Abstract
Glycogen phosphorylase (GP) catalyzes the rate-limiting step in glycogen catabolism and plays a key role in maintaining cellular and organismal glucose homeostasis. GP is the first protein whose function was discovered to be regulated by reversible protein phosphorylation, which is controlled by phosphorylase kinase (PhK) and protein phosphatase 1 (PP1). Here, we report that lysine acetylation negatively regulates GP activity by both inhibiting enzyme activity directly and promoting dephosphorylation. Acetylation of GP Lys470 enhances its interaction with the PP1 substrate targeting subunit, GL, and PP1, thereby promoting GP dephosphorylation and inactivation. We show that GP acetylation is stimulated by glucose and insulin and inhibited by glucagon. Our results provide molecular insights into the intricate regulation of the classical GP and a functional cross-talk between protein acetylation and phosphorylation.
Keywords: Acetylation, phosphorylation, metabolism, insulin, glycogen phosphorylase
Glycogen phosphorylase (GP) catalyzes phosphorolytic cleavage of glycogen to produce glucose-1-phosphate for glucose-dependent tissues when body glucose is scarce. GP activity plays an important role in glucose homeostasis and glycogen metabolism. Defects in glycogen synthesis and breakdown in liver, muscle and other glucose-dependent tissues often cause glycogen storage diseases (Stegelmeier et al., 1995). For example, McArdle disease is caused by mutations in muscle GP and patients with this disorder are intolerance in exercise and show early fatigue (Andreu et al., 2007; Tang et al., 2003). Due to their critical roles in glucose homoeostasis, regulations of glycogen synthase (GS) and GP activities have been extensively investigated in hoping of finding therapeutic strategy for type II diabetes.
GP was the first allosteric enzyme to be discovered by Carl and Gerty Cori who demonstrated that GP existed in two interconvertible forms, referred subsequently to as an active a form or inactive b form, that are regulated by adenosine monophosphate (AMP) (Cori and Cori, 1936). GP is activated by the binding of AMP and IMP, whereas it is inhibited by the binding of ATP and glucose-6-phosphate (Barford et al., 1991; Barford and Johnson, 1989). These allosteric controls provide excellent means of GP activity regulation in response to energy and metabolic status. Moreover, GP activity is tightly regulated by reversible phosphorylation and dephosphorylation. In addition to allosteric regulation, GP is also regulated by post translational modifications (Johnson, 1992). In fact, GP was also the first protein discovered to be regulated by reversible protein phosphorylation (Fischer and Krebs, 1955; Sutherland and Wosilait, 1955), which exemplifies a signal transduction pathway by phosphorylation cascades. Under high serum glucose conditions, release of insulin indirectly activates protein phosphatase-1 (PP1), which dephosphorylates serine-15 and converts the active a form of GP to unphosphorylated inactive b form, leading to the inhibition of glycogen breakdown (Browner and Fletterick, 1992). Conversely, when glucose concentration is low, glucagon triggers a cascade of signal transduction that activates protein kinase A (PKA) which phosphorylates and activates phosphorylase kinase (PhK) which, in turn, activates GP by phosphorylating serine-15 and leads to increased glycogen breakdown and ultimately higher glucose levels.
PP1 regulates glycogen metabolism by inhibiting GP activity and activating glycogen synthase (GS) activity. The hepatic glycogen binding subunit GL is a glycogen metabolizing scaffold protein that binds to PP1, glycogen, GS and GP (Armstrong et al., 1998). GL targets PP1 to glycogen, where it dephosphorylates and inhibits GP, in addition to dephosphorylating and activating GS, thereby increasing glycogen synthesis and reducing glucose output (Alemany and Cohen, 1986). Therefore, the phosphorylation status of GP is critically regulated by its interaction with GL.
Lysine acetylation has emerged as a common regulatory mechanism of diverse cellular processes, including in metabolism (Choudhary et al., 2009; Kim et al., 2006; Wang et al., 2010; Zhao et al., 2010). Acetylation modulates enzymes involved in fatty acid metabolism, urea cycle, TCA cycle and gluconeogenesis via different mechanisms such as inhibition, activation, and protein destabilization (Guan and Xiong, 2011; Kim and Yang, 2011). In the present study, we show that acetylation inhibits GP activity by promoting its dephosphorylation. This is accomplished by an acetylation-induced interaction between GP and the PP1 targeting subunit GL. Our study provides an example of cross-talk between acetylation and phosphorylation in regulation of a key enzyme of the glycogen metabolism in response to different physiologic conditions.
RESULTS
Acetylation negatively regulates GP catalytic activity
In an attempt to profile liver protein acetylation, we previously enriched acetylated peptides of human liver proteins by affinity purification (Zhao et al., 2010). Among the many liver acetylated proteins identified, two peptides were found to contain acetylated lysine 470 (K470) and lysine 796 (K796) of GP (Figure S1A). To confirm GP acetylation, we expressed Flag-tagged GP in Chang’s liver cells and then treated cells with nicotinamide (NAM) and trichostatin A (TSA), two commonly used deacetylase inhibitors that inhibit all four classes of deacetylases (Xu et al., 2007). GP was found to be acetylated and its acetylation was significantly elevated (approximately 2 fold) after NAM and TSA treatment (Figure 1A). Mutation of both K470 and K796 (GPK2R) dramatically decreased GP acetylation (Figure 1B), indicating that K470 and K796 are the major, if not the sole, acetylation sites in GP.
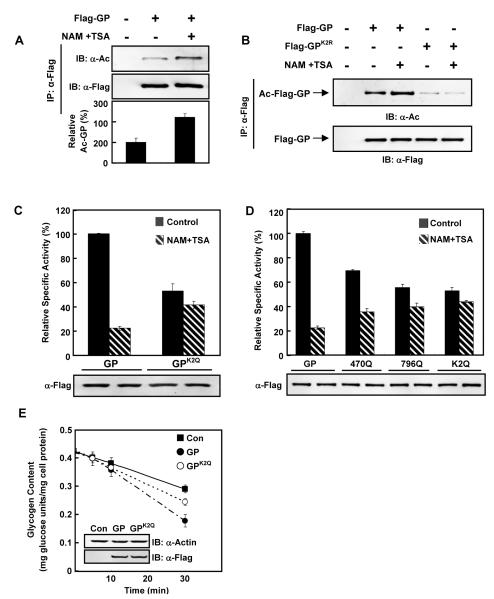
Figure 1. GP activity is negatively regulated by acetylation.
(A) GP acetylation is increased by deacetylase inhibitor. Flag-tagged GP was expressed in Chang’s cells with or without nicotinamide and trichostatin A (NAM+TSA) treatment. Acetylation levels of Flag-beads purified GP were blotted with pan-anti-acetyllysine antibody (α-AcK). IB and IP denote immunoblotting and immunoprecipitation, respectively.
(B) K470 and K796 are the major acetylation sites in GP. Ectopically expressed Flag-GP and GPK2R in Chang’s cells were immunopurified and immuno-blotted with pan-anti-acetyllysine antibody.
(C) GP catalytic activity is negatively regulated by acetylation. Both wild type GP and GPK2Q were expressed in Chang’s liver cells with or without NAM+TSA treatment. Catalytic activity of affinity purified GP proteins were determined and normalized to protein levels. Activity of wild type GP under no treatment condition was set as 100%.
(D) The acetylation target K470 and K796 are important for GP inhibition by the deacetylase inhibitor treatment. GP, GPK470Q, GPK796Q and GPK2Q were each expressed in Chang’s liver cells with (hatched bars) or without (solid bars) NAM+TSA treatment as indicated. Specific activity of each purified enzyme was determined.
(E) Wild-type GP degrades glycogen faster than K2Q mutant. Wild type GP and mutant GPK2Q were expressed at similar levels in Chang’s liver cells maintained in regular DMEM medium. The medium was replaced by glucose-free DMEM at time 0 and the glycogen degradation rate was measured. All error bars represent standard deviation (SD). n = 3 for each experimental group.
