Background: Galectin-3 PTMs are involved in tumorigenicity of prostate cancer.
Results: Phosphorylation of galectin-3 by c-Abl and dephosphorylation by PTEN serve as shut off/on switch for its cleavage by PSA.
Conclusion: Galectin-3 cleavage by PSA may play a role during prostate cancer progression.
Significance: The ratio of phosphorylated/nonphosphorylated galectin-3 may be a complimentary indicator in addition to PSA level in prostate cancer patients.
Keywords: Carbohydrate-binding Protein, Galectin, Kallikrein, Prostate Cancer, Protein Phosphorylation
Abstract
Galectin-3 is a chimeric carbohydrate-binding protein, which interacts with cell surface carbohydrate-containing molecules and extracellular matrix glycoproteins and has been implicated in various biological processes such as cell growth, angiogenesis, motility, and metastasis. It is expressed in a wide range of tumor cells and is associated with tumor progression. The functions of galectin-3 are dependent on its localization and post-translational modifications such as cleavage and phosphorylation. Recently, we showed that galectin-3 Tyr-107 is phosphorylated by c-Abl; concomitantly, it was also shown that galectin-3 can be cleaved at this site by prostate-specific antigen (PSA), a chymotrypsin-like serine protease, after Tyr-107, resulting in loss of galectin-3 multivalency while preserving its carbohydrate binding activity. Galectin-3 is largely a monomer in solution but may form a homodimer by self-association through its carbohydrate recognition domain, whereas, in the presence of a ligand, galectin-3 polymerizes up to pentamers utilizing its N-terminal domain. Oligomerization is a unique feature of secreted galectin-3, which allows its function by forming ordered galectin-glycan structures, i.e. lattices, on the cell surface or through direct engagement of specific cell surface glycoconjugates by traditional ligand-receptor binding. We questioned whether Tyr-107 phosphorylation by c-Abl affects galectin-3 cleavage by PSA. The data suggest a role for galectin-3 in prostate cells associated with increased activity of c-Abl kinase and loss of phosphatase and tensin homologue deleted on chromosome 10 (PTEN) activity. In addition, the ratio of phosphorylated/dephosphorylated galectin-3 might be used as a complementary value to that of PSA for prognosis of prostate cancer and a novel therapeutic target for the treatment of prostate cancer.
Introduction
Galectin-3 is one of the animal lectins belonging to the galectin family that includes a total of 15 proteins. Human galectin-3 is an ∼30-kDa carbohydrate-binding protein comprising 250 amino acid residues. Galectin-3 has three different domains: a short N-terminal domain that contains two serine phosphorylation sites at positions 6 and 12 (1) and regulates cellular targeting, a collagen α-like domain that is cleavable by proteases and contains a few serines and tyrosines that may be phosphorylated (2), and a C-terminal domain composed of 130 amino acids that contains a single carbohydrate recognition domain (CRD)2 (3).
Galectin-3 is the only member of the galectin family that can form homodimers and homopentamers through intermolecular interactions involving the N-terminal domain (4). Galectin-3 multimers can cross-link cell surface glycoconjugates (glycoproteins or glycolipids) causing activation of cell signaling pathways. They can also mediate cell-cell and cell-extracellular matrix adhesion by serving as a bridge to bind cells to each other and to extracellular matrix proteins (5). The function of galectin-3 is dependent on post-translational modifications such as phosphorylation and proteolytic cleavage as well as on its multivalency (2, 6–13).
Because the main function of galectin-3 is scaffolding of glycoconjugates performed intracellularly or extracellularly, any PTMs of galectin-3 would change these interactions. For example, the cleavage of collagen-like sequence of galectin-3 by MMPs and other proteases changes the affinity of the lectin to the glycoconjugates and self-association of the galectin-3 molecules (14–17); similarly, phosphorylation of serine 6 significantly changes the interaction of galectin-3 with its ligands (6). Both the carbohydrate recognition domain and the N-terminal domain were shown to be involved in formation of galectin-3 multimers.
Intracellularly, galectin-3 is predominantly localized in the cytoplasm, and although no consensus signal sequence for either secretion or nuclear translocation was found in galectin-3, it may be secreted and/or translocated to the nucleus via a nonclassical pathway (18–21). The intracellular and extracellular galectin-3 has different functions, which are determined by the pattern of post-translational modifications as well as the different set of ligands.
Galectin-3 plays multiple roles in cancer pathogenesis, proliferation, and metastasis. Not only the intensity of galectin-3 expression but also its intracellular distribution and extracellular concentration were found to be important for cancer progression. In human prostate cancer, galectin-3 expression was reported to be down-regulated with progressive stages (22). We demonstrated that although the levels of intact galectin-3 decreased, the levels of galectin-3 cleaved by proteases increased with progression of the prostate cancer (23).
Recently, we identified Tyr-107 as one of the targets of c-Abl and Arg kinases and demonstrated that the presence of phosphorylated galectin-3 increases cell motility and alters cell morphology (2). Saraswati et al. (29) showed that active prostate-specific antigen (PSA) cleaves galectin-3 between amino acid Tyr-107 and Gly-108.
