Background: Scavenger receptor A (SR-A) is implicated in the development of autoimmunity.
Results: Deficiency of SR-A, anti-SR-A antibody, and small molecule inhibitors (SMIs) block antigen processing.
Conclusion: Two SMIs (sennoside B and tannic acid) that reduce antigen transfer and T cell immunity were identified.
Significance: SR-A-mediated antigen trafficking is blocked by SMIs, leading to reduced T cell responses.
Keywords: Antigen Presentation, Cell Surface Receptor, Dendritic Cells, Macrophages, Scavenger Receptor, B Cells
Abstract
B cell acquisition and presentation of specific autoantigens (auto-Ags) are thought to play an important and complex role in autoimmunity development. We previously identified scavenger receptor A (SR-A) as an early target in altering B cell-mediated autoimmunity. SR-A is highly expressed on professional antigen-presenting cells such as macrophages (MΦs) and dendritic cells (DCs). In this study, we demonstrate that SR-A is responsible for controlling B cell interactions with DCs/MΦs to promote Ag transfer from B cells to DCs/MΦs. We established a high-throughput ELISA-based screen to identify novel SR-A inhibitors, the specificity of which was determined by dose dependence and Biacore surface plasmon resonance testing. We identified small molecule inhibitors (SMIs) able to reduce SR-A-mediated Ag transfer in human cells. In particular, the SMIs prevented SR-A-positive cells from accumulating/loading Ag over time. Furthermore, we determined that one SMI, sennoside B, can reduce SR-A-mediated capture of B cells. Finally, SMI-mediated decreases in Ag transfer or accumulation reduced T cell proliferation in vitro and in vivo. These observations demonstrate that B cell-DC/MΦ interactions are conducive to promoting Ag trafficking between these cell types via SR-A. Inhibitors of SR-A may provide a novel therapeutic strategy in ameliorating autoimmune disease development.
Introduction
Current understanding suggests that, when environmental or signaling cues are conducive, the first antigen-presenting cell subset to acquire antigen (Ag)3 can have a directing role in shaping immune response outcome (1, 2). Although innate systems ensure that some foreign Ags are detected and targeted quickly, most Ags require a more fine-tuned trafficking pathway dependent on Ag type and anatomical location. We have previously characterized the role Ag-presenting B cells play in triggering autoimmunity and routing specific Ags to other professional Ag-presenting cells (1, 3, 4). Through the expression of unique B cell receptors, B cells can acquire specific Ag that is present at a low concentration to amplify Ag-specific immunity (5–7).
In vivo studies using mice transgenic for a specific B cell receptor (anti-4-hydroxy-3-nitrophenyl (NP)) enabled us to control B cell Ag acquisition and to track subsequent Ag transfer to other professional Ag-presenting cells (5). Parallel experiments with human B cell and macrophage (MΦ) cell lines and primary cells support this mechanism in the development of human immune or autoimmune responses (8). We also demonstrated that the B cell requires direct contact with scavenger receptor A (SR-A) on the surface of a MΦ or dendritic cell (DC) when presenting and transferring Ag. Moreover, SR-A-mediated trafficking between B cells and MΦs is often more efficient than conventional phagocytosis of Ag by MΦs (8). Finally, autoimmune-prone MRL mice deficient in SR-A develop lower titers of autoantibodies and less pathology relative to wild-type littermates.4
In addition to SR-A being implicated in Ag trafficking in systemic lupus erythematosus, it is also known to be important in ligand uptake in cardiovascular disease, cell adhesion, cell-cell interactions, phagocytosis, host defense, and cell activation (8–17). These diverse properties suggest that SR-A is not a typical static receptor, and different environmental cues or cell interactions likely activate diverse biological SR-A functions. These characteristics make SR-A an attractive target for modulating important immunological pathways relevant to the development of immune-mediated diseases.
In this study, we sought to identify novel small molecule inhibitors (SMIs) of SR-A that may alter the biology of Ag (or auto-Ag) trafficking and subsequent immunity. We screened bioactive SMI libraries and examined candidates that significantly reduce Ag transfer from B cells to MΦs ex vivo in human cell lines and in vivo in mice. Herein, we report novel SR-A-specific SMIs that prevent or reduce cell-cell interactions between B cells and DCs/MΦs and alter the pathway of Ag processing and downstream B and T cell immunity. Importantly, by effectively reducing B cell-to-DC/MΦ Ag transfer, these SMIs can decrease overall T cell proliferation. We propose that these novel SR-A-specific SMIs will be effective in modulating Ag trafficking and reducing the induction of autoimmunity.