Next, the functional significance of acetylation in GP catalytic activity was investigated. For GP assay, we adapted a published protocol (Jones and Wright, 1970) and confirmed that under the conditions used in our experiments, the assay was linear to GP enzyme concentrations and displayed a typical Michaelis-Menten response to glycogen concentrations (Figure S1B, S1C). Treatment with deacetylase inhibitors decreased the specific activity of GP by 75% (Figure 1C), suggesting that GP activity is negatively regulated by acetylation. Moreover, when both K470 and K796 of GP were changed to acetylation mimetic glutamines (GPK2Q), the GPK2Q mutant displayed significantly lower (about 55%) specific activity than the wild type. Interestingly, the GPK2Q was no longer inhibited by deacetylase inhibitor treatment (Figure 1C), indicating that the K470 and K796 in GP are the primary acetylation sites for its enzymatic inhibition. To clarify the individual contribution of K470 and K796 acetylation to GP activity, we generated single lysine to glutamine substitution on either K470 (GPK470Q) or K796 (GPK796Q). GPK470Q and GPK796Q had about 30% and 45% less specific activity than wild type GP, respectively (Figure 1D), suggesting that acetylation of either K470 or K796 contributed to GP inhibition. Notably, unlike GPK2Q, which had negligible response to deacetylase inhibitor treatment, both GPK470Q and GPK796Q activities were still partially inhibited by NAM and TSA treatment (Figure 1D), suggesting that both K470 and K796 acetylation contribute to GP catalytic activity regulation and these two acetylation sites may function additively.
Because GP can form homodimers, we investigated whether endogenous GP might interfere with our assays by forming heterodimers with the transfected mutant GP and whether the mutant GP could dominantly inhibit wild type GP. In the transfected cells, the ectopically expressed GP was much higher (about 10 folds) than the endogenous protein GP (Figure S1D), indicating that endogenous GP should have negligible effect on our assay results. Moreover, we purified WT homodimer, WT-K2R heterodimer, and K2R homodimer and assayed their catalytic activity. The WT-K2R heterodimer exhibited activity that was the average of wild type and K2R homodimers (Figure S1E), showing that the K2R mutant has no dominant inhibition on wild type GP. Together, our results suggest that K470 and K796 are two major acetylation sites in GP responsible for its inhibition by acetylation. To further test the effect of acetylation on GP activity and glycogen metabolism, we determined cellular glycogen content in response to NAM and TSA treatment. Consistent with an inhibitory effect of acetylation on GP activity, we found that inhibition of deacetylases by NAM and TSA increased cellular glycogen levels (Figure S1F). Next, we determined glycogen hydrolysis in cells expressing different GP mutants. Expression of wild type GP promoted a faster glycogen hydrolysis than the GPK2Q mutant (Figure 1E), further supporting a lower enzymatic activity of the acetylation mimetic mutant GPK2Q.
GP acetylation and activity is regulated by glucose, insulin, and glucagon
Given that glucose concentration is a key factor in GP regulation, we determined GP acetylation under different glucose concentrations. The acetylation level of ectopically expressed GP was low when cells were maintained in glucose free medium, and increased significantly with elevated glucose concentrations in a dose dependent manner by as much as 4 folds (Figure 2A), suggesting that acetylation of GP was up-regulated by glucose. Moreover, acetylation of endogenous GP in L02 human hepatocytes was increased by glucose (Figure 2B, S2A). NAM and TSA treatment of cells cultured in high glucose medium could further increase the acetylation level of endogenous GP (Figure 2B), showing that deacetylation of GP occurs even under high glucose.
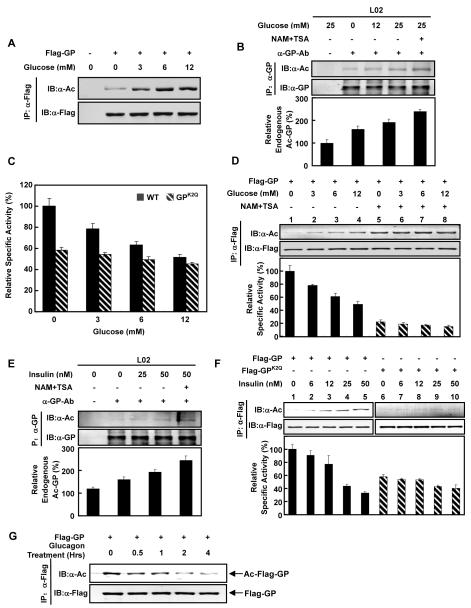
Figure 2. Regulation of GP acetylation by glucose, insulin, and glucagon.
(A) Glucose increases GP acetylation. Acetylation levels of GP ectopically expressed in Chang’s cells maintained in different glucose concentrations were probed by anti-acetyllysine antibody.
(B) Glucose and deacetylase inhibitor increase endogenous GP acetylation. Human hepatic L02 cells were treated with various concentrations of glucose and deacetylase inhibitors as indicated. Endogenous GP was immunoprecipitated with a GP antibody. Acetylation levels of GP were determined by western blot.
(C) Glucose decreases GP activity through acetylation. Relative specific activity of GP (solid bars) and GPK2Q (hatched bars) expressed in Chang’s cells maintained in different glucose concentrations were determined. Specific activity of GP from glucose free medium was arbitrarily set as 100.
(D) Deacetylase inhibitor treatment blocks the glucose inhibition on GP activity. Relative specific activities for GP expressed in Chang’s cells maintained in different glucose concentrations with (hatched bars) and without NAM+TSA treatment (solid bars) were determined.
(E) Insulin and deacetylase inhibitor increase endogenous GP acetylation. Human hepatic L02 cells were treated with increasing concentrations of insulin and treated with deacetylase inhibitors as indicated. Acetylation of immunoprecipitated endogenous GP was detected by western blot.
(F) Insulin decreases GP activity through acetylation. Flag-GP (solid bars) and Flag-GPK2Q (hatched bars) were expressed in Chang’s cells and treated with increasing concentrations of insulin. GP acetylation and activity were determined.
(G) Glucagon decreases GP acetylation. Acetylation levels of Flag-GP expressed in Chang’s cells treated with 10nM glucagon for different time periods (as indicated) were determined. All error bars represent standard deviation (SD). n = 3-4 for each experimental group.
Next, the effect of glucose on GP activity was determined. As shown in Figure 2C, the activity of wild type GP was significantly inhibited by increasing concentrations of glucose while GPK2Q was largely refractory to inhibition by glucose (Figure 2C). These results suggest that glucose inhibits GP activity through K470 and K796 acetylation. To further test the function of acetylation in mediating glucose-induced GP inhibition, we measured the effect of glucose on GP activity after NAM and TSA treatment. We hypothesized that since the inhibition of deacetylases would increase GP acetylation, high glucose may not have a significant effect on GP activity in the presence of deacetylase inhibitors. As expected, glucose increased GP acetylation and decreased GP activity in the absence of deacetylase inhibitors (Figure 2D, lanes 1-4). However, in the presence of deacetylase inhibitors, glucose had little effect on GP activity and did not further increase the elevated GP acetylation (Figure 2D, lanes 5-8). These results further support that acetylation plays a major role in GP inhibition in response to glucose.
Both insulin and glucagon are important signals that regulate GP activity and glycogen metabolism in opposite manners (Lok et al., 1994; Massague and Guinovart, 1977). We tested whether these two hormones could regulate GP acetylation. In L02 human hepatocytes, the acetylation of endogenous GP was increased with insulin treatment in time and dose-dependent manners (Figure 2E, S2B). Moreover, acetylation of ectopically expressed GP increased within 30 minutes of insulin addition (Figure S2C). We then examined the combined effect of both glucose and insulin. GP acetylation in cells cultured in the presence of both glucose and insulin was higher than cells cultured in either glucose or insulin (Figure S2D). Consistent with the stimulatory effect of insulin on GP acetylation, we found that insulin inhibited GP activity in a dose-dependent manner (Figure 2F, lanes 1-5). These effects required K470 and K796, as the GPK2Q mutant showed little acetylation and its catalytic activity did not respond to insulin treatment (Figure 2F, lanes 6-10). Contrary to insulin treatment, glucagon decreased GP acetylation in a time-dependent manner (Figure 2G). Together, these results suggest that insulin and glucagon may regulate GP activity by affecting K470 and K796 acetylation.
