Abstract
Individuals with body dysmorphic disorder (BDD) are preoccupied with perceived defects in appearance. Preliminary evidence suggests abnormalities in global and local visual information processing. The objective of this study was to compare global and local processing in BDD subjects and healthy controls by testing the face inversion effect, in which inverted (upside-down) faces are recognized more slowly and less accurately relative to upright faces. Eighteen medication-free subjects with BDD and 17 matched, healthy controls performed a recognition task with sets of upright and inverted faces on a computer screen that were either presented for short duration (500 msec) or long duration (5000 msec). Response time and accuracy rates were analyzed using linear and logistic mixed effects models, respectively. Results indicated that the inversion effect for response time was smaller in BDD subjects than controls during the long duration stimuli, but was not significantly different during the short duration stimuli. Inversion effect on accuracy rates did not differ significantly between groups during either of the two durations. Lesser inversion effect in BDD subjects may be due to greater detail-oriented and piecemeal processing for long duration stimuli. Similar results between groups for short duration stimuli suggest that they may be normally engaging configural and holistic processing for brief presentations. Abnormal visual information processing in BDD may contribute to distorted perception of appearance; this may not be limited to their own faces, but to others’ faces as well.
Keywords: body dysmorphic disorder, inverted faces, face inversion effect, face processing, global and local
Introduction
Body Dysmorphic Disorder (BDD) is a psychiatric disorder in which individuals are preoccupied with an imagined defect of appearance or are excessively concerned about a slight physical abnormality, which causes distress and/or functional impairment (American Psychiatric Association., 2000). BDD affects approximately 1-2% of the population (Faravelli et al., 1997; Koran, Abujaoude, Large, & Serpe, 2008; Otto, Wilhelm, Cohen, & Harlow, 2001; Rief, Buhlmann, Wilhelm, Borkenhagen, & Brahler, 2006 ), and is associated with high lifetime rates of psychiatric hospitalization (48%) (Phillips & Diaz, 1997a) and suicide attempts (22-27.5%) (Phillips et al., 2005; Phillips et al., 1997a; Phillips & Menard, 2006; Veale et al., 1996). Previous studies estimate that 27-60% are delusional in their beliefs (Mancuso, Knoesen, & Castle, in press; Phillips, 2004). Individuals with BDD tend to engage in repetitive and compulsive behaviors such as checking their appearance in the mirror and scrutinizing details of others’ appearances to compare to their own (Phillips, 2005). Despite the prevalence and severity of the disorder, relatively little is known about the pathophysiology underlying various symptom domains.
Clinical observation and neuropsychological data suggest that abnormal information processing may underscore the apparent perceptual abnormalities in BDD. Clinically, they often focus on details of their appearance, frequently involving their faces, at the expense of global or configural aspects. A neuropsychological study using the Rey-Osterrieth Complex Figure Test demonstrated that BDD patients performed poorly relative to controls due to differences in organizational strategies including selective recall of details instead of larger organizational design features (Deckersbach et al., 2000). Individuals with BDD may also have abnormalities in own-face processing, as evidenced by a study in which they perceived distortions that were not actually present (Yaryura-Tobias et al., 2002). Moreover, studies by Buhlmann et al. (2004, 2006) found abnormalities in emotional face processing in BDD consisting of recognition biases and/or misinterpretation of faces that were perceived as contemptuous or otherwise negative (Buhlmann, Etcoff, & Wilhelm, 2006; Buhlmann, McNally, Etcoff, Tuschen-Caffier, & Wilhelm, 2004). Whether these recognition biases or misinterpretations are the result of abnormalities in visual processing is not clear.
In general, face processing is an important function of the brain, underscored by how critical it is for social functioning. Efficient face processing relies both on featural and configural information (Moscovitch, Winocur, & Behrmann, 1997). Featural information about faces includes details such as skin smoothness, blemishes, lines, hair texture, etc. and is conveyed by high spatial frequency information (Norman & Ehrlich, 1987; Schyns & Oliva, 1999). Configural information includes basic spatial relationships of features (e.g. eyes above the mouth), distances between features, and holistic elements (i.e. the face as one percept) (Maurer, Le Grand, & Mondloch, 2002) and is conveyed by low spatial frequency information (Costen, Parker, & Craw, 1996; Sergent, 1985). At short viewing durations lower spatial frequencies are primarily processed (Breitmeyer, 1975; Breitmeyer & Ganz, 1977), as they occur on a faster timescale than processing of high spatial frequencies (Peyrin, Mermillod, Chokron, & Marendaz, 2006; Schyns & Oliva, 1994). In addition, no more than two eye fixations typically occur for exposure durations of 500 msec or less, while detail processing is serial in nature and requires multiple eye fixations and therefore longer viewing times (Castelhano, Mack, & Henderson, 2009; Hsiao & Cottrell, 2008).
In healthy adults, viewing inverted faces impairs recognition, which is believed to be the result of disrupted configural processing. Yin (1969) first reported that inversion of faces, but not objects, disrupted behavioral performance on a recognition task (Yin, 1969). This “face inversion effect” is defined as the difference in performance between upright and inverted photographs of faces (Farah, Tanaka, & Drain, 1995; Leder & Bruce, 2000; Valentine, 1988). This most likely occurs because the presence of a general structure or uniform set of features allows for a configural template for efficient processing of different faces, although variations exist between individual faces (Freire, Lee, & Symons, 2000). However, this template does not apply when faces are inverted; individuals may then have to rely more on the faces’ component parts. The face inversion effect is eliminated in healthy controls when they are forced to learn the faces in terms of their component parts and then asked to recognize them when inverted (Farah et al., 1995). This supports the idea that the face inversion effect is dependent on holistic processing of upright images.