EXPERIMENTAL PROCEDURES
Mice
C57BL/6.Cg-Msr1tm1Csk/J (SR-A−/−), C57BL/6, B10.BR, BALB/c, and MRL/MpJ-Faslpr/2J (MRL) mice were obtained from The Jackson Laboratory. MRL mice were crossed with the SR-A−/− mice for >10 generations. Transgenic (Tg) mice expressing a surface membrane-restricted IgM gene (mIgM, also referred to as anti-NP), VH186.2, which partly encodes specificity for NP, were developed as described previously (18). B10.BR and Tg mice expressing a pigeon cytochrome c (PCC) MHC class II-restricted T cell receptor were provided by Dr. Joseph Craft (Yale University). All animals were maintained in pathogen-free facilities at the Yale Animal Resources Center. Animal studies were approved by the Yale University Institutional Animal Care and Use Committee.
High-throughput ELISA Screening of SMIs of SR-A
Recombinant human SR-A (rhSR-A; R&D Systems 2708-MS) was plated at 1 μg/ml in 50 mm carbonate buffer (Sigma C30411), pH 9.6, overnight at 4 °C in high-binding 384-well plates (Nunc MaxiSorp 460518). Plates were washed three times and then blocked with TBS, 5% powdered milk, and 0.02% NaN3 for 1 h at room temperature. Plates were washed four times, and either Me2SO or SMI (in Me2SO) was added to the plates at 10 μm for 1 h at room temperature. SMI libraries included the MicroSource GenPlus library (960 compounds), the National Institutes of Health Clinical Collection (445 compounds), and the MicroSource Natural Compounds library (800 compounds). Acetylated LDL (AcLDL)-biotin (0.016 μg/ml ; Kalen Pharmaceuticals C7502), a ligand of SR-A, or goat anti-macrophage scavenger receptor type 1 polyclonal antibody (1:20,000; Millipore AB5486) was added overnight at 4 °C. Plates were then washed four times, and either ExtrAvidin-alkaline phosphatase (0.5 μg/ml; Sigma E2636) or alkaline phosphatase-conjugated donkey anti-goat IgG antibody (1:500; Sigma A4187) was used to detect AcLDL or the anti-SR-A antibody, respectively. Plates were washed four times; fluorescein diphosphate (10 μm; AnaSpec 85300) in 50 mm Tris-HCl, pH 9.0, 10 mm MgCl2, and 10 mm glycine was added to the wells; and fluorescein product was detected.
In Vitro Ag Transfer from B Cells to MΦs
2 × 106 human THP-1 cells were incubated with 10 μm phorbol 12-myristate 13-acetate (PMA) for 24 h. BJAB human Burkitt lymphoma cells (a gift provided by Dr. Charlie Benson, Georgia State University) were blocked with human Fc receptor blocking reagent (Miltenyi) and treated with 10 μg/ml Alexa Fluor 488-conjugated F(ab′)2 anti-human IgG antibody (Invitrogen), as a model B cell receptor-binding Ag, for 45 min as described previously (8). Cells were then washed extensively and added to the THP-1 MΦs in the presence of 50 μm SMI or Me2SO. 12 h later, cells were trypsinized, stained for CD11b and CD19, and analyzed by flow cytometry (BD FACSCalibur). CD11b+ and CD19− cells were quantified for Alexa Fluor 488-labeled Ag.
In Vivo Ag Transfer from B Cells to MΦs
8-Week-old C57BL/6 mice were intraperitoneally injected with 1 ml of thioglycolate. 48 h later, mice were injected with an SMI: tannic acid (18.5 mg/kg), sennoside B (9.3 mg/kg), hematein (3.3 mg/kg), cetrimonium (5 mg/kg), cetylpyridinium (0.5 mg/kg), or benzalkonium (4 mg/kg). Tg anti-NP B cells were enriched from spleen and lymph nodes (STEMCELL Technologies 19754) from a Balb/c background (H-2d haplotype-specific) to function as Ag-presenting cells. These anti-NP B cells were treated with mouse Fc receptor blocking reagent (Miltenyi), followed by treatment with 10 μg/ml NP-PCC-Alexa Fluor 488-labeled Ag for 45 min. 2 h after SMI treatment, the C57BL/6 mice were intraperitoneally injected with 1 × 107 of the Ag-treated B cells. The C57BL/6 mice are H-2b haplotype, ensuring that Ag transfer from H-2d anti-NP B cells is haplotype-mismatched to the C57BL/6 T cells. This restricts initial Ag transfer from B cells to resident C57BL/6 MΦs or DCs in a non-haplotype-dependent manner. 16 h after injecting Ag-treated B cells, the mice were sacrificed and subjected to peritoneal lavage. Harvested cells were stained for F4/80 or CD11c and CD19 and analyzed by flow cytometry to quantify Ag transfer to resident MΦs or DCs.