Acetylation decreases GP phosphorylation
It is well-characterized that GP is activated by PhK-mediated serine phosphorylation at Ser15 (Nolan et al., 1964). To investigate the mechanism of acetylation in regulating GP activity, we looked into a possible cross-talk between acetylation and phosphorylation by determining GP serine phosphorylation under different conditions that are known to affect GP acetylation levels. We found that deacetylase inhibitor treatment increased GP acetylation and at the same time decreased Ser15 phosphorylation by 70% (Figure 3A). Similar results were observed when we utilized a pan-phosphoserine antibody to detect the GP serine phosphorylation level after NAM and TSA treatment (Figure S3A). The inverse relationship between endogenous GP acetylation and phosphorylation in responding to deacetylase inhibitor was also examined in L02 human hepatocytes and freshly isolated mouse primary hepatocytes. In both hepatocytes, endogenous GP showed a significant increase in acetylation and a concomitant decrease in Ser15 phosphorylation upon NAM and TSA treatment (Figure 3B). Similar results were also observed in freshly isolated mouse skeletal muscle cells (Figure S3B). These observations demonstrate that acetylation negatively regulates GP phosphorylation.
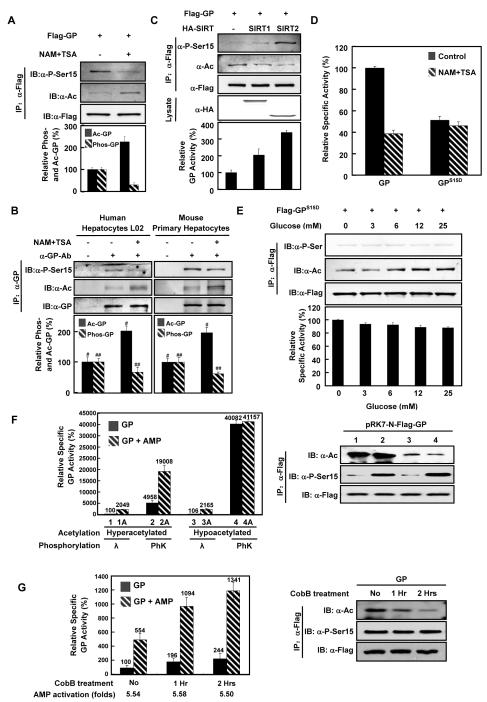
Figure 3. Acetylation decreases GP Ser-15 phosphorylation.
(A) Deacetylase inhibitor treatment inhibits GP phosphorylation. Flag-GP was expressed in Chang’s liver cells and treated with or without NAM+TSA. Acetylation and GP-Ser15 phosphorylation levels of Flag-GP proteins were determined by western blot.
(B) Deacetylase inhibitor treatment increases acetylation and decreases phosphorylation of hepatic GP. Human hepatic L02 cells and mouse primary hepatocytes were treated with or without NAM+TSA as indicated. Acetylation and Ser-15 phosphorylation of GP proteins were determined by western blot (L02: p#=0.0094, n=3; p##=0.0107, n=3. Primary hepatocytes: p#=0.0090, n=3; p##=0.0270, n=3).
(C) Co-expression of deacetylases reduces GP acetylation and increases GP phosphorylation. Flag-GP was expressed alone or co-expressed with Sirt1 and Sirt2 in Chang’s liver cells as indicated. Acetylation and Ser-15 phosphorylation of affinity purified GP were measured. Relative specific activities of GP were determined by normalizing GP activity against GP protein.
(D) GP Ser-15 is required for deacetylase inhibitor-induced GP inactivation. Wild type GP and GPS15D mutant GP were expressed in Chang’s liver cells, with or without NAM+TSA treatment. Activities of affinity purified GP were determined.
(E) The effect of glucose on GP activity but not acetylation depends on Ser15. GPS15D transfected Chang’s liver cells were treated with different concentrations of glucose. Acetylation, phosphorylation, and relative activity of GPS15D were determined. Activity of GP from glucose free medium was set as 100% arbitrarily.
(F) Effect of acetylation and phosphorylation on GP activity and activation by AMP. Hyperacetylated GP was immunoprecipitated from Flag-GP-transfected Chang’s liver cells maintained in high glucose (25mM) medium supplemented with NAM+TSA. Hypoacetylated GP was immunoprecipitated from Flag-GP transfected cells cultured in glucose free medium without NAM+TSA. The immunopurified GP was incubated with lambda phosphatase (λ) or phosphorylase kinase (PhK) in appropriate buffers for 2 hours. Both phosphatase and kinase were removed by washing with PBS and the treated GP was measured for enzyme activity with (hatched bars) or without AMP (5mM, solid bars) as indicated. Relative enzyme activity was normalized against GP protein. Ser15 phosphorylation and lysine acetylation levels were determined by western blotting.
(G) GP allosteric activation by AMP is not affected by acetylation. Flag-GP were purified from Chang’s liver cells and then treated for 1 or 2 hours with buffer or CobB as indicated. Relative activity of purified GP was measured in the absence (solid bars) or presence of 5mM AMP (hatched bars) and normalized by protein quantity. All error bars represent standard deviation (SD). n = 3 for each experimental group.
To provide direct evidence that acetylation regulates GP phosphorylation, we examined GP phosphorylation status when GP was co-expressed with deacetylases. When GP was co-expressed with SIRT1 and SIRT2, two cytosolic deacetylases, an evident decrease in GP acetylation and concomitant increase in GP Ser15 phosphorylation were observed with SIRT2 being more potent in deacetylating GP and increasing Ser15 phosphorylation (Figure 3C). Collaborating with these findings, the catalytic activity of GP was also activated by either SIRT1 or SIRT2 co-expression. This result indicates a causal role of acetylation in modulating GP phosphorylation.
To further elucidate the relationship between GP acetylation and phosphorylation, we determined the effect of deacetylase inhibitors on the activity of GPS15D, which had Ser15 replaced by an aspartic acid and displayed a lower activity (Buchbinder et al., 1997). Interestingly, the activity of GPS15D mutant was not inhibited by deacetylase inhibitor treatment whereas the wild type GP was potently inhibited by a similar treatment (Figure 3D). Of note, glucose still increased the acetylation of GPS15D mutant (Figure 3E). Therefore, Ser15 phosphorylation is not required for high glucose-stimulated GP acetylation; rather, acetylation may down-regulate Ser-15 phosphorylation and activity of GP.
Because the inhibitory effect of acetylation on phosphorylation, the above data was unable to show whether acetylation may directly affect GP activity. To address this question, we prepared GP with or without acetylation and with or without phosphorylation. Hyperacetylated GP was obtained by expressing Flag-GP in Chang’s liver cells maintained in high glucose (25mM) medium supplemented with NAM and TSA, while hypoacetylated GP was obtained by glucose free medium without NAM and TSA. The immunopurified GP proteins were treated with lambda phosphatase or PhK in vitro. After verifying both the acetylation and Ser15 phosphorylation of GP by western blotting (right panel, Figure 3F), GP activity was determined. We found that phosphorylation by GP kinase (PhK) dramatically increased GP activity regardless whether GP was hyperacetylated (lane 1 vs. 2, left panel, Figure 3F) or hypoacetylated (lanes 3 vs. 4). Acetylation did not affect GP activity when GP was hypophosphorylated (lanes 1 vs. 3). However, when GP was hyperphosphorylated, acetylation exhibited an inhibitory effect on GP in vitro (lanes 2 vs. 4). These results indicate that acetylation inhibits GP only when it is hyperphosphorylated and active.