Another study demonstrated that, given longer exposure to the stimuli, subjects experienced an increased ability to recognize inverted faces (Barton, Keenan, & Bass, 2001). This suggests that configural, or global, processing provides an advantage over component, or local, processing only given stimuli with shorter presentation times, and that longer times may allow for part decomposition.
Clinical observation and neuropsychological and neuroimaging studies suggests the hypothesis that individuals with BDD may have an abnormal propensity towards detail processing, and/or abnormalities in configural/holistic processing. If so, then they may also demonstrate less of a face inversion effect than healthy controls. To our knowledge, no study to date has tested the face inversion effect in BDD subjects.
The objective of this study was to investigate the face inversion effect in a cohort with BDD compared to healthy controls, in order to understand global (holistic) and local (detailed) processing of faces. The experiment included both short and long duration of stimuli in order to test differential abnormalities in global and/or local processing, respectively. BDD subjects and healthy controls engaged in a recognition task consisting of upright and inverted faces, and response times and accuracy were recorded and analyzed. We hypothesized that individuals with BDD would have less of an inversion effect on response time and accuracy than controls for long duration stimuli, reflecting a greater propensity for local visual processing, but not for the short duration, reflecting no differences for global processing at short stimulus times.
Method
Participants
Eighteen right-handed BDD patients and 17 healthy controls, matched for gender, age, handedness, and years of education, were recruited from the community and participated in the study. The investigation was carried out in accordance with the latest version of the Declaration of Helsinki and the study design was reviewed and approved by the Institutional Review Board at UCLA. We obtained informed consent of the participants after fully explaining the nature of the procedures. All BDD subjects met the Diagnostic and Statistical Manual (DSM-IV) criteria for Body Dysmorphic Disorder, using the Body Dysmorphic Disorder Module (Phillips, Atala, & Pope, 1995), a reliable and standard diagnostic module modeled after the Structured Clinical Interview for DSM. All BDD subjects were required to have a Body Dysmorphic Disorder version of the Yale-Brown Obsessive-Compulsive Disorder Scale (BDD-YBOCS) score of ≥20. The BDD-YBOCS is a validated scale that is a widely-used standard to evaluate symptom severity in BDD, with a range of scores from 0 to 48 (Phillips et al., 1997c). It has excellent interrater and test-retest reliability (intra-class correlation coefficients for total score=0.99 and 0.88, respectively), internal consistency (Cronbach’s alpha=0.80), and convergent validity (r=.55 with the CGI) (Phillips et al., 1997b). In addition to the BDD-YBOCS, we also administered the Hamilton Depression Rating Scale-17 (Hamilton, 1960) and the Hamilton Anxiety Rating Scale (Hamilton, 1969), which have similar standards for validity and reliability. We performed a clinical psychiatric evaluation on all participants and administered the Mini International Neuropsychiatric Inventory (MINI) (Sheehan et al., 1998) to screen for comorbid diagnoses. Subjects with comorbidity of any current (or lifetime for psychotic disorders and bipolar disorder) Axis I disorders were excluded, with the exception of major depressive disorder, dysthymia, and generalized anxiety disorder. As depression and anxiety are so frequently comorbid in this population, we believed it would not be a representative sample to exclude these. We excluded subjects whom the investigator judged were suicidal. Other exclusion criteria for both subjects and controls were active substance abuse, current neurological disorder with or without medication, pregnancy, or any current medical disorder that may affect cerebral metabolism. All subjects had normal or corrected-to-normal visual acuity, as verified by the Snellen eye chart.
Stimuli
Stimuli consisted of grayscale photographs of neutral-expression faces of men and women of average attractiveness, from the Psychological Image Collection at Stirling (http://pics.psych.stir.ac.uk/). We obtained attractiveness ratings of the face stimuli from a separate set of 8 healthy controls, and eliminated the two highest and two lowest outlier faces, to minimize interference in processing that might result from the BDD subjects reacting emotionally to very attractive or unattractive faces. The mean attractiveness ratings for the faces (±SD) on a Likert scale of 0-10 was 3.95±0.88. Photos were cropped to an oval to remove clothing and hair.
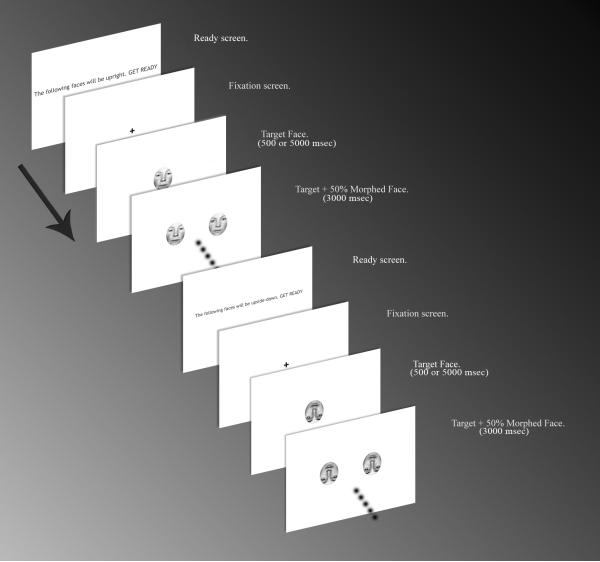
We also created “incorrect selection” faces by morphing of each of the 28 individual “correct selection” faces 50% with another gender-matched face using FantaMorph (Abrosoft http://www.fantamorph.com/), and then equalizing the luminance (Fig.1). This created morphed faces that contained both featural and configural changes that were halfway between the “correct selection” face and a different person’s face.
