fitzpatrick DA, Higgins DG, Thiele DJ. The copper regulon of the human fungal pathogen Cryptococcus neoformans H99. Mol Microbiol. 2011;81:1560–1576. doi: 10.1111/j.1365-2958.2011.07794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty CP. Host-pathogen interactions: the role of iron. J Nutr. 2007;137:1341–1344. doi: 10.1093/jn/137.5.1341. [DOI] [PubMed] [Google Scholar]
- Dollwet HS, JRJ Historic uses of copper compounds in medicine. Trace elements in medicine. 1985;2:80–87. [Google Scholar]
- Driscoll JA, Brody SL, Kollef MH. The epidemiology, pathogenesis and treatment of Pseudomonas aeruginosa infections. Drugs. 2007;67:351–368. doi: 10.2165/00003495-200767030-00003. [DOI] [PubMed] [Google Scholar]
- Ehrensberger KM, Bird AJ. Hammering out details: regulating metal levels in eukaryotes. Trends Biochem Sci. 2011;36:524–531. doi: 10.1016/j.tibs.2011.07.002. [DOI] [PubMed] [Google Scholar]
- EPA. EPA registers copper-containing alloy products. 2008 [Google Scholar]
- Espariz M, Checa SK, Audero ME, Pontel LB, Soncini FC. Dissecting the Salmonella response to copper. Microbiology. 2007;153:2989–2997. doi: 10.1099/mic.0.2007/006536-0. [DOI] [PubMed] [Google Scholar]
- Festa RA, Jones MB, Butler-Wu S, Sinsimer D, Gerads R, Bishai WR, Peterson SN, Darwin KH. A novel copper-responsive regulon in Mycobacterium tuberculosis. Mol Microbiol. 2011;79:133–148. doi: 10.1111/j.1365-2958.2010.07431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis MS, Thomas CJ. Mutants in the CtpA copper transporting P-type ATPase reduce virulence of Listeria monocytogenes. Microb Pathog. 1997;22:67–78. doi: 10.1006/mpat.1996.0092. [DOI] [PubMed] [Google Scholar]
- Garcia-Rivera J, Tucker SC, Feldmesser M, Williamson PR, Casadevall A. Laccase expression in murine pulmonary Cryptococcus neoformans infection. Infect Immun. 2005;73:3124–3127. doi: 10.1128/IAI.73.5.3124-3127.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold B, Deng H, Bryk R, Vargas D, Eliezer D, Roberts J, Jiang X, Nathan C. Identification of a copper-binding metallothionein in pathogenic mycobacteria. Nat Chem Biol. 2008;4:609–616. doi: 10.1038/nchembio.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossoehme N, Kehl-Fie TE, Ma Z, Adams KW, Cowart DM, Scott RA, Skaar EP, Giedroc DP. Control of copper resistance and inorganic sulfur metabolism by paralogous regulators in Staphylococcus aureus. J Biol Chem. 2011;286:13522–13531. doi: 10.1074/jbc.M111.220012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn TR, Macfarlane S, Phillips LI. Difficulties in the neonatal diagnosis of Menkes' kinky hair syndrome--trichopoliodystrophy. Clin Pediatr (Phila) 1984;23:514–516. doi: 10.1177/000992288402300915. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JM. The importance of free radicals and catalytic metal ions in human diseases. Mol Aspects Med. 1985;8:89–193. doi: 10.1016/0098-2997(85)90001-9. [DOI] [PubMed] [Google Scholar]
- Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291:881–884. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- Jungmann J, Reins HA, Lee J, Romeo A, Hassett R, Kosman D, Jentsch S. MAC1, a nuclear regulatory protein related to Cu-dependent transcription factors is involved in Cu/Fe utilization and stress resistance in yeast. EMBO J. 1993;12:5051–5056. doi: 10.1002/j.1460-2075.1993.tb06198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehl-Fie TE, Skaar EP. Nutritional immunity beyond iron: a role for manganese and zinc. Curr Opin Chem Biol. 2010;14:218–224. doi: 10.1016/j.cbpa.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr KG, Snelling AM. Pseudomonas aeruginosa: a formidable and ever-present adversary. J Hosp Infect. 2009;73:338–344. doi: 10.1016/j.jhin.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Kim BE, Nevitt T, Thiele DJ. Mechanisms for copper acquisition, distribution and regulation. Nat Chem Biol. 2008;4:176–185. doi: 10.1038/nchembio.72. [DOI] [PubMed] [Google Scholar]
- Kim JS, Kim MH, Joe MH, Song SS, Lee IS, Choi SY. The sctR of Salmonella enterica serova Typhimurium encoding a homologue of MerR protein is involved in the copper-responsive regulation of cuiD. FEMS Microbiol Lett. 2002;210:99–103. doi: 10.1111/j.1574-6968.2002.tb11166.x. [DOI] [PubMed] [Google Scholar]