GP is also regulated by allosteric effect, including activation of inactive b form by AMP. To explore the effect of acetylation on allosteric regulation of GP, we determined the activity of hypo- vs. hyperacetylated GP in response to AMP. We found that AMP induced a similar activation to both the hyperacetylated (lanes 1 vs. 1A, 20.5 folds, Figure 3F) and hypoacetylated GP (lanes 3 vs. 3A, 20.4 folds) when GP was hypophosphorylated, indicating that acetylation does not directly affect allosteric activation of GP by AMP. However, when GP was fully activated (hypoacetylated/hyperphosphorylated), AMP could not activate GP further (lanes 4 vs. 4A). To provide more direct evidence to determine the effect of acetylation on allosteric regulation, we treated immunopurified GP with recombinant deacetylase CobB in vitro and measured its allosteric activation by AMP after confirming the decrease of acetylation (right panel, Figure 3G). We found that AMP activated GP equally regardless of the levels of GP’s acetylation (5.54 folds, 5.58 folds, and 5.50 folds, left panel, Figure 3G). We therefore conclude that acetylation does not directly affect allosteric activation of GP by AMP.
Physiological stimuli regulate GP acetylation-induced dephosphorylation
The notion that acetylation negatively regulates GP-Ser15 phosphorylation was further pursued by analyzing acetylation and phosphorylation levels in response to physiological stimuli. We found that glucose increased GP acetylation and decreased phosphorylation in a dose-dependent manner (Figure 4A), indicating an inverse relationship between GP acetylation and phosphorylation. Moreover, insulin treatment increased GP acetylation and decreased GP phosphorylation (Figure 4B), whereas glucagon treatment decreased GP acetylation and increased GP phosphorylation (Figure 4C). A time-dependent inverse correlation between acetylation and phosphorylation in GP was observed in response to glucose, insulin, and glucagon (Figure 4D, S4A-B), supporting that acetylation may inhibit GP phosphorylation. It is worth noting that glucagon can regulate GP activity through a direct signaling pathway. Of note, although Ser15 phosphorylation occurred rapidly and was evident as early as 30 minutes after glucagon treatment, it increased continuously throughout the 4 hours of experimental duration (Figure 2G, 4D). Thus, it is possible that deacetylation may not be required for the acute GP phosphorylation by glucagon but rather play a role to maintain the phosphorylated and active GP in the active state (Figure S7).
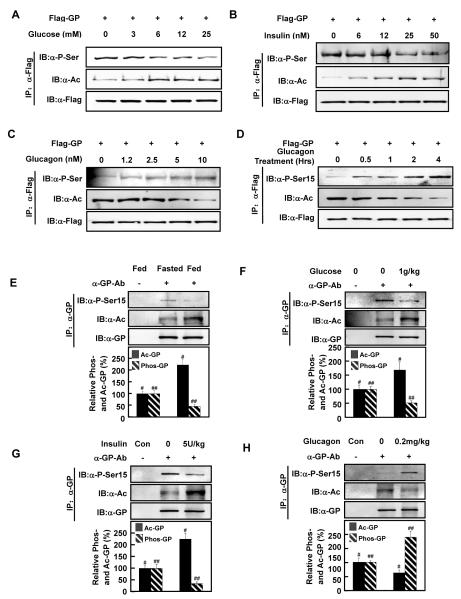
Figure 4. GP acetylation and phosphorylation are inversely regulated by physiological stimuli.
(A-C) The effect of glucose, insulin, and glucagon on GP acetylation and phosphorylation. Flag-GP expressing Chang’s cells were treated with different conditions as indicated. Acetylation and serine phosphorylation of Flag-GP were determined by western blotting.
(D) Time course of glucagon on GP acetylation and phosphorylation. Flag-GP expressing Chang’s liver cells were treated with glucagon (10nM) for different times as indicated. Levels of GP acetylation and Ser15 phosphorylation were determined by western blotting.
(E) Fasting decreases GP acetylation and increases GP phosphorylation in mouse liver. Mice were fasted overnight before sacrifice, and GP proteins were immunoprecipitated from the liver. Acetylation and Ser15 phosphorylation levels of GP were determined by western blot (p#=0.0021, n=3; p##=0.0064, n=3).
(F, G) Both glucose and insulin increase acetylation and decrease phosphorylation of GP in mouse liver. Glucose (1g/kg) or insulin (5U/kg) was intraperitoneally injected into overnight fasted mice. At 30 min post injection, mice were sacrificed and liver samples were harvested. GP proteins were immunoprecipitated from the liver, and acetylation and Ser15 phosphorylation levels of GP proteins were determined by western blot (Glucose: p#=0.0191, n=3; p##=0.0013, n=3. Insulin: p#=0.0021, n=3; p##=0.0036, n=3).
(H) Glucagon decreases acetylation and increases phosphorylation of GP in mouse liver. Glucagon (0.2mg/kg) was intraperitoneally injected into fed mice, and the mice were sacrificed at 30 min post injection. GP protein was immunoprecipitated from liver, and acetylation and Ser phosphorylation were determined by western blotting (p#=0.0200, n=3; p##=0.0100, n=3). All error bars represent standard deviation (SD). n = 3 for each experimental group.
We next examined the response of Ser15 phosphorylation in GPK2R, the non-acetylatable form of GP, to different glucose concentrations. Contrary to wild type GP, which showed increased acetylation and decreased Ser15 phosphorylation by glucose, Ser15 phosphorylation of GPK2R was largely unaffected by increasing glucose concentrations (Figure S4C), indicating an essential role of acetylation in regulating Ser15 phosphorylation. Notably, Ser15 phosphorylation remained constitutively high in GPK2R regardless the glucose concentrations, suggesting that the deacetylated GP favors being phosphorylated at Ser15. This notion was supported by the observation that GPK2Q, the acetylation mimetic mutant, not only showed a very weak phosphorylation level but its phosphorylation was unresponsive to varying glucose concentrations (Figure S4D). Similarly, insulin did not influence the phosphorylation levels of either GPK2Q or GPK2R (Figure S4E). Collectively, the above data indicate that acetylation of K470 and K796 are required for GP phosphorylation regulation by glucose and insulin.
To obtain in vivo data to support the inverse relationship between GP acetylation and Ser15 phosphorylation, mouse experiments were performed. Upon feeding, a state that causes high glucose or high insulin, mouse liver GP acetylation level was high, whereas the level of Ser15 phosphorylation was low (Figure 4E, S4F). After overnight fasting, hepatic GP acetylation was decreased, while hepatic Ser15 phosphorylation was inversely increased. Furthermore, when mice were intraperitoneally injected with glucose (1g/kg), insulin (5U/kg), or glucagon (0.2mg/kg), in line with our observations in vitro, glucose and insulin injection increased endogenous GP acetylation and decreased Ser15 phosphorylation (Figure 4F, 4G, S4G, S4H). In contrast, glucagon injection decreased endogenous GP acetylation and increased Ser15 phosphorylation in mouse livers (Figure 4H, S4I). Together, these data indicate an inverse co-regulation of GP acetylation and Ser15 phosphorylation, and the regulation of these two posttranslational modifications under physiological conditions.