In the present study, we demonstrate that phosphorylation by c-Abl at the Tyr-107 residue of galectin-3 blocks its cleavage by PSA and affects extracellular functions of galectin-3, leading to increased angiogenesis, chemotaxis, and heterotypic aggregation. We show that dephosphorylation of galectin-3 Tyr(p)-107 by phosphatase and tensin homologue deleted on chromosome 10 (PTEN), which is frequently down-regulated in progressive prostate cancer and is associated with gain of function and oncogenic signaling (24), allows the cleavage by PSA and inhibits its function.
MATERIALS AND METHODS
Cell Lines and Antibodies
The human prostate cancer cell line LNCaP C4-2B (LNCaP) was purchased from Urocor (Oklahoma City, OK) and maintained in RPMI 1640 medium (Invitrogen) supplemented with 10% fetal bovine serum (FBS; Atlanta Biologicals). Bovine adrenal microvascular endothelial cells (BAMEC) were a gift from Dr. D. Banerjee (University of Puerto Rico, San Juan, Puerto Rico) and cultured in medium consisting of minimal essential medium with Earle's salts and l-glutamine (Invitrogen) supplemented with 10% fetal bovine serum (HyClone, Logan, UT) and antibiotics (Mediatech). Human prostate cancer PC3M cells were a gift from Dr. Fidler (University of Texas MD Anderson Cancer Center, Houston, TX) and cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS (Atlanta Biologicals). All cells were maintained in a humidified chamber with 95% air and 5% CO2 at 37 °C. The cells were grown to near confluence and detached from the monolayer with 0.25% trypsin and 2 mm EDTA for 2 min at 37 °C. The use of cell lines was approved by the Human Investigation Committee, Wayne State University, Detroit, MI.
Customized polyclonal rabbit anti-galectin-3 antibody against the recombinant whole molecule was created by Zymed Laboratories Inc. (South San Francisco, CA). Monoclonal rat anti-galectin-3 M3/38 antibody was isolated from the supernatant of hybridoma TIB-166 (American Type Culture Collection, Manassas, VA). Phospho-tyrosine blot was performed with anti-Tyr(P) antibody coupled with IRDye 800 (Rockland Immunochemicals, Gilbertsville, PA). Mouse anti-β-actin antibody was purchased from Sigma-Aldrich.
Plasmid Constructs and Purification of Recombinant Proteins
Galectin-3 was PCR-amplified from a pcDNA-Gal-3 wild type vector and subcloned into pVitB (modified pcDNA6) as a BamHI-EcoRI fragment. Full-length galectin-3 and 1–107 and 108–250 human galectin-3 were subcloned into the pET30as (modified pET30a) vector as a BamHI-XhoI fragment and overexpressed in Escherichia coli at 20 °C. The expression construct introduced a His tag to the protein. The soluble protein was purified by nickel-agarose affinity chromatography. The protein was concentrated in a buffer containing 20 mm Tris (pH 7.9), 25% (v/v) glycerol, 10 mm DTT, 2 mm EDTA and stored at −80 °C. The His tags were not removed from the protein.
c-Abl Kinase Assay
c-Abl kinase assay was performed using the HTScan Abl1 kinase assay kit (Cell Signaling Technology) according to the manufacturer's instructions.
Three-dimensional Growth and Tube Formation Assay
To analyze in vitro angiogenesis and interactions between epithelial and endothelial cell formation of tubular networks by co-cultured BAMEC and LNCaP cells, a three-dimensional growth and tube formation assay was performed as described (25). Matrigel (10 μl) was added to each chamber of the slide (μ-Slide, ibidi, Martinsried, Germany) and gelled by a 30-min incubation at room temperature, after which 15,000 BAMEC and 5000 LNCaP cells were plated onto the gel in 50 μl of the Eagle's minimal essential complete medium. In some chambers, full-length galectin-3 or its fragments were added. After 36 h, three-dimensional structures were observed under phase contrast microscope and photographed. Images were acquired with an Olympus (Melville, NY) IX71 microscope supporting a Hamamatsu ORCA-ER video camera. Tube formation image analysis was done using the web-based Image Analysis WinTube module of Wimasis online software.
Western Blot Analysis
Cells were grown up to 80% confluence, and whole-cell lysates were prepared in lysis buffer (20 mm Tris-HCl (pH 7.4), 0.1% SDS (Fisher Scientific), 1.0% Triton X-100, 0.25% sodium deoxycholate, 1 mm EGTA, 1 mm EDTA, 5 mm sodium fluoride, 1 mm sodium orthovanadate, 1 mm phenylmethylsulfonyl fluoride, 1 μg/ml leupeptin, 1 μg/ml pepstatin, 1 μg/ml aprotinin (Sigma). An equal amount of protein was loaded on the gel, resolved by 10% SDS-PAGE, and electroblotted onto polyvinylidene difluoride membrane (Immobilon® FL, Millipore). Membranes were quenched in a solution of TBS containing 0.1% casein and 0.1% Tween 20 for 60 min or in 2% gelatin from cold water fish skin (teleostean gelatin) in the case of phospho-blots (Sigma) in TBST (TBS containing 0.1% Tween 20) on a rotary shaker. Blots were incubated with the appropriate primary antibodies, washed, and then incubated with the appropriate secondary antibodies conjugated with IRDye 800 (Rockland Immunochemicals) or Alexa Fluor 680 (Invitrogen) for 30 min at room temperature. After incubation with both the primary and the secondary antibodies, membranes were washed four times with TBST at 5-min intervals. Immunoblots were visualized, and the density of each band was quantitated using the Odyssey infrared imaging system and Odyssey application software (LI-COR Biosciences, Lincoln, NE).
