Abstract
The success of the RAF protein kinase inhibitor vemurafenib for the treatment of BRAF-mutant metastatic melanoma has produced another poster child for the promise of personalized medicine. However, the results of a recent study also reveal unexpected pitfalls in the application of signal transduction-targeted therapies.
The era of personalized cancer medicine is upon us. The cancer patient's genome can now be interrogated for specific genetic alterations to guide the application of therapies specifically targeted to those alterations. A dramatic therapeutic advance in this area is the BRAF-selective inhibitor vemurafenib, which has provided a significant improvement in overall survival compared to the previous standard of care for metastatic melanoma (Chapman et al., 2011). However, recent findings with vemurafenib and other protein kinase inhibitors demonstrate that the new era of signal transduction-targeted therapies is handicapped by some of the same issues that have plagued traditional cytotoxic drugs.
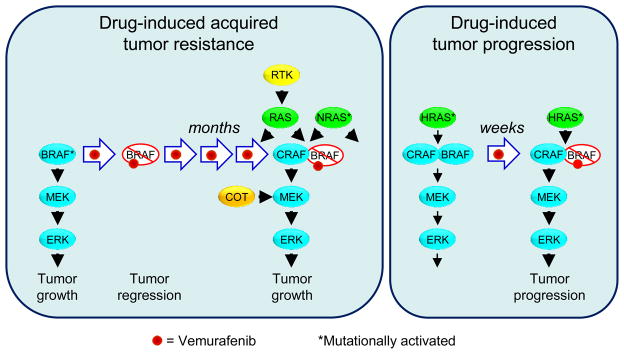
One key distinction between targeted versus cytotoxic therapies is decreased normal cell toxicity. Symptoms such as the classic myelosuppression associated with many cytotoxic antineoplastics are not as limiting with targeted agents, whose therapeutic effects are typically achievable at doses lower than those conferring myelosuppression or other dose-limiting toxicities. However, rapidly acquired cancer cell resistance shortens the duration of treatment response. For example, although the initial response to vemurafenib is impressive, with a response rate of ~50% and significant survival benefit, tumor resistance usually occurs within 2–18 months of initial treatment. Multiple mechanisms of resistance have been described, including mutational activation of NRAS or receptor tyrosine kinase-mediated activation of RAS, both leading to CRAF-dependent activation of MEK-ERK signaling (Figure 1) (Johannessen et al., 2010; Nazarian et al., 2010). Thus, as for cytotoxic drugs, combinations of targeted therapies will be needed, both to enhance the initial response and to reduce the subsequent onset of drug resistance. Such combinations may also have advantages in blocking the existing tumor without inducing or allowing new ones to appear.
Figure 1. Consequences of RAF Inhibitor Therapy.
Cellular responses to vemurafenib treatment in BRAF-mutant melanoma or RAS-mutant skin epithelial cells. In both settings, concurrent treatment with a MEK inhibitor may decrease the onset of these mechanisms of tumor resistance or tumor progression.
(A) Resistance mechanisms for BRAF-mutant melanomas. inactivation of BRAF(V600E) mutant by RAF inhibitor initially leads to inhibition of MEK-ERK signaling. However, tumor cells can develop resistance by multiple mechanisms, including upregulated expression of receptor tyrosine kinases (RTK) or mutational activation of NRAS leading to CRAF-dependent activation of MEK or by overexpression of the COT/TPL2 serine/threonine protein kinase, a direct activator of MEK1/2.
(B) Mutationally activated RAS forms a complex with a BRAF/CRAF heterodimer. RAF inhibitor binds preferentially to BRAF and inactivates it, but also causes transactivation of the associated CRAF, enhancing MEK-ERK activation and cellular proliferation.
