Abstract
Background
Intravenous delivery of volatile fluorinated anesthetics has a number of potential advantages when compared to the current inhalation method of administration. We reported previously that the IV delivery of sevoflurane can be achieved through an emulsion composed of a linear fluorinated diblock copolymer, a stabilizer, and the anesthetic. However, this original emulsion was subject to particle size growth that would limit its potential clinical utility. We hypothesized that the use of bulkier fluorous groups and smaller poly(ethylene glycol) moieties in the polymer design would result in improved emulsion stability while maintaining anesthetic functionality.
Methods
The authors prepared emulsions incorporating sevoflurane, perfluorooctyl bromide as a stabilizing agent, and combinations of linear fluorinated diblock copolymer and a novel dibranched fluorinated diblock copolymer. Emulsion stability was assessed using dynamic light scattering. The ability of the emulsions to induce anesthesia was tested in vivo by administering them intravenously to fifteen male Sprague-Dawley rats and measuring loss of the forepaw righting reflex.
Results
20% (volume/volume) sevoflurane emulsions incorporating mixtures of dibranched- and linear diblock copolymers had improved stability, with those containing an excess of the dibranched polymers displaying stability of particle size for over one year. The ED50s for loss of forepaw righting reflex were all similar, and ranged between 0.55 and 0.60 ml/kg body weight.
Conclusions
Hemifluorinated dibranched polymers can be used to generate exceptionally stable sevoflurane nanoemulsions, as required of formulations intended for clinical use. Intravenous delivery of the emulsion in rats resulted in induction of anesthesia with rapid onset and smooth and rapid recovery.
Introduction
Altering the way with which drugs are administered has the potential to change their pharmacokinetic and even pharmacodynamic properties.1–3 Fluorinated anesthetics are currently administered through inhalation. However, intravenous delivery of these anesthetics has a number of potential advantages when compared with inhalation.4–6 In principle, IV delivery could eliminate the need for a vaporizer 4–6 and allow more rapid changes in anesthetic depth.7 Indeed, by eliminating the equilibration steps between the vaporizer outflow and the breathing circuit and between the alveoli and the blood, anesthetic induction and recovery have been shown to be extremely rapid – as rapid for induction with intravenous emulsified isoflurane as for intravenous propofol, with even more rapid recovery than from propofol.8
Because of these potential benefits, the intravenous administration of fluorinated anesthetics has been investigated for more than 40 years. Early efforts of direct IV delivery of methoxyflurane and halothane led to lethal damage for humans and animals.9–13 Lipid emulsions such as Intralipid have been tested for IV delivery of volatile anesthetics without adverse effects in animals.8,14–18 However, the fluorophilic nature of these anesthetics,19 with limited solubility in aqueous or lipid phases, has prevented the use of classic lipids and surfactants in the preparation of stable emulsions. Furthermore, only small concentrations of emulsified fluorinated anesthetic (3–8% volume/volume) can be achieved by using Intralipid8,17,18,20–22 or fluorocarbons such as Oxygent, a second-generation blood substitute.23
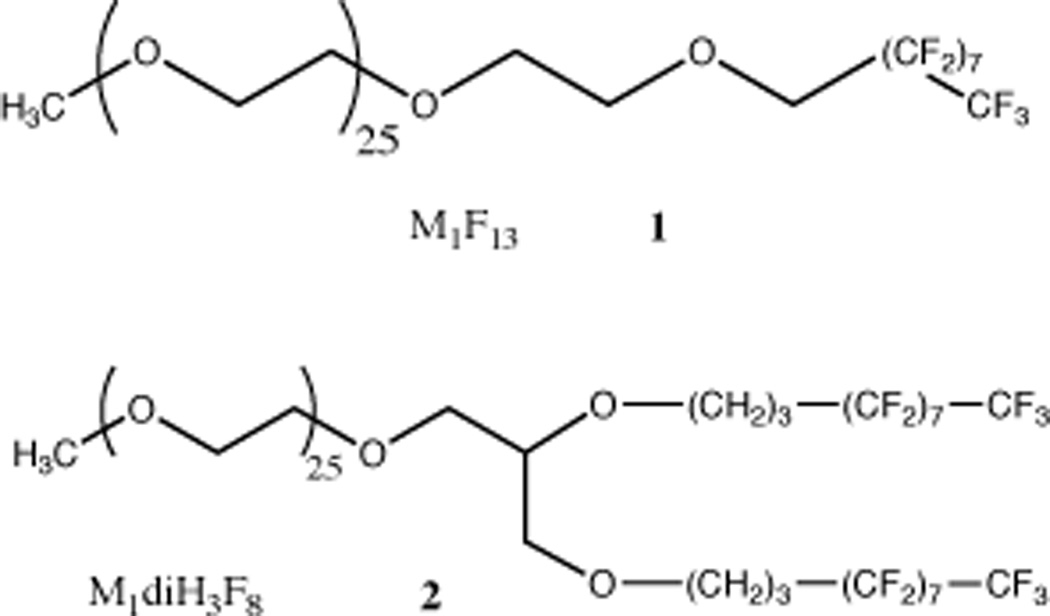
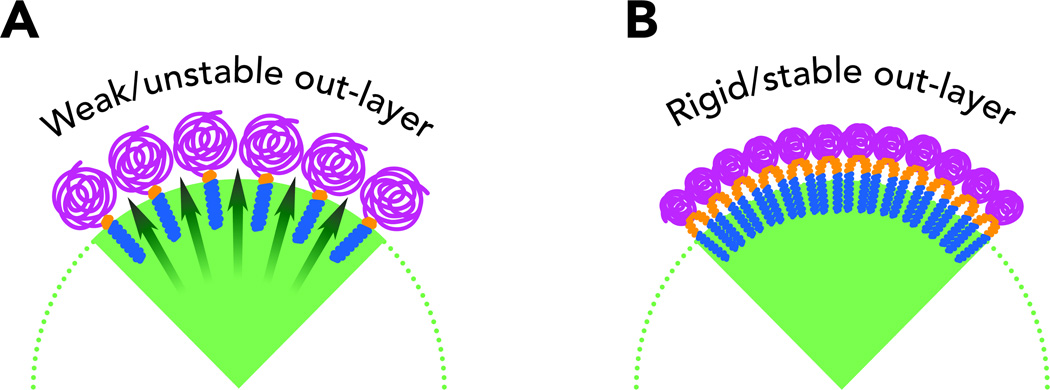
