Abstract
Lipids, particularly phospholipids, are fundamental to central nervous system (CNS) tissue architecture and function. Endogenous polyunsaturated fatty acid chains of phospholipids possess cis-double bonds each separated by one methylene group. These phospholipids are very susceptible to free-radical attack and oxidative modifications. A combination of analytical methods including different versions of chromatography and mass spectrometry allows obtaining detailed information on the content and distribution of lipids and their oxidation products thus constituting the newly emerging field of oxidative lipidomics. It is becoming evident that specific oxidative modifications of lipids are critical to a number of cellular functions, disease states and responses to oxidative stresses. Oxidative lipidomics is beginning to provide new mechanistic insights into traumatic brain injury (TBI) which may have significant translational potential for development of therapies in acute CNS insults. In particular, selective oxidation of a mitochondria-specific phospholipid, cardiolipin, has been associated with the initiation and progression of apoptosis in injured neurons thus indicating new drug discovery targets. Further, imaging mass-spectrometry represents an exciting new opportunity for correlating maps of lipid profiles and their oxidation products with structure and neuropathology. This review is focused on these most recent advancements in the field of lipidomics and oxidative lipidomics based on the applications of mass-spectrometry and imaging mass-spectrometry as they relate to studies of phospholipids in TBI.
Lipids, particularly phospholipids, are indispensible parts of cell membranes where they play the major structural role as parts of non-raft-organized bilayer and protein annulus zones, and as precursors of diverse regulators of intra- and extracellular metabolism. The distinction of polyunsaturated fatty acid residues from less unsaturated fatty acids – that significantly defines their roles in membranes - is the presence of a repeating =CH-CH(2)-CH= unit that produces an extremely flexible structure rapidly isomerizing through conformational states (Wassall and Stillwell 2008). This essentiality of polyunsaturated phospholipids is also associated with the vulnerability of membranes to oxidative modifications and damage. The development of contemporary mass spectrometry (MS) designated the major breakthroughs in our understanding of structure-activity relationships of different membrane lipids. More specifically, the technological advancements facilitated the emergence of a new field of research and knowledge – lipidomics - opening remarkable opportunities for sensitive quantitative and structural analysis of individual molecular species of phospholipids and their role in cellular metabolism. This review is focused on the most recent advancements in the field of lipidomics and oxidative lipidomics based on the applications of mass-spectrometry and imaging mass-spectrometry as it relates to studies of phospholipids in traumatic brain injury (TBI).
Diversity of Brain Lipids
Lipids are fundamental to central nervous system (CNS) tissue architecture and function. This is evident on a gross level based on lipid content and tissue dry weight, where CNS tissue has the highest lipid content next to adipose tissue (Han 2007) and is further supported by the fact that brain lipids constitute more than half of the dry weight in human brain (Piomelli et al. 2007). Brain lipid composition and metabolism change during development and these qualities can vary with anatomical region (Rouser et al. 1971). CNS tissue contains a diverse variety of complex lipids including neutral lipids (such as cholesterol and acylglycerols), glycolipids (such as galactosylceramide and gangliosides) and phospholipids (such as phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, sphingolipids, etc). Due to the fact that phospholipids are the major building blocks of plasma and intracellular membranes, they account for approximately 25% of the dry weight of the adult rat brain (Yusuf 1992). Phospholipids are precursors of important signaling molecules such as neuroprotectins and resolvins, which are formed by multistage oxygenation of docosahexaenoic acid and eicosapentaenoic acid (Marcheselli et al. 2003). In addition to their role in membrane architecture and signaling, phospholipids also play a critical role in sub-cellular organelle function.
Phospholipids consist of a glycerol backbone, fatty acid chains, and a phosphoester-connected headgroup (Figure 1). Because of the hydrophobic nature of their fatty acid chains, most phospholipids are found in various cellular and sub-cellular membranes with their hydrophilic headgroups exposed to the aqueous environment. Different headgroups define each phospholipid class and their properties (http://www.lipidmaps.org/). Different classes of phospholipids will have various combinations of fatty acid chains that can be esterified to the sn-1 and sn-2 positions on the glycerol backbone. Fatty acids can be released by phospholipase A from phospholipids and have important roles in cell signaling and metabolism as shown in Figure 2. There are several fatty acid residues that are more common than others in brain phospholipids (Table1) (Fahy et al. 2005; Fahy et al. 2009). Just among the eight most common fatty acid chains in brain, this gives 82=64 possible molecular species for each class. Cardiolipin (CL), a mitochondria specific phospholipid, on the other hand, has four fatty acid chains which allows for the formation of a diverse number of possible molecular species, usually in excess of one hundred (Bayir et al. 2007; Cheng et al. 2008; Tyurin et al. 2008b).
Figure 1.
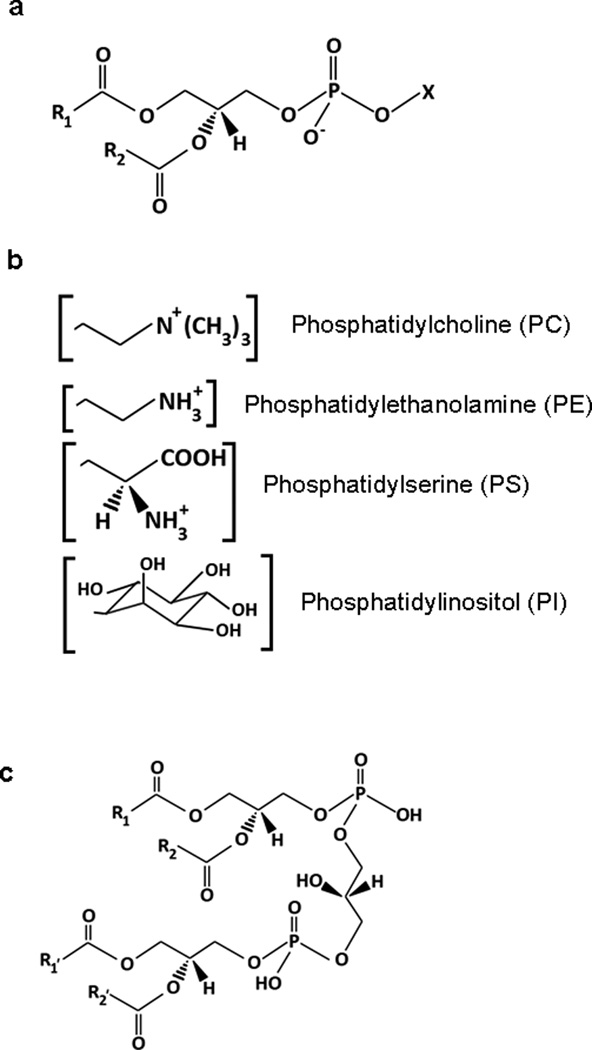
Glycerophospholipid structure. (a) Fatty acyl chains in the sn-1 (R1) and sn-2 (R2) positions attach directly to the glycerol backbone, while the head group (X) attaches through the phosphate group. Although fatty acyl linkages are shown, both ether and plasmalogen (vinyl ether) bonds can occur in the sn-1 position. (b) Several common glycerophospholipid headgroups. (c) Cardiolipin (CL) structure. Four fatty acyl chains (sn-1, sn-2, sn-1’, sn-2’) can provide much molecular diversity.
Figure 2.

Fatty acids can be released by phospholipase A from phospholipids and have important roles in cell signaling and metabolism.
Table 1.
Common endogenous fatty acid chains and their nomenclature (with oxidizable marked)
| 12:0 | Dodecanoic | Lauroyl | saturated | non-oxidizable |
| 14:0 | Tetradecanoic | Myristoyl | saturated | non-oxidizable |
| 16:0 | Hexadecanoic | Palmitoyl | saturated | non-oxidizable |
| 18:0 | Octadecanoic | Stearoyl | saturated | non-oxidizable |
| 18:1 (n-9) | 9-Octadeceanoic | Oleoyl | monounsaturated | non-oxidizable |
| 18:2 (n-6) | 9,12-Octadecadienoic | Linoleoyl | polyunsaturated | oxidizable |
| 20:4 (n-6) | 5,8,11,14-Eicosatetraenoic | Arachidonoyl | polyunsaturated | oxidizable |
| 22:5 | 7,10,13,16,19-Docosapentaenoic | Docosapentaenoyl | polyunsaturated | oxidizable |
| 22:6 (n-3) | 4,7,10,13,16,19-Docosahexenoic | Docosahexaenoyl | polyunsaturated | oxidizable |
The shorthand notation is given by number of carbons followed by number of double bonds. Thus “PC(16:0/16:0)” is dipalmitoyl phosophatidylcholine, and “PC(32:0)” is a combination of any two saturated chains that add up to 32 carbons. All the chains that posses two or more double bonds form conjugated systems and thus are potential targets for oxidative modification.
Phospholipids contain a mixture of polyunsaturated, monounsaturated and saturated fatty acid chains. Usually, polyunsaturated fatty acid residues are located in the sn-2 position whereas saturated ones are in the sn-1 position of phospholipids. Under physiological conditions monounsaturated and saturated fatty acids do not undergo oxidation. Endogenous polyunsaturated chains on sn-2 position possess cis-double bonds each separated by one CH2 group. These hydrogens are very reactive to free-radical attack, making polyunsaturated fatty acids subject to oxidative modification (Huvaere et al. 2010). While the proportion of highly oxidizable polyunsaturated sn-2 fatty acid residues with 4-, 5-, and 6-double bonds in the brain varies according to class, these highly oxidizable substrates are always in abundance compared to the actual presence of peroxidized phospholipids under normal physiological conditions and even after TBI or chronic disease conditions (Tyurin et al.; Benjamins et al. 2006; Bayir et al. 2007; Tyurin et al. 2008a). This availability of peroxidation substrates is not usually a limiting factor that determines specificity of phospholipid peroxidation (Bayir et al. 2007; Tyurin et al. 2008b).
The distribution and molecular species of phospholipids in the brain varies depending on the developmental stage, anatomical, cellular and subcellular localization. For example, the major species of phospholipids in synaptosomes in postnatal day (PND) 17 rat consist of phosphatidylcholine (PC) and phosphatidylethanolamine (PE) (Bayir et al. 2007) followed by phosphatidylserine (PS) and phosphatidylinositol (PI). The major molecular species of PC in turn are PC(16:0/16:0), PC(16:0/18:1), and PC(16:1/20:3) (Tyurin et al.). Imaging mass spectrometry (IMS) studies further show that PC(18:0/18:1) localizes mostly to the gray matter while PC(16:0/16:0) localizes mostly to the white matter (Wang et al. 2007; Wang et al. 2008).
