Abstract
The goal of the present study was to elucidate the role of DARPP-32 (dopamine- and cyclic adenosine 3′-5′-monophosphate-regulated phosphoprotein, 32 kDa) in retinal function. We examined mouse and rat retinas for the presence of DARPP-32 by immunocytochemistry. In both rodent retinas DARPP-32 immunoreactivity was localized to horizontal and AII amacrine neurons and to the Mueller glial cells, using immuno-double labelling. Additional unidentified neurons in the amacrine cell layer also showed DARPP-32 immunoreactivity. Using mice entrained to a 12–12 h light–dark cycle, we found that exposure to light presented during the dark phase significantly enhanced phosphorylation of DARPP-32 at threonine (Thr) 34 and phosphorylation of the ionotropic glutamate receptor subunit GluR1 at serine (Ser) 845, as measured by immunoblots. However, light also increased Ser 845-GluR1 phosphorylation in DARPP-32-knockout mice. When a dopamine D1 receptor antagonist was injected into the eye prior to light exposure, phosphorylation of both Thr 34-DARPP-32 and Ser 845-GluR1 was significantly reduced. These data indicate that DARPP-32 participates in dopamine-mediated modifications of retinal function. We also tested for a possible circadian rhythm of Thr 34- and Thr 75-DARPP-32 and Ser 845-GluR1 expression. No significant circadian rhythm of either DARPP-32 or GluR1 phosphorylation was found.
Keywords: D1 dopamine receptor, dopamine, glutamate, mouse, phosphorylation, rat
Introduction
The dopamine and cyclic adenosine 3′-5′monophosphate-regulated phosphoprotein, 32 kDa (DARPP-32), has been studied intensively in the vertebrate CNS. Initially DARPP-32 was shown to be phosphorylated through a signalling cascade that begins with activation of the D1 dopamine receptor, leading to increased cAMP production, activation of protein kinase A and phosphorylation of DARPP-32 at threonine (Thr) 34 (Walaas et al., 1983). In this phosphorylated state DARPP-32 is a potent inhibitor of protein phosphatase 1 (Hemmings et al., 1984). More recent work demonstrates that DARPP-32 has multiple phosphorylation sites acted upon by a variety of kinases and resulting in different cascades. For example, phosphorylation at Thr 75 converts DARPP-32 into an inhibitor of protein kinase A (Bibb et al., 1999; reviewed in Svenningsson et al., 2004). Thus DARPP-32 can either promote or inhibit protein phosphorylation.
Although dopamine-mediated modulation of mammalian retinal physiology is well documented (reviewed in Witkovsky, 2004), and the presence of DARPP-32 in some mammalian retinas has been established (Hemmings & Greengard, 1986; Meister et al., 1991; Partida et al., 2004), the specific retinal cell types containing DARPP-32 are only partially identified, and the pathways and mechanisms through which DARPP-32 participates in retinal function are as yet unstudied. Early investigations in the CNS (Ouimet et al., 1984) showed that DARPP-32 was associated with neurons possessing D1 dopamine receptors. In the mammalian retina, dopamine D1 receptors are distributed widely among second- and third-order retinal neurons, including horizontal, bipolar and amacrine cells (Veruki & Wässle, 1996; Nguyen-Legros et al., 1997; Li et al., 2004). Each of these neuronal classes occurs in multiple subtypes, yet which subtypes colocalize a D1 receptor and DARPP-32 is poorly understood. Partida et al. (2004) demonstrated, in the rat retina, that the AII amacrine cell, an integral neuron of the pathway for transmitting rod information, was immunoreactive for DARPP-32. Consistent with this finding, the gap junctions that join neighbouring AII amacrines are uncoupled by dopamine, acting through a D1 receptor (Hampson et al., 1992). An earlier immunocytochemical study that examined cat, monkey and rat retinas (Meister et al., 1991) found DARPP-32 associated with Mueller glial cells and retinal neurons of unidentified subtypes. Mouse retinas have so far not been explored in relation to DARPP-32.
In the present study we examined DARPP-32 localization by immunocytochemistry in mouse and rat retinas. We also utilized a genetically modified mouse in which the DARPP-32 protein has been deleted [DARPP-32-knockout (KO) mouse; Fienberg et al., 1998], to explore whether DARPP-32 is obligatory for the phosphorylation of certain target proteins. In wild-type (WT) rat and mouse retinas, the cell groups containing DARPP-32 included horizontal cells, AII amacrine cells, Mueller glial cells and some amacrines of unidentified subtypes. In addition we looked for a possible circadian rhythm of DARPP-32 expression and examined the effect of light exposure on DARPP-32 phosphorylation at different threonine residues. Our results contribute to the understanding of where in the retina DARPP-32 is located and also indicate strongly that DARPP-32 participates in light-dependent signalling in mammalian retinas.
Materials and methods
Animals
All animal handling and anaesthetic procedures were approved by the Animal Care and Use committees of the respective institutions (New York University School of Medicine, Columbia University, Rockefeller University). C57BL/6 adult male mice and DARPP-32-KO adult male mice on a C57BL/6 background (Fienberg & Greengard, 2000) were bred at Rockefeller University and housed in translucent propylene cages in a 12 : 12 h light–dark (L/D) cycle for at least 3 weeks before use. For immunocytochemical procedures, the mice were anaesthetized deeply with 200 mg/kg pentobarbital and perfused intracardially with 50 mL of 0.9% saline, followed by 100 mL of 4% paraformaldehyde in 0.2 M phosphate buffer (PB), pH 7.2. After perfusion, the eyes were removed, hemisected and the lens removed, then postfixed for an additional 1 h in buffered paraformaldehyde, followed by 3 × 20-min washes in phosphate-buffered saline (PBS).
Adult Sprague-Dawley albino rats were obtained from a commercial supplier and maintained on a 12 : 12 h L/D cycle. Rats were killed 3–4 h after light onset by exposure to 100% CO2 for ~30 s until the withdrawal response to a leg pinch had disappeared, then decapitated and the eyes enucleated. After removal of the cornea and lens, the posterior pole of the eye was fixed for 1 h at room temperature in buffered paraformaldehyde, then washed 3 × 20 min in PB. During all fixation and wash procedures the eye tissues were rotated at 10 r.p.m. on a rotating table. Both mouse and rat eyes were cryoprotected by overnight immersion, at 4 °C, in 30% sucrose solution, then frozen-sectioned at 14–16 μm. After drying, the sections were kept at −20 °C until used.
Immunocytochemistry
Two anti-DARPP-32 antibodies were tested: a polyclonal rabbit anti-DARPP-32 for which the antigen was a keyhole limpet hemocyanin-coupled 15-amino acid peptide from human DARPP-32 surrounding Thr 34 (1 : 1000, #2302; Cell Signalling Technology, Danvers, MA, USA), and a monoclonal mouse anti-DARPP-32 raised against complete bovine DARPP-32 (1 : 1000–1 : 10 000; C24a-6a; Ouimet et al., 1984; Hemmings & Greengard, 1986). For colocalization studies we utilized mouse anticalbindin (1 : 1000, C-8666; Sigma-Aldrich, St Louis, MO, USA), rabbit anticalbindin (1 : 2000, AB 1778; Chemicon, Temecula CA, USA), rabbit antidisabled-1 (Dab1; 1 : 20 000, AB5840; Chemicon), rabbit antiglutamine synthetase (1 : 20,000–1 : 50 000, G2781; Sigma-Aldrich) mouse antiparvalbumin (1 : 1000, P-3171; Sigma-Aldrich) and rabbit antimelanopsin (1 : 1000; a generous gift from Dr M. Rollag, Uniformed Services University of the Health Sciences). The secondary antibodies were Alexa 488 goat antimouse (1 : 400; Molecular Probes, Eugene, OR, USA) and Cy3 goat antirabbit (1 : 200; Jackson ImmunoResearch, West Grove, PA, USA).