Fig. 1.
Example presentation sequence of experimental stimuli.
Procedure
Subjects were tested while seated in front of a computer screen using a chin rest to standardize the distance from the screen (50 cm, corresponding to a visual angle of 5.7° × 4.7° for the face stimuli). We presented stimuli for this delayed matching-to-sample task with the program MacStim 3.0 (White Ant Occasional Publishing, West Melbourne, Australia), which also recorded the response time (RT) and the specific responses for accuracy calculations.
The subjects viewed a single target face, and then were shown a second screen that included the “correct selection” face and the “incorrect selection” morphed face (Figs. 1 and 2). The selection faces were randomized to appear either on the right or the left. Subjects made their selections with the left or right button (1 or 2) on the keyboard to indicate which of the two selection faces was the same as the target face, and were instructed to make their selections as rapidly and as accurately as possible. At the beginning of the experiment subjects were given a standardized set of instructions and practiced with a set of 4 faces with 500 msec target face presentation time.
Fig. 2.

Mean response times in seconds for BDD and healthy controls for upright and inverted faces, for long and short duration stimulus times.
*p=0.0279
There was a 1000 millisecond interstimulus blank screen between target and selection face screens. There were 4 different blocks of conditions in which subjects performed the task, with 28 faces in each block:
target face (500 msec) and selection faces (3000 msec) upright
target face (500 msec) and selection faces (3000 msec) inverted
target face (5000 msec) and selection faces (3000 msec) upright
target face (5000 msec) and selection faces (3000 msec) inverted
There were four possible experimental orders. All orders presented the two 500 millisecond target face stimuli conditions before the 5000 millisecond target face stimuli in order to prevent biasing of short presentation time processing by detail encoding that otherwise could occur if longer presentation time blocks appeared first. However, whether the upright or inverted faces appeared first or second varied for both stimuli times. Within the BDD and healthy control groups, the order of presentation was counterbalanced using a modified Latin-square design to account for potential order effects.
Data Analysis
The “inversion effect” is defined as the mean difference in response times or accuracy between the trials presenting inverted faces and those presenting upright faces (Farah et al., 1995; Leder et al., 2000; Valentine, 1988). Our primary hypothesis involved differences in inversion effects between the BDD and control groups. We compared the inversion effects between the groups in each of the 5000 msec and 500 msec periods and also contrasted the two durations, thereby assessing whether the estimated difference in inversion effects between BDD and control interacted with duration.
We applied linear and logistic mixed effects models with a random subject effect to the repeated measures on response times and accuracy, respectively. Mixed models account for the correlation induced by obtaining multiple measurements on subjects, thereby increasing power to test hypotheses about fixed effects, providing flexibility with respect to handling of missing data, and complex covariance structures. In order to test the hypotheses of interest, the models fit separate means to the four experimental conditions in each of the BDD and control groups. Overall the models included factors for BDD/control group, experimental condition, and their interaction. A main effect of gender was not significant and did not change our conclusions regarding the hypotheses of interest; we have therefore omitted it from the final model in order to conserve power and maximize stability of the estimates. We also explored learning during the task, whereby subjects respond faster and/or more accurately over the course of the experimental condition, through the inclusion of fixed effects for trial. Inference on specific hypotheses regarding face inversion effects and their interaction with stimuli duration and BDD/control group were assessed through t-tests on linear combinations of the fixed effects. Response times were analyzed on the log scale. All reported p-values are two-sided.
Results
Demographics and Psychometrics
Table 1 summarizes the demographic and psychometric data for both groups. One BDD subject had comorbid MDD, two had GAD, four had both MDD and GAD, and one had both dysthymic disorder and GAD. The BDD symptoms were the primary concern in every subject. All 18 BDD subjects had preoccupations with perceived facial defects.
Table 1.
Demographics and psychometric scoresa
| BDD group (N=18) |
Control group (N=17) |
p valueb | |
|---|---|---|---|
| Age | 28.56±6.7 | 28.12±5.4 | 0.833 |
| Gender (F/M) | 10/8 | 10/7 | 1 |
| Years of education | 15.5±2.7 | 16.76±2.3 | 0.14 |
| BDD-YBOCS score | 28.94±5.4 | N/A | N/A |
| HDRS score | 10.5±7.5 | 1.35±1.5 | <0.001 |
| HARS score | 12.39±8.1 | 1.47±1.3 | <0.001 |
Abbreviations: BDD: body dysmorphic disorder; BDD-YBOCS: BDD version of the Yale-Brown Obsessive-Compulsive Scale (scores range from 0-48); HDRS: 17-item Hamilton Depression Rating Scale (scores range from 0-53); HARS: Hamilton Anxiety Rating Scale (scores range from 0-56)
Data are given as mean±SD unless otherwise indicated.