In addition to the anatomical (both cellular and sub-cellular) and developmental differences, phospholipid class differences, differences in diversity within each class (especially in the case of CL) and susceptibility to oxidation need to be considered and assessed. Therefore, the demands for detailed analysis of lipid molecular species in brain tissue are daunting. Subtle differences in fatty acid chains can impart drastically different oxidative properties to phospholipids and only certain species of phospholipids appear to play a role in oxidative signaling (Greenberg et al. 2006; Hazen 2008). Therefore, a technique that provides both molecular and spatial information of phospholipid species becomes mandatory.
Lipidomics and Mass Spectrometry
Lipidomics is a “systems-based study of all lipids, the molecules with which they interact, and their function within the cell” (Watson 2006). Lipidomics integrates various ”front end” liquid chromatography (LC) methods used for the separation of lipids and multiple “back end” technologies such as MS for their detection, identification and characterization. The field of lipidomics has experienced a heightened interest in the last several years due to its critical role in health and disease (Han and Gross 2003; Tyurin et al. 2008a).
Mass spectrometry – the most powerful contemporary analytical approach in lipidomics - is based on the detection of ions (as charged lipid molecules) after their separation within an electromagnetic field. Typically, MS instruments consist of an ion source, a mass analyzer and a detector. MS as a scientific technique has existed at least since J.J. Thompson's discovery of isotopes at the turn of the 20th century, and plays a prominent role in diverse chemical fields such as petroleum, nuclear, environmental, forensic and archeological chemistry (Dass 2007; Hillenkamp and Peter-Katalinic 2007; Ekman 2009). It was not until the development of soft ionization techniques in the late 1980's that the field of biological MS blossomed (Griffiths 2008). Soft ionization allowed the direct examination of intact biomolecules without splitting them into multiple fragments. Gas chromatography-MS (GC-MS) has also been used for many years in the field of lipidomics. However, GC-MS usually requires chemical derivatization for phospholipid analyses which prevents detailed structural information from being determined (Peterson and Cummings 2006). Soft ionization techniques provide information about molecular identity, composition, and oxidation state. The two most prominent ionization techniques are Electrospray Ionization (ESI) and Matrix-Assisted Laser Desorption Ionization (MALDI) (Karas and Hillenkamp 1988; Fenn et al. 1989). They can detect intact peptides, proteins, oligonucleotides, and phospholipids in situ without requiring labeling or chemical derivatization. With tandem instruments and analyses (MS/MS), structural information by selective fragmentation can also be obtained (Domingues et al. 2008). Through IMS, information about the spatial mapping of specific molecular species of phospholipids directly from tissue slices can be realized (Hillenkamp and Peter-Katalinic 2007). Given the remarkable opportunities offered by different versions of MS analysis in neuro-lipidomics, the major emphasis of this review will be placed on the major principles and new developments in this area.
ESI and MALDI
Electrospray requires the sample to be in a liquid state. The sample is sent into the mass analyzer from a heated capillary as a steady stream of charged droplets. Solvent molecules are evaporated during inlet, leaving charged analyte molecules to enter the instrument in the gas phase. During inlet, the sample rapidly transits from ambient pressure and high temperature to high vacuum. This could present a problem with the analysis of oxidized lipids (see below). This method of sample inlet is most often coupled to “continuous scanning” analyzers such as ion traps and/or quadrupoles. It is essential to remove excess salts and buffers prior to ESI analysis, although most methods of lipid extraction include a step that performs this and thus the extract can be analyzed directly by ESI (Han and Gross 2003; Pulfer and Murphy 2003). Frequently, the effluent from a LC column is directly coupled to a mass spectrometer containing an ESI source allowing continuous analysis by LC-MS.
MALDI analysis requires the sample to be dried or otherwise turned into a condensed state. A “matrix” compound, which is usually a small organic molecule exhibiting a strong absorption at a laser light wavelength, is added in large excess to the analyte of interest. DHB (2,5-dihydroxybenzoic acid) has often been used as a matrix for phospholipid analysis (Schiller et al. 2004; Fuchs et al. 2007; Fuchs and Schiller 2008). The matrix is either mixed with the sample (“dried droplet”) or deposited over the sample (“overspotting”). The laser (usually in the UV range) is then pulsed across the sample. With each pulse, the matrix absorbs the laser light. This added energy desorbs it and nearby analyte molecules off the surface in a plume. The laser intensity is regulated to be high enough to excite the matrix compound but low enough to avoid damaging the sample. Rapid charge-transfer reactions take place during or immediately after desorption (Karas et al. 2000). Since the ions are formed in discrete packets with each laser pulse, MALDI is most often coupled to a Time-of-flight (TOF) analyzer. A distinct and significant advantage of MALDI in analysis of oxidatively modified lipids is that the sample is kept at ambient temperature and under high vacuum during analysis, minimizing oxidative artifacts. A disadvantage is that matrix clusters will sometimes interfere with the detection of lower-mass phospholipds, which limits the range of low mass ions that can routinely be detected. MALDI is also used as a rapid “pre-screening” technique prior to the more extensive ESI-LC-MS. A simple MALDI-MS analysis can take as little as a few seconds of instrument time, compared to minutes or hours for full ESI-LC-MS analyses. However, chromatographic separation of lipid classes is sometimes required for complete analysis of mixtures (see below) (Fuchs et al. 2007).
Mass Accuracy, resolution and sensitivity
If different ionization methods determine what samples can be examined, then different mass analyzers determine how well minimal differences in mass-values can be distinguished and identified as individual species of phospholipids (Table 2). For example, the Bruker Daltonik Apex FT-ICR (Ion Cyclotron Resonance) analyzer with a MALDI source can differentiate a mono-oxidation state (+15.9959 Da exact mass) from a structure containing the addition of one carbon and four hydrogens (+16.0313 Da) (Ishida et al. 2004). In theory MALDI and ESI can be used with any analyzer, although most instruments are coupled to one type for their lifetime.
Table 2.
Comparison of Mass Spectrometry instrumentation
| Ionization Method | Resolution for Imaging MS | Mass Range |
|---|---|---|
| ESI | Not possible | Less than 40 kDa |
| DESI | 100–200 microns | Less than 40 kDa |
| MALDI | 10–30 microns | 400 Da – 200+ kDa(2) |
| ME-SIMS | 1–10 microns | Less than 2500 Da |
| SIMS | sub-micron | Less than 1000 Da |
| Analyzer | Mass Resolution(1) | Structural Information |
|---|---|---|
| FT-ICR, Orbitrap | 0.01 – 0.05 Da | Best (MSn) |
| Linear Ion Trap | 0.5 – 1.0 Da | Very Good (MSn) |
| Triple Quadrupole | 0.5 – 1.0 Da | Good (MS/MS with precursor and neutral loss scans) |
| TOF (reflector, TOF/TOF) | 0.1 – 0.2 Da | Good (MS/MS by PSD and CID) |
| TOF (linear) | 1.0 – 3.0Da (but much worse at high masses) | Poor (prompt decay only) |
These values are taken from multiple examples in the literature (see text for exact citations) and are based on what a typical laboratory can routinely achieve. They are meant as a rough guide only. Labs that are experts in a given technique probably can achieve better. Different samples will give different quality of results, and also there is variation in performance within each equipment classification depending on model and manufacturer. (ESI: electrospray ionization, DESI: desorption electrospray ionization, MALDI: matrix assisted laser desorption ionization, SIMS: secondary-ion mass Spectrometry, ME-SIMS: matrix-enhanced SIMS, FT-ICR: Fourier-transform ion cyclotron resonance mass spectrometry, TOF: time of flight, PSD: post source decay, CID: collision induced dissociation).
1: Assumed for a typical 750 Da Phospholipid, different masses will give different resolutions
2: Matrix clusters tend to put a lower limit on MALDI, very much dependent on choice of matrix and amount used
All soft ionization techniques depend on charge transfer, and different analytes have different proton affinities. Acidic phospholipids will tend to take negative charges, and basic ones positive charges. Both MALDI and ESI can analyze either positively or negatively charged ions (positive and negative modes), and the resulting lipid spectra look drastically different in different modes (Han and Gross 2003; Schiller et al. 2007; Fuchs et al. 2009). Therefore, direct quantitative comparisons between different classes of lipids based on signal intensities are not practical. However, relative changes in abundances are easily detectable, and thus comparisons between different samples can be performed. This is seen in “shotgun lipidomics” for identification and relative quantification of multiple molecular species (Han et al. 2006; Han et al. 2007). Addition of an internal standard of the same lipid class (which will thus ionize with similar efficiency) allows for adequate quantification.
Lipid mixtures can give signal suppression, especially with MALDI analyses. If one analyte is in high excess over another, then competition for, or suppression of, available charges may take place. This will reduce or eliminate the signal from the less abundant analyte. This is important for two reasons: first, oxidized lipids are in relatively low abundance versus non-oxidized (see below), and second, anionic lipids that may be key targets of oxidative damage, such as CL and PS, are usually in lower abundance than zwitter-ionic lipids (like PC).
Suppression of anionic lipids by PC in MALDI has been known for many years, and hampers direct analysis of anionic lipids in mixtures (Petkovic et al. 2001; Schiller et al. 2002). Off-line separation and collection of the lipids by TLC or LC prior to analysis is one alternative, although the time and effort required to do this eliminates the advantage of speed that MALDI offers over ESI (Fuchs et al. 2009). Suppression can become a problem when performing IMS for anionic lipids in tissue, since prior chromatographic separation is not possible. However, there are several MALDI-based approaches that do not use off-line separation and collection that can be utilized. One approach is the use of disposable pipette-tip solid phase columns (Emerson et al. 2010). This allows PC to be selectively removed in a simple and rapid way. Another is on-target washing to selectively remove some phospholipids, although this has mainly been used for IMS applications (Seeley et al. 2008). Yet another approach directly combines MALDI and TLC. The lipids are separated on the TLC plate, which is then sprayed with matrix and directly analyzed by MALDI (Fuchs et al. 2007; Stübiger et al. 2009). This not only eliminates sample loss and the time consuming step of sample collection, but also utilizes software developed for IMS to detect subtle differences in mobility resulting from different fatty acyl chains (Goto-Inoue et al. 2009). A final approach is to optimize the matrix for detection of different classes of phospholipids (Sun et al. 2008; Teuber et al. 2010).
Oxidative Lipidomics
Oxidative lipidomics is a part of lipidomics and includes: identification and structural characterization of oxidatively modified lipids by MS, quantitative analysis of their content and identification of the mechanisms and pathways through which they interact with other molecules within the cell and contribute to cellular functions. Membranes require polyunsaturated lipids for their structural organization and optimal function. Consequently, membrane structure/function can be compromised when vulnerable lipids are subjected to oxidative modification and degradation. As shown in Figure 3, propagation of free radical lipid peroxidation occurs through abstraction of hydrogen from a lipid molecule. This reaction happens much more readily in polyunsaturated lipids vs. saturated or monounsaturated lipids. In other words, free radical lipid oxidation is specific towards the degree of polyunsaturation of lipids rather than chemical nature of their polar headgroups. With the newly emerging field of oxidative lipidomics, specific oxidative modifications of lipids now appear to be critical to a number of cellular functions, disease states and responses to oxidative stresses. However, the field has been somewhat hampered with regard to the assessment of specific oxidized species of lipids, due to the difficulty in their detection. There are several main reasons for this:
Figure 3.