Chemotaxis
This assay was performed using a Boyden chamber (Neuroprobe, Cabin John, MD). In the lower chamber, the chemo-attractant Matrigel (BD Biosciences, Bedford, MA) alone or mixed with various concentrations of recombinant galectin-3, galectin-3 1–107, or galectin-3 108–250 was added. LNCaP (5 × 104) cells suspended in basic DMEM were loaded in the upper chamber. The two chambers were separated by a polycarbonate filter of 5-micron pore size and incubated in a 37 °C tissue culture incubator for 5 h, after which the filter was removed, the cells on top of the filter were wiped off, and the migrated cells were fixed, stained using Protocol Hema 3 stain set (Fisher Scientific), and counted under microscope. Each assay was carried out in triplicate.
Wound-healing Assay
A wound-healing assay was performed according to a published protocol (25).
Statistical Analysis
Data experiments are expressed as mean ± S.D. of three independent experiments. Comparisons between the groups were determined by using the one-way analysis of variance test using an on-line calculator.3 p < 0.05 was considered statistically significant.
RESULTS
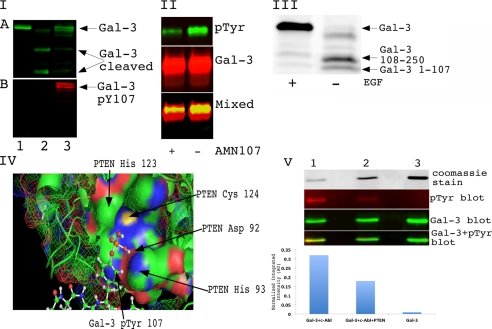
Recently, we demonstrated that galectin-3 could be phosphorylated by c-Abl at Tyr-79, -107, and -118 (2). PSA was demonstrated to cleave galectin-3 between Tyr-107 and Gly-108 and produce a functionally active, monovalent lectin (29). Because Tyr-107 is the major phosphorylation site, we checked the ability of active PSA to cleave galectin-3 phosphorylated on Tyr-107. Wild type galectin-3 was purified and used as a substrate for active c-Abl kinase in an in vitro assay. Active PSA was added to untreated (unphosphorylated) and treated (phosphorylated) galectin-3. After incubation at room temperature for 2 h, the presence of cleaved protein and the phosphorylation status of galectin-3 were checked by Western blot analysis. Our experiment confirmed the published data (29) that unphosphorylated galectin-3 can be digested by active PSA and also demonstrated that phosphorylation of Tyr-107 blocks this cleavage (Fig. 1, panel I).
FIGURE 1.
Phosphorylation of galectin-3 on Tyr-107 regulates its cleavage by PSA. Panel I, phosphorylation on Tyr-107 (pY107) blocks the cleavage of Gal-3 by PSA in vitro. An equal amount of recombinant galectin-3 wild type was loaded on the gel. Lane 1, galectin-3. Lane 2, PSA-treated galectin-3. Lane 3, galectin-3 was phosphorylated by c-Abl and treated with PSA. The reaction was stopped by adding sample buffer, resolved on a 10% SDS-PAGE gel, and immunoblotted using anti-galectin-3 antibody (A) or anti-Tyr(P) (pTyr) antibody (B). Panel II, tyrosine phosphorylation of galectin-3 in vivo is blocked by c-Abl inhibitor. PC3M cells were treated with 150 ng/ml EGF and 50 ng/ml PDGF for 8 h. One plate was also treated with 1 μm AMN107 (c-Abl inhibitor). Conditioned media were collected, and galectin-3 was immunoprecipitated with TIB166 antibody. Samples were resolved using 10% SDS-PAGE gel and immunoblotted with anti-phosphotyrosine antibody (top panel) anti-galectin-3 (HL31) (middle panel). The bottom panel represents overlapping Tyr(P) and galectin-3 blots loaded and run on an SDS-PAGE gel. Panel III, EGF treatment blocks cleavage of galectin-3 in conditioned medium of co-cultured LNCaP and PC3M cells. LNCaP and PC3M cells were co-cultured for 24 h. One plate was treated with 150 ng/ml EGF for 8 h. Conditioned medium was collected and concentrated using Millipore filters with a 3-kDa cutoff. 50 μg of total protein was loaded and run on a gradient (4–20%) SDS-PAGE gel. After transfer, membrane was blotted with polyclonal HL31 antibody, which can recognize multiple epitopes on galectin-3, for 2 h at room temperature. Panel IV, docking of Gal-3 phosphorylated on Tyr-107 with PTEN. Autodock4 (Scripps Research Institute) was used to dock the phosphorylated version of Gal-3 and PTEN. His-123, Cys-124, Asp-92, and His-93 represent amino acids from the catalytic active site and form the PTEN HCXXGXXR motif, the Cys-124 and Arg-130 residues are essential for catalysis, and the His-123 residue is important for the conformation of the P loop. Panel V, dephosphorylation of galectin-3 Tyr(P)-107 with PTEN. Lane 1, wild type recombinant galectin-3 phosphorylated with c-Abl. Lane 2, phosphorylated galectin-3 treated with PTEN for 2 h at 30 °C. Lane 3, recombinant untreated galectin-3. the top panel represents 15% of the sample mixture run on 10% SDS-PAGE and visualized with Coomassie Blue stain. The rest of the samples were resolved using 10% SDS-PAGE gel and immunoblotted with anti-phosphotyrosine antibody (second panel) and anti-galectin-3 (HL31) antibody (third panel). The bottom panel represents overlapping Tyr(P) and galectin-3 blots. The normalized integrated intensity was calculated as band integrated intensity. AU, arbitrary units.