Confocal Microscopy and Ag Quantification
Peritoneal exudate cells (PECs) from MRL SR-A−/− and MRL+/+ mice were plated in 35-mm glass bottom imaging chambers (MatTek) at 6 × 106 cells/chamber and stained with 5 μm CellTracker Orange CMRA (Invitrogen C34551). The adherent PECs were then treated with 20 μm Me2SO or SMI. Anti-NP B cells were stained with 5 μm CellTrace Far Red DDAO-SE (Invitrogen C34553) and treated with 10 μg/ml NP-PCC-Alexa Fluor 488. These cells were then washed extensively and added to the adherent PECs. Using a ×40 objective, images of 10 random fields in each chamber were acquired using the same imaging parameters. Images were then analyzed by Velocity software (PerkinElmer Life Sciences), and cells were quantified based on the level of fluorescently tagged Ag acquired from B cells. Adherent PECs were demarcated into regions of interest based on positive CytoTracker Orange staining. The mean pixel intensity (PI) of Alexa Fluor 488-labeled Ag fluorescence within the cell boundary was calculated. A background region of interest was distinguished for each frame, and the mean background signal was subtracted from the fluorescent PI signals per cell. Based on mean Ag PI levels, PECs were categorized into four different groups: negative = 0 PI; + = 0–25 PI; ++ = 50–75 PI; and ++++ = 75 PI and above. PI-positive cells reflect the level of acquired Ag. This same system and analysis were used to analyze PMA-treated adherent THP-1 MΦs incubated with Ag-treated BJAB B cells.
Biacore Binding Assay
500 μg/ml rhSR-A was amine-coupled to dextran/gold-coated CM5 sensor chips (GE Healthcare BR-1005-30) using an amine-coupling kit (GE Healthcare BR-1000-50) for experiments performed on a Biacore T100 surface plasmon resonance (SPR) system. Different concentrations of protein were bound to the chip depending on the number of protein injections, and bound rhSR-A was quantified prior to each experiment. Me2SO or SMI (20 μm) in HBS-P+ buffer (GE Healthcare BR-1006-71) was injected over the Biacore chip at a flow rate of 100 μl/min to measure binding to coated rhSR-A. Human BJAB cells (2 × 106 cells/ml) were washed two times, resuspended in buffer, and injected over the chip at a flow rate of 30 μl/min. B cell binding to plated rhSR-A was detected and quantified.
In Vivo T Cell Proliferation Assay
Transgenic anti-NP B cells were enriched from spleen and lymph nodes and incubated with Fc receptor blocking reagent. The cells were then treated with non-fluorescent NP-PCC Ag and washed extensively to remove excess Ag. T cells were enriched (STEMCELL Technologies 19752) from B10.BR and Tg mice expressing a PCC MHC class II-restricted T cell receptor and stained with 5 μm carboxyfluorescein succinimidyl ester. Recipient B10.BR mice were injected with thioglycolate, and 48 h later, mice were injected with either sennoside B (9.3 mg/kg) or Me2SO. 2 h later, mice were injected with 7–10 × 106 enriched Tg T cells and 10 × 106 Ag-treated anti-NP B cells. Again, an MHC mismatch control was used to ensure that Ag transfer occurred initially only from Ag-treated B cell (H-2d) to resident B10.BR MΦs or DCs (H-2k), which could then present Ag to the Tg anti-PCC B10.BR T cells (H-2k). 72 h later, mice were sacrificed and subjected to peritoneal lavage. The isolate was stained for CD4+ cells and analyzed by flow cytometry to quantify the proliferation of CFSE-labeled T cells.
RESULTS
SR-A Is Required for Ag Transfer between B Cells and DCs/MΦs
Based on prior studies from our laboratory, mice genetically deficient in SR-A were evaluated for interactions between B cells and MΦs. PECs from wild-type MRL+/+ and MRL SR-A−/− mice were incubated with Ag-pulsed Tg anti-NP B cells. Over time, MRL+/+ PECs broadly acquired fluorescent Ag from the Ag-presenting B cells as assessed by flow cytometry and confocal microscopy. The MRL SR-A−/− cells acquired significantly lower levels of Ag compared with the MRL+/+ cells (Fig. 1A).
FIGURE 1.
SR-A is required for Ag transfer. A, SR-A-positive PECs acquired significant amounts of Ag from Ag-presenting B cells relative to SR-A-deficient PECs that received little to no Ag when analyzed by flow cytometry. B, anti-SR-A is an effective ligand capable of inhibiting Ag transfer between human cells. PMA-activated human THP-1 cells treated with anti-SR-A antibody received significantly less Ag from Ag-presenting BJAB cells relative to untreated THP-1 cells when analyzed by flow cytometry.
We next tested a specific antibody for SR-A that is able to block or inhibit receptor activity to evaluate the role of SR-A in Ag transfer in human cells. Human THP-1 cells were activated with PMA to an adherent state and then incubated with anti-SR-A antibody. Ag-pulsed human BJAB cells were added to the THP-1 cells. Similar to the SR-A-deficient mice, inhibiting SR-A receptor activity reduced fluorescent Ag transfer compared with untreated THP-1 cells (Fig. 1B). Therefore, we were able to utilize two separate systems to evaluate SR-A-mediated Ag transfer in SR-A-positive or SR-A-deficient mice and in human cells treated or untreated with anti-SR-A blocking antibody.