Peroxidation of membrane phospholipids.
Abundance
Lipid hydroperoxides exist in low abundance. Model systems produce large amounts of lipid hydroperoxides in comparison to what is usually seen within biological systems. These manipulations are usually done with individual (single) lipid species, where the mole percents of oxidized species produced are abundant and easily detected. However, in biological systems, the insults (ie. those causing the production of reactive oxygen species (ROS) which can attack and damage lipids), usually result in minor but very critical amounts of oxidized lipid products.
Stability
The general consensus is that lipid hydroperoxides have some degree of chemical and thermal instability, making the low abundance of these species in biological systems even more difficult to detect. Chemical instability may include the reaction of peroxides with trace metals in one’s “system”, thus promoting further oxidation and degradation. One way around this problem is to chemically derivatize the oxidized species. However, as with any manipulation, this may result in significant loss of the oxidized species in question.
Oxidative propagation
Once formed, lipid hydroperoxides can act as propagating molecules for further oxidations. Other fates of peroxyl radicals include the formation of cyclic peroxides and endoperoxides. In addition, peroxidation of PUFA may produce mixtures of shorter fatty acids with aldehyde, keto, hydroxy or epoxy functional groups. Existence of these species will increase lipid diversity and reduce the likelihood of detecting intact peroxide species at any given moment in time.
Inherent complexity of brain cardiolipin
Unlike many tissues where only a few major species of cardiolipin exist, brain tissue cardiolipin is extremely diverse with respect to the number of CL species as mentioned above. A typical CL spectrum of murine brain exhibits approximately 8–10 major CL clusters, most of which have 8–10 individual CL species associated with them (Bayir et al. 2007; Kiebish et al. 2008c). Figure 4 shows typical spectra of brain CL obtained by ESI and MALDI-MS in comparison to lung and heart CL. Because of this structural diversity, detecting hydroperoxide species of CL by MS is challenging, since masses associated with oxidized species are likely to overlap the natural brain CL species present (Figure 5). While CL plays a critical role in mitochondrial membrane architecture, membrane fluidity and electron transport chain function, these functions, by themselves, cannot account for all of the CL species present in brain tissue. The high degree of diversity seen in brain CL species most likely represents/suggests additional signaling/biological and other yet to be identified roles for brain CL. Interestingly, decreased number of molecular species of CL was observed in brain tumors compared to normal tissue (Kiebish et al. 2008b; Kiebish et al. 2008c; Kiebish et al. 2009). Shotgun lipidomics studies have revealed that synaptic mitochondria had lower levels of CL than nonsynaptic mitochondria (Kiebish et al. 2008a) however there is insufficient information on specific localization (mapping) and alterations of this particular phospholipid species in brain tissue has been reported, especially after acute brain injury. Thus, assessing the alterations in CL species and their associated oxidative events becomes necessary.
Figure 4.

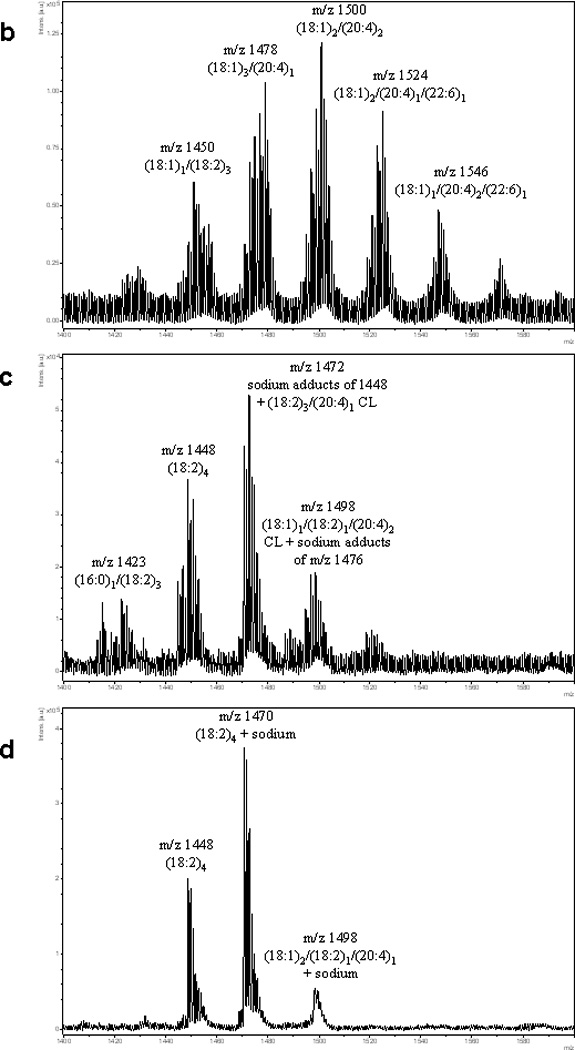
MS Analysis of CL from brain, lung and heart. (a) LC-ESI-MS analysis of CL from murine brain. A murine brain lipid extract was separated by normal-phase HPLC to assess phospholipid classes. CL, which eluted with the 10–12min retention time window, displayed 8 to 10 major clusters of mass ions. Each cluster contains approximately 8–10 different species of CL. For simplicity, fatty acyl chain analysis is shown for only one of the major mass ions in each cluster in panels 4a–4d as determined by MS/MS analysis. Other fatty acyl chain combinations are present. In some cases, multiple fatty acyl combinations were detected for CL species of the same mass. Two additional CL clusters (m/z 1404 and 1592) were detected by the LC-ESI-MS method as compared to MALDI analysis. (b) MALDI analysis of murine brain CL. A murine brain lipid extract was analyzed by negative ion mode MALDI using DHB as a matrix compound. A series of mass ions with a similar degree of complexity is seen for CL using this platform. (c) MALDI analysis of CL species from murine lung in negative ion mode. DHB was utilized as the matrix. A mitochondria enriched fraction from murine lung was extracted for CL analysis. A reduced number of CL species exists as compared to brain tissue. In the absence of any LC separation, sodium adducts are also present. TLCL and TLCL + sodium adducts are the major species. (d) MALDI analysis of CL from bovine heart in negative ion mode. DHB was utilized as the matrix. TLCL dominates as the major CL species running as the (M-H)− ion as well as its sodium adduct. Other minor CL species are also present.
Figure 5.

MALDI-TOF MS (negative mode) of pure CL(18:2)4 oxidized in vitro. Starting from one molecular species (tetralinoleoyl-cardiolipin (TLCL), 1448.9 m/z) will produce many combinations of hydroxy- (+16 Da) and hydroperoxy- (+32 Da) products.
Application of oxidative lipidomics approach to TBI
Mass spectrometry has been employed by our group and a number of other groups to detect and analyze oxidized lipid products. However, these studies have been met with some difficulty due to the points mentioned above. In addition, the ability to detect the loss of a hydroperoxy or hydroxy group by various mass spectrometric scanning/fragmentation techniques has been met with limited success.
This problem is actually two fold. First, the species of interest must be present in sufficient concentration to perform any type of fragmentation. As stated above, peroxidized lipids exist in low abundance to begin with. Second, the group of interest must be able to be fragmented at the expense of all other fragmentations, that is, it must be one of the only groups lost. Since it requires more energy to “dissociate” an OH or OOH group than it does to fragment the remainder of the phospholipid in question, one cannot scan for losses of 16 or 32 Da very easily as other fragmentations within the phospholipid structure will also occur. Taking all of the above points together, detection of brain lipid hydroperoxides becomes an extremely challenging task.
Lipid peroxidation has been widely studied in TBI (Lewen et al. 2000; Roof and Hall 2000; Bazan 2006). A number of lipid peroxidation markers such as F2-isoprostane and malondialdehyde, have been shown to increase in brain tissue, serum and cerebrospinal fluid (CSF) after experimental and clinical TBI (Hoffman et al. 1996; Tyurin et al. 2000; Kasprzak et al. 2001; Bayir et al. 2002; Singh et al. 2006; Seifman et al. 2008). Several studies suggest that lipid oxidation markers might be associated with outcome after clinical TBI. Increased levels of highly oxidizable PUFA in CSF were associated with worse clinical outcome in adults with severe TBI (Pilitsis et al. 2003). Increased levels of thiobutiric acid reactive substances (TBARS) in CSF correlate with TBI severity in adults with contusion (Kasprzak et al. 2001). Although these markers give an idea about the scale of lipid peroxidation they do not indicate the source of lipid being oxidized. The latter is important for design of targeted therapeutic interventions.
We applied oxidative lipidomics and analyzed oxidation of lipids in brain after TBI and in neurons exposed to proapopoptotic agents. Unexpectedly, the profiles of lipid oxidation did not correlate with the abundance of PUFA residues in them. Highly abundant phospholipids, such as PC and PE, were not substrates for oxidation whereas minor anionic phospholipids were the preferred substrates for oxidation (Bayir et al. 2007; Tyurin et al. 2008a). In particular, mitochondrial specific CL and extramitochondrial PS accumulated most of the oxygenated fatty acid residues. This suggests that TBI-induced phospholipid oxidation does not follow the “polyunsaturation rule” hence likely does not occur via random free radical mechanisms. So what then may be the mechanisms catalyzing peroxidation of CL and PS? Based on previous studies, it is possible that cell damage and execution of an apoptotic program are the major causes for lipid peroxidation (Tyurin et al. 2008a). If so, it is logical to expect that CL and PS will be involved in oxidation reactions proceeding as a part of the apoptotic program. As it has been shown earlier, cytochrome c acts as the catalyst of CL peroxidation early in mitochondria-dependent apoptosis (Kagan et al. 2005). Subsequently cytochrome c released into the cytosol participates in the catalysis of PS peroxidation. TBI is accompanied by significant neuronal apoptosis: thus it is likely that nonrandom peroxidation of phospholipids in the injured brain reflects the peroxidation processes occurring in apoptotic neurons. In support of this interpretation are the data demonstrating that selective oxidation of CL and PS in neurons is triggered during staurosporine-induced apoptosis (Tyurin et al. 2008b) (Figure 6a). The significance of these findings is that CL peroxidation is essential for the release of proapoptotic factors from mitochondria into the cytosol (Kagan et al. 2005). Moreover PS peroxidation is a part of the cascade of events ultimately leading to PS oxidation and externalization—signals required for recognition of apoptotic cells by professional phagocytes such as macrophages and microglia in the brain.
Figure 6.