In an effort to develop a formulation sufficiently concentrated for possible clinical use, we previously created a nanoemulsion composed of sevoflurane, perfluorooctyl bromide serving as a stabilizing agent, and a semifluorinated diblock copolymer serving as an interfacial agent, and reported that it could be used to dissolve sevoflurane in water with concentrations up to 20% volume/volume.24 This formulation was tested in vivo in rats and shown to induce general anesthesia with very rapid onset and recovery. However, as we report here, our follow-up studies revealed insufficient long-term stability. The main mechanism of destabilization in these emulsions is that of Ostwald ripening, in which smaller particles dissolve as larger particles grow. This mechanism eventually leads to emulsions with particles too big to be used safely. The emulsions we originally prepared and studied used semifluorinated linear diblock copolymers similar to M1F13 (Figure 1, molecule 1).24 In an attempt to reduce particle growth as much as possible, we designed a new hemifluorinated dibranched polymer M1diH3F8 (Figure 1, molecule 2). The main feature of these polymers is the doubling in size and volume of the perfluorinated moiety. We hypothesized that this change in design would allow us to prepare nanoemulsions in which the individual nanodroplets composed of a mixture of sevoflurane and perfluorooctyl bromide would be surrounded by a more dense and compact shell of polymer (Figure 2). The synthesis and physicochemical characterization of these new polymers have been described elsewhere.25 Here, we report on the ability of these novel polymers to provide exceptionally stable concentrated emulsions of sevoflurane, in which no significant ripening is observed over one year, and on their ability to induce anesthesia in vivo.
Figure 1.
Chemical structures of fluoropolymers. Molecule 1: M1F13. The number following the letter M indicates the molecular weight of the poly(ethylene glycol). Thus, M1 indicates a methyl-capped poly(ethylene glycol) of average molecular weight 1,000. The number after the F indicates the number of carbon atoms in the fluorocarbon chains. Molecule 2: M1diH3F8. The number after the H indicates the number of carbon atoms composing the intermediate hydrocarbon segment
Figure 2.
Schematic structure of a section of a fluorous emulsion nanoparticle. (A) The original lineal diblock copolymer forms a polymer shell around the fluorous particle that allows easy diffusion of anesthetic molecule in solution. This phenomenon leads to the growth of the nanoparticles volume and destabilizes the emulsion. (B) The use of a smaller poly(ethylene glycol) and a much bulkier fluorous tail leads to a more compact polymer shell around the fluorous nanoparticle. The rate of diffusion of anesthetic molecules in solution is reduced and the emulsion is stabilized.
Materials and methods
Materials
Sevoflurane was purchased from Abbott Labs (N. Chicago, IL) and perfluorooctyl bromide was obtained from SynQuest Laboratories, Inc (Alachua, FL). Sodium chloride (NaCl) was purchased from Fisher Scientific (Fair Lawn, NJ). High purity water (MilliQ, Millipore, Billerica, MA) was used during the studies.
Preparation of 20% (volume/volume) sevoflurane emulsion
Polymer aqueous solutions were prepared by first dissolving the fluoropolymers (500 mg) in saline (0.9% sodium chloride solution, 11.9 ml). Sevoflurane (3.4 ml) and perfluorooctyl bromide (1.7 ml) were added to the aqueous solution, for a total volume of 17 ml. The mixture was initially homogeneized with a high speed homogenizer (Power Gen 500, Fisher Scientific, Hampton, NH) for 1 min at 21,000 rpm at room temperature. The crude emulsion was then microfluidized with a Microfluidizer (model 110 S, Microfluidics Corp., Newton, MA) for 1 min under 5,000 psi at 15°C maintained through a cooling bath. The resulting nanoemulsion was filtered through a 0.45 µm nylon syringe filter. Nanoemulsions were stored in 15 ml sterile centrifuge tubes (Corning Inc., Corning, NY) at 4°C. We prepared several sevoflurane emulsions with various composition ratios of the two fluorous surfactants: M1diH3F8/ M1F13 (0/100, 28/72, 54/46, 77/23 and 100/0, weight/weight) and evaluated their physicochemical stability by analyzing particle sizes over one year.