- Kreuder J, Otten A, Fuder H, Tumer Z, Tonnesen T, Horn N, Dralle D. Clinical and biochemical consequences of copper-histidine therapy in Menkes disease. Eur J Pediatr. 1993;152:828–832. doi: 10.1007/BF02073380. [DOI] [PubMed] [Google Scholar]
- Lim SY, Joe MH, Song SS, Lee MH, Foster JW, Park YK, Choi SY, Lee IS. CuiD is a crucial gene for survival at high copper environment in Salmonella enterica serovar Typhimurium. Mol Cells. 2002;14:177–184. [PubMed] [Google Scholar]
- Lin X, Huang JC, Mitchell TG, Heitman J. Virulence attributes and hyphal growth of C. neoformans are quantitative traits and the MATalpha allele enhances filamentation. PLoS Genet. 2006;2:e187. doi: 10.1371/journal.pgen.0020187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Tewari RP, Williamson PR. Laccase protects Cryptococcus neoformans from antifungal activity of alveolar macrophages. Infect Immun. 1999;67:6034–6039. doi: 10.1128/iai.67.11.6034-6039.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Ramesh A, Ma Z, Ward SK, Zhang L, George GN, Talaat AM, Sacchettini JC, Giedroc DP. CsoR is a novel Mycobacterium tuberculosis copper-sensing transcriptional regulator. Nat Chem Biol. 2007;3:60–68. doi: 10.1038/nchembio844. [DOI] [PubMed] [Google Scholar]
- Macomber L, Imlay JA. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc Natl Acad Sci U S A. 2009;106:8344–8349. doi: 10.1073/pnas.0812808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macomber L, Rensing C, Imlay JA. Intracellular copper does not catalyze the formation of oxidative DNA damage in Escherichia coli. J Bacteriol. 2007;189:1616–1626. doi: 10.1128/JB.01357-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marais F, Mehtar S, Chalkley L. Antimicrobial efficacy of copper touch surfaces in reducing environmental bioburden in a South African community healthcare facility. J Hosp Infect. 2010;74:80–82. doi: 10.1016/j.jhin.2009.07.010. [DOI] [PubMed] [Google Scholar]
- McDonough JA, Hacker KE, Flores AR, Pavelka MS, Jr, Braunstein M. The twin-arginine translocation pathway of Mycobacterium smegmatis is functional and required for the export of mycobacterial beta-lactamases. J Bacteriol. 2005;187:7667–7679. doi: 10.1128/JB.187.22.7667-7679.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikolay A, Huggett S, Tikana L, Grass G, Braun J, Nies DH. Survival of bacteria on metallic copper surfaces in a hospital trial. Appl Microbiol Biotechnol. 2010;87:1875–1879. doi: 10.1007/s00253-010-2640-1. [DOI] [PubMed] [Google Scholar]
- Nathan CF, Shiloh MU. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc Natl Acad Sci USA. 2000;97:8841–8848. doi: 10.1073/pnas.97.16.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odermatt A, Suter H, Krapf R, Solioz M. An ATPase operon involved in copper resistance by Enterococcus hirae. Annals of the New York Academy of Sciences. 1992;671:484–486. doi: 10.1111/j.1749-6632.1992.tb43836.x. [DOI] [PubMed] [Google Scholar]
- Olafson RW, McCubbin WD, Kay CM. Primary- and secondary-structural analysis of a unique prokaryotic metallothionein from a Synechococcus sp. cyanobacterium. Biochem J. 1988;251:691–699. doi: 10.1042/bj2510691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman D, Cavet JS. Copper homeostasis in bacteria. Adv Appl Microbiol. 2008;65:217–247. doi: 10.1016/S0065-2164(08)00608-4. [DOI] [PubMed] [Google Scholar]
- Osman D, Waldron KJ, Denton H, Taylor CM, Grant AJ, Mastroeni P, Robinson NJ, Cavet JS. Copper homeostasis in Salmonella is atypical and copper-CueP is a major periplasmic metal complex. J Biol Chem. 2010;285:25259–25268. doi: 10.1074/jbc.M110.145953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outten FW, Huffman DL, Hale JA, O'Halloran TV. The independent cue and cus systems confer copper tolerance during aerobic and anaerobic growth in Escherichia coli. J Biol Chem. 2001;276:30670–30677. doi: 10.1074/jbc.M104122200. [DOI] [PubMed] [Google Scholar]
- Outten FW, Outten CE, Hale J, O'Halloran TV. Transcriptional activation of an Escherichia coli copper efflux regulon by the chromosomal MerR homologue, cueR. J Biol Chem. 2000;275:31024–31029. doi: 10.1074/jbc.M006508200. [DOI] [PubMed] [Google Scholar]