(a) Comparison of the abundance of major phospholipid (PL) classes with their oxidation. Profiles of PL and PL-hydroperoxides in control and apoptotic primary cortical neurons. PL content is expressed as percentage of total PL and shown in green scale. PL-hydroperoxides are presented as percentage of PL and shown in purple scale. Highly abundant PL, such as phosphatidylcholine (PC) and phosphatidylethanolamine (PE) were not substrates for oxidation whereas minor anionic PL, cardiolipin (CL) and phosphatidylserine (PS) were the preferred substrates for oxidation in neurons triggered to undergo staurosporine-induced apoptosis. PI: phosphatidylinositol. (b) CL oxidation and cytochrome c release during neuronal apoptosis. Cytochrome c forms a high-affinity complex with CL; the complex exerts strong peroxidase activity towards bound CL and causes its peroxidation. Accumulation of CL oxidation products leads to the release of proapoptotic factors, including cytochrome c into cytosol. Alpha synuclein (Syn), an abundant lipid-binding protein of synaptic terminals, interacts with anionic lipids (including CL) and cytochrome c to form a triple complex, Syn/CL/cytochrome c, which acts as a catalytically competent peroxidase that covalently cross-links Syn with cytochrome c into hetero-oligomers. This prevents death signaling effects of the cytochrome c in the cytosol.
Cardiolipin cytochrome c interactions and apoptosis
If the hypothesis that nonrandom oxidation of phospholipids is true, then one can assume that inhibition of CL peroxidation may lead to suppression of apoptosis and protection against TBI. Doubly negatively charged CL with its four fatty acid residues anchoring it to the hydrophobic core of the lipid bilayer, is located exclusively in the inner mitochondrial membrane (Hatch 1998; McMillin and Dowhan 2002). In the inner mitochondrial membrane, CLs interact and are essential for normal functioning of many intrinsic proteins, including major electron transport complexes. Formation of respiratory super-complexes, “respirasomes”, requires the presence of CLs. In particular, the formation of respiratory supercomplexes III–IV is dependent on “gluing” by CLs (Schagger 2002). Our previous work demonstrated that cytochrome c forms a high-affinity complex with CL; the complex exerts strong peroxidase activity towards bound CL and causes its selective peroxidation. Early in apoptosis, translocation of CL takes place resulting in its appearance in the outer mitochondrial membrane (Kagan et al. 2005). Consequently, significant amounts of CL become available for the interactions with cytochrome c, one of the major proteins of the intermembrane space. In contrast to many other hemoproteins, normally, all six of the coordination positions in heme iron of cytochrome c are occupied, thus preventing its interactions with small ligands such as hydrogen peroxide (H2O2), and nitric oxide (NO•) (Stellwagen 1968). By contrast, cytochrome c bound to CL exerts an entirely different conformation, with partial unfolding of the protein and a weakened/ruptured Fe-Met80 bond (Tuominen et al. 2002; Bernad et al. 2004). The heme site of CL-modified cytochrome c allows access to H2O2 and small organic peroxides, conferring catalytic peroxidase competence on the protein (Theorell and Åkesson 1941; Stellwagen 1968). Most importantly, thus formed mitochondrial complex of cytochrome c with CL acts as a potent CL-specific peroxidase and generates CL hydroperoxides (CL-OOH). The catalytic mechanisms of CL oxidation include the formation of protein-derived (tyrosyl) radicals detectable by low-temperature electron paramagnetic resonance (EPR) spectroscopy as well as by immuno-spin trapping.
The mechanisms of CL transmembrane redistribution and exposure at the contact sites between inner and outer mitochondrial membranes are not well understood. Several proteins have been implicated with the apoptotic transmigration of CL such as truncated Bid (tBid), one of the BH3-only members of the Bcl-2 family of proteins, phospholipid scramblase-3 (PLS-3), and mitochondrial isoforms of creatine kinase (MtCK) and nucleoside diphosphate kinase (NDPK-D) (Liu et al. 2003; Gonzalvez et al. 2005; Tyurin et al. 2007; Schlattner et al. 2009). tBid has a CL binding domain and in tBid treated mitochondria, CL redistributes from inner leaflet to other leaflet of inner mitochondrial membrane and also appears in the outer mitochondrial membrane (Gonzalvez et al. 2005; Tyurin et al. 2007). This in turn causes Bak/Bax oligomerization and cytochrome c release. Moreover, CL acts as an activation platform for the caspase-8 that is responsible for cleavage of Bid to form tBid (Schug and Gottlieb 2009).Activation of caspase-8 and cleavage of Bid has been reported in neurons and glia after experimental and clinical TBI (Franz et al. 2002; Zhang et al. 2003). Recently it has been shown that Caspase-8 translocates to mitochondria where it oligomerizes and gets activated (Gonzalvez et al. 2008). Furthermore, this association of caspase-8 with mitochondria depends on the presence of CL. Whether caspase-8 also interacts with CL-OOH is not known.
Several small molecule inhibitors have been proposed as potential regulators of cytochrome c/CL peroxidase activity. Their action may be directed towards different stages of the peroxidase reaction such as i) production of H2O2 as a source of oxidation equivalents ii) formation of cytochrome c/CL complexes and iii) inhibition of the peroxidase activity of cytochrome c in the complex. Among small molecule inhibitors of the first category, we explored the potential protective effects of a mitochondria-targeted stable nitroxyl radical - 2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPO). While oxygen radical-scavenging substances such as nitroxides are well known to reduce the intracellular ROS levels and have been shown to confer neuroprotection after TBI (Deng-Bryant et al. 2008), the high millimolar concentrations required for their significant therapeutic effects limit their clinical applications. We discovered that it is possible to engineer bacterial membrane targeting antibiotic gramicidin to deliver 4-amino TEMPO (GS-TEMPO) to the mitochondrial membrane and markedly increase 4-amino TEMPO’s potency (Wipf et al. 2005). One of these compounds, XJB-5-131, has been shown to inhibit CL peroxidation and improve survival after lethal hemorrhagic shock and irradiation (Macias et al. 2007; Jiang et al. 2008). We have recently shown that GS-TEMPO is preferentially partitioned in neuronal mitochondria and displays neuroprotective effects in vitro and in vivo (Ji et al. 2009).
There are also endogenous regulators that inhibit cytochrome c mediated neuronal apoptosis. We have shown that alpha synuclein (Syn), an abundant lipid-binding protein of synaptic terminals, interacts with anionic lipids (including CL) and cytochrome c to form a triple complex, Syn/CL/cytochrome c, which acts as a catalytically competent peroxidase that covalently cross-links Syn with cytochrome c into hetero-oligomers (Bayir et al. 2009). The covalent conjugation of Syn with cytochrome c in into aggregates prevents death signaling effects of the cytochrome c in the cytosol (Figure 6b). In line with this, accumulation of Syn has been described in axonal swellings and neuronal cytoplasm in the traumatized brain in mice and in humans (Newell et al. 1999; Uryu et al. 2003; Ikonomovic et al. 2004). Recent reports indicate that Syn can translocate to mitochondria, especially during oxidative or metabolic stress conditions (Cole et al. 2008). Corroborating this, accumulation of co-localized Syn-cyt c hetero-oligomers in mitochondria of cells exposed to oxidative stress was observed (Bayir et al. 2009). Since Syn has CL binding capacity, mice lacking Syn show reduced brain CL content with increased saturated fatty acids in CL molecular species and decreased activity of linked complex I/III in mitochondria (Ellis et al. 2005; Barcelo-Coblijn et al. 2007). There has not been a study evaluating deficiency of Syn in TBI. Given the fact that mitochondrial damage and impairment of bioenergetics occurs after injury (Singh et al. 2006; Pandya et al. 2007), Syn deficiency may worsen outcome after TBI.
Imaging lipids in the brain
In spite of the innovative nature of biochemical MS techniques described above, topographical evaluations of lipids and their oxidation events in cells and tissues remain obscure. Extracts of whole-brain tissue will, of course, not have spatial resolution. Since a small amount of tissue is required to be extracted for LC-MS, lipid extractions from progressively finer cuts of tissue could give regional localization. Also, laser microdissection has been used for a few non-lipid analytes, giving high spatial resolution. With the advent of IMS, studies of the topography of proteins and particularly small molecules, such as drugs and lipids, are now possible (McDonnell and Heeren 2007; Heeren et al. 2009) (Figure 6). Recently, this topic has been also reviewed in special. In our view, MALDI has the best combination of sensitivity and spatial resolution for oxidatively modified lipids, but other techniques such as Desorption Electrospray Ionisation (DESI), Secondary-Ion MS (SIMS) and Matrix-Enhanced SIMS (ME-SIMS) have advantages over MALDI in some areas (Table 2). Proper tissue preparation is essential for subsequent imaging mass spectrometry. Unfortunately, most archived tissues are not usable for IMS (see below), but ongoing work is being devoted to determine just what information can be obtained with them (Nirmalan et al. 2008; Mange et al. 2009).
DESI involves sputtering a stream of charged solvent droplets onto a surface, desorbing and ionizing. Unlike SIMS or MALDI, it is conducted at atmospheric pressure under ambient conditions, and requires no sample preparation (Takáts et al. 2004; Manicke et al. 2008; Girod et al. 2010). Its spatial resolution of approximately 200 microns is worse than SIMS or MALDI, but it is a much milder ionization method and has also been used for lipid imaging of the rat brain (Manicke et al. 2008; Daiki et al. 2010).
SIMS involves “sputtering” of a tightly focused beam of high-energy ions onto a surface, and analyzing the resulting secondary ions generated from the surface (Jungnickel et al. 2005). MALDI has a practical limitation in spatial resolution to the width of the laser beam (10–30 microns), while SIMS can give sub-micron resolution (Benabdellah et al. 2010). This means that MALDI can image lipids in cells or small groups of cells, while SIMS can image at the sub-cellular level. However, SIMS tends to produce uncontrolled fragmentation of lipids above 1000 Da, which makes it very difficult to obtain information on mammalian CL (Benabdellah et al. 2010). A modification of this method is Matrix-Enhanced SIMS (ME-SIMS), where a matrix is applied to the tissue as in MALDI. This results in less fragmentation of lipids, but at the expense of poorer spatial resolution, making it an intriguing mix of the best features of MALDI and SIMS (McDonnell et al. 2005; Fitzgerald et al. 2010).
MALDI-based IMS was originally developed for detection of peptides and proteins in intact tissue slices by Richard Caprioli and Pierre Chaurand (Caprioli et al. 1997). Several MS laboratories now perform MALDI-IMS, although preparation of the tissue prior to analysis is just as important as the MS instrumentation (McDonnell and Heeren 2007). One requirement for this technique is that the tissue must be fresh frozen, and neither formalin fixed nor optimal cutting temperature polymer (OCT)-embedded. MALDI-MS techniques optimized to detect lipids will also detect OCT, and the latter signal will overwhelm the spectrum (Puolitaival et al. 2008; Burnum et al. 2009; Ridenour et al. 2010). Care must be taken during tissue cutting that only minimal OCT is used – just enough to fix the sample to the cryostat block – and that the blade never touches OCT. Washing with alcohol as part of the standard tissue fixation for IMS of proteins will de-lipidate the tissue, preventing IMS of lipids (Chaurand et al. 2008). Therefore, most archived histology samples (embedded blocks of fixed tissue and stained slides) are not able to be subsequently analyzed by IMS for lipids. There is ongoing work in the field to determine what information can be obtained out of archived samples, although success with protein analysis may be more likely than with lipids (Nirmalan et al. 2008; Mange et al. 2009). However, selective washing which removes salts or some lipids is an intriguing possibility for improving IMS analysis of other, particularly less abundant, lipid species (Puolitaival et al. 2008).