Slides were washed 3 × 10 min in PBS, then 30 min in blocking solution (PBS containing 0.1% Na azide, 0.3% Triton X-100 and 10 mg/mL bovine serum albumin). Thereafter the primary antibody or a mixture of primary antibodies was diluted with blocking solution and left for 16–20 h at room temperature. After 3 × 10 min washes in PBS, the secondary antibodies were applied for 2 h. The slides then were washed 3 × 10 min in PBS and the coverslips mounted in VectaShield (Vector Laboratories, Burlingame, CA, USA).
Sections were examined in a Nikon PM800 confocal microscope (Nikon, Melville, NY, USA) equipped with a digital camera controlled by the Spot software program (Diagnostic Instruments, Inc., Sterling Hgts., MI, USA). Digital files were processed in Adobe PhotoShop 7.0 (Adobe Systems, San Jose, CA, USA). Auto-Quant software, which carries out iterated image deconvolutions based on an estimated point spread function, was used to improve the quality of confocal images (Auto-Quant Imaging, Watervliet, NY, USA).
Drug injections
We utilized the specific dopamine D1 receptor antagonist SCH 23390 (Tocris, Ellisville, MO, USA). A 1 mM stock solution was made in PBS and frozen. Mice were anaesthetized with a 0.2 mL injection i.p. of a mixture of ketamine and xylazine. In complete darkness, 1 μL of 200 μM SCH 23390 was injected into the left eye of an anaesthetized mouse and 1 μL of PBS was injected into the right eye, using an infrared imaging system. Further details of this experiment are given in Results. After drug application and exposure to light the mice were decapitated and the heads frozen on dry ice. Subsequently the tissues were allowed to warm to just above freezing, the eyes removed and the retinas dissected out and refrozen on dry ice for processing by immunoblotting, as described below.
Circadian rhythmicity
For tests of a circadian rhythm in retinal DARPP-32 phosphorylation, WT and DARPP-32-KO C57BL/6 mice were maintained in the Rockefeller University animal quarters on a 12 : 12 h L/D cycle. L/D-entrained mice were placed in complete darkness (DD) for 48 h. On the third day of DD, mice were killed at 4-h intervals (corresponding to circadian times [CT ] 4, 8, 12, 16, 20 and 24 h) by focused microwave irradiation (4.5–5.0 kW for 1.4 s), using a small animal microwave (Muromachi Kikai, Tokyo, Japan).
Immunoblotting
Retinas were dissected free and immediately refrozen on dry ice for processing. Retinal tissues were sonicated in boiling 1% sodium dodecyl sulphate solution, and boiled for an additional 10 min. Small aliquots of the homogenate were retained for protein determination by the BCA protein assay method (Pierce, Rockford, IL, USA) using bovine serum albumin as a standard. Equal amounts of protein (20 μg) were loaded onto 12% acrylamide gels and the proteins separated by SDS-PAGE, then transferred to nitrocellulose membranes (0.2 μm; Schleicher & Schuell, Keene, NH, USA) by the method of Towbin et al. (1979). The membranes were immunoblotted with phosphospecific antibodies against Thr 34-DARPP-32, Thr 75-DARPP-32 or serine (Ser) 845-ionotropic glutamate receptor subunit 1 (GluR1; Cell Signalling Technology) or using either the monoclonal or polyclonal anti-DARPP-32 antibodies described above (monoclonal, 1 : 7500 dilution; polyclonal, 1 : 5000 dilution). Identical results were obtained with the two anti-DARPP-32 antibodies. We also tested a pan anti-GluR1 antibody (Cell Signalling Technology). Antibody binding was revealed by incubation with goat antimouse horseradish peroxidase-linked IgG (1 : 6000–8000; Pierce) and the ECL immunoblotting detection system (GE Healthcare BioSciences, Piscataway, NJ, USA). Chemiluminescence was detected by autoradiography using DuPont NEN autoradiography film (Perkin Elmer, Wellesley, MA, USA). Levels of DARPP-32 or GluR1 phosphoproteins were normalized by reference to the total amount of DARPP-32 or GluR1 protein, respectively. Two-way ANOVA with a post hoc Tukey test was used to evaluate the experimental data for statistical significance.
Results
Differences in DARPP-32 immunoreactivity (IR) between mouse and rat retinas
The first two questions we addressed were the comparison of mouse and rat retinas for DARPP-32 IR and the identification of the subtypes of retinal neuron or glial cell showing DARPP-32 IR.
The retinal cell types showing DARPP-32 IR were identical in mouse and rat retinas, although some staining differences were noted depending on the identity and the dilution of the primary antibody, as described below. In the mouse retina, using a monoclonal anti-DARPP-32 antibody at a dilution of 1 : 1000, the most prominently labelled cell group (Fig. 1a) was situated in the distal portion of the inner nuclear layer (inl) corresponding in position to retinal horizontal cells. Immunostaining of presumed horizontal cell processes at the level of the outer plexiform layer (opl) was noted (Fig. 1a, white arrow). Another class of immunolabelled cell was located at the border of inl and the inner plexiform layer (ipl), a retinal location at which primarily amacrine cells are located Fig. 1a, black arrows). In the middle of the inl we observed additional, faintly stained, cell bodies of irregular shape; moreover, fine vertically directed immunostained processes were observed in the photoreceptor layer (Fig. 1a, asterisk). Both of these are features of Mueller glial cells.
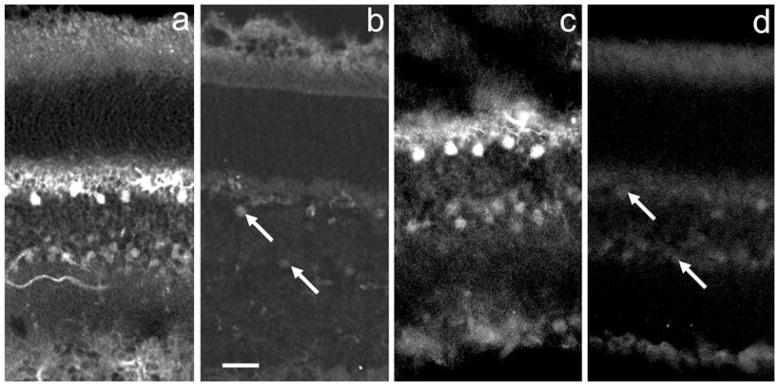
Fig. 1.
DARPP-32 IR in mouse and rat retinas. Panels (a) and (c) show, respectively, the patterns of immunostaining in WT mouse and rat retinas using a monoclonal anti-DARPP antibody at 1 : 1000 dilution. Panels (b) and (d) illustrate that immunostaining is completely blocked by preincubation of the primary antibody with recombinant DARPP-32. Retinal layers labelled in (b) apply to all panels: phot, photoreceptor layer; opl, outer plexiform layer; inl, inner nuclear layer; ipl, inner plexiform layer; gcl, ganglion cell layer. In (a) the asterisk in the photoreceptor layer indicates distal processes of glial Mueller fibers, the white arrow indicates a horizontal cell body and the black arrows point to amacrine cell bodies. In (c) the asterisk indicates Mueller cell processes, the downward pointing arrow marks a Mueller cell body and the rightward slanting arrows point to amacrine cell bodies. Staining of Mueller cell basal processes is noted at the vitreal (lower) edge of the retina in both (a) and (c). Scale bar, 10 μm (applies to all panels).