two-sample t-test for all comparisons except gender (X2 test)
Response Time Analysis (Fig. 3 and Table 2)
Table 2. Estimated Inversion Effects on Response Times.
a. Estimated inversion effect for each group and stimulus duration. For the 5000 msec duration, we averaged over the 28 trials because of the learning effect during the inverted 5000 msec period. b. Contrasts testing specific hypotheses of interest on inversion effects. a.-b. Estimated % change: estimates from log-transformed outcomes are interpreted as percent changes in the original msec scale, e.g. (a.) for BDD 5000 msec, response times on inverted faces are 23% longer on average than those of upright faces; (b.) the inversion effect in BDD 5000 msec is 8% shorter than that of control 5000 msec.
| a. Inversion Effect | Estimated % Change |
Estimated Log Change |
Std Error (Log scale) |
p-value |
|---|---|---|---|---|
| BDD 5000 msec | + 23% | .206 | .0287 | <.0001 |
| Control 5000 msec | + 34% | .291 | .0295 | <.0001 |
| BDD 500 msec | + 18% | .162 | .0216 | <.0001 |
| Control 500 msec | + 11% | .107 | .0222 | <.0001 |
| b. Inversion Effect Contrasts | Estimated % Change |
Estimated Log Change |
Std Error (Log scale) |
p-value |
| BDD - control during 5000 msec | − 8% | −.0850 | .0387 | .0279 |
| BDD - control during 500 msec | + 6% | .0555 | .0310 | .0733 |
| Difference: BDD - control 5000 - 500 msec |
− 13% | −.141 | .0496 | .0046 |
The inversion effect was evident in both groups, as both BDD and control subjects responded more slowly on average in the inverted phases of the longer and shorter-duration stimuli. In addition, subjects in both the BDD and control groups responded significantly more slowly on average when they were allowed more time in both the inverted and upright phases of the experiment.
The estimated contrasts support our hypotheses that the inversion effect is smaller in the BDD subjects during the longer-duration stimuli (p=.028). Inversion effects during the shorter-duration stimuli are not significantly different between BDD and control groups (p=.073). The contrast between the two BDD-control inversion effects in the long and short periods is also significant (p=.0046). The estimates and contrasts in Table 2 are on the log scale and have interpretations as percent changes in response times on the original msec scale. For example, during the longer-duration stimuli response times on inverted faces are about 23% longer on average for BDD subjects and 34% longer on average for controls.
Accuracy Analysis
Overall, subjects had high rates of face recognition. Table 3 displays the raw data accuracy rates among BDD and control subjects under the four experimental conditions and estimated inversion effects. Accuracy rates are highest during the upright 5000 msec period and lowest during the inverted 500 msec period.
Table 3. Accuracy Rates and Inversion Effects.
Accuracy Rates and Inversion Effects. a. Raw data accuracy rates in the BDD and control groups under the four experimental conditions. A learning effect was not evident so is absent from this model. b. Subject-specific inversion effects on the odds ratio scale (i.e. the odds of a correct response for the inverted faces relative to the upright faces). All odds ratios are significant less than 1 (p-values < .0001), indicating significant inversion effects. However, inversion effects do not differ significantly between BDD and control during either of the two durations (p>0.05).
| a. Experimental Condition | Group | Accuracy Rate in Sample |
|---|---|---|
| Inverted 5000 msec | Control | .86 |
| BDD | .87 | |
| Inverted 500 msec | Control | .72 |
| BDD | .76 | |
| Upright 5000 msec | Control | .94 |
| BDD | .95 | |
| Upright 500 msec | Control | .84 |
| BDD | .88 | |
| b. Inversion Effect | Estimated Odds Ratio | |
| BDD 5000 msec | .32 | |
| Control 5000 msec | .38 | |
| BDD 500 msec | .42 | |
| Control 500 msec | .46 | |
The inversion effects in the logistic model represent log-odds ratios of accurate face recognition in the inversion period compared to the upright period of the same duration. These estimates are all significantly different from 1 (p-values <.0001), meaning that both BDD and control subjects perform considerably better during the upright phases of the experiment (accuracy rates on upright stimuli are about 9% and 16% higher in both group for the longer-duration and shorter-duration stimuli, respectively). The inversion effects do not differ significantly between BDD and control during either of the two durations (p>.05). In order to estimate the main effects of group and experimental condition, we fit a second model with no group by condition interaction. The odds of a correct response for a typical BDD subject are 25% higher than those of a typical control subject, although this odds ratio is not significantly different from 1.
Discussion
This experiment demonstrated that individuals with BDD show less of an inversion effect than healthy controls for long duration (5000 msec) but not short duration (500 msec) stimuli. This difference appeared to be explained by 8.5% faster response times in BDD subjects for the long duration inverted faces stimuli. These findings confirmed our hypothesis with respect to response time but not for accuracy rates, which were not significantly different between groups.
Reduced face inversion effect in individuals with BDD relative to healthy controls for the long duration stimuli may be due to a general propensity to engage in highly detailed and piecemeal processing. In healthy controls, processing of upright faces is believed to occur holistically while presentation of inverted faces engages slower processing of details and components, due to the lack of a holistic “template” for inverted faces (Freire et al., 2000). If individuals with BDD encode details more rapidly than controls whether upright or inverted, this would result in less of a difference in response times for the different orientations.