Emulsion particle size determination
Dynamic light scattering was used to analyze the emulsion particle size. Measurements were performed with a NICOMP 380 ZLS (Particle Sizing Systems, Santa Barbara, CA) equipped with a 639 nm laser positioned at a scattering angle of 90°. The nanoemulsions were diluted at the dynamic light scattering intensity factor of 300 by mixing 20 µl of the emulsion and 3.0 ml of Millipore water. Each particle size measurement was run for 5 min at room temperature and repeated three times. The raw data were analyzed using a Guassian fit with the ZW380 software (v. 1.61A, Particle Sizing Systems). Results were reported as volume weighted average diameters.
Emulsion physical stability
The nanoemulsions were stored at 4°C for periods up to 357 days. The emulsion particle size was analyzed at 1, 2, 7, 14, 21, 28 days and then every two weeks up to 357 days.
Animal studies
All animal studies were approved by the University of Wisconsin Animals Care and Use Committee, Madison, Wisconsin, and were performed in accordance with the guidelines laid out in the Guide for the Care and Use of Laboratory Animals published by the National Research Council.
Dose-response data (ED50) for the intravenous delivery of sevoflurane emulsions were measured on male Spraque-Dawley rats (Harlan Spraque-Dawley, Inc., Indianapolis, IN) weighing approximately 280 g. The rats were received with a jugular catheter surgically implanted by the supplier. The rats were weighed and the emulsion dose was adjusted according to the body weight. Fifteen rats were divided into three groups of five rats and each group was used to test one specific emulsion. The same five rats were also used to study the effect of multiple doses of the emulsions. In all cases, the rats received only one dose of anesthetic per day.
The sevoflurane emulsions were administered by first restraining the rat with a towel. The plug placed at the end of the catheter was then removed and replaced with a 22-gauge needle connected to an insulin-type syringe. To remove the heparin-based fill solution and check that no blockage was obstructing in the catheter, the syringe plunger was slowly withdrawn until blood filled the catheter. The rat was then placed in a transparent cage for observation. The 22-gauge needle was then connected to the syringe containing the sevoflurane emulsions. Forty µl of the sevoflurane emulsion, corresponding to the volume of the catheter, was injected to prime the catheter and then the administration of the emulsion was started. The emulsion injection rate was controlled through an infusion pump (11 plus; Harvard Apparatus, Holliston, MA). A bolus dose was delivered within 20 s regardless of the volume. Immediately after injection, the rat was rolled onto its back and loss of righting reflex (LORR) was evaluated. The time to achieve and to recover from LORR and non-coordination were recorded. When the rat completely recovered from LORR, non-coordination and disorientation (within 3 min in all cases), the catheter was flushed with 0.08 ml of a normal saline solution to remove the residual emulsion and then refilled with 0.08 ml of a heparin-based fill solution. The end of the catheter was sealed with a sterile plug.
In experiments to determine the effective dose for anesthetizing 50% of the population (ED50), doses of the emulsions were administered to the grouped five rats in irregular order. The emulsion starting dose was derived from our previous study in which the ED50 of sevoflurane emulsified with the linear semifluorinated polymer 1 was found to be 0.41 ml /kg.20
Statistical analysis
To calculate ED50 for LORR, we determined the number of rats that lost the righting reflex out of the total number that received intravenous delivery of the emulsions. ED50 values were calculated through nonlinear regression, data were fitted to a sigmoidal dose-response relation using the program Graphpad Prism (ver. 5.01; GraphPad Software, Inc., San Diego, CA), according to the equation:
where X is the logarithm of concentration and Y is the response. 95% confidence intervals of ED50 were also calculated using the program Graphpad Prism (ver. 5.01; GraphPad Software, Inc., San Diego, CA).
Statistical analyses were performed using Student’s unpaired t-test and one-way analysis of variance (ANOVA), as indicated in the text. A p-value of less than 0.05 was considered significant.
Values are presented as mean ± standard deviation.