Early IMS was performed on tissue slides placed on stainless steel or gold MALDI sample holders (Caprioli et al. 1997). However, special glass histology slides that have a very fine coating of Indium-Tin Oxide (ITO) can be used (Chaurand et al. 2004; Altelaar et al. 2007; Vidova et al. 2010). The ITO provides a conductive surface (essential for MALDI), but the thin coating is transparent to light, and thus the tissue can be subsequently stained and examined by light microscopy after IMS. Of course, serial or near-serial sections can also be analyzed with immunohistochemical techniques for comparison with IMS data.
The MALDI matrix needs to be deposited uniformly onto the tissue slice to prevent “hot spots” of matrix clusters or analyte delocalization. The ultimate resolution of IMS depends on several factors including matrix crystal size. Therefore, small homogeneous crystals of matrix are desired (Werner et al. 2010). Methods commonly employed for IMS matrix deposition vary from climate-controlled aerosol chambers (“Image Prep” by Bruker Daltonik) to manual application with TLC sprayers or even commercial airbrushes (Chaurand et al. 2004). Other methods that have been used include sublimation, dry-powder application, and electro-blotting with a modified inkjet printer (Mange et al. 2009). In the end, the “best” method is the one that consistently yields reproducible results for the lipids of interest.
IMS has progressed to include detection of other biomolecules including phospholipids (Schwartz et al. 2003; Caldwell and Caprioli 2005). Recent developments in MALDI have enabled direct detection of lipids as intact molecular species present within cellular membranes. Abundant lipid-related ions are produced from the direct analysis of thin tissue slices (10 µm) when sequential spectra are acquired across a tissue surface that has been coated with a MALDI matrix. With high resolution mass analyzers, the lipid derived ions can often be distinguished from other biomolecules because of cumulative and significant mass defects resulting from the larger proportion of hydrogen (1.007825 Da) present in the fatty acyl chains of lipids (Murphy et al. 2009).
Imaging in lipidomics has been used to visualize intact molecular distributions and determine head group identity, acyl chain length, and degrees of unsaturation on single cell surfaces (Heeren et al. 2009). In our preliminary experiments, we utilized MALDI-IMS in PND 17 rat brain showing spatial distributions of polyunsaturated oxidizable [834.6 m/z (PS(40:6), red)] and monounsaturated non-oxidizable [788.6m/z (PS(36:1), green)] molecular species of PS in addition to ganglioside, GD1 (18:1/18:0) as potassium adduct at 1874 m/z and sulphatide, ST(24:0) at 888.8 m/z (Figure 7). IMS assessments of changes in oxidizable molecular species of phospholipids can be utilized as indirect measures of their likely oxidative modifications caused by brain injury or disease. As can be seen in Figure 8, signal intensity for polyunsaturated oxidizable molecular species of PS [834.6 m/z (PS(40:6), red)] and PI [885.7 m/z PI(38:4), green)] were decreased in the ipsilateral hemisphere compared to contralateral hemisphere after controlled cortical impact in PND 17 rat. Clearly, methodological advancements, particularly with regards to detection of low abundance lipids, will make direct IMS of peroxidized phospholipids possible. In particular, the diversity of CL molecular species and relatively low abundance of each of the CL species in the brain makes the task of their IMS challenging. Development of new methods based on selective photo-sensitization and ionization of CL molecules or products of their light-induced decay may be very promising in this respect. Notably, MS imaging has recently been successfully utilized for mapping of not only abundant species of PC but also its minor lyso-derivatives, lyso-PCs (Koizumi et al.; Hayasaka et al. 2008). Moreover, a recent study showed that several lyso-PCs (mostly lyso-PC(16:0) and (18:0)) were elevated with a decrease in several polyunsaturated PC species such as PC(36:4) in the rat cortex after focal cerebral ischemia (Wang et al.). This suggests that low abundance species of phospholipids such as peroxidized phospholipids, including oxidized CL and PS - are promising candidates for MS imaging.
Figure 7.

(a) MALDI-IMS of brain showing different spatial distributions of different compounds. (Left) Red: Ganglioside GD1(d18:1/18:0) as potassium adduct at 1874 m/z, Blue: PS(36:1) at 788.6 m/z, Green: PI(38:4) at 885.6 m/z. (Right) Red: PS(40:6) at 834.7m/z, Blue: ST(24:0) at 888.8 m/z, Green: PI(38:4)at 885.7 m/z (green). Hippocampus is marked by a rectangle. (b) Negative mode MALDI-IMS (left panel) of 834.6 m/z [PS(40:6), red] and 788.6m/z [PS(36:1), green] showing different localization for different molecular species of PS. This is compared to a serial H&E stain (right panel), showing that fine features of brain architecture can be correlated between MALDI imaging and histology staining at 50 micron resolution. (c) Zoom in on hippocampus.
Figure 8.

(Left) Optical image of injured brain coated with DHB matrix prior to MALDI imaging of highlighted region. Controlled cortical impact was applied to the hemisphere marked. (Right) Imaged areas across hippocampal region of PS(40:6) at 834.7m/z, (red), PI(38:4)at 885.7 m/z (green) along with overlaid area.
Conclusions
Lipid oxidation products play essential regulatory and signaling roles in the CNS. Detailed information on the content and distribution of lipids and their oxidation products can be obtained by a combination of analytical methods including chromatography and MS. Oxidative lipidomics is beginning to provide new mechanistic insights into TBI which may have significant translational potential for development of therapies in acute CNS insults. Finally, lipid imaging represents an exciting new opportunity for correlating maps of lipid profiles and their oxidation products with structure and neuropathology.
Acknowledgment
Supported by grants from NIH (NS061817, NS30318, HL70755, U19 AIO68021, HD05758); NIOSH (OH008282) and the US Army (W81XWH-09-0187).
References
- Altelaar AFM, Luxembourg SL, McDonnell LA, Piersma SR, Heeren RMA. Imaging mass spectrometry at cellular length scales. Nature Protocols. 2007;2:1185–1196. doi: 10.1038/nprot.2007.117. [DOI] [PubMed] [Google Scholar]
- Barcelo-Coblijn G, Golovko MY, Weinhofer I, Berger J, Murphy EJ. Brain neutral lipids mass is increased in alpha-synuclein gene-ablated mice. J Neurochem. 2007;101:132–141. doi: 10.1111/j.1471-4159.2006.04348.x. [DOI] [PubMed] [Google Scholar]
- Bayir H, Kagan VE, Tyurina YY, Tyurin V, Ruppel RA, Adelson PD, Graham SH, Janesko K, Clark RS, Kochanek PM. Assessment of antioxidant reserves and oxidative stress in cerebrospinal fluid after severe traumatic brain injury in infants and children. Pediatr Res. 2002;51:571–578. doi: 10.1203/00006450-200205000-00005. [DOI] [PubMed] [Google Scholar]
- Bayir H, Tyurin VA, Tyurina YY, Viner R, Ritov V, Amoscato AA, Zhao Q, Zhang XJ, Janesko-Feldman KL, Alexander H, Basova LV, Clark RS, Kochanek PM, Kagan VE. Selective early cardiolipin peroxidation after traumatic brain injury: an oxidative lipidomics analysis. Ann Neurol. 2007;62:154–169. doi: 10.1002/ana.21168. [DOI] [PubMed] [Google Scholar]
- Bayir H, Kapralov AA, Jiang J, Huang Z, Tyurina YY, Tyurin VA, Zhao Q, Belikova NA, Vlasova II, Maeda A, Zhu J, Na HM, Mastroberardino PG, Sparvero LJ, Amoscato AA, Chu CT, Greenamyre JT, Kagan VE. Peroxidase mechanism of lipid-dependent cross-linking of synuclein with cytochrome C: protection against apoptosis versus delayed oxidative stress in Parkinson disease. J Biol Chem. 2009;284:15951–15969. doi: 10.1074/jbc.M900418200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan NG. The onset of brain injury and neurodegeneration triggers the synthesis of docosanoid neuroprotective signaling. Cell Mol Neurobiol. 2006;26:901–913. doi: 10.1007/s10571-006-9064-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benabdellah F, Seyer A, Quinton L, Touboul D, Brunelle A, Laprévote O. Mass spectrometry imaging of rat brain sections: nanomolar sensitivity with MALDI versus nanometer resolution by TOF–SIMS. Analytical and Bioanalytical Chemistry. 2010;396:151–162. doi: 10.1007/s00216-009-3031-2. [DOI] [PubMed] [Google Scholar]
- Benjamins JA, Hajra AK, Agranoff BW. Basic Neurochemistry: Molecular, Cellular and Medical Aspects. Elsevier; 2006. Lipids; pp. 33–49. [Google Scholar]
- Bernad S, Oellerich S, Soulimane T, Noinville S, Baron MH, Paternostre M, Lecomte S. Interaction of horse heart and thermus thermophilus type c cytochromes with phospholipid vesicles and hydrophobic surfaces. Biophys J. 2004;86:3863–3872. doi: 10.1529/biophysj.103.025114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnum KE, Cornett DS, Puolitaival SM, Milne SB, Myers DS, Tranguch S, Brown HA, Dey SK, Caprioli RM. Spatial and temporal alterations of phospholipids determined by mass spectrometry during mouse embryo implantation. Journal of Lipid Research. 2009;50:2290–2298. doi: 10.1194/jlr.M900100-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell RL, Caprioli RM. Tissue Profiling by Mass Spectrometry. Mol Cell Proteomics. 2005;4:394–401. doi: 10.1074/mcp.R500006-MCP200. [DOI] [PubMed] [Google Scholar]
- Caprioli RM, Farmer TB, Gile J. Molecular Imaging of Biological Samples: Localization of Peptides and Proteins Using MALDI-TOF MS. Analytical Chemistry. 1997;69:4751–4760. doi: 10.1021/ac970888i. [DOI] [PubMed] [Google Scholar]