In the rat retina, at the same 1 : 1000 dilution of a monoclonal anti-DARPP-32 antibody, strong DARPP-32 immunostaining was seen for cells located in the middle portion of the inl (Fig. 1c, downward pointing arrow). Additional prominent immunostaining was seen at the vitreal surface of the retina, and as fine, vertically orientated fibers in the photoreceptor layer Fig. 1c, asterisk). As noted above, all of these immunolabelled structures are consistent with the anatomy of Mueller glial fibers. Another set of immunoreactive cells was a number of presumed amacrine neurons located at the inl–ipl border (Fig. 1c, right pointing arrows). However, at 1 : 1000 there was no apparent immunostaining of horizontal cells. Our tentative conclusions concerning the identities of the various immunoreactive cell types were probed further by colocalization of characteristic cell marker proteins with DARPP-32 IR, as described below.
Tests of antibody specificity
We ran, in parallel, slides containing primary antibody and with the primary antibody omitted. Omission of the primary antibody resulted in no visible immunostaining (not illustrated). When the primary antibody was preincubated with rat recombinant DARPP-32 protein overnight, immunostaining was completely abolished in both mouse and rat retinas (Fig. 1b and d).
Immunostaining of the mouse retina in which DARPP-32 was genetically deleted is illustrated in Fig. 2. WT (Fig. 2a and c) and KO mouse sections (Fig. 2b and d) were run in parallel and their digital images captured using the same settings. For Fig. 2a and b, the mouse monoclonal anti-DARPP-32 antibody (1 : 1000) was employed, and for Fig. 2c and d, the rabbit polyclonal anti-DARPP-32 antibody (1 : 1000) was tested. It is noteworthy that, with mouse retina as a substrate, the two anti-DARPP-32 antibodies gave similar immunostaining patterns. In both cases it can be seen that, compared to the WT control, the immunostaining of the KO mouse was very substantially reduced but was not completely absent.
Fig. 2.
Comparison of anti-DARPP-32 IR in WT and DARPP-32-KO mouse retinas. (a and c) DARPP-32 IR in WT mouse retinas exposed, respectively, to (a) a mouse monoclonal and (c) a rabbit polyclonal anti-DARPP-32 antibody at 1 : 1000 dilution. (b and d) Immunostaining of the same antibodies in mouse knockout retinas. Retinal layers can be identified by reference to the labels in Fig. 1b. Note that in (c), the plane of the section is oblique. In the WT retinas, horizontal cells and amacrine cells are clearly labelled. In the KO retinas, these two cell types are faintly stained, as indicated by the arrows. Scale bar, 20 μm (applies to all panels).
Western blots were produced from WT and DARPP-32-KO mice, as illustrated in Fig. 3. In contrast to the immunostaining results, in the DARPP-32-KO mouse no band was observed corresponding to the 32-kDa band noted in the WT eye (Fig. 3, lower panels). This difference may indicate a differential sensitivity of the two methods or might be explained by assuming that the anti-DARPP-32 antibody lacks specificity (see Discussion). Figure 3, upper panels, illustrate that GluR1 protein is present in both WT and DARPP-32-KO mouse retinas.
Fig. 3.
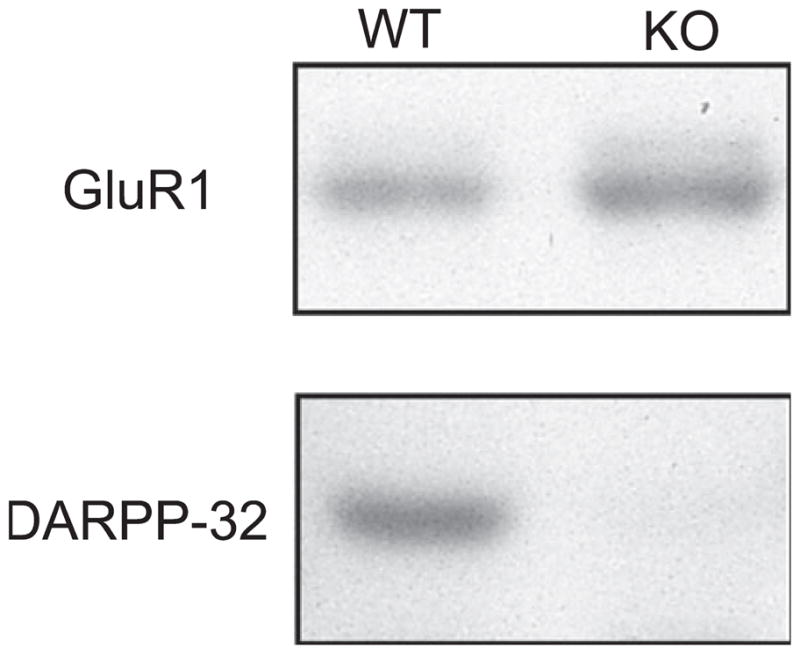
Immunoblots of total retinal DARPP-32 and GluR1 in WT and DARPP-32-KO mice. The upper panels show that GluR1 protein is found in both WT or DARPP-32-KO mice. The lower panels illustrate total retinal DARPP-32 protein in WT mice (left) and in DARPP-32-KO mice (right). DARPP-32 protein was undetectable in DARPP-32-KO mice. See Materials and Methods for details of the procedure.
Effect of antibody dilution on anti-DARPP-32 immunostaining in rat retina
To complete the characterization of DARPP-32 IR in rodent retinas, Fig. 4 illustrates a comparison of rat retinal IR obtained with different dilutions of a rabbit polyclonal anti-DARPP-32 antibody. The results with a monoclonal anti-DARPP-32 antibody at 1 : 1000 were presented above (Fig. 1c). Using a rabbit polyclonal anti-DARPP-32 antibody at dilutions of either 1 : 200 (Fig. 4a), 1 : 500 (Fig. 4b) or 1 : 1000 (Fig. 4c) we obtained somewhat different patterns of DARPP-32 IR. At any dilution, the most prominently labelled cell group was presumed amacrine cells at the inl–ipl border. The size and shape of these neurons, together with the observation that many of them have a vertically orientated primary dendrite that enters the ipl, indicate that these are AII amacrine cells (Partida et al., 2004). At a dilution of 1 : 200 (Fig. 4a) both horizontal and glial Mueller cells showed immunostaining, but at a dilution of 1 : 1000 no horizontal cell IR was evident and that of Mueller cells was weak (Fig. 4c).
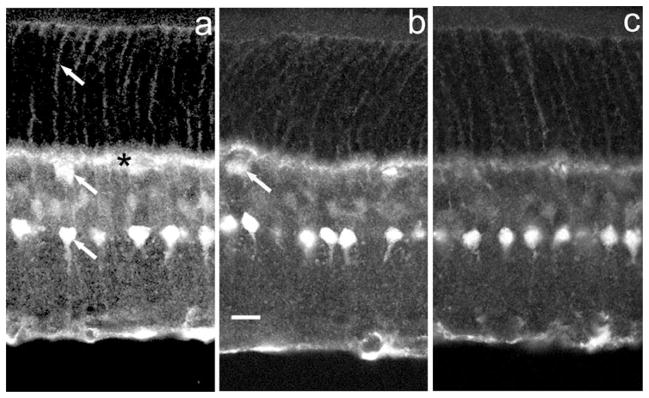
Fig. 4.