Results
Emulsion stability
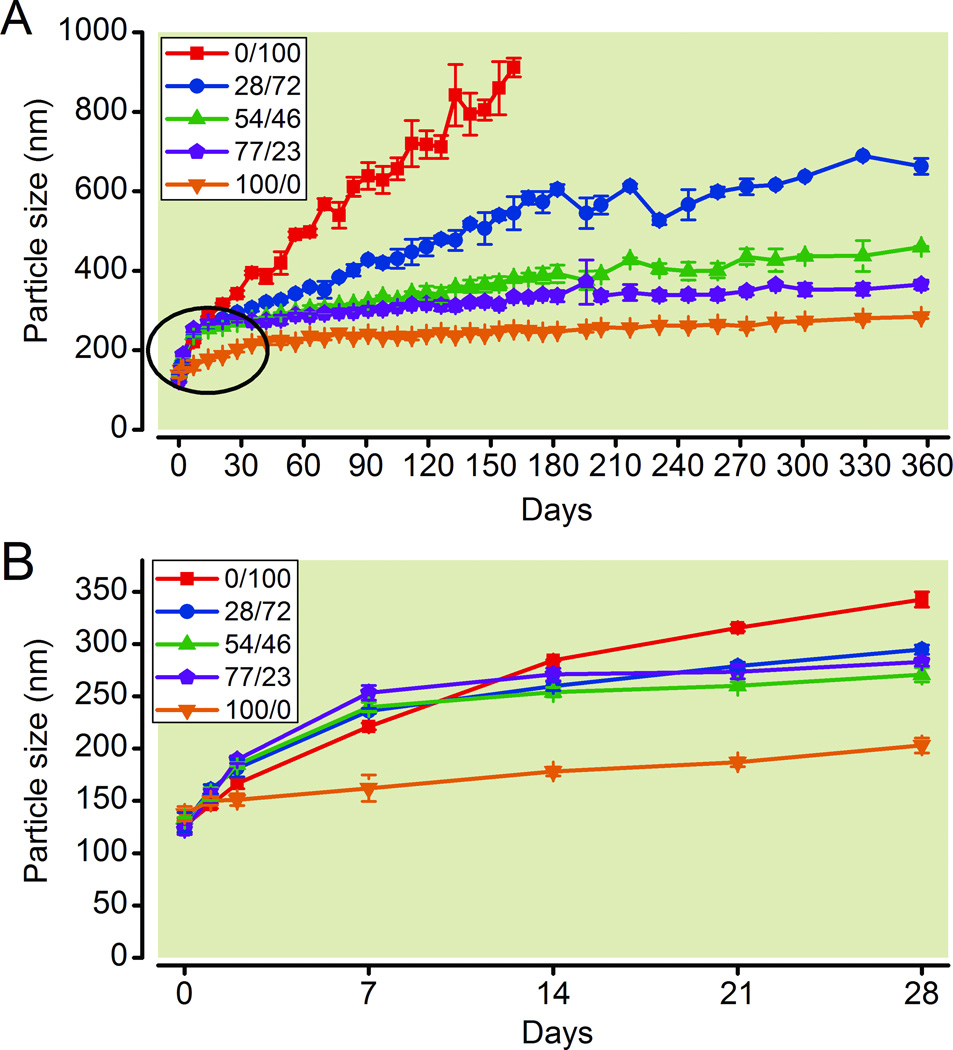
The dibranched polymers of the general molecular formula MzdiHxFy are made through a multistep synthesis and are relatively expensive compounds. Thus, we tested various mixtures of the two most promising polymers, M1F13 and M1diH3F8, to establish whether stable sevoflurane emulsions could be formed by a combination of the relatively inexpensive surfactant M1F13 and the more expensive M1diH3F8. Immediately after preparation, the emulsion particle size did not show significant differences between the various surfactant ratios, and ranged from 126 to 138 nm in diameter. In all cases the emulsion particles grew in size during the first week. This initial increase was followed by slower ripening. The ripening was maximum in the case of M1F13, and minimum in the case of pure M1diH3F8 (Figure 3A). As shown in Figure 3B, the emulsion containing pure M1F13 (0/100) increased to 284 ± 1 nm during the first 14 days, and then the increasing ratio was slower. The emulsions with 28% of M1diH3F8, (28/72), 54% of M1diH3F8 (54/46), and 77% of M1diH3F8 (77/23) increased to 236 ± 2 nm, 240 ± 5 nm and 253 ± 7 nm respectively, during the first seven days. Particle size ripening slowed significantly after the seventh day, and the particle growth rate was greater with increasing M1F13 concentration. Specifically, the particles in the emulsion made by using only M1F13, increased to 912 ± 24 nm during 161 days and sedimentation was then observed. After one year the particle size of the emulsions containing M1diH3F8/M1F13 ratios of 28/72 and 54/46 were 663 ± 20 nm, and 460 ± 2 nm respectively. Sedimentation was observed in both emulsions starting on day 301. The particle size of the emulsion containing an M1diH3F8/M1F13 ratio of 77/23 increased slightly faster than the particles in the emulsion with pure M1diH3F8 during the first seven days. After this time, the rates of ripening of this emulsion and that with pure M1diH3F8 were similar. After one year the 77/23 emulsion contained particles of the average size 365 ± 11, still within a safe therapeutic range according to United States Pharmacopeia Chapter 729 guidelines.26 Remarkably, after one year, the particle size of the emulsion containing pure M1diH3F8 (100/0) was only 285 ± 5 nm. Figures 3A, B show complete growth plots for the five emulsions investigated in this study.
Figure 3.
Particle size of emulsions with varying M1diH3F8 / M1F13 composition ratios. (A) Particle size over 1 yr. (B) Expansion to show details of particle size increase during the first 28 days (encircled in A). Values are mean ± standard deviation (n = 3).