- Chaurand P, Schwartz SA, Billheimer D, Xu BJ, Crecelius A, Caprioli RM. Integrating Histology and Imaging Mass Spectrometry. Analytical Chemistry. 2004;76:1145–1155. doi: 10.1021/ac0351264. [DOI] [PubMed] [Google Scholar]
- Chaurand P, Latham JC, Lane KB, Mobley JA, Polosukhin VV, Wirth PS, Nanney LB, Caprioli RM. Imaging Mass Spectrometry of Intact Proteins from Alcohol-Preserved Tissue Specimens: Bypassing Formalin Fixation. Journal of Proteome Research. 2008;7:3543–3555. doi: 10.1021/pr800286z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Mancuso DJ, Jiang X, Guan S, Yang J, Yang K, Sun G, Gross RW, Han X. Shotgun lipidomics reveals the temporally dependent, highly diversified cardiolipin profile in the mammalian brain: temporally coordinated postnatal diversification of cardiolipin molecular species with neuronal remodeling. Biochemistry. 2008;47:5869–5880. doi: 10.1021/bi7023282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole NB, Dieuliis D, Leo P, Mitchell DC, Nussbaum RL. Mitochondrial translocation of alpha-synuclein is promoted by intracellular acidification. Exp Cell Res. 2008;314:2076–2089. doi: 10.1016/j.yexcr.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daiki A, Kentaro Y, Sen T, Kenzo H. Direct analysis of lipids in mouse brain using electrospray droplet impact/SIMS. Journal of Mass Spectrometry. 2010;45:437–443. doi: 10.1002/jms.1729. [DOI] [PubMed] [Google Scholar]
- Dass C. Fundamentals of contemporary mass spectrometry. Hoboken, N.J: Wiley-Interscience; 2007. [Google Scholar]
- Deng-Bryant Y, Singh IN, Carrico KM, Hall ED. Neuroprotective effects of tempol, a catalytic scavenger of peroxynitrite-derived free radicals, in a mouse traumatic brain injury model. J Cereb Blood Flow Metab. 2008;28:1114–1126. doi: 10.1038/jcbfm.2008.10. [DOI] [PubMed] [Google Scholar]
- Domingues MRM, Reis A, Domingues P. Mass spectrometry analysis of oxidized phospholipids. Chemistry and Physics of Lipids. 2008;156:1–12. doi: 10.1016/j.chemphyslip.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Ekman R. Mass spectrometry : instrumentation, interpretation, and applications. Hoboken, N.J: John Wiley & Sons; 2009. [Google Scholar]
- Ellis CE, Murphy EJ, Mitchell DC, Golovko MY, Scaglia F, Barcelo-Coblijn GC, Nussbaum RL. Mitochondrial lipid abnormality and electron transport chain impairment in mice lacking alpha-synuclein. Mol Cell Biol. 2005;25:10190–10201. doi: 10.1128/MCB.25.22.10190-10201.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson B, Gidden J, Lay JO, Jr, Durham B. A rapid separation technique for overcoming suppression of triacylglycerols by phosphatidylcholine using MALDI-TOF MS. Journal of Lipid Research. 2010 doi: 10.1194/jlr.D003798. in press, jlr.D003798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahy E, Subramaniam S, Murphy RC, Nishijima M, Raetz CRH, Shimizu T, Spener F, van Meer G, Wakelam MJO, Dennis EA. Update of the LIPID MAPS comprehensive classification system for lipids. Journal of Lipid research. 2009;50:S9–S14. doi: 10.1194/jlr.R800095-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahy E, Subramaniam S, Brown HA, Glass CK, Merrill AH, Jr, Murphy RC, Raetz CRH, Russell DW, Seyama Y, Shaw W, Shimizu T, Spener F, van Meer G, VanNieuwenhze MS, White SH, Witztum JL, Dennis EA. A comprehensive classification system for lipids. Journal of Lipid Research. 2005;46:839–862. doi: 10.1194/jlr.E400004-JLR200. [DOI] [PubMed] [Google Scholar]
- Fenn JB, Mann M, Meng CK, Wong SF, Whitehouse CM. Electrospray Ionization for Mass Spectrometry of Large Biomolecules. Science. 1989;246:64–71. doi: 10.1126/science.2675315. [DOI] [PubMed] [Google Scholar]
- Fitzgerald JJD, Kunnath P, Walker AV. Matrix-Enhanced Secondary Ion Mass Spectrometry (ME SIMS) Using Room Temperature Ionic Liquid Matrices. Analytical Chemistry. 2010 doi: 10.1021/ac100133c. in press. [DOI] [PubMed] [Google Scholar]
- Franz G, Beer R, Intemann D, Krajewski S, Reed JC, Engelhardt K, Pike BR, Hayes RL, Wang KK, Schmutzhard E, Kampfl A. Temporal and spatial profile of Bid cleavage after experimental traumatic brain injury. J Cereb Blood Flow Metab. 2002;22:951–958. doi: 10.1097/00004647-200208000-00006. [DOI] [PubMed] [Google Scholar]
- Fuchs B, Schiller J. MALDI-TOF MS Analysis of Lipids from Cells, Tissues, and Body Fluids. In: Quinn PJ, Wang X, editors. Lipids in Health and Disease. Springer Science; 2008. pp. 541–565. [DOI] [PubMed] [Google Scholar]
- Fuchs B, Nimptsch A, Süß R, Schiller J. Lipidomics. Vol 579. Humana Press; 2009. Capabilities and Drawbacks of Phospholipid Analysis by MALDI-TOF Mass Spectrometry; pp. 103–125. [DOI] [PubMed] [Google Scholar]
- Fuchs B, Schiller J, Süß R, Schürenberg M, Suckau D. A direct and simple method of coupling matrix-assisted laser desorption and ionization time-of-flight mass spectrometry (MALDI-TOF MS) to thin-layer chromatography (TLC) for the analysis of phospholipids from egg yolk. Analytical and Bioanalytical Chemistry. 2007;389:827–834. doi: 10.1007/s00216-007-1488-4. [DOI] [PubMed] [Google Scholar]
- Girod M, Shi Y, Cheng J-X, Cooks RG. Desorption Electrospray Ionization Imaging Mass Spectrometry of Lipids in Rat Spinal Cord. Journal of the American Society for Mass Spectrometry. 2010 doi: 10.1016/j.jasms.2010.03.028. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- Gonzalvez F, Schug ZT, Houtkooper RH, MacKenzie ED, Brooks DG, Wanders RJ, Petit PX, Vaz FM, Gottlieb E. Cardiolipin provides an essential activating platform for caspase-8 on mitochondria. J Cell Biol. 2008;183:681–696. doi: 10.1083/jcb.200803129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalvez F, Pariselli F, Dupaigne P, Budihardjo I, Lutter M, Antonsson B, Diolez P, Manon S, Martinou JC, Goubern M, Wang X, Bernard S, Petit PX. tBid interaction with cardiolipin primarily orchestrates mitochondrial dysfunctions and subsequently activates Bax and Bak. Cell Death Differ. 2005;12:614–626. doi: 10.1038/sj.cdd.4401571. [DOI] [PubMed] [Google Scholar]
- Goto-Inoue N, Hayasaka T, Taki T, Gonzalez TV, Setou M. A new lipidomics approach by thin-layer chromatography-blot-matrix-assisted laser desorption/ionization imaging mass spectrometry for analyzing detailed patterns of phospholipid molecular species. Journal of Chromatography A. 2009;1216:7096–7101. doi: 10.1016/j.chroma.2009.08.056. [DOI] [PubMed] [Google Scholar]
- Greenberg ME, Sun M, Zhang R, Febbraio M, Silverstein R, Hazen SL. Oxidized phosphatidylserine-CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J Exp Med. 2006;203:2613–2625. doi: 10.1084/jem.20060370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths J. A Brief History of Mass Spectrometry. Analytical Chemistry. 2008;80:5678–5683. doi: 10.1021/ac8013065. [DOI] [PubMed] [Google Scholar]
- Han X. Neurolipidomics: challenges and developments. Front Biosci. 2007;12:2601–2615. doi: 10.2741/2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Gross RW. Global analyses of cellular lipidomes directly from crude extracts of biological samples by ESI mass spectrometry: a bridge to lipidomics. Journal of Lipid Research. 2003;44:1071–1079. doi: 10.1194/jlr.R300004-JLR200. [DOI] [PubMed] [Google Scholar]
- Han X, Yang K, Yang J, Cheng H, Gross RW. Shotgun lipidomics of cardiolipin molecular species in lipid extracts of biological samples. Journal of Lipid Research. 2006;47:864–879. doi: 10.1194/jlr.D500044-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Yang J, Yang K, Zhao Z, Abendschein DR, Gross RW. Alterations in Myocardial Cardiolipin Content and Composition Occur at the Very Earliest Stages of Diabetes: A Shotgun Lipidomics Study. Biochemistry. 2007;46:6417–6428. doi: 10.1021/bi7004015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch GM. Cardiolipin: biosynthesis, remodeling and trafficking in the heart and mammalian cells (Review) Int J Mol Med. 1998;1:33–41. doi: 10.3892/ijmm.1.1.33. [DOI] [PubMed] [Google Scholar]
- Hayasaka T, Goto-Inoue N, Sugiura Y, Zaima N, Nakanishi H, Ohishi K, Nakanishi S, Naito T, Taguchi R, Setou M. Matrix-assisted laser desorption/ionization quadrupole ion trap time-of-flight (MALDI-QIT-TOF)-based imaging mass spectrometry reveals a layered distribution of phospholipid molecular species in the mouse retina. Rapid Commun Mass Spectrom. 2008;22:3415–3426. doi: 10.1002/rcm.3751. [DOI] [PubMed] [Google Scholar]
- Hazen SL. Oxidized phospholipids as endogenous pattern recognition ligands in innate immunity. J Biol Chem. 2008;283:15527–15531. doi: 10.1074/jbc.R700054200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeren RMA, Smith DF, Stauber J, Kükrer-Kaletas B, MacAleese L. Imaging Mass Spectrometry: Hype or Hope? Journal of the American Society for Mass Spectrometry. 2009;20:1006–1014. doi: 10.1016/j.jasms.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Hillenkamp F, Peter-Katalinic J. MALDI MS. Wiley-VCH: Weinheim; 2007. [Google Scholar]
- Hoffman SW, Roof RL, Stein DG. A reliable and sensitive enzyme immunoassay method for measuring 8-isoprostaglandin F2 alpha: a marker for lipid peroxidation after experimental brain injury. J Neurosci Methods. 1996;68:133–136. doi: 10.1016/0165-0270(96)00014-3. http://www.lipidmaps.org/ [DOI] [PubMed] [Google Scholar]
- Huvaere K, Cardoso DR, Homem-de-Mello P, Westermann S, Skibsted LH. Light-Induced Oxidation of Unsaturated Lipids as Sensitized by Flavins. The Journal of Physical Chemistry B. 2010 doi: 10.1021/jp9121744. in press. [DOI] [PubMed] [Google Scholar]