Next, we tested whether endogenous secreted galectin-3 could be phosphorylated on tyrosine residues. We used PC3M cells to demonstrate that after treatment with EGF and PDGF, tyrosine phosphorylation can be detected on secreted galectin-3. c-Abl inhibitor AMN107 significantly blocked galectin-3 tyrosine phosphorylation, indicating that this is primarily a c-Abl-dependent event (Fig. 1, panel II). We further analyzed whether galectin-3 phosphorylated on Tyr-107 in vivo blocks the cleavage of secreted galectin-3 by PSA. As none of the prostate cancer cell lines that we tested secreted phosphorylated galectin-3 and endogenous PSA, we co-cultured PC3M (secreted galectin-3 Tyr(P)-107 after EGF treatment) and LNCaP cells (secreted endogenous PSA) and collected the conditioned medium after EGF treatment. As expected, based on the results of in vitro experiments, phosphorylation blocks cleavage of galectin-3 in conditioned medium (Fig. 1, panel III). Until now, a few authors (2, 26–28) have confirmed that tyrosine residues on galectin-3 can be phosphorylated; however, to the best of our knowledge, no one has demonstrated a tyrosine phosphatase that can dephosphorylate these residues. Based on in silico docking (Fig. 1, panel IV), we predicted that PTEN can be one of the tyrosine phosphatases that can at least partially be responsible for galectin-3 dephosphorylation. To prove it, we phosphorylated galectin-3 in vitro with active c-Abl and then treated it with PTEN (Fig. 1, panel V). The results suggest that PTEN can be responsible for dephosphorylation of galectin-3 Tyr(P)-107 in vivo.
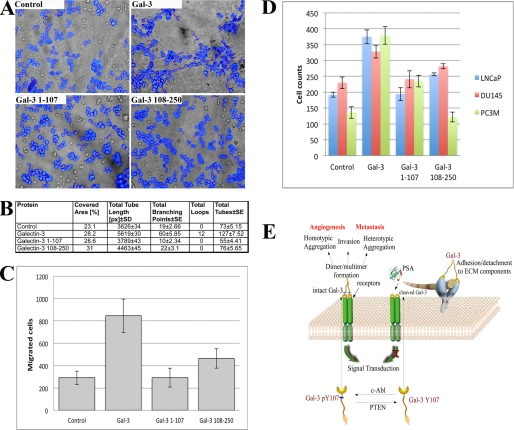
Cleavage of galectin-3 with PSA creates a mixture of two galectin-3 fragments: galectin-3 1–107 containing the NH2-terminal domain with a repeated collagen-like sequence and the functional carbohydrate recognition domain of galectin-3 containing residues 108–250. To understand the physiological relevance of galectin-3 cleavage by PSA, we constructed peptides of amino acid sequences resulting from cleavages at Tyr-107 fused with His tags for purification using nickel-agarose affinity chromatography. We then performed some of the functional assays with which galectin-3 is involved. Three-dimensional co-cultures of epithelial (LNCaP) and endothelial (BAMEC) cells were performed on Matrigel to study the tube formation in the presence of galectin-3 and its fragments.
Full-length galectin-3 added to the medium showed 127 units of tubes and 12 loops, respectively, as compared with 73, 55, and 76 units of tubes and 0 loops by control and 1–107 and 108–250 fragments, respectively (Fig. 2, A and B). Thus, we tested the effect of galectin-3 1–107 and 108–250 fragments as well as full-length galectin-3 on migration of LNCaP cells. A Boyden chamber-based cell migration assay, in which the cells must penetrate a porous polycarbonate filter toward a chemoattractant, demonstrated that migration of LNCaP cells toward Matrigel with added full-length galectin-3 was significantly higher as compared with control (wells without recombinant protein) or either of the two fragments of galectin-3, although we observed increased cell migration toward the galectin-3 CRD domain (Fig. 2C).
FIGURE 2.
Functional significance of galectin-3 cleavage by PSA. A, the effect of recombinant full-length galectin-3 and 1–107 and 108–250 fragments on endothelial cell morphogenesis. Three-dimensional heterotypic co-cultures of BAMEC and LNCaP cells on Matrigel were performed in the presence of 10 μg/ml recombinant galectin-3 and its fragments. B, quantitative evaluation of tube formation assay. C, chemotaxis assay in LNCaP cells. Full-length galectin-3 (10 μg/ml) increases chemotaxis in LNCaP cells as compared with fragments of galectin-3 and control cells. Data points show the mean ± S.E. (n = 3) in each condition. D, prostate cancer cells cell migration in the scratch assay. The assay was performed with LNCaP, Du145, and PC3M cell lines. The scratch assay was done to assess in vitro migration of cultured prostate cancer cells stimulated by full-length galectin-3, galectin-3 1–107, and galectin-3 108–250. A uniform wound was made in each plate using a 1-μl pipette tip. The wound area was observed immediately, and at 24 h after creation, cells were counted. Cells were grown under identical conditions. E, a pathway graphic describing the possible mechanisms for the roles of PSA-resistant galectin-3 in the tumorigenesis and progression of prostate cancer.