Acetylation increases the GP-PP1α interaction
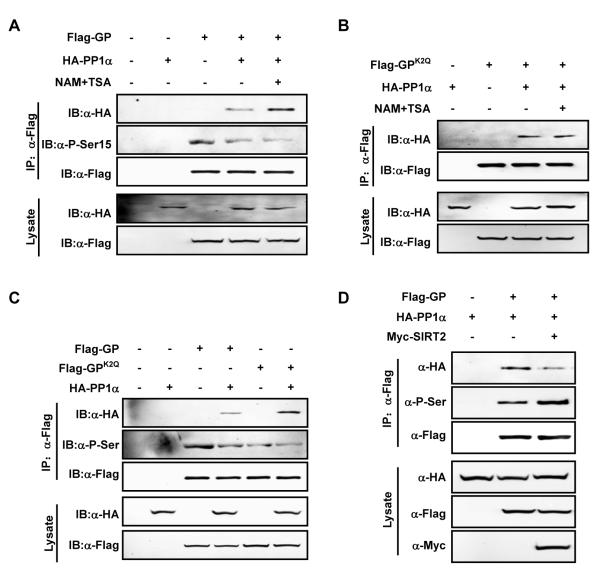
After establishing a causal relationship between acetylation and phosphorylation, a key question is how acetylation modulates GP phosphorylation, which is controlled by the PhK and PP1. The interaction between GP and PP1 is important for GP dephosphorylation. As expected, co-expression of PP1α, the phosphatase that dephosphorylates P-Ser15 of GP, largely abolished the inhibitory effect of deacetylase inhibitor on GP activity (Figure S5A). We thus investigated whether acetylation affected the PP1α-GP interaction. When PP1α and GP were co-expressed in Chang’s cells, the interaction between PP1α and GP was readily detectable (Figure 5A). Interestingly, the PP1α-GP interaction was increased (by more than 100%) after deacetylase inhibitor treatment (Figure 5A), suggesting that acetylation enhances the recruitment of PP1α to GP. Consistent with a role of acetylation in promoting the interaction between PP1α and GP, deacetylase inhibitor treatment did not further increase the interaction between PP1α and GPK2Q (Figure 5B).
Figure 5. Acetylation increases GP-PP1α interaction.
(A) Deacetylase inhibitor treatment enhances GP binding to PP1α and decreases GP phosphorylation. Flag-GP was co-expressed with HA-PP1α in Chang’s liver cells and treated with or without NAM+TSA. GP co-precipitation with PP1α and serine15 phosphorylation was determined by western blot.
(B) Deacetylase inhibitor treatment has no affect on the interaction between GPK2Q and PP1α. Flag-GPK2Q was co-expressed with HA-PP1α in Chang’s liver cells and NAM+TSA treatment was indicated. GPK2Q co-precipitation with PP1α was determined.
(C) GPK2Q binds stronger to PP1α than wild-type GP. HA-PP1α was co-expressed with Flag-GP or Flag-GPK2Q in Chang’s liver cells. PP1α-GP and PP1α-GPK2Q interactions were detected by co-immunoprecipitation. Phosphorylation of GP and GPK2Q were determined by pan-phosphoserine antibody.
(D) Ectopic expression of SIRT2 decreases PP1α-GP binding and increases GP serine phosphorylation. Flag-GP was expressed alone or co-expressed with HA-PP1α or Myc-SIRT2 in Chang’s liver cells as indicated, PP1α-GP interaction and GP serine phosphorylation level were determined. All error bars represent standard deviation (SD). n = 3 for each experimental group.
To further confirm the function of acetylation in enhancing the PP1α-GP interaction, we compared the binding of PP1α to GP and acetylation mimic GPK2Q mutant. When PP1α and GP proteins were co-expressed in Chang’s cells, the amount of PP1α protein co-immunoprecipitated with GPK2Q was about 2-fold more than that associated with the wild type GP protein, suggesting that the acetylation of GP may enhance its with PP1α. Consistently, the phosphorylation level of GPK2Q was about 60% weaker than that of the wild type GP (Figure 5C). Moreover, deacetylase inhibitor treatment decreased Ser15 phosphorylation of GP that was already reduced by PP1α co-expression (Figure 5A, S5B). Furthermore, co-expression of SIRT2 impaired the interaction between GP and PP1α and at the same time stimulated GP serine phosphorylation (Figure 5D). Taken together, the above data support a model that acetylation of K470 and K796 in GP enhances its interaction with PP1α, thereby resulting in GP dephosphorylation and inactivation (Figure 7).
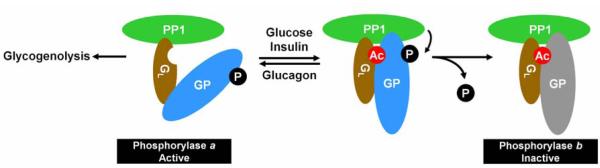
Figure 7. Acetylation negatively regulates GP activity by promoting the binding of phosphatase and the dephosphorylation of GP.
The acetylation of GP is stimulated by glucose and insulin and inhibited by glucagon. The acetylation enhances the binding of GL subunit to GP and dephosphorylation of GP by PP1.
K470 acetylation increases GP-GL interaction
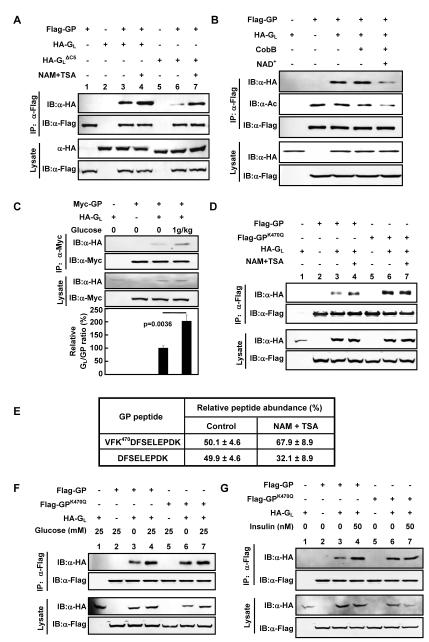
The PP1-GP interaction is mediated by GL, a substrate targeting subunit of PP1 (Armstrong et al., 1998). To determine whether the observed PP1α-GP interaction was resulted from increased PP1α-GL, Flag-PP1α and HA-GL were co-expressed in Chang’s cells, and the interaction between PP1α and GL was determined. We found that the association between PP1α and GL was not altered by deacetylase inhibitor treatment (Figure S6A), indicating that acetylation does not regulate interaction between PP1 and GL. On the other hand, the interaction between GP and GL was increased upon deacetylase inhibitor treatment (Figure 6A, lanes 3,4). GL binds to GP through its C-terminal 269-284 residues and deletion of these 5 residues in GL diminishes its interaction with GP (Kelsall et al., 2007; Pautsch et al., 2008). We thus generated a GLΔC5 mutant by deleting the C-terminal 5 residue deletion of GL, and confirmed that the deletion weakened the binding of GL with GP. Notably, inhibition of deacetylases increased the interaction between GLΔC5 and GP to the level similar to that of wild-type GP (Figure 6A). This result suggests that the acetylation enhances the GP-GL interaction through a novel site in GL that is independent of the C-terminal five residues in GL. Consistently, when GPK2R was co-expressed with GLΔC5 in Chang’s cells, virtually no interaction was detected between them either in the presence or absence of deacetylase inhibitors (Figure S6B). Meanwhile, we characterized another protein, PTG (protein targeting to glycogen), which also functions as a molecular scaffold targeting GP to PP1 (Brady et al., 1997), and investigated whether acetylation regulates GP and PTG interaction. The result demonstrated that GP-PTG binding could also be stimulated by NAM and TSA treatment (Figure S6C).
Figure 6. Acetylation increases GP-GL interaction.
(A) Acetylation enhances GP-GL interaction. Flag-GP was transfected into Chang’s liver cells either alone or with HA tagged GL or GLΔC5. NAM+TSA treatment was indicated. GP-GL and GP-GLΔC5 interactions were determined by co-immunoprecipitation.
(B) Deacetylation of GP decreases GP-GL interaction in vitro. Flag-GP was expressed in HEK293 cells in the presence of deacetylase inhibitor and HA-GL was separately expressed. Both proteins were affinity purified. Flag-GP was deacetylated with purified bacterial deacetylase CobB in vitro as indicated. In vitro binding between Flag-GP and HA-GL was performed. Acetylation level of Flag-GP and the amount of HA-GL co-precipitated by Flag-GP were determined by western.
(C) Glucose increases GP-GL interaction in vivo. Human Myc-GP and HA-GL plasmids were intravenously injected into mice. The injected mice were fasted overnight and then intraperitoneally injected with glucose (1g/kg). The mice were sacrificed at 30 min post injection. GP and GL proteins expressed in the injected mouse liver were immunoprecipitated by Myc beads for Myc-GP. The amount of GP and co-precipitated GL proteins was measured, and the GL/GP ratio was calculated.