DARPP-32 IR in the rat retina at different dilutions of a rabbit polyclonal antibody. (a) 1 : 200 dilution. Immunostaining is seen most strongly in amacrine cells at the inl–ipl border (lowest arrow), in horizontal cell bodies (middle arrow) and horizontal cell processes in the outer plexiform layer (asterisk) and in glial Mueller cells with cell bodies in the inl and distal processes in the photoreceptor layer (uppermost arrow). (b) 1 : 500 dilution. Horizontal cell bodies (arrow) and Mueller cells show reduced immunostaining whereas amacrine cells retain robust immunostaining. (c) 1 : 1000 dilution. Amacrine cells are strongly immunoreactive, but horizontal cell immunostaining is absent and Mueller cell IR is weak.
Co-localization of DARPP-32 immunolabelling with cell marker proteins
Calbindin
The calcium binding protein calbindin is very prominent in mammalian retinal horizontal cells (Haverkamp & Wässle, 2000). Figure 5 illustrates, for the mouse retina, calbindin staining of horizontal cell bodies and processes alone (Fig. 5a), anti-DARPP-32 staining of the same cells (Fig. 5b) and the merged images in Fig. 5c. The yellow colour which fills the horizontal cell bodies and processes attests to the extensive colocalization of these two markers in the mouse retina. Similar results were obtained for rat retina, using a 1 : 200 dilution of the anti-DARPP-32 antibody to reveal the horizontal cells (not illustrated).
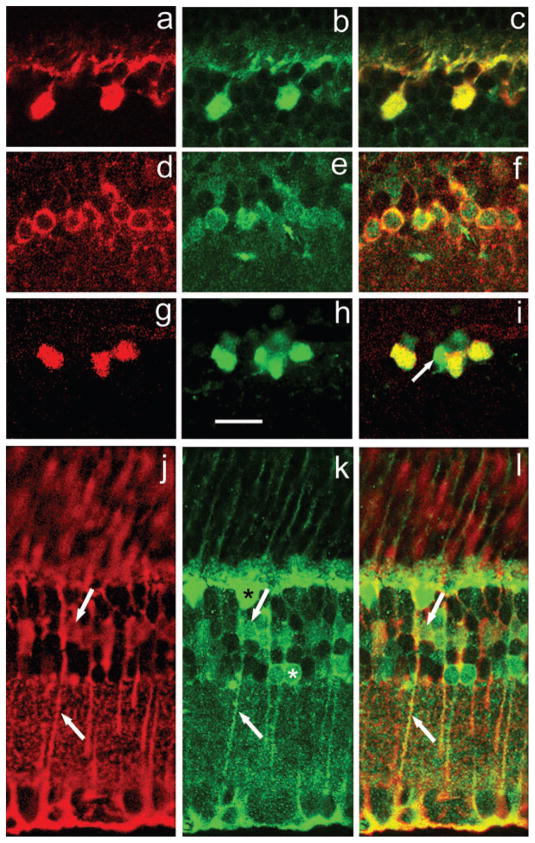
Fig. 5.
Colocalization of DARPP-32 with cell marker proteins. Each horizontal set of three panels illustrates the marker protein in red at left, mouse monoclonal DARPP-32 in green in the middle and the merged image at right. (a–c) Horizontal cells in the mouse retina immunostained with an anticalbindin antibody. (d–f) AII amacrine cells in the mouse retina immunostained with an anti-Dab1 antibody. (g–i) AII amacrine cells in the rat retina immunostained with an antiparvalbumin antibody. The arrow indicates a DARPP-32-positive amacrine cell which does not show parvalbumin IR. (j–l) Glial Mueller cells in the mouse retina immunostained with an antiglutamine synthetase. In each panel, a Mueller cell vertically directed process through the ipl is indicated by an upwards-pointing arrow, and the Mueller cell body in the inl by a downwards-pointing arrow. In (k), an AII amacrine is indicated by a white asterisk, a horizontal cell by a black asterisk. Antibody dilutions: DARPP-32, 1 : 1000; calbindin, 1 : 1000; parvalbumin, 1 : 500; Dab1, 1 : 10 000; glutamine synthetase, 1 : 20 000. Scale bar, 20 μm (applies to all panels).
Parvalbumin and disabled-1 (Dab1)
In the rat, but not the mouse, retina, AII amacrine cells are immunoreactive for parvalbumin (Wässle et al., 1993) whereas in the mouse, but not the rat, retina, AII amacrine cells are immunoreactive for Dab1, a protein involved in retinal development (Rice & Curran, 2000). AII amacrines are distinguished by their high density (MacNeil et al., 1999), their relatively small size (7–10 μm diameter) and their round shape from which a single vertical dendrite emerges that enters the ipl. Figure 5d–f illustrates the colocalization of Dab1 IR and anti-DARPP-32 IR in AII amacrine cells. A corresponding set of immunostainings on rat retina revealed colocalization of parvalbumin and anti-DARPP-32 IR (Fig. 5g–i). However, not all of the DARPP-32-positive neurons at the inl–ipl border were also parvalbumin-positive (Fig. 5i, arrow), indicating that at least one other subtype of presumed amacrine cell also manifests DARPP-32 IR.
Melanopsin
The visual pigment melanopsin is found in a subset of ganglion cells which are light-sensitive (Hattar et al., 2002) and which project to the suprachiasmatic nucleus (Berson et al., 2002), the locus of the master circadian clock. We found that melanopsin-containing ganglion cells did not colocalize DARPP-32 IR (not illustrated).
Glutamine synthetase
In vertebrate retinas, glutamine synthetase is confined to the Mueller glial cells (Vardimon et al., 1986). An antiglutamine synthetase antibody results in immunostaining of both mouse and rat retinal Mueller cells throughout their length, from the vitreal endfoot, through the inner nuclear layer where the nucleus of the Mueller cell is located, to its distal prolongation in the photoreceptor layer. These features are illustrated in a mouse retina in Fig. 5j. Monoclonal anti-DARPP-32 IR is evident in all parts of the Mueller cell, from the vitreal end foot through the fibre portion to the cell body, as noted in Fig. 5k. Colocalization of these two markers is presented in Fig. 5l. Identical results showing colocalization of DARPP-32 and glutamine synthetase IR were obtained for the rat retina (not illustrated).
Light-induced phosphorylation of DARPP-32
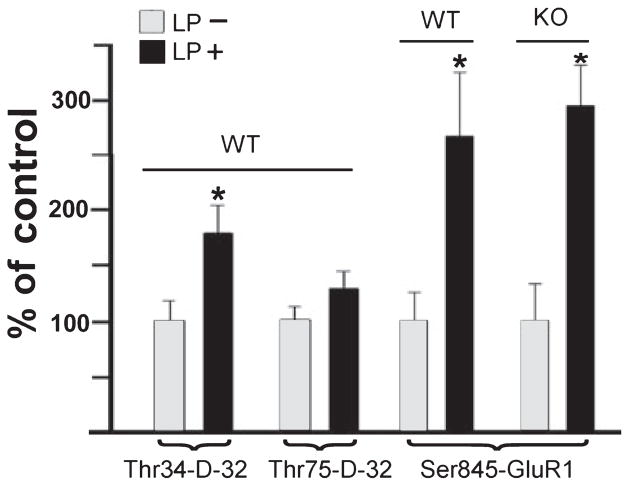
Phosphorylation of DARPP-32 is a crucial step in its activation (reviewed in Svenningsson et al., 2004). We studied the control of DARPP-32 phosphorylation in two ways. One was to attempt to modify the level of DARPP-32 phosphorylation by light. Mice were placed in DD for 48 h after being housed in a 12 : 12 h L/D cycle for 3 weeks. On the third day in DD, beginning at CT 17, the mice were exposed to fluorescent light (300 lux) for 30 min, then killed immediately by microwave irradiation and the retinas harvested. Immunoblots were generated to assay for DARPP-32 phosphorylation at Thr 34 and Thr 75. Figure 6, left side, shows the effects of exposure to light on Thr 34- and Thr 75-DARPP-32 phosphorylation. There was a significant effect of light on phosphorylation of DARPP-32 at Thr 34 but not at Thr 75 (ANOVA F1,29 = 9.16, P = 0.006; Tukey’s test for Thr 34, P = 0.005 and for Thr 75, P > 0.05.