We used LNCaP (null galectin-3), DU145 (stable clone with reduced galectin-3 expression), and PC3M cell lines to examine the effect of full-length galectin-3 and galectin-3 1–107 and galectin-3 108–250 on cancer cell motility using a wound-healing assay. As shown in Fig. 2D, the addition of galectin-3 induced cell motility, whereas galectin-3 1–107 and 108–250 did not show a significantly increased motility as compared with the control. These results suggest that only full-length galectin-3 promotes prostate cancer cell motility.
DISCUSSION
Galectin-3 is a chimeric carbohydrate-binding lectin, which interacts with intracellular glycoproteins, cell surface molecules, and extracellular matrix proteins based on its intra- and extracellular distribution. The investigation of galectin-3 revealed its involvement in various biological phenomena such as cell growth, adhesion, angiogenesis, apoptosis, motility, and metastasis. This protein is widely expressed in various tumor cells, and its expression is correlated with tumorigenesis, tumor progression, and metastasis. The functions of galectin-3 are dependent on its binding partners and its localization and can change as a result of post-translational modifications such as cleavage and phosphorylation. It was shown that galectin-3 can be phosphorylated by casein kinase I at serine residues with serine 6 being the main site phosphorylated. Galectin-3 phosphorylated at serine 6 showed reduced binding to laminin and asialomucin and resulted in a diminished ability of galectin-3 to protect cells from cisplatin-induced apoptosis (1). Glycogen synthase kinase β (GSK-3β) is responsible for phosphorylation of serine 92 and 96, and these phosphorylations are mediated by Axin (29). In our recent study, we demonstrated that galectin-3 could be phosphorylated by c-Abl at Tyr-79, -107, and -118, where Tyr-107 is the major phosphorylation site (2). Galectin-3 is also known to be a substrate for MMP-2 and -9. Cleavage sites of human galectin-3 by MMPs were identified between Gly-32-Ala-33 and Ala-62-Tyr-63 amino acids resulting in 27- and 22-kDa peptides, respectively (12). Recently, it was shown that galectin-3 can be cleaved by the prostate-specific antigen after Tyr-107, and this cleavage destroys galectin-3 multivalency while preserving its carbohydrate binding activity (29). In solution, galectin-3 largely occurs as a monomer. It can also form homodimer by self-association through its CRDs in the absence of its binding ligands. However, in the presence of its carbohydrate binding ligands, galectin-3 can polymerize up to pentamers through its N-terminal domain (4, 30). Oligomerization is a unique feature of surface or secreted galectin-3, which allows formation of ordered galectin-glycan structures, also called lattices, on the cell surface or through direct engagement of specific cell surface glycoconjugates by traditional ligand-receptor interactions (31). Although secreted galectin-3 can interrupt protein interactions by blocking the access of other galectins, its main function is scaffolding of a preferred set of cell surface glycoconjugates, which explains the functional importance of the N terminus of galectin-3. The molecular modeling of the hamster galectin-3 N-terminal domain predicted its role in carbohydrate binding. Moreover, this modeling also predicted that Tyr-102 of hamster galectin-3 (homologous to Tyr-107 of human galectin-3) makes significant contributions to oligosaccharide binding (32).
Based on the available data, we hypothesized that phosphorylation on Tyr-107 may block the cleavage of galectin-3 by PSA. Our experiments confirm this hypothesis, and we suggest a model demonstrating possible functions of galectin-3 in prostate cells with increased activity of c-Abl and Arg kinases.
Although c-Abl fusion proteins have not been detected in solid cancers, recent data indicate that deregulation could be also caused by a genetic mechanism (33). It was also shown that activity of c-Abl increased in PC3 prostate cancer cells overexpressing the Shb adapter protein (34). Galectin-3 secreted from PC3M cells in our experiment was also phosphorylated on tyrosines. Silencing of PTEN and GSK-3β is frequently associated with advanced prostate cancers and likely serves critical roles in promoting androgen receptor and PI3K/Akt gain of function (35–37). We demonstrate here that PTEN can be responsible for dephosphorylation of galectin-3 Tyr(P)-107. We suggest in our model that activation of c-Abl or Arg and loss of PTEN occur during the tumor formation and progression of prostate cancer (Fig. 2E). As a consequence of disrupting the balance between phosphorylation and dephosphorylation, galectin-3 is phosphorylated at Tyr-107 by c-Abl and secreted; phosphorylated galectin-3 is resistant to cleavage by PSA and able to cross-link its cell surface ligands to form lattice-like structures that trigger the initiation of cell surface molecule-associated cell signaling. This cross-linking is associated with extracellular activities of galectin-3 including cell adhesion and signal transduction. However, in noncancerous prostate and in some prostate cancer cases, unphosphorylated galectin-3 is cleaved at tyrosine 107 by PSA, resulting in removal of the N-terminal part including the collagen-like sequence, thus blocking its ability to create the lattices and promote the cancer behavior of the cells. As compared with truncated galectin-3, PSA-resistant intact galectin-3 may exert biological activities through ligand cross-linking to boost the tumorigenesis and progression of prostate cancer.