The interpretation of greater detail-oriented processing in BDD is supported by a previous functional magnetic resonance imaging (fMRI) study that examined brain activation patterns in response to viewing others’ faces (Feusner, Townsend, Bystritsky, & Bookheimer, 2007). The experiment utilized normal, high, and low spatial frequency photographs of other peoples’ faces in order to isolate specific neural pathways responsible for different spatial frequency domains. Individuals with BDD demonstrated abnormal left hemisphere hyperactivity in an extended face-processing network including temporal, parietal and inferior frontal gyrus regions. This occurred even for low spatial frequency images, which normally are processed by the right hemisphere (Hellige, 1996). Predominant left hemisphere activity suggests greater detail encoding and analysis relative to configural processing for faces. Clinically, while individuals with BDD may perceive details accurately (and perhaps more accurately than healthy controls (Stangier, Adam-Schwebe, Muller, & Wolter, 2008)), the perceptual distortions may come from a failure to integrate these details into a visual gestalt.
The reduced face inversion effect on response times for BDD subjects in the current study occurred only for long but not short duration stimuli. The fact that there were minimal and nonsignificant differences in inversion effect on response time for short duration stimuli suggest that BDD subjects and healthy controls may equally engage processing of holistic and configural information, conveyed by low spatial frequency information, when given a brief presentation. Long duration stimuli, on the other hand, may have allowed time for encoding of details, conveyed by high spatial frequency information. If the BDD group has a greater propensity for processing high spatial frequency information (and/or lesser low spatial frequency processing) this would confer an advantage for inverted faces, which require detail processing (Farah et al., 1995). An alternative explanation is that BDD subjects may not have relied on normal holistic/configural processing during the short duration period but instead employed abnormally rapid detail processing, although it is unlikely given the 500 msec timeframe.
Another (possibly related) explanation for differences in inversion effect for long but not short durations is that longer times allow for multiple eye fixations. Only the longer duration stimuli would therefore have allowed sufficient time for visual scanning and subsequent detail encoding. If the BDD group has a greater propensity for visual scanning and subsequent detail encoding, this would confer an advantage for the inverted faces. Short durations stimuli did not allow sufficient time for multiple eye fixations, which may have resulted in normal holistic/configural processing in the BDD group and hence no significant differences from controls. Future studies with eye-tracking would be helpful to determine if this is occurring.
Perceived saliency of facial features may have driven enhanced detail processing in the BDD group and therefore differences in inversion effects. Barton et al. (2001) found that salient features (especially the mouth, for healthy controls) were less susceptible to inversion effects than non-salient features (Barton et al., 2001). Individuals with BDD may find multiple features and details of others’ faces salient (e.g. skin blemishes, lines, irregularities of the contour of the nose) because when viewing others they typically engage in comparisons to their own appearance, of which they typically have multiple concerns (Phillips, 2005). Greater saliency of multiple facial features would then invoke greater part decomposition and detail encoding in the BDD group in this study, consistent with the previous explanations. Only longer duration stimuli may have allowed time for this, resulting in differences in inversion effects between groups.
To our knowledge no previous study has specifically tested the face inversion effect in BDD. However, several studies have investigated face identity recognition of upright faces. Using slightly different versions of face-matching tasks, these studies have demonstrated similar findings as the current study; individuals with BDD do not appear to differ greatly from healthy controls in terms of response time or accuracy with respect to behavioral performance on upright faces (Buhlmann et al., 2004; Feusner, Bystritsky, Hellemann, & Bookheimer, in press; Feusner et al., 2007). However, because these studies all used stimulus durations of several seconds, it is possible that the BDD group may have utilized rapid local processing while controls may have relied primarily on global processing for the same task.
No other studies, to our knowledge, have investigated inverted face processing in disorders related to BDD such as obsessive-compulsive disorder, social phobia, or eating disorders. However, several studies have tested the face inversion effect in individuals with autistic spectrum disorders. Some of these (Bookheimer, Wang, Scott, Sigman, & Dapretto, 2008; Hobson, Ouston, & Lee, 1988; Langdell, 1978; Rose et al., 2007; Tantam, Monaghan, Nicholson, & Stirling, 1989) but not all (Teunisse & de Gelder, 2003) have demonstrated lesser inversion effect relative to healthy controls, attributed to enhanced featural processing and a local bias and/or lesser contextual processing.
Interestingly, children also show reduced face inversion effect, relative to adults, which similarly appears to be associated with a more piecemeal strategy (Aylward et al., 2005; Joseph et al., 2006). This raises the possibility that the abnormalities in visual processing of faces in BDD seen in this study may stem from aberrant neural development.
With respect to accuracy in the current study, both groups demonstrated an inversion effect. However, our hypothesis that there would be less of an inversion effect for the 5000 msec but not the 500 msec condition was not confirmed. A possible ceiling effect for the long duration stimuli may limit our ability to interpret this finding. There may have also been a tradeoff between accuracy and response time, as both were lower across groups for short duration stimuli.
