Background: The SUN domain mediates mechanical linkage across the nuclear envelope.
Results: The structure of the SUN2 protein SUN domain was solved. The structure features important for SUN domain function were identified.
Conclusion: The SUN domain forms a homotrimer. The SUN-KASH domain interaction is required for nuclear migration.
Significance: The study provides insights into how the SUN protein complex functions.
Keywords: Crystallography, Cytoskeleton, Migration, Nuclear Membrane, Structural Biology, KASH Domain, LINC Complex, SUN Domain, Nuclear Envelope, Nuclear Migration
Abstract
The SUN (Sad1-UNC-84 homology) domain is conserved in a number of nuclear envelope proteins involved in nuclear migration, meiotic telomere tethering, and antiviral responses. The LINC (linker of nucleoskeleton and cytoskeleton) complex, formed by the SUN and the nesprin proteins at the nuclear envelope, serves as a mechanical linkage across the nuclear envelope. Here we report the crystal structure of the SUN2 protein SUN domain, which reveals a homotrimer. The SUN domain is sufficient to mediate binding to the KASH (Klarsicht, ANC-1, and Syne homology) domain of nesprin 2, and the regions involved in the interaction have been identified. Binding of the SUN domain to the KASH domain is abolished by deletion of a region important for trimerization or by point mutations associated with nuclear migration failure. We propose a model of the LINC complex, where the SUN and the KASH domains form a higher ordered oligomeric network in the nuclear envelope. These findings provide the structural basis for understanding the function and the regulation of the LINC complex.
Introduction
The nuclear envelope (NE)5 is a membranous structure that defines the boundary between the nucleus and cytoplasm. The NE is composed of the inner and outer nuclear membranes (INM and ONM, respectively). The ONM is contiguous with the endoplasmic reticulum, whereas the INM is associated with the nuclear lamin network through a set of integral transmembrane proteins. The lumen between the INM and ONM is known as the perinuclear space (PNS). The nuclear pore complexes span the double membrane and form the channels that mediate trafficking of macromolecules between the cytoplasm and the nucleus.
The integrity of the NE, as well as the molecular transport across the NE, is essential for normal eukaryotic cell function (1). Defects of NE proteins have been associated with various severe human diseases and anomalies, such as muscular dystrophy and progeria syndrome (2–6). The nuclear lamin network may also interact with pools of ErbB receptors directed to nuclear sites (7–10).
Molecular connections that bridge the cytoplasmic and nuclear cytoskeletal networks across the NE, called the LINC (linker of nucleoskeleton and cytoskeleton) complex, have been defined in recent studies of the SUN (Sad1-UNC-84 homology) proteins and the KASH (Klarsicht, ANC-1, and Syne homology) proteins. ANC-1, an ONM protein involved in nuclear positioning, is associated with the actin network (11). The localization of ANC-1 is dependent upon UNC-84, an INM protein, whereas the localization of UNC-84 itself is dependent upon lamin (12). Based on these findings, it was proposed that UNC-84 could interact with ANC-1 in the PNS via their respective luminal domains (12, 13). Further studies also suggested that the INM-associated SUN proteins function as tethers for the ONM-associated KASH domain-containing proteins (14–20).
KASH proteins contain a single transmembrane anchor and a segment of ∼30 residues that are exposed to the PNS (21–23). Members of this family, such as ANC-1 and UNC-83, have little in common with each other beyond the KASH domain. Nesprin/SYNE-1, -2, and -3 are representative members of the mammalian KASH domain protein family (20, 24, 25).
SUN proteins are characterized by a C-terminal ∼200 amino acid residue SUN domain that is conserved among all eukaryotes including Schizosaccharomyces pombe Sad1, Caenorhabditis elegans UNC-84 and SUN1, and five human SUN proteins (16, 17, 22, 26, 27). Based upon localization patterns, human SUN proteins can be grouped into two subfamilies: SUN1 and SUN2 are integral membrane components of the INM, with their SUN domains extended into the PNS (16, 17, 28), whereas SUN3 and the sperm-associated antigen 4, a potential cancer marker, localize to the endoplasmic reticulum and the ONM (29, 30).
In C. elegans, mutations in the SUN domain of UNC-84 compromise the localization of UNC-83 (31) and ANC-1 and disrupt nuclear migration (11, 13, 27, 31–33). In mammals, SUN2 and nesprin/SYNE mediate nuclear movement in migrating fibroblast cells and during retinal development (34, 35). SUN1 is essential for early embryonic and germ cell development (36) and is also involved in endosome fusion (37). SUN2 is involved in tethering mammalian meiotic telomeres to the nuclear envelope (38). Furthermore, SUN proteins are capable of facilitating the movement of specific chromosomal loci along nuclear membranes by linking the cytoskeleton network to chromosomes via developmentally expressed connector proteins such as Bqt1 and Bqt2 (39, 40). More recently, SUN2 has been identified as an interferon-stimulated gene that plays a role in antiviral response (41).