It was shown that extracellular full-length galectin-3 could increase the motility of cancer cells, mediating metastatic cell adhesion to the endothelium and increased angiogenesis (38–42). To demonstrate whether galectin-3 cleaved by PSA can promote endothelial cell morphogenesis, we added recombinant full-length galectin-3 and 1–107 and 108–250 fragments to co-cultured BAMEC and LNCaP cells on Matrigel. Only full-length galectin-3 induced endothelial cell chemotaxis, facilitating their motility during the initial phase of tube formation, whereas fragments did not show the effect. Here, we show that although galectin-3 induced branching morphogenesis of endothelial cells, galectin-3 1–107 and 108–250 failed to do so. Endothelial cell migration is a prerequisite for angiogenesis. Cell migration requires cytoskeleton reorganization involving phosphorylation of cytoskeleton-associated tyrosine kinases and formation or removal of focal adhesion complexes that are sites of cell substrate contact and where the traction force necessary for cellular movement is generated (43).
When we evaluated the effect of galectin-3 1–107 and 108–250 fragments as well as full-length galectin-3 on migration of LNCaP cells in Boyden chamber-based cell migration assay, we found that migration of LNCaP cells toward Matrigel with added full-length galectin-3 significantly increased as compared with control and two fragments of galectin-3. However, we also observed increased cell migration toward galectin-3 CRD domain as compared with N-terminal domain and control. We suggest that this occurs due to the ability of galectin-3 CRD domain to form homodimers and may function in the same way as galectin-1, which is also known to play a role in tumor angiogenesis, although galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors (44, 45). As we expected, in a scratch assay, cells treated with galectin-3 showed increased motility as compared with control or either of the two fragments of galectin-3.
The results of these experiments confirm that only full-length galectin-3 functions as the factor promoting chemotaxis, morphogenesis, and cell motility. Earlier, we demonstrated that galectin-3 digested with MMP-2 and -9 significantly induced migration and angiogenesis as compared with full-length galectin-3. Because MMP can remove either 32 or 64 amino acids, we presume that rest of the N terminus has an increased ability to oligomerize as compared with full-length galectin-3.
We posit that post-translational modifications of galectin-3 in the cells are but one component of a constellation of factors contributing to cancer disease prevalence and severity. In conclusion, the data suggest that the balance between the level of c-Abl and PTEN activity will determine the galectin-3 phosphorylation on Tyr-107. We speculate that this association might be used as a marker for prognosis and a therapeutic target for the treatment of prostate cancer.
Acknowledgments
We thank Dr. Banerjee (University of Puerto Rico) for the gift of BAMEC and Dr. Fidler (University of Texas MD Anderson Cancer Center, Houston, TX) for the gift of PC3M cells. We thank Victor Hogan for editing the manuscript.
This work was supported, in whole or in part, by a National Institutes of Health grant (to A. R.). This work was also supported by American Cancer Society Grant “The interplay between prostate-specific antigen and galectin-3 during prostate cancer progression” IRG 11-053-01-IRG (to V. B.).
T. W. Kirkman, personal communication.
- CRD
- carbohydrate recognition domain
- PSA
- prostate-specific antigen
- PTEN
- phosphatase and tensin homologue deleted on chromosome 10
- Gal-3
- galectin-3
- MMP
- metalloproteinase
- BAMEC
- bovine adrenal microvascular endothelial cells.
REFERENCES
- 1. Huflejt M. E., Turck C. W., Lindstedt R., Barondes S. H., Leffler H. (1993) L-29, a soluble lactose-binding lectin, is phosphorylated on serine 6 and serine 12 in vivo and by casein kinase I. J. Biol. Chem. 268, 26712–26718 [PubMed] [Google Scholar]
- 2. Balan V., Nangia-Makker P., Jung Y. S., Wang Y., Raz A. (2010) Galectin-3: a novel substrate for c-Abl kinase. Biochim. Biophys. Acta 1803, 1198–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barondes S. H., Cooper D. N., Gitt M. A., Leffler H. (1994) Galectins: structure and function of a large family of animal lectins. J. Biol. Chem. 269, 20807–20810 [PubMed] [Google Scholar]
- 4. Ahmad N., Gabius H. J., André S., Kaltner H., Sabesan S., Roy R., Liu B., Macaluso F., Brewer C. F. (2004) Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. J. Biol. Chem. 279, 10841–10847 [DOI] [PubMed] [Google Scholar]