Findings from this study may have clinical implications. BDD patients typically perceive defects of their own appearance, often a facial feature, which are not noticeable or appear minor to others. They also frequently check appearance features of others to compare to their own (Phillips, 2005). The findings from this study add to converging (although preliminary) evidence suggesting that disordered visual information processing may not be limited to their own face, but may extend to others’ faces as well (Feusner et al., in press; Feusner et al., 2010; Feusner et al., 2007; Yaryura-Tobias et al., 2002). This suggests a more general visual processing diathesis, further supported by abnormal visuospatial performance for complex figures (Deckersbach et al., 2000). However, it is still not clear if this represents an underlying trait that predisposes individuals to BDD, or a result of the illness itself. In these regards, if the reduced face inversion effect for long duration stimuli found in this study reflects faster detail processing than healthy controls, the question remains if this represents an underlying endophenotype that predisposes individuals to BDD, or if it is a result of habitually engaging in selective attention to details, driven by appearance concerns.
In this study, the random effects statistical models incorporate baseline heterogeneity in subjects’ tendencies to respond slower or faster than the average trend. The small sample size limited our ability to obtain precise estimates of both a more complex covariance structure and the fixed effects simultaneously. However, in exploratory models we did detect evidence of a random learning effect during the inverted 5000 msec period and heteroskedastic serial correlation over the four experimental conditions. This random learning effect occurred during what appeared to be the most difficult period for subjects, implying that there is additional variability in how quickly individuals adapt to the conditions beyond the average learning trend in this period. The serial correlation components suggest that an individual’s responses that are closer together in time are more correlated than those spaced further apart; this phenomenon is stronger in the two shorter-duration periods, when subjects have less time to process each face independently. The estimated fixed effects are virtually unchanged in this more complicated covariance model, but the standard errors are somewhat larger. We have chosen to present the parsimonious version in order to preserve power to test our hypotheses of interest, which we believe is more appropriate to the scope of the present experiment, but plan to pursue this issue in future, more highly-powered studies.
There are several limitations in this study that should be considered. The sample size was not sufficient to separately analyze individuals with comorbid depressive disorders, GAD, or both. Also, the sample size and the fact that there were a high number of covariates due to the four different experimental conditions and the learning effect limited our ability to investigate clinical variables such as anxiety and depression as covariates.
In sum, our data suggests that BDD subjects experience less of an inversion effect on response times compared to controls for long, but not short, stimulus durations. Although there are possible alternative explanations, this may provide evidence of a propensity to process faces in a piecemeal, detail-oriented manner, at least when viewed for longer durations. Clinically, abnormal face processing may explain perceptual distortions in the way that individuals with BDD view their own faces, as well as perhaps the faces of others. Further research involving testing of the inversion effect with other tools such as fMRI or event-related potentials may help to illuminate whether differences stem from encoding and/or recognition processes, or some other phenomenon. Additionally, eye-tracking techniques may provide useful analysis of what may behaviorally mediate abnormalities in facial recognition.
Acknowledgments
Funding for this study was provided by the NIMH and the Obsessive-Compulsive Foundation.
Footnotes
Results presented in part at the 16th Annual Obsessive Compulsive Foundation Conference; August 7th, 2009: Minneapolis, MN
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders : DSM-IV-TR. 4th ed American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Aylward EH, Park JE, Field KM, Parsons AC, Richards TL, Cramer SC, Meltzoff AN. Brain activation during face perception: evidence of a developmental change. J Cogn Neurosci. 2005;17:308–19. doi: 10.1162/0898929053124884. [DOI] [PubMed] [Google Scholar]
- Barton JJ, Keenan JP, Bass T. Discrimination of spatial relations and features in faces: Effects of inversion and viewing duration. Br J Psychol. 2001;92(Part 3):527–549. [PubMed] [Google Scholar]
- Bookheimer SY, Wang AT, Scott A, Sigman M, Dapretto M. Frontal contributions to face processing differences in autism: evidence from fMRI of inverted face processing. J Int Neuropsychol Soc. 2008;14:922–32. doi: 10.1017/S135561770808140X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitmeyer BG. Simple reaction time as a measure of the temporal response properties of transient and sustained channels. Vision Res. 1975;15:1411–2. doi: 10.1016/0042-6989(75)90200-x. [DOI] [PubMed] [Google Scholar]