(D) K470 is required for deacetylase inhibitors to stimulate the GP-GL interaction. Flag-GP and Flag-GPK470Q were transfected in Chang’s liver cells, either alone or with HA tagged GL. Cells were treated with or without NAM+TSA. GP-GL interactions were determined by co-immunoprecipitation.
(E) Quantification of GP K470 acetylation. Acetylation levels of K470 of GP expressed in Chang’s liver cells treated with or without NAM+TSA were quantified by iTRAQ.
(F-G) K470 in GP is required for regulation of GP-GL interaction by glucose and insulin. Flag-GP and Flag-GPK470Q were transfected in Chang’s liver cells, either alone or with HA tagged GL. Cells were treated with or without glucose (F) or insulin (G). GP-GL interactions were determined by co-immunoprecipitation. All error bars represent standard deviation (SD). n = 3-4 for each experimental group.
To obtain direct evidence to support a role of acetylation in promoting GP-GL interaction, we performed in vitro deacetylation and binding experiments. Hyperacetylated GP was purified from cells treated with deacetylase inhibitors and the purified GP was deacetylated in vitro by incubating with the NAD+-dependent bacterial deacetylase CobB. We found that CobB treatment decreased GP acetylation and also reduced its interaction with GP-GL in a NAD+-dependent manner (Figure 6B), providing direct biochemical evidence that acetylation of GP enhances its interaction with GL. We extended these experiments in mouse liver. Plasmids encoding epitope tagged GP and GL were intravenously injected into mouse tail veins to express the two proteins in liver. The interaction of the ectopically expressed GP and GL in mouse liver was determined in response to glucose signal. When glucose (1g/kg) was injected intraperitoneally into fasted mice, we found that the hepatic GP-GL interaction was increased significantly (Figure 6C). As expected, glucose injection indeed increased blood glucose concentration (Figure S6D). These results further support the notion that acetylation of GP increases GP-GL interaction in vivo.
We next investigated the importance of K470 and K796 acetylation sites in regulating the GP-GL interaction. HA-G with Flag-GPK470Q or GPK796Q L were co-expressed in Chang’s cells. As a positive control, deacetylase inhibitors increased the interaction between wild type GP and HA-GL (Figure 6D, lanes 3,4). In contrast, a similar treatment did not increase the interaction between Flag-GPK470Q and GL, and the mutant GPK470Q displayed a stronger interaction with GL than the wild type GP (Figure 6D, lanes 6,7). On the other hand, the binding between GPK796Q and GL was increased by NAM+TSA treatment, and GPK796Q showed a basal GL interaction similar to the wild type protein (Figure S6E). These results suggest that the acetylation-enhanced interaction between GP and GL is predominantly mediated by K470, but not K796. We performed iTRAQ mass spectrometry analyses to quantify GP K470 acetylation. Our results showed that as much as 50% of GP was acetylated at K470 in this assay and that inhibition of deacetylases resulted in a increase in the ratio of acetylated K470 versus unacetylated K470 increased from roughly 1:1 to 2:1 (Figure 6E, S6F-I), suggesting that a substantial fraction of K470 in GP is acetylated in the cell.
Finally, to investigate whether the GP-GL interaction is regulated by physiological stimuli, we determined the interaction between GL with either wild-type or K470Q mutant GP in response to glucose concentration and insulin stimulation. We found that the GP-GL interaction was increased by approximately 100% after switching from glucose free medium to 25mM glucose medium (Figure 6F). However, a similar glucose switch did not affect the interaction between GPK470Q and GL. Furthermore, insulin stimulated the interaction between the wild type GP, but not GPK470Q, and GL (Figure 6G). These results support that GP-GL interaction is regulated by K470 acetylation in response to nutrient and hormonal signals.
DISCUSSION
Following the initial discovery of histone acetylation (Allfrey et al., 1964; Phillips, 1963), extensive studies over the last four decades not only have identified the enzymes that catalyze reversible acetylation, the protein lysine acetyltransferases (KATs, formerly termed histone acetyltransferases, HATs) and deacetylases (commonly known as histone deacetylases, or HDACs), but also many non-histone substrates. Until relatively recently, nearly all well-characterized acetylation substrates are nuclear proteins, including transcription factors and co-regulators (Yang and Seto, 2008). Recent studies, in particular genetic studies of various strains of SIRT mutants (Finkel et al., 2009) and proteomic studies of acetylomes in different organisms (Guan and Xiong, 2011; Kim and Yang, 2011), have revealed important and broad roles of acetylation in the regulation of non-transcriptional processes, including especially the regulation of metabolic enzyme activity. In this study we have uncovered an important function of acetylation in the regulation of GP, the key enzyme in glycogen breakdown. Our study has gained new molecular insights into the regulation of this extensively investigated and historically significant enzyme.
Crosstalk between different protein posttranslational modifications (PTMs), such as phosphorylation-targeted protein ubiquitylation (Hunter, 2007), plays important role in coordinating and connecting different cellular processes. As metabolism needs to respond to variety of intra- and extracellular conditions such as cell growth signals and nutrient availability, metabolic enzymes, which have now been found to be frequently modified by acetylation, are expected to be subject to such crosstalk regulation between acetylation and other type of PTMs. We have demonstrated recently that acetylation of phosphoenolpyruvate carboxykinase (PEPCK1), a rate-limiting enzyme in gluconeogenesis, promotes its association with UBR5/EDD1 HECT E3 ligase and thus its degradation in the presence of high glucose (Jiang et al., 2011). The current study adds another distinct example—promoting protein dephosphorylation by facilitating the recruitment of a phosphatase—of crosstalk between acetylation and phosphorylation. Our results support a model where acetylation of GP modulates its phosphorylation status by enhancing GP’s binding with the GL subunit of PP1 phosphatase, thereby stimulating GP dephosphorylation by PP1 and inhibiting GP activity (Figure 7). Although it remains to be elucidated how the acetylation of GP, mostly on K470, facilitates the binding of GL to GP, we speculate that controlling protein-protein interaction by acetylation could be a broad mechanism for acetylation to regulate activities of other metabolic enzymes or proteins.
Beside reversible phosphorylation, another remarkable feature of GP regulation is its interaction with several allosteric effectors, including glucose and AMP, and regulation by hormones, such as insulin and glucagon, thereby allowing cells to integrate different cellular conditions and energy status to the regulation of GP. The finding that GP acetylation and activity are controlled by glucose, insulin and glucagon places acetylation downstream of these physiological stimuli. Binding of glucose shifts GP in liver from a more relaxed and thus active state (R) to a tense and less active state (T). As a result, low glucose would lead to decreased glucose binding and higher GP activity, resulting in an increased production of glucose from glycogen. Under such low glucose condition, GP is hypoacetylated in the cell and its interaction with GL (hence PP1) is weakened, which in turn would increase GP Ser15 phosphorylation and activity (Figure 7). Conversely, in cells with high glucose concentration, GP is hyperacetylated and the acetylation enhances GP’s binding with GL and thus PP1, leading to decreased Ser15-phopshorylation and activity of GP and eventually reduced glycogen degradation. Therefore, it appears that high glucose can down regulate GP activity by two different mechanisms; a conformational change by the direct binding of glucose and acetylation-mediated dephosphorylation. It will be important to determine whether these two regulations operate separately or sequentially, and whether GP conformational change brought by the initial glucose binding facilitates the acetylation.
The role of acetylation in recruiting PP1 clearly is consistent with the inverse correlation between acetylation and phosphorylation of GP. Glucagon can induce a direct signaling pathway to phosphorylate and activate GP in a manner independent of acetylation. However, we speculate that the decrease of GP acetylation induced by glucagon may contribute to the magnitude and duration of GP activation in response to glucagon. Glucagon stimulates GP by activating the GP kinase via the classical phosphorylation cascade and also by dissociating GP phosphatase via acetylation (Figure S7). It should be noted that acetylation appears to have no direct role in GP allosteric activation by AMP although AMP cannot further activate GP when GP is fully active. Although the precise mechanism by which glucose regulates GP acetylation remains to be elucidated, using acetylation machinery to control GP adds another layer of regulation and thus renders cells further versatility in integrating multifaceted signaling pathways and nutrient conditions to this enzyme that is not only central to the glycogen metabolism, but is also interlocked with multiple energy metabolic pathways.