- 5. Barondes S. H., Castronovo V., Cooper D. N., Cummings R. D., Drickamer K., Feizi T., Gitt M. A., Hirabayashi J., Hughes C., Kasai K. (1994) Galectins: a family of animal β-galactoside-binding lectins. Cell 76, 597–598 [DOI] [PubMed] [Google Scholar]
- 6. Mazurek N., Conklin J., Byrd J. C., Raz A., Bresalier R. S. (2000) Phosphorylation of the β-galactoside-binding protein galectin-3 modulates binding to its ligands. J. Biol. Chem. 275, 36311–36315 [DOI] [PubMed] [Google Scholar]
- 7. Mazurek N., Sun Y. J., Liu K. F., Gilcrease M. Z., Schober W., Nangia-Makker P., Raz A., Bresalier R. S. (2007) Phosphorylated galectin-3 mediates tumor necrosis factor-related apoptosis-inducing ligand signaling by regulating phosphatase and tensin homologue deleted on chromosome 10 in human breast carcinoma cells. J. Biol. Chem. 282, 21337–21348 [DOI] [PubMed] [Google Scholar]
- 8. Mazurek N., Sun Y. J., Price J. E., Ramdas L., Schober W., Nangia-Makker P., Byrd J. C., Raz A., Bresalier R. S. (2005) Phosphorylation of galectin-3 contributes to malignant transformation of human epithelial cells via modulation of unique sets of genes. Cancer Res. 65, 10767–10775 [DOI] [PubMed] [Google Scholar]
- 9. Yoshii T., Fukumori T., Honjo Y., Inohara H., Kim H. R., Raz A. (2002) Galectin-3 phosphorylation is required for its anti-apoptotic function and cell cycle arrest. J. Biol. Chem. 277, 6852–6857 [DOI] [PubMed] [Google Scholar]
- 10. Nangia-Makker P., Balan V., Raz A. (2008) Regulation of tumor progression by extracellular galectin-3. Cancer Microenviron. 1, 43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nangia-Makker P., Raz T., Tait L., Hogan V., Fridman R., Raz A. (2007) Galectin-3 cleavage: a novel surrogate marker for matrix metalloproteinase activity in growing breast cancers. Cancer Res. 67, 11760–11768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nangia-Makker P., Wang Y., Raz T., Tait L., Balan V., Hogan V., Raz A. (2010) Cleavage of galectin-3 by matrix metalloproteases induces angiogenesis in breast cancer. Int. J. Cancer 127, 2530–2541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rabinovich G. A., Toscano M. A., Jackson S. S., Vasta G. R. (2007) Functions of cell surface galectin-glycoprotein lattices. Curr. Opin. Struct. Biol. 17, 513–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ochieng J., Green B., Evans S., James O., Warfield P. (1998) Modulation of the biological functions of galectin-3 by matrix metalloproteinases. Biochim. Biophys. Acta 1379, 97–106 [DOI] [PubMed] [Google Scholar]
- 15. Ochieng J., Fridman R., Nangia-Makker P., Kleiner D. E., Liotta L. A., Stetler-Stevenson W. G., Raz A. (1994) Galectin-3 is a novel substrate for human matrix metalloproteinases-2 and -9. Biochemistry 33, 14109–14114 [DOI] [PubMed] [Google Scholar]
- 16. Pelletier I., Sato S. (2002) Specific recognition and cleavage of galectin-3 by Leishmania major through species-specific polygalactose epitope. J. Biol. Chem. 277, 17663–17670 [DOI] [PubMed] [Google Scholar]
- 17. Karlsson A., Follin P., Leffler H., Dahlgren C. (1998) Galectin-3 activates the NADPH-oxidase in exudated but not peripheral blood neutrophils. Blood 91, 3430–3438 [PubMed] [Google Scholar]
- 18. Hughes R. C. (1999) Secretion of the galectin family of mammalian carbohydrate-binding proteins. Biochim. Biophys. Acta 1473, 172–185 [DOI] [PubMed] [Google Scholar]
- 19. Menon R. P., Hughes R. C. (1999) Determinants in the N-terminal domains of galectin-3 for secretion by a novel pathway circumventing the endoplasmic reticulum-Golgi complex. Eur. J. Biochem. 264, 569–576 [DOI] [PubMed] [Google Scholar]
- 20. Nakahara S., Oka N., Wang Y., Hogan V., Inohara H., Raz A. (2006) Characterization of the nuclear import pathways of galectin-3. Cancer Res. 66, 9995–10006 [DOI] [PubMed] [Google Scholar]
- 21. Nakahara S., Hogan V., Inohara H., Raz A. (2006) Importin-mediated nuclear translocation of galectin-3. J. Biol. Chem. 281, 39649–39659 [DOI] [PubMed] [Google Scholar]
- 22. Merseburger A. S., Kramer M. W., Hennenlotter J., Simon P., Knapp J., Hartmann J. T., Stenzl A., Serth J., Kuczyk M. A. (2008) Involvement of decreased galectin-3 expression in the pathogenesis and progression of prostate cancer. Prostate 68, 72–77 [DOI] [PubMed] [Google Scholar]
- 23. Wang Y., Nangia-Makker P., Tait L., Balan V., Hogan V., Pienta K. J., Raz A. (2009) Regulation of prostate cancer progression by galectin-3. Am. J. Pathol. 174, 1515–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim J., Coetzee G. A. (2004) Prostate-specific antigen gene regulation by androgen receptor. J. Cell Biochem. 93, 233–241 [DOI] [PubMed] [Google Scholar]
- 25. Liang C. C., Park A. Y., Guan J. L. (2007) In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2, 329–333 [DOI] [PubMed] [Google Scholar]