- Breitmeyer BG, Ganz L. Temporal studies with flashed gratings: inferences about human transient and sustained channels. Vision Res. 1977;17:861–5. doi: 10.1016/0042-6989(77)90130-4. [DOI] [PubMed] [Google Scholar]
- Buhlmann U, Etcoff N, Wilhelm S. Emotional recognition bias for contempt and anger in body dysmorphic disorder. Journal of Psychiatric Research. 2006;40:105–11. doi: 10.1016/j.jpsychires.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Buhlmann U, McNally R, Etcoff N, Tuschen-Caffier B, Wilhelm S. Emotion recognition deficits in body dysmorphic disorder. Journal of Psychiatric Research. 2004;38:201–6. doi: 10.1016/s0022-3956(03)00107-9. [DOI] [PubMed] [Google Scholar]
- Castelhano MS, Mack ML, Henderson JM. Viewing task influences eye movement control during active scene perception. Journal of Vision. 2009;9 doi: 10.1167/9.3.6. [DOI] [PubMed] [Google Scholar]
- Costen NP, Parker DM, Craw I. Effects of high-pass and low-pass spatial filtering on face identification. Percept Psychophys. 1996;58:602–12. doi: 10.3758/bf03213093. [DOI] [PubMed] [Google Scholar]
- Deckersbach T, Savage C, Phillips K, Wilhelm S, Buhlmann U, Rauch S, Baer L, Jenike M. Characteristics of memory dysfunction in body dysmorphic disorder. Journal of the International Neuropsychological Society. 2000;6:673–81. doi: 10.1017/s1355617700666055. [DOI] [PubMed] [Google Scholar]
- Farah MJ, Tanaka JW, Drain HM. What causes the face inversion effect? J Exp Psychol Hum Percept Perform. 1995;21:628–34. doi: 10.1037//0096-1523.21.3.628. [DOI] [PubMed] [Google Scholar]
- Faravelli C, Salvatori S, Galassi F, Aiazzi L, Drei C, Cabras P. Epidemiology of somatoform disorders: a community survey in Florence. Soc Psychiatry Psychiatr Epidemiol. 1997;32:24–9. doi: 10.1007/BF00800664. [DOI] [PubMed] [Google Scholar]
- Feusner JD, Bystritsky A, Hellemann G, Bookheimer S. Impaired processing of faces with emotional expresions in body dysmorphic disorder. Psychiatry Research. doi: 10.1016/j.psychres.2009.01.016. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feusner JD, Moody T, Townsend J, McKinley M, Hembacher E, Moller H, Bookheimer S. Abnormalities of visual processing and fronto-striatal systems in body dysmorphic disorder. Archives of General Psychiatry. 2010;67:197–205. doi: 10.1001/archgenpsychiatry.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feusner JD, Townsend J, Bystritsky A, Bookheimer S. Visual information processing of faces in body dysmorphic disorder. Arch Gen Psychiatry. 2007;64:1417–25. doi: 10.1001/archpsyc.64.12.1417. [DOI] [PubMed] [Google Scholar]
- Freire A, Lee K, Symons LA. The face-inversion effect as a deficit in the encoding of configural information: direct evidence. Perception. 2000;29:159–70. doi: 10.1068/p3012. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. Diagnosis and rating of anxiety. British Journal of Psychiatry. 1969;3:76–79. [Google Scholar]
- Hellige JB. Hemispheric asymmetry for visual information processing. Acta Neurobiol Exp (Wars) 1996;56:485–97. doi: 10.55782/ane-1996-1151. [DOI] [PubMed] [Google Scholar]
- Hobson R, Ouston J, Lee A. What’s in a face? The case of autism. British Journal of Psychology. 1988;79:441–453. doi: 10.1111/j.2044-8295.1988.tb02745.x. [DOI] [PubMed] [Google Scholar]
- Hsiao JHW, Cottrell G. Two Fixations Suffice in Face Recognition. Psychological Science. 2008;19:998–1006. doi: 10.1111/j.1467-9280.2008.02191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph JE, Gathers AD, Liu X, Corbly CR, Whitaker SK, Bhatt RS. Neural developmental changes in processing inverted faces. Cogn Affect Behav Neurosci. 2006;6:223–35. doi: 10.3758/cabn.6.3.223. [DOI] [PubMed] [Google Scholar]
- Koran LM, Abujaoude E, Large MD, Serpe RT. The prevalence of body dysmorphic disorder in the United States adult population. CNS Spectr. 2008;13:316–22. doi: 10.1017/s1092852900016436. [DOI] [PubMed] [Google Scholar]
- Langdell T. Recognition of faces: an approach to the study of autism. Journal of Child Psychology and Psychiatry. 1978;19:255–268. doi: 10.1111/j.1469-7610.1978.tb00468.x. [DOI] [PubMed] [Google Scholar]
- Leder H, Bruce V. When inverted faces are recognized: the role of configural information in face recognition. Q J Exp Psychol A. 2000;53:513–36. doi: 10.1080/713755889. [DOI] [PubMed] [Google Scholar]
- Mancuso SG, Knoesen NP, Castle DJ. Delusional versus nondelusional body dysmorphic disorder. Comprehensive Psychiatry. doi: 10.1016/j.comppsych.2009.05.001. (in press) [DOI] [PubMed] [Google Scholar]
- Maurer D, Le Grand R, Mondloch CJ. The many faces of configural processing. Trends in Cognitive Sciences. 2002;6:255–260. doi: 10.1016/s1364-6613(02)01903-4. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Winocur G, Behrmann M. What is special about face recognition? Nineteen experiments on a person with visual object agnosia and dyslexia but normal face recognition. Journal of Cognitive Neuroscience. 1997;9:555–604. doi: 10.1162/jocn.1997.9.5.555. [DOI] [PubMed] [Google Scholar]