Dose-response studies of sevoflurane emulsions
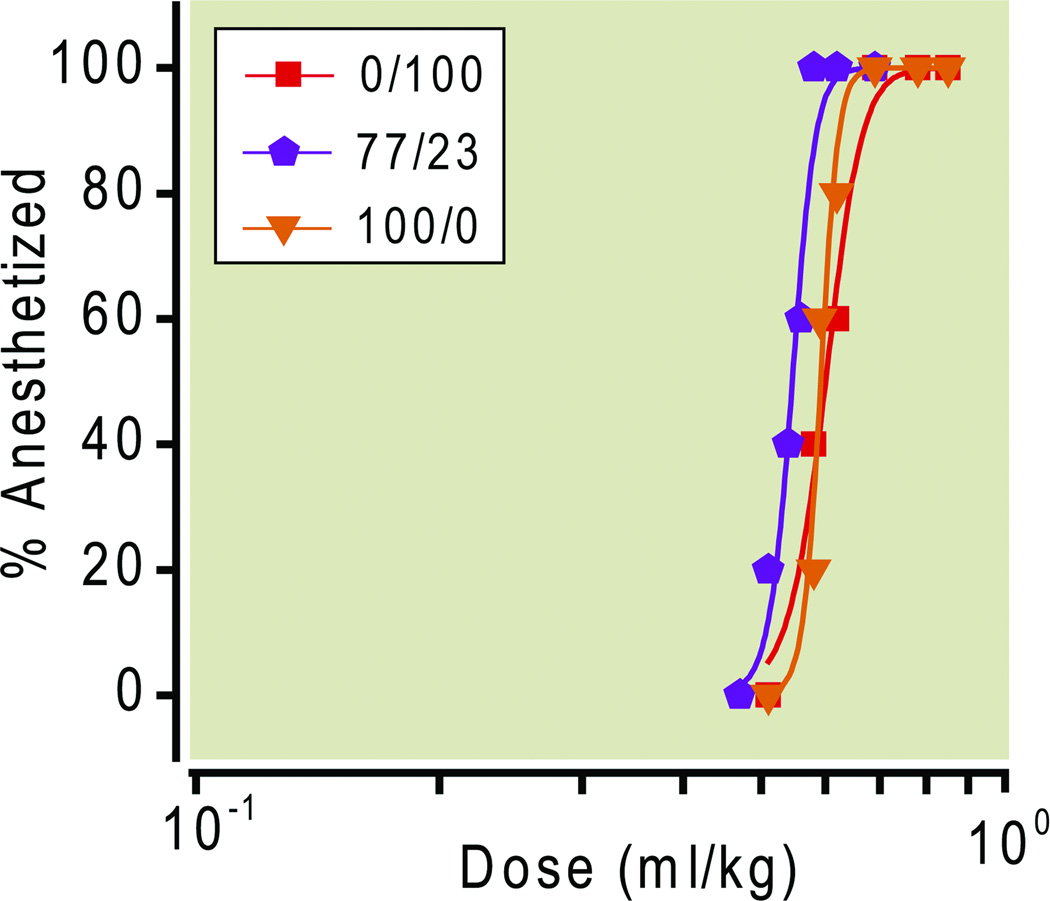
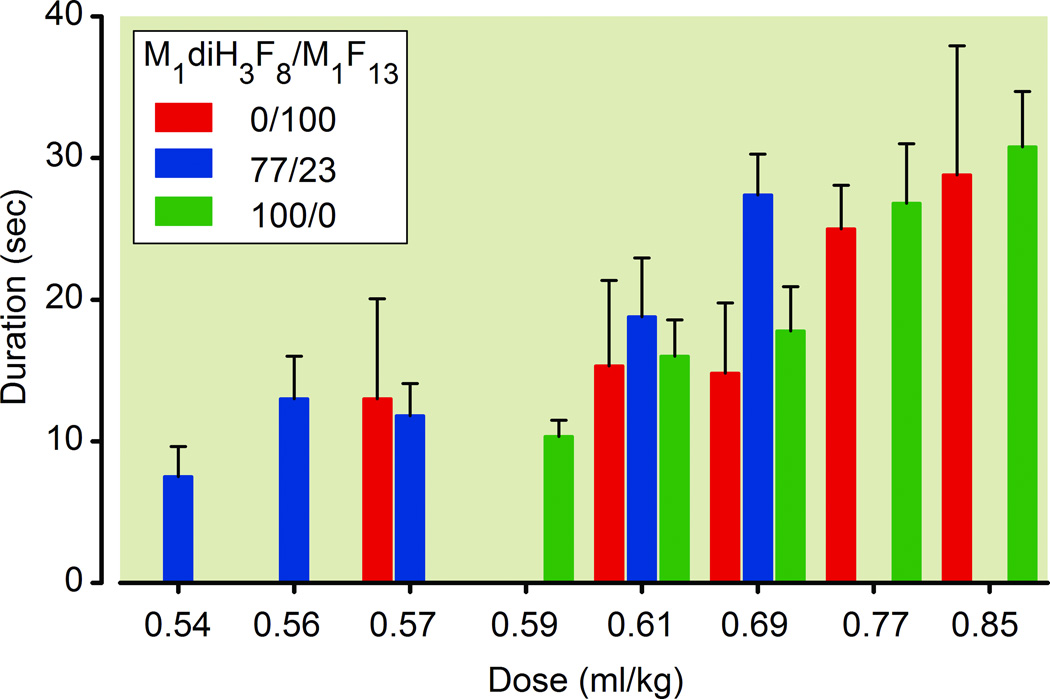
Three different formulations (M1diH3F8/M1F13 ratios of 100/0, 77/23 and 0/100) were chosen for dose-response studies in rats. The dose-response curves for LORR of the three different emulsions are shown in Figure 4. Figure 4 indicates the doses given to the rats and the corresponding ED50 values, which were all similar, ranging from 0.55 to 0.60 ml/kg. In all cases, the lowest administered dose that induced LORR in all rats (i.e. 0.69 ml/kg for M1diH3F8/M1F13 ratios of 100/0 and 0/100, and 0.57 ml/kg for 77/23) produced this effect after 18±1 sec from the start of injection. The duration of anesthesia, as measured from the start of LORR (induction) to recovery from LORR (emergence from anesthesia) ranged from 10 to 30 sec, in a dose-dependent manner (ANOVA) for all three formulations (Figure 5).