It is documented that SR-A mediates scavenging and clearance of a variety of extracellular ligands. This particular biological function might also be related to autoimmune disease development, as apoptotic cells are a potential source of auto-Ags. One important natural ligand that SR-A binds and takes up is AcLDL. We tested AcLDL in the human cell line Ag transfer system and determined that AcLDL did not inhibit Ag transfer (data not shown). Furthermore, competition ELISAs revealed that AcLDL and the anti-SR-A antibody interacted with rhSR-A uniquely and with high avidity (data not shown). Therefore, it is unclear whether there is overlap in ligand uptake and Ag transfer functions or whether the anti-SR-A and AcLDL ligands might affect these SR-A functions separately.
High-throughput Screening Assay Identifies Several SR-A-specific SMIs
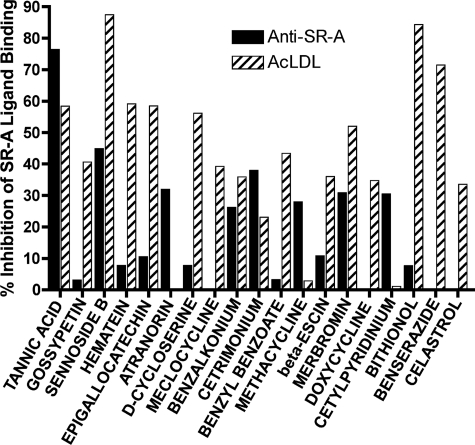
We next sought to identify novel inhibitors of SR-A ligands to modulate SR-A biological activities and ultimately evaluate SR-A in the context of autoimmune disease. The inhibitor libraries consisted of three sets of biologically active compounds. Anti-SR-A antibody and AcLDL were defined as ligands with 100% binding to recombinant SR-A. We then assayed for inhibition of binding of these ligands following SMI treatment. These screens yielded 25 positive inhibitors, which ranged in inhibition from a low of ∼30% to a high of ∼90% (sennoside B, 87.5% inhibition of AcLDL; bithionol, 84.3% inhibition of AcLDL; tannic acid, 76.4% inhibition of anti-SR-A antibody) (Fig. 2).
FIGURE 2.
High-throughput screening identifies effective SMIs of SR-A ligands. SMI libraries were screened to identify inhibitors capable of blocking avid SR-A ligands (anti-SR-A or AcLDL) from binding to the rhSR-A protein. Uninhibited ligands generated a fluorescent signal indicative of 100% binding. SMIs that reduced this signal by at least 25% were considered positive inhibitors.
Positive candidates were further evaluated in a dose-dependent response screen. 13 compounds demonstrated measurable dose-dependent activity in anti-SR-A inhibition, AcLDL inhibition, or both. Several compounds exhibited low IC50 values (Table 1). We next moved forward in analyzing the 13 compounds in biological assays.
SMIs Can Reduce SR-A-mediated Ag Transfer in Human Cells
The SR-A-specific compounds identified were tested in an in vitro live cell assay for their ability to prevent Ag transfer. THP-1 cell line monocytes were PMA-activated to an adherent MΦ state. These cells were then treated with SMI, a corresponding volume of Me2SO, anti-SR-A blocking antibody for positive inhibition control, or a nonspecific antibody (negative inhibition control). Human BJAB cells were treated with Ag, washed extensively, and added to the adherent THP-1 MΦs. 12 h later, cells were stained for either CD19 (to distinguish B cells) or CD11b (to distinguish MΦs) and analyzed by flow cytometry. Six SMIs were able to block fluorescent Ag transfer from B cells to MΦs significantly compared with control treatments (Fig. 3A).
FIGURE 3.
SMIs inhibit Ag transfer in vitro in human cells and in vivo in mice. A, PMA-activated human THP-1 cells were treated with SMIs, anti-SR-A antibody, or Me2SO. Following SMI incubation, human Ag-presenting BJAB cells treated with fluorescent Ag were added to the THP-1 MΦs. Cells were stained for CD11b and CD19 and analyzed for Ag transfer by flow cytometry. Several SMIs inhibited Ag transfer to CD11b+ MΦs more effectively than anti-SR-A antibody, the positive control. B, SMIs inhibit Ag transfer in the peritoneal cavity. C57BL/6 mice were intraperitoneally injected with thioglycolate, followed by SMI or Me2SO as a positive control. SR-A-deficient C57BL/6 mice were used as a negative control for this assay. Mice were then intraperitoneally injected with Ag-presenting anti-NP B cells. PECs were harvested and stained for F4/80 (MΦ marker) and CD11c (DC marker) and analyzed by flow cytometry. Several SMIs reduced Ag transfer to MΦs, DCs, or both cell types. In particular, tannic acid and sennoside B were both as effective as SR-A-deficient cells in reducing Ag transfer to both cell types.