- Palmgren MG, Nissen P. P-type ATPases. Annu Rev Biophys. 2011;40:243–266. doi: 10.1146/annurev.biophys.093008.131331. [DOI] [PubMed] [Google Scholar]
- Petersen C, Moller LB. Control of copper homeostasis in Escherichia coli by a P-type ATPase, CopA, and a MerR-like transcriptional activator, CopR. Gene. 2000;261:289–298. doi: 10.1016/s0378-1119(00)00509-6. [DOI] [PubMed] [Google Scholar]
- Pontel LB, Soncini FC. Alternative periplasmic copper-resistance mechanisms in Gram negative bacteria. Mol Microbiol. 2009;73:212–225. doi: 10.1111/j.1365-2958.2009.06763.x. [DOI] [PubMed] [Google Scholar]
- Prentice AM, Ghattas H, Cox SE. Host-pathogen interactions: can micronutrients tip the balance. J Nutr. 2007;137:1334–1337. doi: 10.1093/jn/137.5.1334. [DOI] [PubMed] [Google Scholar]
- Raimunda D, Gonzalez-Guerrero M, Leeber BW, 3rd, Arguello JM. The transport mechanism of bacterial Cu(+)-ATPases: distinct efflux rates adapted to different function. Biometals. 2011;24:467–475. doi: 10.1007/s10534-010-9404-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees EM, Thiele DJ. From aging to virulence: forging connections through the study of copper homeostasis in eukaryotic microorganisms. Curr Opin Microbiol. 2004;7:175–184. doi: 10.1016/j.mib.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Rensing C, Fan B, Sharma R, Mitra B, Rosen BP. CopA: An Escherichia coli Cu(I)-translocating P-type ATPase. Proc Natl Acad Sci U S A. 2000;97:652–656. doi: 10.1073/pnas.97.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridge PG, Zhang Y, Gladyshev VN. Comparative genomic analyses of copper transporters and cuproproteomes reveal evolutionary dynamics of copper utilization and its link to oxygen. PLoS One. 2008;3:e1378. doi: 10.1371/journal.pone.0001378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible UE, Kaufmann SH. Iron and microbial infection. Nat Rev Microbiol. 2004;2:946–953. doi: 10.1038/nrmicro1046. [DOI] [PubMed] [Google Scholar]
- Schwan WR, Warrener P, Keunz E, Stover CK, Folger KR. Mutations in the cueA gene encoding a copper homeostasis P-type ATPase reduce the pathogenicity of Pseudomonas aeruginosa in mice. Int J Med Microbiol. 2005;295:237–242. doi: 10.1016/j.ijmm.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Shafeeq S, Yesilkaya H, Kloosterman TG, Narayanan G, Wandel M, Andrew PW, Kuipers OP, Morrissey JA. The cop operon is required for copper homeostasis and contributes to virulence in Streptococcus pneumoniae. Mol Microbiol. 2011;81:1255–1270. doi: 10.1111/j.1365-2958.2011.07758.x. [DOI] [PubMed] [Google Scholar]
- Shiloh MU, Nathan CF. Reactive nitrogen intermediates and the pathogenesis of Salmonella and mycobacteria. Curr Opin Microbiol. 2000;3:35–42. doi: 10.1016/s1369-5274(99)00048-x. [DOI] [PubMed] [Google Scholar]
- Smaldone GT, Helmann JD. CsoR regulates the copper efflux operon copZA in Bacillus subtilis. Microbiology. 2007;153:4123–4128. doi: 10.1099/mic.0.2007/011742-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solioz M, Stoyanov JV. Copper homeostasis in Enterococcus hirae. FEMS microbiology reviews. 2003;27:183–195. doi: 10.1016/S0168-6445(03)00053-6. [DOI] [PubMed] [Google Scholar]
- Stoyanov JV, Hobman JL, Brown NL. CueR (YbbI) of Escherichia coli is a MerR family regulator controlling expression of the copper exporter CopA. Mol Microbiol. 2001;39:502–511. doi: 10.1046/j.1365-2958.2001.02264.x. [DOI] [PubMed] [Google Scholar]
- Talaat AM, Lyons R, Howard ST, Johnston SA. The temporal expression profile of Mycobacterium tuberculosis infection in mice. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:4602–4607. doi: 10.1073/pnas.0306023101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitzel GM, Geddie A, De Long SK, Kirisits MJ, Whiteley M, Parsek MR. Survival and growth in the presence of elevated copper: transcriptional profiling of copper-stressed Pseudomonas aeruginosa. J Bacteriol. 2006;188:7242–7256. doi: 10.1128/JB.00837-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaden JT, Lory S, Gardner TS. Quorum-sensing regulation of a copper toxicity system in Pseudomonas aeruginosa. J Bacteriol. 