Figure 4.
ED50 Dose-response curves for the emulsions with the M1diH3F8 / M1F13 composition ratios of ( ) 0/100, ED50 = 0.6; (
) 0/100, ED50 = 0.6; ( ) 77/23, ED50 = 0.55; (
) 77/23, ED50 = 0.55; ( ) 100/0, ED50 = 0.59.
) 100/0, ED50 = 0.59.
Figure 5.
Average duration of loss of righting reflex for rats. Values are mean ± standard deviation (n = 3).
Discussion
The original formulation we developed for the intravenous delivery of sevoflurane allowed us to dissolve up to 20% sevoflurane as an emulsion in saline solution.24 This formulation was tested in vivo and proved to be able to induce and maintain general anesthesia, with a therapeutic Index of 2.6. However, emulsions are not inherently thermodynamically stable. Various physicochemical mechanisms may contribute to particle growth, which may be followed by phase separation. Therefore, as potential pharmaceutical development requires emulsions to be stable for an extended period of time, we were confronted with the need to stabilize our emulsions. As shown in Figure 3, our original formulations exemplified by the emulsion containing only the M1F13 surfactant showed a relatively fast rate of particle growth, not compatible with clinical use.
In our attempts to create an emulsion with improved stability, we hypothesized that changes in the size and shape of the polymer would be able to induce the formation of a more dense and compact polymeric shell around the emulsion particles. This effect, in turn, would stabilize the emulsions by reducing particle growth. The additional protection provided by the new dibranched fluoropolymer did indeed strongly enhance the emulsion stability for up to one year --the longest duration tested (Figure 3).
We performed dose-response (ED50) studies with emulsions with a ratio M1diH3F8/M1F13 of 0/100, 77/23, and 100/0. The three emulsions had similar ED50s, ranging from 0.55 to 0.60 ml/kg. Loss of righting reflex was rapid – within the twenty second injection period – and recovery of righting reflex was also rapid in comparison with other intravenous induction agents such as propofol or isoflurane in lipid emulsion.7, 8 The rapid induction of anesthesia indicates that despite its stability in water, the emulsion releases sevoflurane quickly; the recovery profile is presumably due to both redistribution and elimination of the anesthetic through the lungs.27 The underlying reasons for these ultrarapid kinetics are not known, but we speculate that it relates to the rapid disaggregation of the nanoemulsion into components or structures that have limited carrying capacity, such as micelles.
This rapid pharmacokinetic profile may prove advantageous under certain conditions, such as increasing anesthetic depth immediately before skin incision, or matching the duration of anesthesia to a brief but intense stimulus such as laryngoscopy or insertion of cranial pins. IV delivery of fluorinated ethers also offers a host of other advantages.4,5 Recent reports have shown that protection against ischemia and reperfusion injury can be achieved with concentrations of anesthetic insufficient to cause hypnotic and anesthetic effects.28 The IV delivery of small amounts of concentrated emulsified sevoflurane would be well suited to such applications. However, it must also be acknowledged that the potential benefits of IV administration remain unproven, and that important questions remain regarding the practical utility of IV administration, the optimal approach to measuring and maintaining consistent depth of anesthesia using IV delivery (possibly in combination with inhalational administration), the pharmacokinetic profile and elimination characteristics when administered as a bolus or infusion, the potential to utilize this method for other agents such as desflurane, which boils at room temperature and pressure, and the safety of administration in situations were the airway remains unprotected, such as in a “preconditioning clinic” or Magnetic Resonance Imaging scanner. Similarly, the disposition of the copolymer surfactant and the perfluorinated stabilizing agent also must be addressed by future studies. When administered as an Magnetic Resonance Imaging contrast agent, perfluorooctyl bromide is removed from the circulation primarily by the parenchyma and macrophages of the liver and reticuloendothelial system and ultimately excreted by the lungs through breathing.29 The surfactant may remain assembled as micelles with limited carrying capacity, or dissociate into monomers, but its eventual disposition remains unknown. The present study, demonstrating that exceptional stability of emulsions under storage conditions is nevertheless compatible with rapid onset of action and recovery, is an important step in continuing to develop such formulations that can be used for such studies, and ultimately for possible clinical use.