SMIs Can Reduce SR-A-mediated Ag Transfer in Vivo in Mice
We then tested the efficacy of the SMI function in blocking Ag transfer in vivo. C57BL/6 mice were primed with thioglycolate to induce an accumulation of PECs in the peritoneal cavity. An SR-A-deficient mouse was also used as a control. Mice were intraperitoneally injected with nontoxic doses of SMIs, and 2 h later, Ag-pulsed anti-NP B cells (non-haplotype compatible with C57BL/6 T cells, as described under “Experimental Procedures”) were intraperitoneally injected. Five of the compounds were able to significantly inhibit Ag transfer to MΦs, DCs, or both cell types. Two of the compounds, tannic acid and sennoside B, were as effective in inhibiting Ag transfer to both MΦs and DCs as the SR-A-deficient mouse (Fig. 3B). These data also suggest that SMIs could potentially be used to modulate Ag transfer to different cell types.
Subset of SR-A-positive Cells Can Accumulate Ag Over Time
PECs from either an MRL+/+ mouse or an MRL SR-A-deficient mouse were plated in live imaging chambers and treated with a cell tracker stain. Anti-NP B cells treated with NP-PCC-Alexa Fluor 488-labeled Ag were then added to the PECs, and the chambers were imaged periodically for 16 h. Over time, a greater number of total cells expressing SR-A accumulated some Ag, but interestingly, a subset of these cells accumulated significant amounts of Ag compared with SR-A-deficient cells (Fig. 4, A–D). The two most effective SMIs from the in vivo mouse Ag transfer experiments were evaluated to determine whether they could reduce Ag accumulation in cells similarly to what was observed in SR-A-deficient cells. MRL+/+ PECs were plated and treated with tannic acid or sennoside B. Ag-treated B cells were then added to the PECs, and the cell chambers were imaged at different time points. SR-A-deficient cells were also plated and monitored over time. Images from untreated control, SMI-treated, and SR-A-deficient cells were then analyzed for Ag-positive fluorescent signal (Alexa Fluor 488), reflecting Ag acquired from B cells. Cells were categorized into four groups based on fluorescence intensity (Fig. 5A). Clearly, in the untreated control cells, a subpopulation of cells accumulated significant amounts of Ag compared with the SR-A−/− or SMI-treated cells. The SMIs effectively prevented cells from accumulating or loading Ag in a manner similar to the SR-A-deficient cells.
FIGURE 4.
Population of adherent SR-A-positive cells accumulates significant amounts of Ag. PECs isolated from an MRL SR-A+/+ mouse (A) and an MRL SR-A−/− mouse (B) were plated for live imaging analysis of Ag transfer from Ag-presenting B cells. When PECs were imaged at 15 h, a population of MRL+/+ cells (B) had accumulated significant amounts of Ag relative to the SR-A-deficient cells (D).
FIGURE 5.

Ag transfer and accumulation are reduced by SMIs. A and B, SMIs inhibited Ag accumulation in both a primary mouse PEC population and a human cell line. MRL SR-A−/− PECs were used in the mouse system as a negative control for Ag transfer, whereas anti-SR-A ligand was used as a negative control in the human system. Total Ag-negative and Ag-positive cells were quantified by average PI/cell. In addition to the Ag-negative category of cells, Ag-positive cells were categorized into three different groups based on the fluorescent Ag intensity level.
To determine whether Ag accumulation in cells in a population subset was the result of a particular cell type (i.e. DCs), we tested the THP-1 human monocyte cell line. PMA-activated human THP-1 cells were treated with sennoside B or tannic acid and imaged periodically. Again, rather than a reduction in the total number of cells receiving Ag, the inhibitors prevented more cells from receiving high levels of Ag. Specifically, under untreated conditions, many cells accumulated excess Ag, and the SMIs decreased this population of cells (Fig. 5B). Observing this phenotype in both primary mouse PECs and human cell lines suggests that there are not differences in the SR-A activity/function in the cell subsets acquiring Ag from B cells.
Sennoside B Can Reduce B Cell Capture by SR-A
One mechanism that could explain the observation that SR-A-positive cells accumulate/load Ag compared with SR-A−/− cells is that an SR-A-positive cell is more prone to stochastically interact with a B cell to form a stable cell-cell interaction conducive to Ag transfer. This stable interaction could enable or permit the Ag loading process to occur more frequently. Therefore, we next measured how effective the SMIs are in preventing SR-A and B cell interactions by Biacore SPR analysis. Sennoside B was the most specific in dose-dependent binding to rhSR-A. Furthermore, sennoside B exhibited a low Kd of receptor saturation binding at 4.36 × 10−6 ± 5.3 × 10−7 m, and the SMI maintained steady-state binding beyond 350 s, indicating that the SMI has a high avidity for SR-A. We next evaluated live human B cells flowing over the rhSR-A-coated chip. Untreated rhSR-A protein captured B cells effectively, and interestingly, treatment with sennoside B blocked rhSR-A and reduced interactions between B cells and rhSR-A by 64% (Fig. 6).
FIGURE 6.

Sennoside B inhibits B cell interactions with rhSR-A. rhSR-A that was amine-coupled to the SPR chip effectively captured human BJAB cells in solution flowing over the rhSR-A-coated chip. The B cells were able to form a stable interaction with rhSR-A. When rhSR-A was blocked with sennoside B prior to B cell injection, B cell interactions with rhSR-A were significantly inhibited, indicating that the SMI prevented SR-A interactions with B cells.