- 26. Li X., Ma Q., Wang J., Liu X., Yang Y., Zhao H., Wang Y., Jin Y., Zeng J., Li J., Song L., Li X., Li P., Qian X., Cao C. (2010) c-Abl and Arg tyrosine kinases regulate lysosomal degradation of the oncoprotein galectin-3. Cell Death Differ. 17, 1277–1287 [DOI] [PubMed] [Google Scholar]
- 27. Yamazaki K., Kawai A., Kawaguchi M., Hibino Y., Li F., Sasahara M., Tsukada K., Hiraga K. (2001) Simultaneous induction of galectin-3 phosphorylated on tyrosine residue, p21WAF1/Cip1/Sdi1 and the proliferating cell nuclear antigen at a distinctive period of repair of hepatocytes injured by CCl4. Biochem. Biophys. Res. Commun. 280, 1077–1084 [DOI] [PubMed] [Google Scholar]
- 28. Menon S., Kang C. M., Beningo K. A. (2011) Galectin-3 secretion and tyrosine phosphorylation is dependent on the calpain small subunit, calpain 4. Biochem. Biophys. Res. Commun. 410, 91–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Saraswati S., Block A. S., Davidson M. K., Rank R. G., Mahadevan M., Diekman A. B. (2011) Galectin-3 is a substrate for prostate-specific antigen (PSA) in human seminal plasma. Prostate 71, 197–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang R. Y., Hill P. N., Hsu D. K., Liu F. T. (1998) Role of the C-terminal lectin domain in self-association of galectin-3. Biochemistry 37, 4086–4092 [DOI] [PubMed] [Google Scholar]
- 31. Rabinovich G. A., Toscano M. A. (2009) Turning “sweet” on immunity: galectin-glycan interactions in immune tolerance and inflammation. Nat. Rev. Immunol. 9, 338–352 [DOI] [PubMed] [Google Scholar]
- 32. Barboni E. A., Bawumia S., Henrick K., Hughes R. C. (2000) Molecular modeling and mutagenesis studies of the N-terminal domains of galectin-3: evidence for participation with the C-terminal carbohydrate recognition domain in oligosaccharide binding. Glycobiology 10, 1201–1208 [DOI] [PubMed] [Google Scholar]
- 33. Sirvent A., Benistant C., Roche S. (2008) Cytoplasmic signaling by the c-Abl tyrosine kinase in normal and cancer cells. Biol. Cell 100, 617–631 [DOI] [PubMed] [Google Scholar]
- 34. Davoodpour P., Landström M., Welsh M. (2007) Reduced tumor growth in vivo and increased c-Abl activity in PC3 prostate cancer cells overexpressing the Shb adapter protein. BMC Cancer 7, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mulholland D. J., Dedhar S., Wu H., Nelson C. C. (2006) PTEN and GSK3β: key regulators of progression to androgen-independent prostate cancer. Oncogene 25, 329–337 [DOI] [PubMed] [Google Scholar]
- 36. Dong J. T. (2006) Prevalent mutations in prostate cancer. J. Cell Biochem. 97, 433–447 [DOI] [PubMed] [Google Scholar]
- 37. Chow L. M., Baker S. J. (2006) PTEN function in normal and neoplastic growth. Cancer Lett. 241, 184–196 [DOI] [PubMed] [Google Scholar]
- 38. Nangia-Makker P., Honjo Y., Sarvis R., Akahani S., Hogan V., Pienta K. J., Raz A. (2000) Galectin-3 induces endothelial cell morphogenesis and angiogenesis. Am. J. Pathol. 156, 899–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Newlaczyl A. U., Yu L. G. (2011) Galectin-3: a jack-of-all-trades in cancer. Cancer Lett. 313, 123–128 [DOI] [PubMed] [Google Scholar]
- 40. Zhao Q., Guo X., Nash G. B., Stone P. C., Hilkens J., Rhodes J. M., Yu L. G. (2009) Circulating galectin-3 promotes metastasis by modifying MUC1 localization on cancer cell surface. Cancer Res. 69, 6799–6806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lehr J. E., Pienta K. J. (1998) Preferential adhesion of prostate cancer cells to a human bone marrow endothelial cell line. J. Natl. Cancer Inst. 90, 118–123 [DOI] [PubMed] [Google Scholar]
- 42. Glinsky V. V., Glinsky G. V., Glinskii O. V., Huxley V. H., Turk J. R., Mossine V. V., Deutscher S. L., Pienta K. J., Quinn T. P. (2003) Intravascular metastatic cancer cell homotypic aggregation at the sites of primary attachment to the endothelium. Cancer Res. 63, 3805–3811 [PubMed] [Google Scholar]
- 43. Geiger B., Yamada K. M. (2011) Molecular architecture and function of matrix adhesions. Cold Spring Harb. Perspect. Biol. 3, pii: a005033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Thijssen V. L., Postel R., Brandwijk R. J., Dings R. P., Nesmelova I., Satijn S., Verhofstad N., Nakabeppu Y., Baum L. G., Bakkers J., Mayo K. H., Poirier F., Griffioen A. W. (2006) Galectin-1 is essential in tumor angiogenesis and is a target for antiangiogenesis therapy. Proc. Natl. Acad. Sci. U.S.A. 103, 15975–15980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stillman B. N., Hsu D. K., Pang M., Brewer C. F., Johnson P., Liu F. T., Baum L. G. (2006) Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J. Immunol. 176, 778–789 [DOI] [PubMed] [Google Scholar]