EXPERIMENTAL PROCEDURES
Mouse liver collection and primary hepatocytes and muscle cell isolation
Male BALB/c (4–6 weeks old, 20–25 g) were divided into two groups, fed and overnight fasted. Fasting started from late afternoon and was lasting for 16 hours before experiments. The fasted mice were further subdivided into glucose- and insulin-treated groups, and the fed mice were treated with glucagon. Mice blood glucose levels were measured by Accu-Chek Active Blood Glucose Meter (Roche). At 30 min post injection, mice were sacrificed and liver samples were harvested. In addition, mouse primary hepatocytes and muscle cells were isolated. Please refer to “Supplemental Experimental Procedures” for detailed information. Animal experiments were performed at Fudan Animal Center in accordance with the animal ware fare guidelines.
Glycogen content measurement
Cells were washed twice and then lysed with supersonic. The cell lysate in 1 ml PBS (pH4.8) was heated at a boiling point for 10min to liberate stored glycogen and to inactivate enzymes, which may produce extra glucose. After centrifugation for 15min at 13000rpm, 4U amyloglucosidase (Sigma) was added into the supernatant. The resulting mixture was incubated for 1 hour at 50°C and followed by boiling for 10min at 99°C. The cell lysate without amyloglucosidase was included as a control. Subsequently, the glycogen content was colorimetrically measured using a glucose assay kit (GAGO20, Sigma). The mixtures were incubated for 30 min at 37°C, and absorbance at 540nm was measured by using a UV/Visible spectrophotometer reader (Ultrospec 3100 pro, Amersham Biosciences).
iTRAQ quantification
Flag-GP proteins were expressed in Chang’s liver cells and quantified by iTRAQ following the method modified according to Zhao (Zhao et al., 2010). GP were immunopurified from cells untreated or treated with NAM and TSA, resolved on 10% SDS-PAGE and stained by coomassie blue and sliced. The dye of gel slice was removed by soaking with 50 mM NH4HCO3 and 50% acetonitrile, followed by water wash for twice and removing water by acetonitrile. The gel was dried and digested in 100μl 50mM NH4HCO3 with trypsin (trypsin:protein at 1:30) at 37°C overnight. The trypsin-treated peptides were extracted by a volume containing 50% acetonitrile and 0.1% trifluoroacetic acid (TFA), and then followed by vacuum dry. Standard control peptides and GP samples were separately labeled with different iTRAQ labeling reagents (ABI) as indicated in the Table S1, and then subjected to LTQ-OrbiTrap MS analysis. Quantification of peptides was calculated by comparing relative intensity of the iTRAQ tags.
Cell treatment
TSA (0.5μM) and NAM (5mM) was added to the culture medium 18 and 6 hours before cell harvest, respectively. Glucose free medium was prepared with DMEM base (GIBCO, #11966) and supplemented with glucose (Sigma), insulin (Sigma) and glucagon (Sigma) of different concentration as indicated. Glucose, insulin, and glucagon treatment were carried out by culturing cells in DMEM medium for 24h before desired medium was used to replace DMEM medium.
Glycogen phosphorylase activity assay and CobB treatment
Flag-tagged proteins were expressed in Chang’s liver cells, eluted by flag peptides (Gilson Biochemical) and measured using the method of Jones and Wright (Jones and Wright, 1970). The glycogen phosphorylase activity assay consists of 50mM sodium glycerol-phosphate (pH7.1), 10mM potassium phosphate, 5mM MgCl2, 0.5mM NAD+, 1mM DTT, 1.6 unit phosphoglucomutase, 1.6 unit glucose-6-phosphate dehydrogenase, and 0.2% glycogen in a total volume of 0.3ml. Reaction was started by adding glycogen phosphorylase into the volume and assayed at 25°C. The reaction was monitored by measuring the increase of fluorescence (Ex. 350nm, Em.470nm, HITACHI F-4600 fluorescence spectrophotometer) for NADH generation.
CobB deacetylation treatment were performed by using a method modified elsewhere (Hallows et al., 2006). CobB was expressed in E.coli, purified with nickel beads, and stored at 80°C in 10% glycerol. The CobB deacetylation assay buffer consists of 40mM HEPES (pH7.0), 6mM MgCl2, 1mM NAD+, 1mM DTT in a total volume of 0.1ml. Reaction was started by adding 10μg CobB and GP into the volume and assayed at 37°C for 1 hour.
λ-Phosphatase and phosphorylase kinase treatment
Flag-GP was ectopically expressed in Chang’s liver cells and immunoprecipitated by Flag beads. Subsequently, the beads-linked GP was washed by PBS (pH 7.4) for 3 times before treated with phosphatase and kinase (PhK). The λ-phosphatase assay consists of NEBuffer Pack for Protein MetalloPhosphatases (50mM HEPES, 100mM NaCl, 2mM DTT, 0.01 % Brij 35, pH 7.5) and 1mM MnCl2 in a total volume of 0.4ml. Reaction was started by adding 50U Lambda Protein Phosphatase (#P0753S, NEB) and GP into the volume and assayed at 30°C for 2 hours by shaking. The PhK reaction assay consists of 41mM glycerophosphate, 41mM Tris, 0.2mM CaCl2, 3mM ATP, MgCl2 (pH8.2). Reaction was started by adding 2U PhK (#P2014, Sigma) and GP into the volume and assayed at 30°C for 2 hours by shaking. The reaction was terminated by washing the beads for 3 times by PBS and eluted by flag peptide, and the activity was determined by fluorescence spectrophotometer (Cohen, 1973; Shenolikar et al., 1979).
In vitro binding
Flag-GP purified by Flag beads was subject to in vitro deacetylation by CobB before it was mixed with purified HA-GL. The binding was allowed in 4°C for 4 hours before the beads were washed for three times by PBS. Proteins on beads were denatured by SDS loading buffer and detected by western blot.
Statistical Analysis
Statistics was performed with a two-tailed unpaired Student’s t-test. All data shown represent the results obtained from triplicated independent experiments with standard deviations (mean ± SD). The values of p<0.05 were considered statistically significant.