In conclusion, sevoflurane nanoemulsions containing ratios of dibranched to linear polymer higher than 50% are characterized by a high drug content and an exceptional physicochemical stability. When these formulations were administered intravenously, a predictable and rapidly reversible anesthesia with rapid onset and smooth recovery was induced in rats. These results suggest a potential clinical use for the described sevoflurane emulsions, particularly in situations where an extremely rapid pharmacokinetic profile might improve physiological stability or extremely rapid recovery is desirable.
Final Box Summary Statement.
What we already know about this topic
Intravenous delivery of volatile anesthetics has potential advantages such as more rapid induction and titration of anesthesia compared to inhalation
Previous emulsion formulations of fluorinated anesthetics have met with limited success
What this article tells us that is new
An improved nanoemulsion of sevoflurane in a fluoropolymer shell was shown to induce general anesthesia with rapid onset and recovery in rats
This preparation is a promising lead for the intravenous delivery of volatile anesthetics
Acknowledgments
FUNDING STATEMENT: The authors acknowledge funding from the National Institutes of Health (Bethesda, MD) (grant GM079375, SM, RAP), the Korean Research Foundation (KRF-2008-357-E00065 partial support for J-PJ), and the University of Wisconsin Department of Anesthesiology, Waters Professorship (RAP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allen TM, Hansen CB, Guo LSS. Subcutaneous administration of liposomes: A comparison with the intravenous and intraperitoneal routes of injection. Biochim Biophys Acta. 1993;1150:9–16. doi: 10.1016/0005-2736(93)90115-g. [DOI] [PubMed] [Google Scholar]
- 2.Harivardhan Reddy L, Sharma RK, Chuttani K, Mishra AK, Murthy RS. Influence of administration route on tumor uptake and biodistribution of etoposide loaded solid lipid nanoparticles in Dalton’s lymphoma tumor bearing mice. J Control Release. 2005;105:185–198. doi: 10.1016/j.jconrel.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 3.Tewes F, Munnier E, Antoon B, Ngaboni Okassa L, Cohen-Jonathan S, Marchais H, Douziech-Eyrolles L, Souce M, Dubois P, Chourpa Comparative study of doxorubicin-loaded poly (lactide-co-glycolide) nanoparticles prepared by single and double emulsion methods. Eur J Pharm Biopharm. 2007;66:488–492. doi: 10.1016/j.ejpb.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 4.Lucchinetti E, Schaub MC, Zaugg M. Emulsified intravenous versus evaporated inhaled isoflurane for heart protection: old wine in a new bottle or true innovation? Anesth Analg. 2008;106:1346–1349. doi: 10.1213/ane.0b013e31816d1661. [DOI] [PubMed] [Google Scholar]
- 5.Raphael J, Lynch C., III Intravenous administration of Halogenated Inhaled Anesthetics – Research Tools or real Application? Can. J. Anesth. 2009;56:91–95. doi: 10.1007/s12630-008-9026-1. [DOI] [PubMed] [Google Scholar]
- 6.Smith I. Total Intravenous Anaesthesia. Is It Worth the Cost? CNS Drugs. 2003;17:606–619. doi: 10.2165/00023210-200317090-00001. [DOI] [PubMed] [Google Scholar]
- 7.Yang XL, Ma HX, Yang ZB, Liu AJ, Luo NF, Zhang WS, Wang L, Jiang XH, Li J, Liu J. Comparison of minimum alveolar concentration between intravenous isoflurane lipid emulsion and inhaled isoflurane in dogs. Anesthesiology. 2006;104:482–487. doi: 10.1097/00000542-200603000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Zhou J, Luo N, Liang X, Liu J. The efficacy and safety of intravenous emulsified isoflurane in rats. Anesth Analg. 2006;102:129–134. doi: 10.1213/01.ane.0000189612.24147.07. [DOI] [PubMed] [Google Scholar]
- 9.Cascorbi HF, Helrich M, Krantz JC, Jr, Baker LR, Rozman RS, Rudo FG. Hazards of methoxyflurane emulsions in man. Anesth Analg. 1968;47:557–559. [PubMed] [Google Scholar]
- 10.Kopriva C, Lowenstein E. An anesthetic accident: Cardiovascular collapse from liquid halothane delivery. Anesthesiology. 1969;30:246–247. [PubMed] [Google Scholar]
- 11.Sandison JW, Sivapragasam S, Hayes JA, Woo-Ming MO. An experimental study of pulmonary damage associated with intravenous injection of halothane in dogs. Br J Anaest. 1970;42:419–423. doi: 10.1093/bja/42.5.419. [DOI] [PubMed] [Google Scholar]
- 12.Sutton J, Harrison GA, Hickie JB. Accidental intravenous injection of halothane. Br J Anaest. 1971;43:513–519. doi: 10.1093/bja/43.5.513. [DOI] [PubMed] [Google Scholar]