- Ikonomovic MD, Uryu K, Abrahamson EE, Ciallella JR, Trojanowski JQ, Lee VM, Clark RS, Marion DW, Wisniewski SR, DeKosky ST. Alzheimer's pathology in human temporal cortex surgically excised after severe brain injury. Exp Neurol. 2004;190:192–203. doi: 10.1016/j.expneurol.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Ishida M, Yamazaki T, Houjou T, Imagawa M, Harada A, Inoue K, Taguchi R. High-resolution analysis by nano-electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry for the identification of molecular species of phospholipids and their oxidized metabolites. Rapid Communications in Mass Spectrometry. 2004;18:2486–2494. doi: 10.1002/rcm.1650. [DOI] [PubMed] [Google Scholar]
- Ji J, Wipf P, Tyurin V, Alexander H, Kochanek P, Kagan VE, Bayir H. Mitochondrial targeting of antioxidants with antiapoptotic action in pediatric TBI. J Neurotrauma. 2009;26:A62. [Google Scholar]
- Jiang J, Belikova NA, Hoye AT, Zhao Q, Epperly MW, Greenberger JS, Wipf P, Kagan VE. A mitochondria-targeted nitroxide/hemigramicidin S conjugate protects mouse embryonic cells against gamma irradiation. Int J Radiat Oncol Biol Phys. 2008;70:816–825. doi: 10.1016/j.ijrobp.2007.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungnickel H, Jones EA, Lockyer NP, Oliver SG, Stephens GM, Vickerman JC. Application of TOF-SIMS with Chemometrics To Discriminate between Four Different Yeast Strains from the Species Candida glabrata and Saccharomyces cerevisiae. Analytical Chemistry. 2005;77:1740–1745. doi: 10.1021/ac048792t. [DOI] [PubMed] [Google Scholar]
- Kagan VE, Tyurin VA, Jiang J, Tyurina YY, Ritov VB, Amoscato AA, Osipov AN, Belikova NA, Kapralov AA, Kini V, Vlasova II, Zhao Q, Zou M, Di P, Svistunenko DA, Kurnikov IV, Borisenko GG. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol. 2005;1:223–232. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- Karas M, Hillenkamp F. Laser desorption ionization of proteins with molecular masses exceeding 10,000 daltons. Analytical Chemistry. 1988;60:2299–2301. doi: 10.1021/ac00171a028. [DOI] [PubMed] [Google Scholar]
- Karas M, Glückmann M, Schäfer J. Ionization in matrix-assisted laser desorption/ionization: singly charged molecular ions are the lucky survivors. Journal of Mass Spectrometry. 2000;35:1–12. doi: 10.1002/(SICI)1096-9888(200001)35:1<1::AID-JMS904>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Kasprzak HA, Wozniak A, Drewa G, Wozniak B. Enhanced lipid peroxidation processes in patients after brain contusion. J Neurotrauma. 2001;18:793–797. doi: 10.1089/089771501316919157. [DOI] [PubMed] [Google Scholar]
- Kiebish MA, Han X, Cheng H, Seyfried TN. In vitro growth environment produces lipidomic and electron transport chain abnormalities in mitochondria from non-tumorigenic astrocytes and brain tumours. ASN Neuro. 2009;1 doi: 10.1042/AN20090011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiebish MA, Han X, Cheng H, Chuang JH, Seyfried TN. Brain mitochondrial lipid abnormalities in mice susceptible to spontaneous gliomas. Lipids. 2008a;43:951–959. doi: 10.1007/s11745-008-3197-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiebish MA, Han X, Cheng H, Chuang JH, Seyfried TN. Cardiolipin and electron transport chain abnormalities in mouse brain tumor mitochondria: lipidomic evidence supporting the Warburg theory of cancer. J Lipid Res. 2008b;49:2545–2556. doi: 10.1194/jlr.M800319-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiebish MA, Han X, Cheng H, Lunceford A, Clarke CF, Moon H, Chuang JH, Seyfried TN. Lipidomic analysis and electron transport chain activities in C57BL/6J mouse brain mitochondria. J Neurochem. 2008c;106:299–312. doi: 10.1111/j.1471-4159.2008.05383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi S, Yamamoto S, Hayasaka T, Konishi Y, Yamaguchi-Okada M, Goto-Inoue N, Sugiura Y, Setou M, Namba H. Imaging mass spectrometry revealed the production of lyso-phosphatidylcholine in the injured ischemic rat brain. Neuroscience. 168:219–225. doi: 10.1016/j.neuroscience.2010.03.056. [DOI] [PubMed] [Google Scholar]
- Lewen A, Matz P, Chan PH. Free radical pathways in CNS injury. J Neurotrauma. 2000;17:871–890. doi: 10.1089/neu.2000.17.871. [DOI] [PubMed] [Google Scholar]
- Liu J, Dai Q, Chen J, Durrant D, Freeman A, Liu T, Grossman D, Lee RM. Phospholipid scramblase 3 controls mitochondrial structure, function, and apoptotic response. Mol Cancer Res. 2003;1:892–902. [PubMed] [Google Scholar]
- Macias CA, Chiao JW, Xiao J, Arora DS, Tyurina YY, Delude RL, Wipf P, Kagan VE, Fink MP. Treatment with a novel hemigramicidin-TEMPO conjugate prolongs survival in a rat model of lethal hemorrhagic shock. Ann Surg. 2007;245:305–314. doi: 10.1097/01.sla.0000236626.57752.8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mange A, Chaurand P, Perrochia H, Roger P, Caprioli RM, Solassol J. Liquid chromatography-tandem and MALDI imaging mass spectrometry analyses of RCL2/CS100-fixed, paraffin-embedded tissues: proteomics evaluation of an alternate fixative for biomarker discovery. J Proteome Res. 2009;8:5619–5628. doi: 10.1021/pr9007128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manicke NE, Wiseman JM, Ifa DR, Cooks RG. Desorption Electrospray Ionization (DESI) Mass Spectrometry and Tandem Mass Spectrometry (MS/MS) of Phospholipids and Sphingolipids: Ionization, Adduct Formation, and Fragmentation. Journal of the American Society for Mass Spectrometry. 2008;19:531–543. doi: 10.1016/j.jasms.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Marcheselli VL, Hong S, Lukiw WJ, Tian XH, Gronert K, Musto A, Hardy M, Gimenez JM, Chiang N, Serhan CN, Bazan NG. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem. 2003;278:43807–43817. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- McDonnell LA, Heeren RMA. Imaging mass spectrometry. Mass Spectrometry Reviews. 2007;26:606–643. doi: 10.1002/mas.20124. [DOI] [PubMed] [Google Scholar]
- McDonnell LA, Piersma SR, Altelaar AFM, Mize TH, Luxembourg SL, Verhaert PDEM, van Minnen J, Heeren RMA. Subcellular imaging mass spectrometry of brain tissue. Journal of Mass Spectrometry. 2005;40:160–168. doi: 10.1002/jms.735. [DOI] [PubMed] [Google Scholar]
- McMillin JB, Dowhan W. Cardiolipin and apoptosis. Biochim Biophys Acta. 2002;1585:97–107. doi: 10.1016/s1388-1981(02)00329-3. [DOI] [PubMed] [Google Scholar]
- Murphy RC, Hankin JA, Barkley RM. Imaging of lipid species by MALDI mass spectrometry. J Lipid Res. 2009;50 Suppl:S317–S322. doi: 10.1194/jlr.R800051-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell KL, Boyer P, Gomez-Tortosa E, Hobbs W, Hedley-Whyte ET, Vonsattel JP, Hyman BT. Alpha-synuclein immunoreactivity is present in axonal swellings in neuroaxonal dystrophy and acute traumatic brain injury. J Neuropathol Exp Neurol. 1999;58:1263–1268. doi: 10.1097/00005072-199912000-00007. [DOI] [PubMed] [Google Scholar]
- Nirmalan NJH, Patricia, Selby PJ, E Banks R. Mining the archival formalin-fixed paraffin-embedded tissue proteome: opportunities and challenges. Molecular BioSystems. 2008;4:712–720. doi: 10.1039/b800098k. [DOI] [PubMed] [Google Scholar]
- Pandya JD, Pauly JR, Nukala VN, Sebastian AH, Day KM, Korde AS, Maragos WF, Hall ED, Sullivan PG. Post-Injury Administration of Mitochondrial Uncouplers Increases Tissue Sparing and Improves Behavioral Outcome following Traumatic Brain Injury in Rodents. J Neurotrauma. 2007;24:798–811. doi: 10.1089/neu.2006.3673. [DOI] [PubMed] [Google Scholar]
- Peterson BL, Cummings BS. A review of chromatographic methods for the assessment of phospholipids in biological samples. Biomedical Chromatography. 2006;20:227–243. doi: 10.1002/bmc.563. [DOI] [PubMed] [Google Scholar]
- Petkovic M, Schiller J, Müller M, Benard S, Reichl S, Arnold K, Arnhold J. Detection of Individual Phospholipids in Lipid Mixtures by Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry: Phosphatidylcholine Prevents the Detection of Further Species. Analytical Biochemistry. 2001;289:202–216. doi: 10.1006/abio.2000.4926. [DOI] [PubMed] [Google Scholar]
- Pilitsis JG, Coplin WM, O'Regan MH, Wellwood JM, Diaz FG, Fairfax MR, Michael DB, Phillis JW. Free fatty acids in cerebrospinal fluids from patients with traumatic brain injury. Neurosci Lett. 2003;349:136–138. doi: 10.1016/s0304-3940(03)00803-6. [DOI] [PubMed] [Google Scholar]
- Piomelli D, Astarita G, Rapaka R. A neuroscientist's guide to lipidomics. Nat Rev Neurosci. 2007;8:743–754. doi: 10.1038/nrn2233. [DOI] [PubMed] [Google Scholar]
- Pulfer M, Murphy RC. Electrospray mass spectrometry of phospholipids. Mass Spectrometry Reviews. 2003;22:332–364. doi: 10.1002/mas.10061. [DOI] [PubMed] [Google Scholar]
- Puolitaival SM, Burnum KE, Cornett DS, Caprioli RM. Solvent-Free Matrix Dry-Coating for MALDI Imaging of Phospholipids. Journal of the American Society for Mass Spectrometry. 2008;19:882–886. doi: 10.1016/j.jasms.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridenour WB, Kliman M, McLean JA, Caprioli RM. Structural Characterization of Phospholipids and Peptides Directly from Tissue Sections by MALDI Traveling-Wave Ion Mobility-Mass Spectrometry. Analytical Chemistry. 2010;82:1881–1889. doi: 10.1021/ac9026115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roof RL, Hall ED. Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone. J Neurotrauma. 2000;17:367–388. doi: 10.1089/neu.2000.17.367. [DOI] [PubMed] [Google Scholar]