2010;192:2557–2568. doi: 10.1128/JB.01528-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thieme D, Neubauer P, Nies DH, Grass G. Sandwich hybridization assay for sensitive detection of dynamic changes in mRNA transcript levels in crude Escherichia coli cell extracts in response to copper ions. Appl Environ Microbiol. 2008;74:7463–7470. doi: 10.1128/AEM.01370-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tree JJ, Ulett GC, Ong CL, Trott DJ, McEwan AG, Schembri MA. Trade-off between iron uptake and protection against oxidative stress: deletion of cueO promotes uropathogenic Escherichia coli virulence in a mouse model of urinary tract infection. J Bacteriol. 2008;190:6909–6912. doi: 10.1128/JB.00451-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno H, Arya S. Neuronal and vascular disorders of the brain and spinal cord in Menkes kinky hair disease. Am J Med Genet Suppl. 1987;3:367–377. doi: 10.1002/ajmg.1320280542. [DOI] [PubMed] [Google Scholar]
- Waggoner DJ, Drisaldi B, Bartnikas TB, Casareno RL, Prohaska JR, Gitlin JD, Harris DA. Brain copper content and cuproenzyme activity do not vary with prion protein expression level. J Biol Chem. 2000;275:7455–7458. doi: 10.1074/jbc.275.11.7455. [DOI] [PubMed] [Google Scholar]
- Wagner D, Maser J, Lai B, Cai Z, Barry CE, 3rd, Honer Zu Bentrup K, Russell DG, Bermudez LE. Elemental analysis of Mycobacterium avium-, Mycobacterium tuberculosis- and Mycobacterium smegmatis-containing phagosomes indicates pathogen-induced microenvironments within the host cell's endosomal system. J Immunol. 2005;174:1491–1500. doi: 10.4049/jimmunol.174.3.1491. [DOI] [PubMed] [Google Scholar]
- Walton FJ, Idnurm A, Heitman J. Novel gene functions required for melanization of the human pathogen Cryptococcus neoformans. Mol Microbiol. 2005;57:1381–1396. doi: 10.1111/j.1365-2958.2005.04779.x. [DOI] [PubMed] [Google Scholar]
- Ward SK, Abomoelak B, Hoye EA, Steinberg H, Talaat AM. CtpV: a putative copper exporter required for full virulence of Mycobacterium tuberculosis. Mol Microbiol. 2010;77:1096–1110. doi: 10.1111/j.1365-2958.2010.07273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SK, Hoye EA, Talaat AM. The global responses of Mycobacterium tuberculosis to physiological levels of copper. J Bacteriol. 2008;190:2939–2946. doi: 10.1128/JB.01847-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman SR, Hacham M, Hu G, Zhu X, Park YD, Shin S, Panepinto J, Valyi-Nagy T, Beam C, Husain S, et al. Role of a CUF1/CTR4 copper regulatory axis in the virulence of Cryptococcus neoformans. J Clin Invest. 2007;117:794–802. doi: 10.1172/JCI30006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg ED. Nutritional immunity. Host's attempt to withold iron from microbial invaders. JAMA : the journal of the American Medical Association. 1975;231:39–41. doi: 10.1001/jama.231.1.39. [DOI] [PubMed] [Google Scholar]
- Weinberg ED. Iron availability and infection. Biochim Biophys Acta. 2009;1790:600–605. doi: 10.1016/j.bbagen.2008.07.002. [DOI] [PubMed] [Google Scholar]
- White C, Lee J, Kambe T, Fritsche K, Petris MJ. A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity. J Biol Chem. 2009;284:33949–33956. doi: 10.1074/jbc.M109.070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolschendorf F, Ackart D, Shrestha TB, Hascall-Dove L, Nolan S, Lamichhane G, Wang Y, Bossmann SH, Basaraba RJ, Niederweis M. Copper resistance is essential for virulence of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2011;108:1621–1626. doi: 10.1073/pnas.1009261108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi-Iwai Y, Serpe M, Haile D, Yang W, Kosman DJ, Klausner RD, Dancis A. Homeostatic regulation of copper uptake in yeast via direct binding of MAC1 protein to upstream regulatory sequences of FRE1 and CTR1. J Biol Chem. 1997;272:17711–17718. doi: 10.1074/jbc.272.28.17711. [DOI] [PubMed] [Google Scholar]
- Zhu X, Williamson PR. A CLC-type chloride channel gene is required for laccase activity and virulence in Cryptococcus neoformans. Mol Microbiol. 2003;50:1271–1281. doi: 10.1046/j.1365-2958.2003.03752.x. [DOI] [PubMed] [Google Scholar]
- Zhu X, Williamson PR. Role of laccase in the biology and virulence of Cryptococcus neoformans. FEMS Yeast Res. 2004;5:1–10. doi: 10.1016/j.femsyr.2004.04.004. [DOI] [PubMed] [Google Scholar]