Fig. 6.
Light-induced phosphorylation of DARPP-32 and GluR1. Mice were conditioned to an L/D cycle for 3 weeks, then placed in DD for 48 h. On day 3 of DD, mice were exposed to 300 lux fluorescent light for 30 min beginning at CT 17, then killed. Immunoblots of retinal tissues were processed using phosphospecific antibodies for Thr 34- and Thr 75-DARPP-32 and for Ser 845-GluR1. Data were normalized to the total amount of DARPP-32 or GluR1 in the retinal sample. The left hand WT group shows that exposure to a light pulse (LP+) significantly increased Thr 34-DARPP-32 over that seen in retinas of mice kept in darkness but killed at the same time (LP–). The same treatment did not significantly increase Thr 75-DARPP-32. The two right-hand groups show that a light pulse significantly increased phosphorylation at the Ser 845-GluR1 in both WT and DARPP-32-KO mice. *P < 0.05.
Light-induced phosphorylation of GluR1
The ionotropic glutamate receptors of the α-amino-3 hydroxy-5-methyl-4-isoxazolepropionate (AMPA) subtype mediate fast excitatory transmission in the CNS. Although we found that the density of GluR1 sites is much lower in retina than in striatum (not illustrated), GluR1 is detectable in the retinas of both WT and KO mice (Fig. 3).
In the mammalian brain, the subunits GluR1 and GluR4 of AMPA receptors are phosphorylated by several kinases, including protein kinase A (Roche et al., 1996; Mammen et al., 1997; Carvalho et al., 1999). Following its own phosphorylation at Thr 34, DARPP-32 participates in the GluR1 phosphorylation process by preventing dephosphorylation through an inhibition of protein phosphatase-1. We utilized the same retinal tissues studied for DARPP-32 phosphorylation (Fig. 6, left side) to examine GluR1 phosphorylation at Ser 845. As illustrated in Fig. 6, right side, in WT mice an exposure to light during the subjective dark phase more than doubled the level of Ser 845-GluR1 phosphorylation. However, augmentation by light of Ser 845-GluR1 phosphorylation was also noted for the DARPP-32-KO mice, indicating the occurrence of both DARPP-32-dependent and DARPP-32-independent pathways for Ser 845-GluR1 phosphorylation. A two-way ANOVA of this data gave the following values: F1.14 = 2.14, P < 0.001 for the effects of light and P = 0.26 for WT vs. KO, indicating no significant effect of genotype. The post hoc Tukey test gave values of P = 0.01 for WT and 0.006 for KO.
Effects of a D1 dopamine antagonist on light-induced phosphorylation of DARPP-32 and GluR1
The experiment of Fig. 6 does not directly implicate dopamine in the light-induced cascade leading to Thr 34-DARPP-32 and Ser 845-GluR1 phosphorylation. On the other hand, it is well known that exposure to light increases dopamine release (reviewed in Witkovsky et al., 2004), thereby activating D1 receptors coupled to adenylate cyclase, resulting in increased cAMP production and activation of PKA. We therefore tested whether a D1 dopamine antagonist could interfere with the effects of light on phosphorylation of DARPP-32 and/or GluR1. WT and DARPP-32-KO mice were maintained for at least 3 weeks on a 12 : 12 h L/D cycle. Animals were anaesthetized in the light just before lights-off (n = 4 for each test group). After 1.5 h in darkness, the left eye was injected with a specific dopamine D1 antagonist, SCH 23390, and the right eye with PBS. Twenty minutes later the mouse was exposed to room light for 20 min, then killed and the retinas harvested for immunoblotting (see Materials and Methods for more details). As documented in Fig. 7, left side, exposure to SCH 23390 significantly reduced the amount of light-induced Thr 34-DARPP-32 phosphorylation relative to the vehicle-treated eye of the same animal. Using samples from the same retinas, in the WT mice, exposure to SCH 23390 also significantly reduced the degree of light-induced phosphorylation of Ser 845-GluR1 phosphorylation relative to that measured in PBS-injected control eyes (Fig. 7, right side, WT, two-way ANOVA: F1,12 = 11.414, P = 0.008. Tukey test, P = 0.03 for the Thr 34 and P = 0.05 for the Ser 845). On the other hand, in DARPP-32-KO mice there was no statistically significant difference in the level of Ser 845-GluR1 phosphorylation between drug- and vehicle-injected eyes (Fig. 7, right side, KO).
Fig. 7.
Interaction of light and a D1 dopamine antagonist on DARPP-32 and GluR1 phosphorylation. WT and DARPP-32-KO mice conditioned to an L/D cycle received an intraocular injection of the D1 dopamine antagonist SCH 23390 in the left eye and vehicle in the right eye in darkness, 1.5 h after lights off. After an additional 20 min in darkness the mice were exposed to room light for 20 min, then killed and the retinas immediately frozen and processed for immunoblots (see Materials and Methods for further details). The left hand WT group shows, for WT mice, that pretreatment with SCH 23390 significantly reduced Thr 34-DARPP-32 relative to vehicle-injected controls, whereas Thr 75-DARPP-32 was not significantly affected. The two groups on the right illustrate that pretreatment with SCH 23390 significantly reduced light-stimulated Ser 845-GluR1relative to vehicle-injected control in WT but not in DARPP-32-KO mice. *P < 0.05.
Tests of circadian rhythmicity
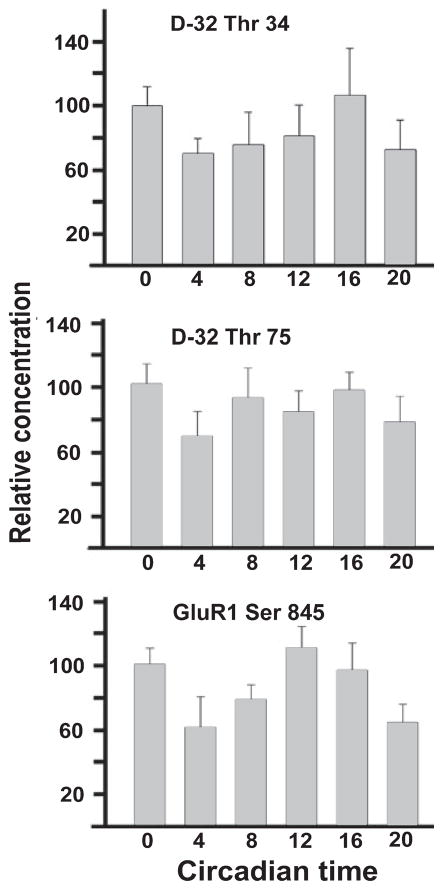
We looked for a possible circadian variation in DARPP-32 phosphorylation at Thr 34 and Thr 75 sites and for Ser 845-GluR1 phosphorylation. For this experiment, four mice were killed at each of six time points at 4-h intervals beginning at CT 0. There was no statistically significant rhythmic variation in phosphorylation at either Thr 34 or Thr 75 through the circadian cycle (Fig. 8, upper and middle panels). Similarly, no circadian rhythmicity was evident in total DARPP-32 or total GluR1 protein (not illustrated). With regard to the phosphorylation of Ser 845-GluR1, no statistically significant rhythmicity was noted although peak phosphorylation at CT 12 was significantly higher than at CT 20, and just missed being significantly higher (P = 0.06) than that seen at CT 4. (Fig. 8, lowest panel). Thus there is a suggestion that Ser 845-GluR1 phosphorylation rises during subjective day and falls during subjective night.