Supplementary Material
ACKNOWLEDGEMENT
We thank the members of the Fudan MCB laboratory for discussions throughout this study. We appreciate Dr. P.T.Cohen for providing the GP Ser15 phosphoantibody. This work was supported by the 985 Program, 973 Program (Grant No. 2009CB918401, 2011CB910600, 2012CB910101, 2012CB910103) and New Century Talent from the Chinese Ministry of Education (Grant No. NCET-09-0315), NSFC (Grant No. 30971485/C0706, 31030042, 31071192), Shanghai key project (Grant No., 09JC1402300, 11JC1401100), and the Shanghai Leading Academic Discipline Project, project number B110, and NIH grants to Y.X. and K.L.G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alemany S, Cohen P. Phosphorylase a is an allosteric inhibitor of the glycogen and microsomal forms of rat hepatic protein phosphatase-1. FEBS Lett. 1986;198:194–202. doi: 10.1016/0014-5793(86)80404-5. [DOI] [PubMed] [Google Scholar]
- Allfrey VG, Faulkner R, Mirsky AE. Acetylation and Methylation of Histones and Their Possible Role in the Regulation of Rna Synthesis. Proc Natl Acad Sci U S A. 1964;51:786–794. doi: 10.1073/pnas.51.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreu AL, Nogales-Gadea G, Cassandrini D, Arenas J, Bruno C. McArdle disease: molecular genetic update. Acta Myol. 2007;26:53–57. [PMC free article] [PubMed] [Google Scholar]
- Armstrong CG, Doherty MJ, Cohen PT. Identification of the separate domains in the hepatic glycogen-targeting subunit of protein phosphatase 1 that interact with phosphorylase a, glycogen and protein phosphatase 1. Biochem J. 1998;336(Pt 3):699–704. doi: 10.1042/bj3360699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barford D, Hu SH, Johnson LN. Structural mechanism for glycogen phosphorylase control by phosphorylation and AMP. J Mol Biol. 1991;218:233–260. doi: 10.1016/0022-2836(91)90887-c. [DOI] [PubMed] [Google Scholar]
- Barford D, Johnson LN. The allosteric transition of glycogen phosphorylase. Nature. 1989;340:609–616. doi: 10.1038/340609a0. [DOI] [PubMed] [Google Scholar]
- Brady MJ, Printen JA, Mastick CC, Saltiel AR. Role of protein targeting to glycogen (PTG) in the regulation of protein phosphatase-1 activity. Journal of Biological Chemistry. 1997;272:20198. doi: 10.1074/jbc.272.32.20198. [DOI] [PubMed] [Google Scholar]
- Browner MF, Fletterick RJ. Phosphorylase: a biological transducer. Trends Biochem Sci. 1992;17:66–71. doi: 10.1016/0968-0004(92)90504-3. [DOI] [PubMed] [Google Scholar]
- Buchbinder JL, Luong CB, Browner MF, Fletterick RJ. Partial activation of muscle phosphorylase by replacement of serine 14 with acidic residues at the site of regulatory phosphorylation. Biochemistry. 1997;36:8039–8044. doi: 10.1021/bi9704820. [DOI] [PubMed] [Google Scholar]
- Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- Cohen P. The Subunit Structure of Rabbit-Skeletal-Muscle Phosphorylase Kinase, and the Molecular Basis of Its Activation Reactions. European Journal of Biochemistry. 1973;34:1–14. doi: 10.1111/j.1432-1033.1973.tb02721.x. [DOI] [PubMed] [Google Scholar]
- Cori CF, Cori GT. Mechansim of formation of hexosemonophosphate in muscle and isolation of a new phosphate ester. Proc. Soc. Exp. Biol. Med. 1936;34:702–705. [Google Scholar]
- Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460:587–591. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer EH, Krebs EG. Conversion of phosphorylase b to phosphorylase a in muscle extracts. J Biol Chem. 1955;216:121–132. [PubMed] [Google Scholar]
- Guan KL, Xiong Y. Regulation of intermediary metabolism by protein acetylation. Trends Biochem Sci. 2011;36:108–116. doi: 10.1016/j.tibs.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallows WC, Lee S, Denu JM. Sirtuins deacetylate and activate mammalian acetyl-CoA synthetases. Proc Natl Acad Sci U S A. 2006;103:10230–10235. doi: 10.1073/pnas.0604392103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. The age of crosstalk: phosphorylation, ubiquitination, and beyond. Mol Cell. 2007;28:730–738. doi: 10.1016/j.molcel.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Jiang W, Wang S, Xiao M, Lin Y, Zhou L, Lei Q, Xiong Y, Guan K-L, Zhao S. Degradation via Recruiting the UBR5 Ubiquitin Ligase. Mol Cell. PEPCK1;43:33–44. doi: 10.1016/j.molcel.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LN. Glycogen phosphorylase: control by phosphorylation and allosteric effectors. FASEB J. 1992;6:2274–2282. doi: 10.1096/fasebj.6.6.1544539. [DOI] [PubMed] [Google Scholar]
- Jones TH, Wright BE. Partial purification and characterization of glycogen phosphorylase from Dictyostelium discoideum. J Bacteriol. 1970;104:754–761. doi: 10.1128/jb.104.2.754-761.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsall IR, Munro S, Hallyburton I, Treadway JL, Cohen PT. The hepatic PP1 glycogen-targeting subunit interaction with phosphorylase a can be blocked by C-terminal tyrosine deletion or an indole drug. FEBS Lett. 2007;581:4749–4753. doi: 10.1016/j.febslet.2007.08.073. [DOI] [PubMed] [Google Scholar]
- Kim GW, Yang XJ. Comprehensive lysine acetylomes emerging from bacteria to humans. Trends Biochem Sci. 2011;36:211–220. doi: 10.1016/j.tibs.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Kim SC, Sprung R, Chen Y, Xu Y, Ball H, Pei J, Cheng T, Kho Y, Xiao H, Xiao L, et al. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol Cell. 2006;23:607–618. doi: 10.1016/j.molcel.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Lok S, Kuijper JL, Jelinek LJ, Kramer JM, Whitmore TE, Sprecher CA, Mathewes S, Grant FJ, Biggs SH, Rosenberg GB, et al. The human glucagon receptor encoding gene: structure, cDNA sequence and chromosomal localization. Gene. 1994;140:203–209. doi: 10.1016/0378-1119(94)90545-2. [DOI] [PubMed] [Google Scholar]
- Massague J, Guinovart JJ. Insulin control of rat hepatocyte glycogen synthase and phosphorylase in the absence of glucose. FEBS Lett. 1977;82:317–320. doi: 10.1016/0014-5793(77)80610-8. [DOI] [PubMed] [Google Scholar]
- Nolan C, Novoa WB, Krebs EG, Fischer EH. Further Studies on the Site Phosphorylated in the Phosphorylase B to a Reaction. Biochemistry. 1964;3:542–551. doi: 10.1021/bi00892a013. [DOI] [PubMed] [Google Scholar]
- Pautsch A, Stadler N, Wissdorf O, Langkopf E, Moreth W, Streicher R. Molecular recognition of the protein phosphatase 1 glycogen targeting subunit by glycogen phosphorylase. J Biol Chem. 2008;283:8913–8918. doi: 10.1074/jbc.M706612200. [DOI] [PubMed] [Google Scholar]
- Phillips DM. The presence of acetyl groups of histones. Biochem J. 1963;87:258–263. doi: 10.1042/bj0870258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenolikar S, Cohen PTW, Cohen P, Nairn AC, Perry SV. The Role of Calmodulin in the Structure and Regulation of Phosphorylase Kinase from Rabbit Skeletal Muscle. European Journal of Biochemistry. 1979;100:329–337. doi: 10.1111/j.1432-1033.1979.tb04175.x. [DOI] [PubMed] [Google Scholar]
- Stegelmeier BL, Molyneux RJ, Elbein AD, James LF. The lesions of locoweed (Astragalus mollissimus), swainsonine, and castanospermine in rats. Vet Pathol. 1995;32:289–298. doi: 10.1177/030098589503200311. [DOI] [PubMed] [Google Scholar]
- Sutherland EW, Jr., Wosilait WD. Inactivation and activation of liver phosphorylase. Nature. 1955;175:169–170. doi: 10.1038/175169a0. [DOI] [PubMed] [Google Scholar]
- Tang NL, Hui J, Young E, Worthington V, To KF, Cheung KL, Li CK, Fok TF. A novel mutation (G233D) in the glycogen phosphorylase gene in a patient with hepatic glycogen storage disease and residual enzyme activity. Mol Genet Metab. 2003;79:142–145. doi: 10.1016/s1096-7192(03)00068-4. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhang Y, Yang C, Xiong H, Lin Y, Yao J, Li H, Xie L, Zhao W, Yao Y, et al. Acetylation of metabolic enzymes coordinates carbon source utilization and metabolic flux. Science. 2010;327:1004–1007. doi: 10.1126/science.1179687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu WS, Parmigiani RB, Marks PA. Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene. 2007;26:5541–5552. doi: 10.1038/sj.onc.1210620. [DOI] [PubMed] [Google Scholar]
- Yang XJ, Seto E. Lysine acetylation: codified crosstalk with other posttranslational modifications. Mol Cell. 2008;31:449–461. doi: 10.1016/j.molcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Xu W, Jiang W, Yu W, Lin Y, Zhang T, Yao J, Zhou L, Zeng Y, Li H, et al. Regulation of Cellular Metabolism by Protein Lysine Acetylation. Science. 2010;327:1000–1004. doi: 10.1126/science.1179689. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.