Decreasing Ag Transfer or Accumulation with SMI Treatment Reduces T Cell Proliferation in Vitro and in Vivo
Because sennoside B was effective in reducing Ag transfer and loading in both mouse and human cells ex vivo and in mice in vivo, we wanted to determine whether this effect translated to a reduction in T cell proliferation. Mouse PECs were plated and treated with SMIs. Ag-treated anti-NP B cells were then added to the PECs along with T cells (haplotype mismatch between these B and T cells as described under “Experimental Procedures”). This approach ensured that any T cell proliferation observed reflected Ag transfer only from DCs/MΦs to T cells and not from B cells to T cells. Sennoside B was effective in reducing T cell proliferation in vitro (data not shown).
We next examined in vivo T cell proliferation in mice. Intraperitoneal thioglycolate injections were administered 48 h in advance. Following SMI treatment, Ag-treated anti-NP B cells and carboxyfluorescein succinimidyl ester-labeled T cells were intraperitoneally injected. 72 h later, harvested peritoneal cells were stained for CD4 and analyzed by flow cytometry. Significant reductions in T cell proliferation were observed when mice were treated with sennoside B compared with Me2SO-treated control mice (Fig. 7).
FIGURE 7.

Sennoside B inhibits T cell proliferation. B10.BR mice were injected with thioglycolate and, 48 h later, injected with sennoside B to inhibit Ag transfer or with Me2SO as a control. Ag-loaded B cells were intraperitoneally co-injected with Tg PCC-specific carboxyfluorescein succinimidyl ester-labeled T cells. After 3 days, peritoneal cells were harvested and analyzed for carboxyfluorescein succinimidyl ester-based T cell proliferation. Mice treated with sennoside B demonstrated significantly lower levels of T cell proliferation compared with Me2SO-treated mice.
DISCUSSION
In this study, we identified several novel and specific inhibitors of SR-A. Our initial high-throughput screening generated candidate compounds effective in blocking strong avidity ligands to SR-A. These molecules were then tested in dose-dependent titrations in a solid-state ELISA format to determine IC50 values. Specific SMIs were further tested in multiple biological systems for efficacy. Biological effectiveness in reducing Ag transfer between B cells and DCs/MΦs ex vivo in both human and mouse cells and in vivo in mice provided compelling evidence that the SMIs selected were active and specific for SR-A. The ability of sennoside B to reduce T cell proliferation indicates that this SMI is effective in modulating an entire immunological pathway and may prove beneficial in quelling a systemic autoimmune response.
Interestingly, the most promising SMIs identified, tannic acid and sennoside B, were reported previously to have biological activity in autoimmune disease, cancer, and atherosclerosis. Administration of tannic acid to mice, for example, has been demonstrated to suppress inflammation and joint damage in rheumatoid arthritis (19). Another study in mice demonstrated that tannic acid not only possesses its own scavenging activity of free radicals but the compound also directly acts upon and reduces negative effects of the cytochrome P450 pathway implicated in DNA mutagenesis and cancer (20). Importantly, tannic acid was also recently used in several human clinical studies, and it was demonstrated to reduce the ability of Escherichia coli to adhere in the urinary tract, thereby reducing infection (21, 22).
Sennoside B is a natural compound that is currently used in humans for gastrointestinal complications. Specifically, this compound can function as a laxative, and it is sometimes used acutely prior to colonoscopy. However, it has also been demonstrated to have anti-inflammatory or immunomodulatory effects, which include reducing paw edema in rats and decreasing spleen size in animal models (23, 24). Bacterial colonization of the gut has been illustrated to alter the course of type 1 diabetes and rheumatoid arthritis autoimmunity in both humans and animal models (25, 26).
Sennoside B demonstrated the highest degree of specificity for SR-A in the sensitive Biacore SPR binding assay, and it was also effective in reducing B cell interactions with rhSR-A in the SPR system. Understanding how sennoside B affects the mucosal intestinal immune system could provide important clues to the SR-A pathway and the biological implications of this drug as an immunomodulatory compound. In addition, sennoside B has been demonstrated to inhibit PDGF receptor signaling and cell proliferation induced by PDGF-BB in human osteosarcoma cells (27).
The structures of both sennoside B and tannic acid exhibit polyanionic characteristics, an attribute common among SR-A ligands. Sennoside B not only demonstrated specificity for SR-A but the compound was also biologically active in reducing Ag transfer in mouse and human systems. However, based on the complexity and diversity of SR-A function, it is possible that some of the other SMIs with less pronounced effects are biologically active in unique ways. For example, an important recent study revealed a role for SR-A in counterbalancing Toll-like receptor cell activation to essentially suppress a DC/MΦ-mediated immune response (28). This suppressive activity, which likely involves endocytic regulation of SR-A, could be favorable in protecting against autoimmunity.