As a component of the LINC complex, SUN1 and SUN2 both interact with the KASH domain of ONM proteins and provide direct mechanical transduction between the cytoskeleton and the nuclear interior (14, 15, 17, 30, 42). Disruption of this structure not only perturbs the organization of the actin and intermediate filament networks but also impairs the transmission of intracellular forces (43).
In yeast, cytoplasmic microtubules are mechanically coupled to the nuclear heterochromatin through the molecular chain containing the SUN-KASH complex (44). Deletion of the endogenous Sad1 protein is lethal to S. pombe (26). SUN proteins are an essential part of the molecular link coupling the centrosome and nuclear chromatin in mammals (45). SUN proteins are thought essential for telomere migration during homologous chromosome pairing (38, 39, 46–48). Thus, the SUN proteins are involved in aspects of both mitosis and meiosis.
In this study, we have resolved the structure of the SUN domain of SUN2. In addition, we have identified the structural interface involved in the interaction between the SUN domain and the luminal region of the nesprin 2 KASH domain. We now propose a new molecular model that provides insights into how SUN2 and, as a component of the LINC complex, modulates the mechanical transduction linkage of cytoplasmic and nuclear cytoskeletal networks.
EXPERIMENTAL PROCEDURES
Protein Expression and Purification
Human SUN2 SUN domain was cloned, expressed, and purified as described in the supplemental text.
Crystallization, Data Collection, and Structure Determination
The crystal structure of SUN domain was determined using multiple isomorphous replacement method as described in the supplemental text. The statistics for data collection and structure determination are summarized in Table 1.
TABLE 1.
Statistics of data collection and refinement
NA, not applicable.
| Native | K2PtCl4 | LuCl3 | |
|---|---|---|---|
| Data collection | |||
| Space group | R32 | R32 | R32 |
| Cell dimensions | |||
| a, b, c (Å) | 78.92, 78.92, 197.92 | 79.82, 79.82, 199.66 | 79.39, 79.39, 199.48 |
| α, β, γ (°) | 90.00, 90.00, 120.00 | 90.00, 90.00, 120.00 | 90.00, 90.00, 120.00 |
| Resolution (Å) | 2.39 (2.48–2.39)a | 2.800 (2.90–2.800) | 2.45 (2.54–2.45) |
| Rsym or Rmerge | 0.055 (0.216) | 0.179 (0.939) | 0.124 (0.697) |
| I/σI | 29.22 (7.02) | 15.46 (2.38) | 17.03 (2.94) |
| Completeness (%) | 98.9 (93.7) | 100.0 (100.0) | 100.0 (100.0) |
| Redundancy | 6.8 (5.3) | 13.5 (13.0) | 7.7 (7.4) |
| Refinement | |||
| Resolution (Å) | 40.0–2.39 | ||
| No. reflections | 8425 | ||
| Rwork/Rfree | 0.2107 / 0.2589 | ||
| No. atoms | |||
| Protein | 1542 | ||
| Ligand/ion | NA | ||
| Water | 59 | ||
| B-factors | |||
| Protein | 40.01 | ||
| Ligand/ion | NA | ||
| Water | 45.83 | ||
| Root mean square deviations | |||
| Bond lengths (Å) | 0.028 | ||
| Bond angles (°) | 2.136 | ||
a The values in parentheses are for the highest resolution shell.
Structural Modeling
The structure of the nesprin 2G luminal domain and the SUN-KASH complex were modeled as described in the supplemental text.
Cell Culture and Transfection
The human embryonic kidney 293T cells were cultured in DMEM supplemented with 10% FBS. The cells were transfected with GFP-SUN2 or FLAG-SUN2 constructs using the FuGENE 6 reagent (Roche Applied Science). Two days after transfection, the cell lysates were prepared in the Nonidet P-40 lysis buffer (120 mm NaCl, 25 mm Tris-HCl, pH 7.4, 0.5% Nonidet P-40) supplemented with a protease inhibitor mixture (Roche Applied Science). Following centrifugation, the supernatant was subjected to incubation with glutathione-Sepharose beads loaded with either GST or GST-Nsp2C proteins. The proteins bound to the beads were separated by SDS-PAGE and subjected to Western blot analysis.
Isothermal Titration Calorimetry (ITC) Analysis
The experiment was carried out following standard protocol as described in the supplemental text.