That chemotherapy can both cure and cause cancer is not a new concept. Conventional cytotoxic chemotherapy has long been known to contribute to the development of secondary cancers that can arise a decade or more after completion of successful therapy. These cancers reflect the inherent DNA damaging and carcinogenic properties of standard cytotoxics. Now, Su and colleagues (Su et al., 2012) describe an unexpected consequence of vemurafenib therapy, accelerated and enhanced occurrence of skin tumors. A side effect of vemurafenib treatment is the rapid appearance of well-differentiated cutaneous squamous cell carcinomas (SCC) and keratoacanthomas in approximately 15–30% of melanoma patients. Taking cues from previous experimental studies in animal models of carcinogenesis, Su et al. asked whether activated mutants of RAS genes might be associated with these tumors. In the classical two-stage carcinogenesis mouse model, a single treatment with the carcinogen 7,12-dimethylbenz[α]anthracene (DMBA), followed by multiple treatments with the tumor promoter 12-O-tetradecanoylphorbol-13-acetate (TPA), causes formation of squamous-cell skin lesions, some of which progress to malignant carcinomas. In these carcinomas, there is a high incidence of HRAS mutational activation, characteristically at codon 61 encoding glutamate (Q61) (Balmain et al., 1984). Similarly, repeated DMBA treatment of rabbits causes formation of keratoacanthomas with HRAS Q61 mutations (Leon et al., 1988). Spontaneously occurring human skin tumors have also been reported to harbor HRAS mutants (Oberholzer et al., 2012).
Su et al. analyzed 35 cutaneous SCC or keratoacanthomas arising in a total of 23 vemurafenib-treated melanoma patients and identified RAS mutations in 60% of them: 16 harbored HRAS mutations (2 at G12, two at G13, 12 at Q61), one harbored NRAS G12, and four harbored KRAS G12 mutations (Su et al., 2012). Similarly, Oberholzer and colleagues (Oberholzer et al., 2012), found a 30% frequency of HRAS mutation in 10 tumors from vemurafenib-treated patients, compared with a 3% frequency in spontaneously occurring skin tumors.
Recent cell culture and mouse studies have revealed the ability of RAF-selective inhibitors both to efficiently block ERK activation and growth in BRAF(V600E)-mutant, RAS-wild type melanomas. In contrast RAF inhibitors caused a paradoxical activation, rather than inactivation, of ERK signaling in BRAF-wild type, RAS-mutant cancer cells (Figure 1) (Hatzivassiliou et al., 2010; Heidorn et al., 2010; Poulikakos et al., 2010). In the latter cells, the persistently GTP-bound RAS associates with BRAF/CRAF heterodimers. Vemurafenib binding to the wild type BRAF inhibits BRAF but causes transactivation of CRAF, leading to MEK and ERK activation rather than inhibition. Using high levels of RAF inhibitor that block both BRAF and CRAF, or alternatively using MEK inhibitors to block downstream of RAF, could prevent this paradoxical activation of the pathway. Su et al. hypothesized that the same paradoxical action mechanism originally modeled in cell culture also occurred in the patient, and used cell culture and mouse model studies to test this hypothesis.
One particularly compelling analysis applied vemurafenib in the DMBA/TPA carcinogenesis mouse model. Control DMBA/TPA-treated mice developed skin tumors at the expected rate, whereas concurrent treatment with vemurafenib accelerated the time of onset (Su et al., 2012). Vemurafenib treatment did not increase the frequency of skin lesions, nor was treatment with DMBA and vemurafenib sufficient to induce skin tumors in the absence of TPA. Thus, vemurafenib does not appear to act as a cancer-causing agent. The RAF inhibitor neither promoted RAS mutation nor replaced TPA to function as a tumor promoter, but simply accelerated the progression of already existing, but subclinical, mutant RAS-containing lesions. This conclusion is consistent with the clinical observation that the median onset of skin lesions was 10 weeks after initiation of treatment. In addition, combination treatment with both vemurafenib and a selective MEK1/2 inhibitor in DMBA/TPA-treated mice resulted a 91% reduction in tumor formation, supporting the authors' main take-home message that the combined use of RAF and MEK inhibitors may prevent the accelerated appearance of cutaneous squamous-cell carcinomas or keratoacanthomas in patients treated for other cancers.