Fig. 8.
Test of circadian rhythmicity in DARPP-32 and GluR1 phosphory-lation. WT mice were conditioned to an LD cycle for 3 weeks before being placed in DD. On day 3 of DD mice were killed in darkness at 4-h intervals (CT 0, CT 4 etc.) and the retinas processed for immunoblotting. Data in each panel are normalized to the value at CT 0. Top to bottom, panels show, respectively, variations in Thr 34-DARPP-32, Thr 75-DARPP-32 and Ser 845-GluR1 relative phosphoprotein concentrations as a function of circadian time (CT). No significant variation was seen for DARPP-32 phosphorylation at either threonine site. GluR1 phosphorylation appeared to rise during subjective day (CT 0–12) and fall during subjective night (CT 12–24). The mean level of GluR1-Ser 845 at CT 12 was significantly different (P = 0.05) from that at CT 20 and just missed being significantly different (P = 0.06) from that at CT 4.
Discussion
Main findings of the current study
The main results of our study are, first, to document the presence of DARPP-32 in the mouse retina. Second, in both mouse and rat retinas we established that DARPP-32 was present in horizontal and AII amacrine neurons and in the principal glial cell of the retina, the Mueller cell. DARPP-32 IR was absent, however, in melanopsin-containing ganglion cells. The blockage of DARPP-32 IR by preincubation of the antibody with synthetic DARPP-32 protein and the absence of a DARPP-32 signal in immunoblots from DARPP-32-KO mice confirm the immunocytochemical results. Third, we found that light stimulates phosphorylation of Thr 34-DARPP-32. Our fourth finding was that light-induced phosphorylation of Thr 34-DARPP-32 is at least partly dependent on dopamine, as it was significantly reduced when the eye was exposed to a D1 dopamine receptor antagonist prior to light exposure. Finally, we found that light increased phosphorylation of the GluR1 AMPA receptor subunit at its Protein kinase A-dependent Ser 845 site. No circadian rhythm was seen in either total DARPP-32 or GluR1 protein, or in the phosphorylated forms of these proteins.
Our data extend earlier immunocytochemical data (Meister et al., 1991; Partida et al., 2004) showing that mammalian retinas express DARPP-32 IR in Mueller glial cells and a subtype of amacrine cell known as the AII amacrine cell. We confirmed the presence of DARPP-32 in these cells and showed in addition that retinal horizontal cells and some amacrine cells of unknown subtype express DARPP-32 IR. The apparent differences in anti-DARPP-32 immunostaining pattern in rat retina between Partida et al. (2004) and our data are attributable to antibody dilution. With the Cell Signalling polyclonal anti-DARPP-32 antibody utilized by Partida et al. (2004) at a dilution of 1 : 1000 we obtained the pattern they reported in which only AII amacrines showed prominent IR but, at dilutions of 1 : 200–1 : 500, immunostaining was additionally seen in horizontal neurons and Mueller glial cells (cf. Fig. 4).
One problematical finding was the presence of residual DARPP-32 immunostaining in the retinas of DARPP-32-KO mice (Fig. 2). This datum contrasts with the absence of DARPP-32 IR in the striatum in the same strain of DARPP-32-KO mice (Fienberg & Greengard, 2000), when tested with the same monoclonal anti-DARPP-32 antibody used in the present study. In addition, retinal DARPP-32 IR was completely blocked by preincubation of the primary antibody with DARPP-32 protein (Fig. 1) and absent in immunoblots of retina (Fig. 3). An isoform of DARPP-32, called t-DARPP (i.e. truncated DARPP), was reported in gastric cancer tissue (El-Rifai et al., 2002). This shortened isoform lacks the Thr 34 site which we have shown to be present in retina (see Fig. 6). Moreover, residual immunostaining is present in DARPP-32-KO mice using a polyclonal anti-DARPP-32 antibody (Fig. 2d) whose epitope is the region around Thr 34, a region lacking in t-DARPP, so it is unlikely that this isoform accounts for the residual staining. Correspondingly, there was no band in our Western blots corresponding to t-DARPP (not illustrated). We assume provisionally that the residual immunostaining is nonspecific.
Colocalization of dopamine D1 receptor and DARPP-32
Given the dependence of Thr 34-DARPP-32 phosphorylation on a dopamine D1 receptor, one would expect that the retinal cell types in which DARPP-32 was found would also possess D1 receptors. The evidence for this colocalization is strongest in the retinal horizontal cell. In mammalian retinas, dopamine D1 receptors have been localized to the horizontal cell by immunocytochemistry (Veruki & Wässle, 1996) and by RT-PCR (Li et al., 2004). He et al. (1994) found that dopamine uncoupled horizontal cells, a response mediated by a D1 dopamine receptor. Comparable evidence for the AII amacrine cell is not yet complete. Although an immunocytochemical probe failed to localize a dopamine D1 receptor to the AII amacrine cell (Veruki & Wässle, 1996), there is good physiological evidence that dopamine, acting through a D1 receptor, uncouples AII amacrine cells (Hampson et al., 1992). As Veruki & Wässle (1996) suggested, it may be that the relevant dopamine receptor on the AII amacrine neuron is of a different subtype, e.g. type D5, which is not recognized by the antibody but still responds to dopamine D1 ligands. With regard to the Mueller glial cell, Kubrusly et al. (2005) demonstrated that Mueller cells cultured from chicken retina have functional D1 dopamine receptors. Comparable data for mammalian retinal Mueller cells are still lacking.
In summary, there is good, if somewhat incomplete, evidence that the three classes of retinal cell in which we found DARPP-32 IR also possess dopamine D1 receptors.
DARPP-32 phosphorylation and its functional significance
We found that exposure to light during subjective night increased DARPP-32 phosphorylation at Thr 34, but not at Thr 75. Thr 34 phosphorylation activates DARPP-32 to become an inhibitor of protein phosphatase-1, resulting in increased phosphorylation of proteins. Light is known to induce an increase in dopamine synthesis and turnover in mammalian retinas (Iuvone et al., 1978; Morgan & Kamp, 1982); this turnover is greatest at the transition from night to day (Nir et al., 2000). Our result thus suggests that a light-induced increase in extracellular dopamine results in increased phosphorylation of Thr 34-DARPP-32.
The finding that light increases DARPP-32 phosphorylation does not, however, necessarily implicate dopamine in the signalling cascade that induces phosphorylation. Our additional experiment to test this point was to inject the eye with a specific dopamine D1 antagonist, SCH 23390, prior to exposing the eye to light. This resulted in a significant fall in DARPP-32 phosphorylation of ~40% relative to the control eye injected with vehicle. Although this result clearly implicates dopamine, it also suggests the possibility that some additional pathway not involving dopamine could lead to DARPP-32 phosphorylation. An obvious possibility is that light increases cAMP production, possibly by activating peptides such as vasoactive intestinal polypeptide (VIP) and pituitary adenylate cyclase-activating peptide, which stimulate cAMP production. In this regard, Lasater et al. (1983) found that VIP strongly activates cAMP synthesis in horizontal cell suspensions collected from fish retinas. Moreover, it has been demonstrated that VIP phosphorylates Thr 34-DARPP-32 (Snyder et al., 1992).