A finding in our study indicates that a population of SR-A-positive cells can accumulate Ag in much greater quantities over time compared with SR-A−/− or SMI-treated cells. Based on the complex nature of SR-A, it is possible that this SMI effect can occur in multiple ways. Our results demonstrate that prolonged stable interaction between Ag-presenting B cells and MΦs or DCs allows single cells to acquire significant levels of transferred Ag.
Herein, we report the identification of several small molecules that inhibit SRA biological activity by altering Ag processing pathways and subsequent B and T cell responses, including a reduction in T cell proliferation. Our findings have broad implications in understanding the immunological events leading to a focused immune response directed toward a select group of foreign or self Ags. Examining these small molecules in spontaneous models of systemic lupus erythematosus to determine effects on the induction of systemic lupus erythematosus disease onset will be the next step in characterizing these SMIs for SR-A in the context of autoimmunity.
Acknowledgments
We thank Michael Norcia (Yale Small Molecule Discovery Center), Ewa Folta-Stogniew (Yale Keck Institute; Biacore T100 system, supported by National Institutes of Health Grant RR026992-0110), and Renelle Gee for assistance in this project.
This work was supported, in whole or in part, by National Institutes of Health Grant AR41032. This work was also supported by an Alliance for Lupus Research Target Identification in Lupus award (to M. J. M.) and by Leif and Adrienne Heimbold with a generous contribution from the Heimbold Foundation.
M. R. Raycroft and M. J. Mamula, unpublished data.
- Ag
- antigen
- NP
- 4-hydroxy-3-nitrophenyl
- MΦ
- macrophage
- SR-A
- scavenger receptor A
- rhSR-A
- recombinant human SR-A
- DC
- dendritic cell
- SMI
- small molecule inhibitor
- Tg
- transgenic
- PCC
- pigeon cytochrome c
- AcLDL
- acetylated LDL
- PMA
- phorbol 12-myristate 13-acetate
- PEC
- peritoneal exudate cell
- PI
- pixel intensity
- SPR
- surface plasmon resonance.
REFERENCES
- 1. Yan J., Harvey B. P., Gee R. J., Shlomchik M. J., Mamula M. J. (2006) B cells drive early T cell autoimmunity in vivo prior to dendritic cell-mediated autoantigen presentation. J. Immunol. 177, 4481–4487 [DOI] [PubMed] [Google Scholar]
- 2. Tzehoval E., De Baetselier P., Ron Y., Tartakovsky B., Feldman M., Segal S. (1983) Splenic B cells function as immunogenic antigen-presenting cells for the induction of effector T cells. Eur. J. Immunol. 13, 89–94 [DOI] [PubMed] [Google Scholar]
- 3. Yan J., Wolff M. J., Unternaehrer J., Mellman I., Mamula M. J. (2005) Targeting antigen to CD19 on B cells efficiently activates T cells. Int. Immunol. 17, 869–877 [DOI] [PubMed] [Google Scholar]
- 4. Yan J., Mamula M. J. (2002) B and T cell tolerance and autoimmunity in autoantibody transgenic mice. Int. Immunol. 14, 963–971 [DOI] [PubMed] [Google Scholar]
- 5. Harvey B. P., Gee R. J., Haberman A. M., Shlomchik M. J., Mamula M. J. (2007) Antigen presentation and transfer between B cells and macrophages. Eur. J. Immunol. 37, 1739–1751 [DOI] [PubMed] [Google Scholar]
- 6. Lanzavecchia A. (1990) Receptor-mediated antigen uptake and its effect on antigen presentation to class II-restricted T lymphocytes. Annu. Rev. Immunol. 8, 773–793 [DOI] [PubMed] [Google Scholar]
- 7. Pierce S. K., Morris J. F., Grusby M. J., Kaumaya P., van Buskirk A., Srinivasan M., Crump B., Smolenski L. A. (1988) Antigen-presenting function of B lymphocytes. Immunol. Rev. 106, 149–180 [DOI] [PubMed] [Google Scholar]
- 8. Harvey B. P., Quan T. E., Rudenga B. J., Roman R. M., Craft J., Mamula M. J. (2008) Editing antigen presentation: antigen transfer between human B lymphocytes and macrophages mediated by class A scavenger receptors. J. Immunol. 181, 4043–4051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yokota T., Ehlin-Henriksson B., Hansson G. K. (1998) Scavenger receptors mediate adhesion of activated B lymphocytes. Exp. Cell Res. 239, 16–22 [DOI] [PubMed] [Google Scholar]
- 10. Platt N., Gordon S. (2001) Is the class A macrophage scavenger receptor (SR-A) multifunctional? The mouse's tale. J. Clin. Invest. 108, 649–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Plüddemann A., Mukhopadhyay S., Gordon S. (2006) The interaction of macrophage receptors with bacterial ligands. Expert Rev. Mol. Med. 8, 1–25 [DOI] [PubMed] [Google Scholar]