Well-differentiated skin lesions are easily identified, simple to remove surgically, and not metastatic. However, their appearance does raise a significant concern that tumors below the skin and thus not so evident may also be accelerated. While the predominant RAS mutation seen in the accelerated lesions of vemurafenib-treated melanoma patients was HRAS Q61, other mutations detected included HRAS G12, HRAS G13, NRAS G12 and KRAS G12 (Su et al., 2012). Thus, the paradoxical mechanism is not restricted to HRAS or to a particular activating mutation. Mutations in KRAS are very early events in colorectal and pancreatic cancers, where mutant KRAS can be found even in histologically normal tissue. Would long-term RAF inhibitor treatment in the adjuvant setting also accelerate progression of these cancers? Vemurafenib treatment of mutant KRAS-driven mouse models of colorectal and pancreatic cancers would provide a critical assessment of this possibility.
Finally, one impressive success in advancing personalized medicine in cancer treatment is the now FDA-mandated requirement to exclude patients with KRAS G12 or KRAS G13 mutant colorectal cancers from treatment with anti-epidermal growth factor receptor (EGFR) therapy, to spare these patients from treatment that is ineffective or worse. With improved development of diagnostic procedures for noninvasive detection of KRAS mutations in stool or blood, perhaps the presence of KRAS lesions will similarly be a marker to exclude patients from treatment with RAF inhibitors. Despite the conceptually different foundations for the application of cytotoxic drugs versus molecularly targeted therapies, the wily cancer cell continues to blur the distinction. Personalized medicine will have to become even wilier to successfully defeat the cancer enemy within.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balmain A, Ramsden M, Bowden GT, Smith J. Activation of the mouse cellular Harvey-ras gene in chemically induced benign skin papillomas. Nature. 1984;307:658–660. doi: 10.1038/307658a0. [DOI] [PubMed] [Google Scholar]
- Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzivassiliou G, Song K, Yen I, Brandhuber BJ, Anderson DJ, Alvarado R, Ludlam MJ, Stokoe D, Gloor SL, Vigers G, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431–435. doi: 10.1038/nature08833. [DOI] [PubMed] [Google Scholar]
- Heidorn SJ, Milagre C, Whittaker S, Nourry A, Niculescu-Duvas I, Dhomen N, Hussain J, Reis-Filho JS, Springer CJ, Pritchard C, Marais R. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209–221. doi: 10.1016/j.cell.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannessen CM, Boehm JS, Kim SY, Thomas SR, Wardwell L, Johnson LA, Emery CM, Stransky N, Cogdill AP, Barretina J, et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010;468:968–972. doi: 10.1038/nature09627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon J, Kamino H, Steinberg JJ, Pellicer A. H-ras activation in benign and self-regressing skin tumors (keratoacanthomas) in both humans and an animal model system. Mol Cell Biol. 1988;8:786–793. doi: 10.1128/mcb.8.2.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, Chen Z, Lee MK, Attar N, Sazegar H, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468:973–977. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberholzer PA, Kee D, Dziunycz P, Sucker A, Kamsukom N, Jones R, Roden C, Chalk CJ, Ardlie K, Palescandolo E, et al. RAS mutations are associated with the development of cutaneous squamous cell tumors in patients treated with RAF inhibitors. J Clin Oncol. 2012;30:316–321. doi: 10.1200/JCO.2011.36.7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464:427–430. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su F, Viros A, Milagre C, Trunzer K, Bollag G, Spleiss O, Reis-Filho JS, Kong X, Koya RC, Flaherty KT, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012;366:207–215. doi: 10.1056/NEJMoa1105358. [DOI] [PMC free article] [PubMed] [Google Scholar]