Our data also established that light induced a sharp increase in the phosphorylation of the AMPA GluR1 subunit at Ser 845, a PKA-dependent process, although other kinases are also active at this site (Roche et al., 1996). Light induced about the same degree of Ser 845-GluR1 phosphorylation in WT and DARPP-32-KO mice, again indicating that not all of this phosphorylation is DARPP-32-dependent. We found, however, when SCH 23390 was administered to the eye prior to light exposure, that Ser 845-GluR1 phosphorylation was significantly reduced relative to control eyes injected with vehicle alone. On the other hand, neither our experimental drug treatments nor exposure to light significantly altered the total retinal amount of either DARPP-32 or GluR1.
Unlike the brain areas (e.g. striatum and hippocampus) in which GluR1 is a predominant subunit of AMPA receptors (Walaas et al., 1983), in the mammalian retina GluR2/3 and 4 are more prevalent than GluR1 (Qin & Pourcho, 1996; Grünert et al., 2002). Thus although we have shown that GluR1 is phosphorylated in a light-and DARPP-32-dependent fashion, it is probable that other AMPA subunits also are subject to this sort of regulation. With regard to the two subtypes of retinal neuron that were the focus of our study, the mammalian retinal horizontal cell lacks GluR1 but possesses GluR2/3 and GluR4 subunits (Morigiwa & Vardi, 1999; Haverkamp et al., 2001). The AII amacrine cell of rat retina is reported to possess GluR1 subunits (Gründer et al., 2000), although the same neuronal class in cat, rabbit and monkey retinas contains GluR2/3 and GluR4 but not GluR1 subunits (Qin & Pourcho, 1999; Ghosh et al., 2001). Dopamine, acting on horizontal cells through a D1 receptor, increases the time the AMPA receptor spends in the open state (Knapp & Dowling, 1987). As the GluR4 subunit studied in brain is phosphorylated by multiple kinases, including protein kinase A (Carvalho et al., 1999), potentially GluR4 is a target of dopamine and DARPP-32 activation, a promising topic for future study.
In summary, we have shown not only that DARPP-32 is present in particular subtypes of retinal neuron and in its principal glial cell but also that it plays a role in regulating glutamatergic transmission via phosphorylation of certain ionotropic glutamate receptors. In the retina, dopamine has been implicated in multiple mechanisms affecting signal transmission, leading to the general conclusion that dopamine helps prepare the retina for daytime vision by enhancing signal transmission through cone-dependent circuits (reviewed in Witkovsky, 2004). Given the findings of the present study indicating that, in rodent retinas, dopamine works at least partly through DARPP-32, it will be important to identify the targets of DARPP-32 activation. In this way it will be possible to relate the functions of DARPP-32 to basic questions of retinal physiology, such as the changes induced in retinal circuits by light and dark adaptation. As a final point, the retina carries information about light signals to the suprachiasmatic nucleus, the master circadian clock. Although we found in this study that the melanopsin-containing ganglion cells which project to the suprachiasmatic nucleus lack DARPP-32, there is evidence from a study comparing WT with DARPP-32-KO mice (Yan et al., 2006) that DARPP-32 influences the retinal pathway carrying photic information that resets the circadian clock.
Acknowledgments
Supported by the Richard H. Chartrand Fdn. (P.W.) Vetenskapsrådet and Hjärnfonden (P.S.) and NS037919 (R.S.). We thank Dr Paul Greengard for providing the monoclonal anti-DARPP-32 antibody and the DARPP-32-knockout animals.
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate
- CT
circadian time
- Dab1
disabled-1
- DARPP-32
dopamine- and cyclic adenosine 3′-5′-monophosphate-regulated phosphoprotein, 32 kDa
- DD
complete darkness
- GluR1
ionotropic glutamate receptor subunit 1
- inl
inner nuclear layer
- ipl
inner plexiform layer
- IR
immunoreactivity
- KO
knockout
- L/D
light–dark
- opl
outer plexiform layer
- PB
phosphate buffer
- PBS
phosphate-buffered saline
- Ser
serine
- Thr
threonine
- VIP
vasoactive intestinal polypeptide
- WT
wild-type
References
- Berson DM, Dunn FA, Motoharu T. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- Bibb JA, Snyder GL, Nishi A, Yan Z, Meijer L, Fienberg AA, Tsai LH, Kwon YT, Girault JA, Czernik AJ, Huganir RL, Hemmings HC, Jr, Nairn AC, Greengard P. Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signaling in neurons. Nature. 1999;402:669–671. doi: 10.1038/45251. [DOI] [PubMed] [Google Scholar]
- Carvalho AL, Kameyama K, Huganir RL. Characterization of phosphorylation sites on the glutamate receptor 4 subunit of the AMPA receptors. J Neurosci. 1999;19:4748–4754. doi: 10.1523/JNEUROSCI.19-12-04748.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Rifai W, Smith MF, Jr, Li G, Beckler A, Carl VS, Montgomery E, Knnutila S, Moskaluk CA, Frierson HF, Jr, Powell SM. Gastric cancers overexpress DARPP-32 and a novel isoform, t-DARPP. Cancer Res. 2002;62:4061–4064. [PubMed] [Google Scholar]
- Fienberg AA, Greengard P. The DARPP-32 knockout mouse. Brain Res Brain Res Rev. 2000;31:313–319. doi: 10.1016/s0165-0173(99)00047-8. [DOI] [PubMed] [Google Scholar]
- Fienberg AA, Hiroi N, Mermelstein PG, Song WJ, Snyder GL, Nishi A, Cheramy A, O’Callaghan JP, Miller DB, Cole DG, Corbett R, Haile CN, Cooper DC, Onn SP, Grace AA, Ouimet CC, White FJ, Hyman SE, Surmeier DJ, Girault JA, Nestler EJ, Greengard P. DARPP-32: regulator of the efficacy of dopaminergic neurotransmission. Science. 1998;281:838–842. doi: 10.1126/science.281.5378.838. [DOI] [PubMed] [Google Scholar]
- Ghosh KK, Haverkamp S, Wässle H. Glutamate receptors in the rod pathway of the mammalian retina. J Neurosci. 2001;21:8636–8647. doi: 10.1523/JNEUROSCI.21-21-08636.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gründer T, Kohler K, Guenther E. Distribution and developmental regulation of AMPA receptor subunit proteins in rat retina. Invest Ophthal Vis Sci. 2000;41:3600–3606. [PubMed] [Google Scholar]
- Grünert J, Haverkamp S, Fletcher EL, Wässle H. Synaptic distribution of ionotropic glutamate receptors in the inner plexiform layer of the primate retina. J Comp Neurol. 2002;447:138–151. doi: 10.1002/cne.10220. [DOI] [PubMed] [Google Scholar]
- Hampson EGCM, Vaney DI, Weiler R. Dopaminergic modulation of gap junction permeability between amacrine cells in mammalian retina. J Neurosci. 1992;12:4911–4922. doi: 10.1523/JNEUROSCI.12-12-04911.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp S, Grunert U, Wässle H. The synaptic architecture of AMPA receptors at the cone pedicle of the primate retina. J Neurosci. 2001;21:2488–2500. doi: 10.1523/JNEUROSCI.21-07-02488.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp S, Wässle H. Immunocytochemical analysis of the mouse retina. J Comp Neurol. 2000;424:1–23. [PubMed] [Google Scholar]
- He S, Weiler R, Vaney DI. Endogenous dopaminergic modulation of horizontal cell gap junctions in mammalian retina. Proc R Soc Lond B Biol Sci. 1994;255:67–72. doi: 10.1098/rspb.1994.0010. [DOI] [PubMed] [Google Scholar]