- 13.Kawamoto M, Suzuki N, Takasaki M. Acute pulmonary edema after intravenous liquid halothane in dogs. Anesth Analg. 1992;74:747–752. doi: 10.1213/00000539-199205000-00021. [DOI] [PubMed] [Google Scholar]
- 14.Johannesson G, Alm P, Biber B, Lennander O, Werner O. Halothane dissolved in fat as an intravenous anaesthetic to rats. Acta Anaesthesiol Scand. 1984;28:381–384. doi: 10.1111/j.1399-6576.1984.tb02081.x. [DOI] [PubMed] [Google Scholar]
- 15.Biber B, Johannesson G, Lennander O, Martner J, Sonander H, Werner O. Intravenous infusion of halothane dissolved in fat: Haemodynamic effects in dogs. Acta Anaesthesiol Scand. 1984;28:385–389. doi: 10.1111/j.1399-6576.1984.tb02082.x. [DOI] [PubMed] [Google Scholar]
- 16.Musser JB, Fontana JL, Mongan PD. The anesthetic and physiologic effects of an intravenous administration of a halothane lipid emulsion (5% vol/vol) Anesth Analg. 1999;88:671–675. doi: 10.1097/00000539-199903000-00038. [DOI] [PubMed] [Google Scholar]
- 17.Eger RP, MacLeod BA. Anaesthesia by intravenous emulsified isoflurane in mice. Can J Anaesth. 1995;42:173–176. doi: 10.1007/BF03028273. [DOI] [PubMed] [Google Scholar]
- 18.Chiari PC, Pagel PS, Tanaka K, Krolikowski JG, Ludwig LM, Trillo RA, Puri N, Kersten JR, Warltier DC. Intravenous emulsified halogenated anesthetics produce acute and delayed preconditioning against myocardial infarction in rabbits. Anesthesiology. 2004;101:1160–1166. doi: 10.1097/00000542-200411000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Gladysz JA, Curran DP, Horvath IT. Handbook of Fluorous Chemistry. Weinheim, Germany: Wiley-VCH; 2004. pp. 95–97. [Google Scholar]
- 20.Johannesson G, Alm P, Biber B, Lennander O, Werner O. Halothane dissolved in fat as an intravenous anaesthetic to rats. Acta Anaesthesiol Scand. 1984;28:381–384. doi: 10.1111/j.1399-6576.1984.tb02081.x. [DOI] [PubMed] [Google Scholar]
- 21.Biber B, Johannesson G, Lennander O, Martner J, Sonander H, Werner O. Intravenous infusion of halothane dissolved in fat: Haemodynamic effects in dogs. Acta Anaesthesiol Scand. 1984;28:385–389. doi: 10.1111/j.1399-6576.1984.tb02082.x. [DOI] [PubMed] [Google Scholar]
- 22.Musser JB, Fontana JL, Mongan PD. The anesthetic and physiologic effects of an intravenous administration of a halothane lipid emulsion (5% vol/vol) Anesth Analg. 1999;88:671–675. doi: 10.1097/00000539-199903000-00038. [DOI] [PubMed] [Google Scholar]
- 23.Cuignet OY, Baele PM, Van Obbergh LJ. A second-generation blood substitute (perflubron emulsion) increases the blood solubility of modern volatile anesthetics. Anesth Analg. 2002;95:368–372. doi: 10.1097/00000539-200208000-00023. [DOI] [PubMed] [Google Scholar]
- 24.Fast JP, Perkins MG, Pearce RA, Mecozzi S. Fluoropolymer-based emulsions for the intravenous delivery of sevoflurane. Anesthesiology. 2008;109:651–656. doi: 10.1097/ALN.0b013e31818630ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parlato MC, Jee JP, Teshite M, Mecozzi S. Synthesis, characterization and applications of hemifluorinated dibranched amphiphiles. J Org Chem. 2011;76:6584–6591. doi: 10.1021/jo200835y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.USP Chapter<729>. Globule size distribution in injectable lipid emulsions. Vol. 31. US Pharmacopoeial Forum; 2005. pp. 1448–1453. [Google Scholar]
- 27.Kharasch ED, Thummel KE. Identification of cytochrome P450 2E1 as the predominant enzyme catalyzing human liver microsomal defluorination of sevoflurane, isoflurane, and methoxyflurane. Anesthesiology. 1993;79:795–807. doi: 10.1097/00000542-199310000-00023. [DOI] [PubMed] [Google Scholar]
- 28.Lucchinetti E, Ambrosio S, Aguirre J, Herrmann P, Härter L, Keel M, Meier T, Zaugg M. Sevoflurane Inhalation at Sedative Concentrations Provides Endothelial Protection against Ischemia-Reperfusion Injury in Humans. Anesthesiology. 2008;106:262–268. doi: 10.1097/00000542-200702000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Giraudeau C, Djemaï B, Ghaly MA, Boumezbeur F, Mériaux S, Robert P, Port M, Robic C, Le Bihan D, Lethimonnier F, Valette J. High sensitivity 19F MRI of a perflurooctyl bromide emulsion: application to a dynamic biodistribution study and oxygen tension mapping in the mouse liver and spleen. NMR Biomed. 2011 doi: 10.1002/nbm.1781. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]