- Norman J, Ehrlich S. Spatial frequency filtering and target identification. Vision Res. 1987;27:87–96. doi: 10.1016/0042-6989(87)90145-3. [DOI] [PubMed] [Google Scholar]
- Otto MW, Wilhelm S, Cohen LS, Harlow BL. Prevalence of body dysmorphic disorder in a community sample of women. Am J Psychiatry. 2001;158:2061–3. doi: 10.1176/appi.ajp.158.12.2061. [DOI] [PubMed] [Google Scholar]
- Peyrin C, Mermillod M, Chokron S, Marendaz C. Effect of temporal constraints on hemispheric asymmetries during spatial frequency processing. Brain Cogn. 2006;62:214–20. doi: 10.1016/j.bandc.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Phillips KA. Psychosis in body dysmorphic disorder. J Psychiatr Res. 2004;38:63–72. doi: 10.1016/s0022-3956(03)00098-0. [DOI] [PubMed] [Google Scholar]
- Phillips KA. The Broken Mirror. Oxford University Press; New York: 2005. [Google Scholar]
- Phillips KA, Atala KD, Pope HG. Diagnostic instruments for body dysmorphic disorder. New Research Program and Abstracts. American Psychiatric Association 148th Annual Meeting; Miami American Psychiatric Association; 1995. p. 157. [Google Scholar]
- Phillips KA, Coles ME, Menard W, Yen S, Fay C, Weisberg RB. Suicidal ideation and suicide attempts in body dysmorphic disorder. J Clin Psychiatry. 2005;66:717–25. doi: 10.4088/jcp.v66n0607. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Diaz SF. Gender differences in body dysmorphic disorder. J Nerv Ment Dis. 1997a;185:570–7. doi: 10.1097/00005053-199709000-00006. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Hollander E, Rasmussen SA, Aronowitz BR, DeCaria C, Goodman WK. A severity rating scale for body dysmorphic disorder: development, reliability, and validity of a modified version of the Yale-Brown Obsessive Compulsive Scale. Psychopharmacol Bull. 1997b;33:17–22. [PubMed] [Google Scholar]
- Phillips KA, Hollander E, Rasmussen SA, Aronowitz BR, DeCaria C, Goodman WK. A severity rating scale for body dysmorphic disorder: development, reliability, and validity of a modified version of the Yale-Brown Obsessive Compulsive Scale. Psychopharmacology Bulletin. 1997c;33:17–22. [PubMed] [Google Scholar]
- Phillips KA, Menard W. Suicidality in body dysmorphic disorder: a prospective study. Am J Psychiatry. 2006;163:1280–2. doi: 10.1176/appi.ajp.163.7.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rief W, Buhlmann U, Wilhelm S, Borkenhagen A, Brahler E. The prevalence of body dysmorphic disorder: a population-based survey. Psychological Medicine. 2006;36:877–85. doi: 10.1017/S0033291706007264. [DOI] [PubMed] [Google Scholar]
- Rose FE, Lincoln AJ, Lai Z, Ene M, Searcy YM, Bellugi U. Orientation and affective expression effects on face recognition in Williams syndrome and autism. J Autism Dev Disord. 2007;37:513–22. doi: 10.1007/s10803-006-0200-4. [DOI] [PubMed] [Google Scholar]
- Schyns P, Oliva A. Dr. Angry and Mr. Smile: when categorization flexibly modifies the perception of faces in rapid visual presentations. Cognition. 1999;69:243–65. doi: 10.1016/s0010-0277(98)00069-9. [DOI] [PubMed] [Google Scholar]
- Schyns PG, Oliva A. From blobs to boundary edges - evidence for time-scale-dependent and spatial-scale-dependent scene recognition. Psychological Science. 1994;5:195–200. [Google Scholar]
- Sergent J. Influence of task and input factors on hemispheric involvement in face processing. J Exp Psychol Hum Percept Perform. 1985;11:846–61. doi: 10.1037//0096-1523.11.6.846. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Harnett-Sheehan K, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar G. The Mini International Neuropsychiatric Interview (M.I.N.I.): The Development and Validation of a Structured Diagnostic Psychiatric Interview. Journal of Clinical Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Stangier U, Adam-Schwebe S, Muller T, Wolter M. Discrimination of facial appearance stimuli in body dysmorphic disorder. J Abnorm Psychol. 2008;117:435–43. doi: 10.1037/0021-843X.117.2.435. [DOI] [PubMed] [Google Scholar]
- Tantam D, Monaghan L, Nicholson H, Stirling J. Autistic children’s ability to interpret faces: a research note. Journal of Child Psychology and Psychiatry. 1989;30:623–630. doi: 10.1111/j.1469-7610.1989.tb00274.x. [DOI] [PubMed] [Google Scholar]
- Teunisse JP, de Gelder B. Face processing in adolescents with autistic disorder: the inversion and composite effects. Brain Cogn. 2003;52:285–94. doi: 10.1016/s0278-2626(03)00042-3. [DOI] [PubMed] [Google Scholar]
- Valentine T. Upside-down faces: a review of the effect of inversion upon face recognition. Br J Psychol. 1988;79(Pt 4):471–91. doi: 10.1111/j.2044-8295.1988.tb02747.x. [DOI] [PubMed] [Google Scholar]
- Veale D, Boocock A, Gournay K, Dryden W, Shah F, Willson R, Walburn J. Body dysmorphic disorder. A survey of fifty cases. British Journal of Psychiatry. 1996;169:196–201. doi: 10.1192/bjp.169.2.196. [DOI] [PubMed] [Google Scholar]
- Yaryura-Tobias J, Neziroglu F, Chang R, Lee S, Pinto A, Donohue L. Computerized perceptual analysis of patients with body dysmorphic disorder. CNS Spectrums. 2002;7:444–446. doi: 10.1017/s1092852900017958. [DOI] [PubMed] [Google Scholar]
- Yin R. Looking at upside-down faces. Journal of Experimental Psychology. 1969;81:141–145. [Google Scholar]