- Hemmings HC, Jr, Greengard P. DARPP-32, a dopamine- and adenosine 3′:5′-monophosphate-regulated phosphoprotein: regional, tissue and phylogenetic distribution. J Neurosci. 1986;6:1469–1481. doi: 10.1523/JNEUROSCI.06-05-01469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings HC, Jr, Greengard P, Tung HY, Cohen P. DARPP-32, a dopamine-regulated neuronal phosphoprotein, is a potent inhibitor of protein phosphatase-1. Nature. 1984;310:503–505. doi: 10.1038/310503a0. [DOI] [PubMed] [Google Scholar]
- Iuvone PM, Galli CL, Garrison-Gund CK, Neff NH. Light stimulates tyrosine hydroxylase activity and dopamine synthesis in retinal amacrine neurons. Science. 1978;202:901–902. doi: 10.1126/science.30997. [DOI] [PubMed] [Google Scholar]
- Knapp AG, Dowling JE. Dopamine enhances excitatory amino acid-gated conductances in cultured retinal horizontal cells. Nature. 1987;325:437–439. doi: 10.1038/325437a0. [DOI] [PubMed] [Google Scholar]
- Kubrusly RC, da Cunha MC, Reis RA, Soares H, Ventura AL, Kurtenbach E, de Mello MC, de Mello FG. Expression of functional receptors and transmitter enzymes in cultured Muller cells. Brain Res. 2005;1038:141–149. doi: 10.1016/j.brainres.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Lasater EM, Watling KJ, Dowling JE. Vasoactive intestinal peptide alters membrane potential and cyclic nucleotide levels in retinal horizontal cells. Science. 1983;221:1070–1072. doi: 10.1126/science.6308770. [DOI] [PubMed] [Google Scholar]
- Li H, Gaughwin P, Li N, He S. Localization of dopamine D1-receptor to A-type horizontal cells in the rabbit retina by single cell RT-PCR. Neurosci Lett. 2004;355:146–148. doi: 10.1016/j.neulet.2003.10.042. [DOI] [PubMed] [Google Scholar]
- MacNeil MA, Heussy JK, Dacheux RF, Raviola E, Masland RH. The shapes and numbers of amacrine cells: matching of photofilled with golgi-stained cells in the rabbit retina and comparison with other mammalian species. J Comp Neurol. 1999;413:305–326. [PubMed] [Google Scholar]
- Mammen AL, Kameyama K, Roche KW, Huganir RL. Phosphorylation of the α-amino-3-hydroxy-5-methylisoxazole-4-proprionic acid receptor GluR1 subunit by calcium/calmodulin-dependent kinase II. J Biol Chem. 1997;272:32528–32533. doi: 10.1074/jbc.272.51.32528. [DOI] [PubMed] [Google Scholar]
- Meister B, Arvidsson J, Hemmings HC, Jr, Greengard P, Hökfelt T. Dopamine- and adenosine-3′:5′-monophosphate (cAMP)-regulated phosphoprotein of Mr 32,000 (DARPP-32) in the retina of cat, monkey and human. Neurosci Lett. 1991;131:66–70. doi: 10.1016/0304-3940(91)90338-t. [DOI] [PubMed] [Google Scholar]
- Morgan WW, Kamp CW. Postnatal development of the light response of the dopaminergic neurons in the rat retina. J Neurochem. 1982;39:283–285. doi: 10.1111/j.1471-4159.1982.tb04737.x. [DOI] [PubMed] [Google Scholar]
- Morigiwa K, Vardi N. Differential expression of ionotropic glutamate receptor subunits in the outer retina. J Comp Neurol. 1999;405:173–184. doi: 10.1002/(sici)1096-9861(19990308)405:2<173::aid-cne3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Nguyen-Legros J, Simon A, Caillé I, Bloch B. Immunocytochemical localization of dopamine D1 receptors in the retina of mammals. Vis Neurosci. 1997;14:545–551. doi: 10.1017/s0952523800012207. [DOI] [PubMed] [Google Scholar]
- Nir I, Haque R, Iuvone PM. Diurnal metabolism of dopamine in the mouse retina. Brain Res. 2000;870:118–125. doi: 10.1016/s0006-8993(00)02409-4. [DOI] [PubMed] [Google Scholar]
- Ouimet CC, Miller PE, Jiemmings HC, Jr, Walaas SI, Greengard P. Darpp-32, a dopamine- and adenosine 3′:5′-monophosphate-regulated phosphoprotein enriched in dopamine-innervated brain regions. J Neurosci. 1984;4:111–124. doi: 10.1523/JNEUROSCI.04-01-00111.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partida GJ, Lee SC, Haft-Candell L, Nichols GS, Ishida AT. DARPP-32-like immunoreactivity in AII amacrine cells of rat retina. J Comp Neurol. 2004;480:251–263. doi: 10.1002/cne.20330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P, Pourcho RG. Distribution of AMPA-selective glutamate receptor subunits in the cat retina. Brain Res. 1996;710:303–307. doi: 10.1016/0006-8993(95)01476-4. [DOI] [PubMed] [Google Scholar]
- Qin P, Pourcho RG. AMPA-selective glutamate receptor subunits, GluR2 and GluR4 in the cat retina: an immunocytochemical study. Vis Neurosci. 1999;16:1105–1114. doi: 10.1017/s0952523899166100. [DOI] [PubMed] [Google Scholar]
- Rice DS, Curran T. Disabled-1 is expressed in type AII amacrine cells in the mouse retina. J Comp Neurol. 2000;424:327–338. doi: 10.1002/1096-9861(20000821)424:2<327::aid-cne10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Roche KW, O’Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–1188. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- Snyder GL, Girault JA, Chen JY, Czernik AJ, Kebabian JW, Nathanson JA, Greengard P. Phosphorylation of DARPP-32 and protein phosphatase inhibitor-1 in rat choroids plexus: regulation by factors other than dopamine. J Neurosci. 1992;12:3071–3083. doi: 10.1523/JNEUROSCI.12-08-03071.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P. DARPP-32: an integrator of neurotransmission. Annu Rev Pharmacol Toxicol. 2004;44:269–296. doi: 10.1146/annurev.pharmtox.44.101802.121415. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehlin T, Gordon J. Electrophorectic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardimon L, Fox LE, Moscona AA. Developmental regulation of glutamine synthetase and carbonic anhydrase II in neural retina. Proc Natl Acad Sci USA. 1986;83:9060–9064. doi: 10.1073/pnas.83.23.9060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veruki ML, Wässle H. Immunohistochemical localization of dopamine D1 receptors in rat retina. Eur J Neurosci. 1996;8:2286–2297. doi: 10.1111/j.1460-9568.1996.tb01192.x. [DOI] [PubMed] [Google Scholar]
- Walaas SID, Aswad W, Greengard P. A dopamine- and cyclic AMP-regulated phosphoprotein enriched in dopamine-innervated brain regions. Nature. 1983;301:69–71. doi: 10.1038/301069a0. [DOI] [PubMed] [Google Scholar]
- Wässle H, Grunert U, Röhrenbeck J. Immunocytochemical staining of AII-amacrine cells in the retina with antibodies against parvalbumin. J Comp Neurol. 1993;332:407–420. doi: 10.1002/cne.903320403. [DOI] [PubMed] [Google Scholar]
- Witkovsky P. Dopamine and retinal function. Doc Ophthal. 2004;108:17–40. doi: 10.1023/b:doop.0000019487.88486.0a. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Veisenberger E, Haycock JW, Akopian A, Garcia-Espana A, Meller E. Activity-dependent phosphorylation of tyrosine hydroxylase in dopaminergic neurons of the rat retina. J Neurosci. 2004;24:4242–4249. doi: 10.1523/JNEUROSCI.5436-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Bobula JM, Svenningsson P, Greengard P, Silver R. DARPP-32 involvement in the photic pathway of the circadian system. J Neurosci. 2006;26:9434–9438. doi: 10.1523/JNEUROSCI.2538-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]