- Rouser G, Yamamoto A, Kritchevsky G. Cellular membranes. Structure and regulation of lipid class composition species differences, changes with age, and variations in some pathological states. Arch Intern Med. 1971;127:1105–1121. doi: 10.1001/archinte.127.6.1105. [DOI] [PubMed] [Google Scholar]
- Schagger H. Respiratory chain supercomplexes of mitochondria and bacteria. Biochim Biophys Acta. 2002;1555:154–159. doi: 10.1016/s0005-2728(02)00271-2. [DOI] [PubMed] [Google Scholar]
- Schiller J, Süß R, Petkovic M, Zschörnig O, Arnold K. Negative-ion matrix-assisted laser desorption and ionization time-of-flight mass spectra of complex phospholipid mixtures in the presence of phosphatidylcholine: a cautionary note on peak assignment. Analytical Biochemistry. 2002;309:311–314. doi: 10.1016/s0003-2697(02)00308-1. [DOI] [PubMed] [Google Scholar]
- Schiller J, Suss R, Fuchs B, Muller M, Zschornig O, Arnold K. MALDI-TOF MS in lipidomics. Frontiers in Bioscience. 2007;12:2568–2579. doi: 10.2741/2255. [DOI] [PubMed] [Google Scholar]
- Schiller J, Süß R, Arnhold J, Fuchs B, Leßig J, Müller M, Petkovic M, Spalteholz H, Zschörnig O, Arnold K. Matrix-assisted laser desorption and ionization time-of-flight (MALDI-TOF) mass spectrometry in lipid and phospholipid research. Progress in Lipid Research. 2004;43:449–488. doi: 10.1016/j.plipres.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Schlattner U, Tokarska-Schlattner M, Ramirez S, Bruckner A, Kay L, Polge C, Epand RF, Lee RM, Lacombe ML, Epand RM. Mitochondrial kinases and their molecular interaction with cardiolipin. Biochim Biophys Acta. 2009;1788:2032–2047. doi: 10.1016/j.bbamem.2009.04.018. [DOI] [PubMed] [Google Scholar]
- Schug ZT, Gottlieb E. Cardiolipin acts as a mitochondrial signalling platform to launch apoptosis. Biochim Biophys Acta. 2009;1788:2022–2031. doi: 10.1016/j.bbamem.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Schwartz SA, Reyzer ML, Caprioli RM. Direct tissue analysis using matrix-assisted laser desorption/ionization mass spectrometry: practical aspects of sample preparation. Journal of Mass Spectrometry. 2003;38:699–708. doi: 10.1002/jms.505. [DOI] [PubMed] [Google Scholar]
- Seeley EH, Oppenheimer SR, Mi D, Chaurand P, Caprioli RM. Enhancement of Protein Sensitivity for MALDI Imaging Mass Spectrometry after Chemical Treatment of Tissue Sections. Journal of the American Society for Mass Spectrometry. 2008;19:1069–1077. doi: 10.1016/j.jasms.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifman MA, Adamides AA, Nguyen PN, Vallance SA, Cooper DJ, Kossmann T, Rosenfeld JV, Morganti-Kossmann MC. Endogenous melatonin increases in cerebrospinal fluid of patients after severe traumatic brain injury and correlates with oxidative stress and metabolic disarray. J Cereb Blood Flow Metab. 2008;28:684–696. doi: 10.1038/sj.jcbfm.9600603. [DOI] [PubMed] [Google Scholar]
- Singh IN, Sullivan PG, Deng Y, Mbye LH, Hall ED. Time course of post-traumatic mitochondrial oxidative damage and dysfunction in a mouse model of focal traumatic brain injury: implications for neuroprotective therapy. J Cereb Blood Flow Metab. 2006;26:1407–1418. doi: 10.1038/sj.jcbfm.9600297. [DOI] [PubMed] [Google Scholar]
- Stellwagen E. Carboxymethylation of horse heart ferricytochrome c and cyanferricytochrome c. Biochemistry. 1968;7:2496–2501. doi: 10.1021/bi00847a008. [DOI] [PubMed] [Google Scholar]
- Stübiger G, Pittenauer E, Belgacem O, Rehulka P, Widhalm K, Allmaier G. Analysis of human plasma lipids and soybean lecithin by means of high-performance thin-layer chromatography and matrix-assisted laser desorption/ionization mass spectrometry. Rapid Communications in Mass Spectrometry. 2009;23:2711–2723. doi: 10.1002/rcm.4173. [DOI] [PubMed] [Google Scholar]
- Sun G, Yang K, Zhao Z, Guan S, Han X, Gross RW. Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometric Analysis of Cellular Glycerophospholipids Enabled by Multiplexed Solvent Dependent Analyte-Matrix Interactions. Analytical Chemistry. 2008;80:7576–7585. doi: 10.1021/ac801200w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takáts Z, Wiseman JM, Gologan B, Cooks RG. Mass Spectrometry Sampling Under Ambient Conditions with Desorption Electrospray Ionization. Science. 2004;306:471–473. doi: 10.1126/science.1104404. [DOI] [PubMed] [Google Scholar]
- Teuber K, Schiller J, Fuchs B, Karas M, Jaskolla TW. Significant sensitivity improvements by matrix optimization: a MALDI-TOF mass spectrometric study of lipids from hen egg yolk. Chemistry and Physics of Lipids. 2010 doi: 10.1016/j.chemphyslip.2010.04.005. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- Theorell H, Åkesson Å. Studies on Cytochrome c. II. The Optical Properties of Pure Cytochrome c and Some of Its Derivatives. J. Am. Chem. Soc. 1941;63:1812–1818. [Google Scholar]
- Tuominen EK, Wallace CJ, Kinnunen PK. Phospholipid-cytochrome c interaction: evidence for the extended lipid anchorage. J Biol Chem. 2002;277:8822–8826. doi: 10.1074/jbc.M200056200. [DOI] [PubMed] [Google Scholar]
- Tyurin VA, Tyurina YY, Osipov AN, Belikova NA, Basova LV, Kapralov AA, Bayir H, Kagan VE. Interactions of cardiolipin and lyso-cardiolipins with cytochrome c and tBid: conflict or assistance in apoptosis. Cell Death Differ. 2007;14:872–875. doi: 10.1038/sj.cdd.4402068. [DOI] [PubMed] [Google Scholar]
- Tyurin VA, Tyurina YY, Kochanek PM, Hamilton R, DeKosky ST, Greenberger JS, Bayir H, Kagan VE. Oxidative lipidomics of programmed cell death. Methods Enzymol. 2008a;442:375–393. doi: 10.1016/S0076-6879(08)01419-5. [DOI] [PubMed] [Google Scholar]
- Tyurin VA, Tyurina YY, Borisenko GG, Sokolova TV, Ritov VB, Quinn PJ, Rose M, Kochanek P, Graham SH, Kagan VE. Oxidative stress following traumatic brain injury in rats: quantitation of biomarkers and detection of free radical intermediates. J Neurochem. 2000;75:2178–2189. doi: 10.1046/j.1471-4159.2000.0752178.x. [DOI] [PubMed] [Google Scholar]
- Tyurin VA, Tyurina YY, Ritov VB, Lysytsya A, Amoscato AA, Kochanek PM, Hamilton R, Dekosky ST, Greenberger JS, Bayir H, Kagan VE. Oxidative lipidomics of apoptosis: quantitative assessment of phospholipid hydroperoxides in cells and tissues. Methods Mol Biol. 610:353–374. doi: 10.1007/978-1-60327-029-8_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyurin VA, Tyurina YY, Feng W, Mnuskin A, Jiang J, Tang M, Zhang X, Zhao Q, Kochanek PM, Clark RS, Bayir H, Kagan VE. Mass-spectrometric characterization of phospholipids and their primary peroxidation products in rat cortical neurons during staurosporine-induced apoptosis. J Neurochem. 2008b;107:1614–1633. doi: 10.1111/j.1471-4159.2008.05728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uryu K, Giasson BI, Longhi L, Martinez D, Murray I, Conte V, Nakamura M, Saatman K, Talbot K, Horiguchi T, McIntosh T, Lee VM, Trojanowski JQ. Age-dependent synuclein pathology following traumatic brain injury in mice. Exp Neurol. 2003;184:214–224. doi: 10.1016/s0014-4886(03)00245-0. [DOI] [PubMed] [Google Scholar]
- Vidova V, Novak P, Strohalm M, Pol J, Havlicek V, Volny M. Laser Desorption-Ionization of Lipid Transfers: Tissue Mass Spectrometry Imaging without MALDI Matrix. Analytical Chemistry. 2010 doi: 10.1021/ac100661h. in press. [DOI] [PubMed] [Google Scholar]
- Wang H-YJ, Jackson SN, Woods AS. Direct MALDI-MS Analysis of Cardiolipin from Rat Organs Sections. Journal of the American Society for Mass Spectrometry. 2007;18:567–577. doi: 10.1016/j.jasms.2006.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H-YJ, Post SNJJ, Woods AS. A minimalist approach to MALDI imaging of glycerophospholipids and sphingolipids in rat brain sections. International Journal of Mass Spectrometry. 2008;278:143–149. doi: 10.1016/j.ijms.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HY, Liu CB, Wu HW, Kuo JS. Direct profiling of phospholipids and lysophospholipids in rat brain sections after ischemic stroke. Rapid Commun Mass Spectrom. 24:2057–2064. doi: 10.1002/rcm.4620. [DOI] [PubMed] [Google Scholar]
- Wassall SR, Stillwell W. Docosahexaenoic acid domains: the ultimate non-raft membrane domain. Chem Phys Lipids. 2008;153:57–63. doi: 10.1016/j.chemphyslip.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Watson AD. Thematic review series: Systems Biology Approaches to Metabolic and Cardiovascular Disorders. Lipidomics: a global approach to lipid analysis in biological systems. Journal of Lipid Research. 2006;47:2101–2111. doi: 10.1194/jlr.R600022-JLR200. [DOI] [PubMed] [Google Scholar]
- Werner B, Oliver S, Daniel E, Bernhard S. Matrix vapor deposition/recrystallization and dedicated spray preparation for high-resolution scanning microprobe matrix-assisted laser desorption/ionization imaging mass spectrometry (SMALDI-MS) of tissue and single cells. Rapid Communications in Mass Spectrometry. 2010;24:355–364. doi: 10.1002/rcm.4401. [DOI] [PubMed] [Google Scholar]
- Wipf P, Xiao J, Jiang J, Belikova NA, Tyurin VA, Fink MP, Kagan VE. Mitochondrial targeting of selective electron scavengers: synthesis and biological analysis of hemigramicidin-TEMPO conjugates. J Am Chem Soc. 2005;127:12460–12461. doi: 10.1021/ja053679l. [DOI] [PubMed] [Google Scholar]
- Yusuf HKM. Understanding the brain and its development: A chemical approach. World Scientific Publishing Co. Pte. Ltd.; 1992. pp. 147–152. [Google Scholar]
- Zhang X, Graham SH, Kochanek PM, Marion DW, Nathaniel PD, Watkins SC, Clark RS. Caspase-8 expression and proteolysis in human brain after severe head injury. Faseb J. 2003;17:1367–1369. doi: 10.1096/fj.02-1067fje. [DOI] [PubMed] [Google Scholar]