- 12. Santiago-García J., Mas-Oliva J., Innerarity T. L., Pitas R. E. (2001) Secreted forms of the amyloid-β precursor protein are ligands for the class A scavenger receptor. J. Biol. Chem. 276, 30655–30661 [DOI] [PubMed] [Google Scholar]
- 13. Goldstein J. L., Basu S. K., Brunschede G. Y., Brown M. S. (1976) Release of low density lipoprotein from its cell surface receptor by sulfated glycosaminoglycans. Cell 7, 85–95 [DOI] [PubMed] [Google Scholar]
- 14. Wang X. Y., Facciponte J., Chen X., Subjeck J. R., Repasky E. A. (2007) Scavenger receptor A negatively regulates antitumor immunity. Cancer Res. 67, 4996–5002 [DOI] [PubMed] [Google Scholar]
- 15. Harshyne L. A., Zimmer M. I., Watkins S. C., Barratt-Boyes S. M. (2003) A role for class A scavenger receptor in dendritic cell nibbling from live cells. J. Immunol. 170, 2302–2309 [DOI] [PubMed] [Google Scholar]
- 16. Marañón C., Desoutter J. F., Hoeffel G., Cohen W., Hanau D., Hosmalin A. (2004) Dendritic cells cross-present HIV antigens from live as well as apoptotic infected CD4+ T lymphocytes. Proc. Natl. Acad. Sci. U.S.A. 101, 6092–6097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Suzuki H., Kurihara Y., Takeya M., Kamada N., Kataoka M., Jishage K., Ueda O., Sakaguchi H., Higashi T., Suzuki T., Takashima Y., Kawabe Y., Cynshi O., Wada Y., Honda M., Kurihara H., Aburatani H., Doi T., Matsumoto A., Azuma S., Noda T., Toyoda Y., Itakura H., Yazaki Y., Kodama T. (1997) A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature 386, 292–296 [DOI] [PubMed] [Google Scholar]
- 18. Chan O. T., Hannum L. G., Haberman A. M., Madaio M. P., Shlomchik M. J. (1999) A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J. Exp. Med. 189, 1639–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shukla M., Gupta K., Rasheed Z., Khan K. A., Haqqi T. M. (2008) Consumption of hydrolyzable tannin-rich pomegranate extract suppresses inflammation and joint damage in rheumatoid arthritis. Nutrition 24, 733–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krajka-Kuniak V., Baer-Dubowska W. (2003) The effects of tannic acid on cytochrome P450 and phase II enzymes in mouse liver and kidney. Toxicol. Lett. 143, 209–216 [DOI] [PubMed] [Google Scholar]
- 21. Howell A. B., Botto H., Combescure C., Blanc-Potard A. B., Gausa L., Matsumoto T., Tenke P., Sotto A., Lavigne J. P. (2010) Dosage effect on uropathogenic Escherichia coli anti-adhesion activity in urine following consumption of cranberry powder standardized for proanthocyanidin content: a multicentric randomized double blind study. BMC Infect. Dis. 10, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Juthani-Mehta M., Perley L., Chen S., Dziura J., Gupta K. (2010) Feasibility of cranberry capsule administration and clean-catch urine collection in long-term care residents. J. Am. Geriatr. Soc. 58, 2028–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guarize L., da Costa J. C., Dutra L. B., Mendes R. F., Lima I. V., Scio E. (2011) Anti-inflammatory, laxative and intestinal motility effects of Senna macranthera leaves. Nat. Prod. Res. 1, 1–13 [DOI] [PubMed] [Google Scholar]
- 24. Hueza I. M., Latorre A. O., Raspantini P. C., Raspantini L. E., Mariano-Souza D. P., Guerra J. L., Górniak S. L. (2007) Effect of Senna occidentalis seeds on immunity in broiler chickens. J. Vet. Med. A Physiol. Pathol. Clin. Med. 54, 179–185 [DOI] [PubMed] [Google Scholar]
- 25. Musso G., Gambino R., Cassader M. (2011) Interactions between gut microbiota and host metabolism predisposing to obesity and diabetes. Ann. Rev. Med. 62, 361–380 [DOI] [PubMed] [Google Scholar]
- 26. Scheinecker C., Smolen J. S. (2011) Rheumatoid arthritis in 2010: from the gut to the joint. Nat. Rev. Rheumatol. 7, 73–75 [DOI] [PubMed] [Google Scholar]
- 27. Chen Y. C., Chang C. N., Hsu H. C., Chiou S. J., Lee L. T., Hseu T. H. (2009) Sennoside B inhibits PDGF receptor signaling and cell proliferation induced by PDGF-BB in human osteosarcoma cells. Life Sci. 84, 915–922 [DOI] [PubMed] [Google Scholar]
- 28. Guo C., Yi H., Yu X., Hu F., Zuo D., Subjeck J. R., Wang X. Y. (2012) Absence of scavenger receptor A promotes dendritic cell-mediated cross-presentation of cell-associated antigen and antitumor immune response. Immunol. Cell. Biol. 90, 101–108 [DOI] [PMC free article] [PubMed] [Google Scholar]