RESULTS
Crystal Structure of SUN Domain
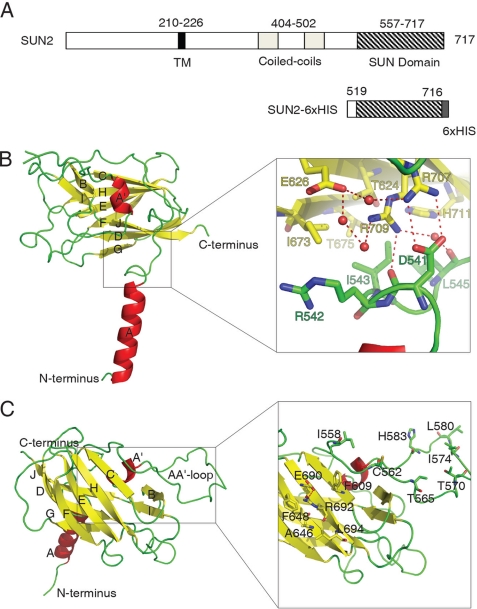
To gain structural insights into the evolutionarily conserved SUN domain, we crystallized the C-terminal region of human SUN2 protein (Val19–His716) and resolved its molecular structure at 2.4 Å resolution (Fig. 1 and Table 1). The structure of the SUN domain is characterized by a “stalk” region that consists of an α-helix and a leaflike subdomain that is formed by a β-sheet sandwich (Fig. 1 and supplemental Fig. S1). The α-helix is N-terminal to the β-sandwich, forming an angle of ∼80° between the axis of the helix and the broad face of the sandwich. The β-sandwich mainly consists of a three-stranded β-sheet JDG and a four-stranded β-sheet CHEF. This jelly roll architecture is brought to a closure by the insertion of an extra α-helix A′ and a mini β-sheet BI. The highly extended AA′ loop further seals the opening of the β-sandwich by spanning its cleft.
FIGURE 1.
Structure of the SUN domain monomer. A, schematic representation of the SUN2 C-terminal fragment that was used for the structure study. TM, transmembrane region. B and C, views of SUN domain monomer from two different directions. The secondary structural elements are labeled alphabetically from N terminus to C terminus. The right panel in B is the zoomed in image of the juncture area connecting the β-sandwich to the α-helix. The red balls represent water molecules involved in a hydrogen network (dashed lines, same below). The right panel in C shows a potential protein binding site located on the molecular surface of the β-sheet CHEF and the extended AA′ loop.
The leaf subdomain is attached to the stalk subdomain through a junction that is formed between the surface of the β-sheet JDG and a loop-turn immediately following the C-terminal end of the α-helix A (Fig. 1). Within the junction, numerous hydrogen bonds and electrostatic and hydrophobic interactions are utilized to tighten the linkage between the two subdomains. For example, residues Arg542, Ile543, and Leu545 from the helical stalk side form hydrophobic or van der Waals contacts with residues Thr624, Ile673, Thr675, and His711 from the sandwich leaf side (Fig. 1B). Two arginine residues Arg707 and Arg709 on the JDG β-sheet form hydrogen bonds with the side chain and the main chain of residue Asp541 located on the tip of the stalk.
A salient feature of the SUN domain lies in its molecular surface of the β-sheet CHEF and the AA′ loop that forms the upper side of the leaf. A group of hydrophobic residues cluster together, forming an extended hydrophobic patch including Phe609, Phe648, and Leu694 (Fig. 1C). A pair of polar residues, Glu690 and Arg692, joins the patch by forming a hydrogen bond that stands between the phenyl rings of residues Phe609 and Phe648, where the cation-pi effect may further stabilize the unique side chain conformations of this cluster of residues. We propose that this patch may function as a site for protein-protein interaction, such as for binding to the KASH domain (see “Discussion”).
Homotrimeric Association of SUN Domain
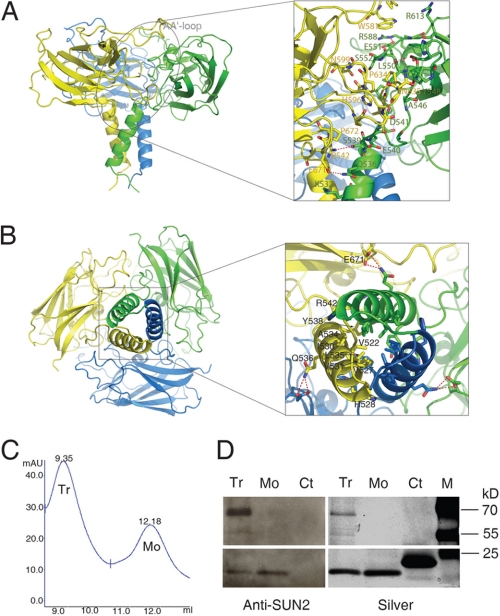
Previous studies indicate that the SUN1 and SUN2 proteins can undergo homo- or heteropolymerization, which involves both the SUN domain and the adjacent coiled coil regions (42, 45, 49, 50). Our current crystallographic study has revealed that the SUN domain alone is sufficient to form a homotrimer (Fig. 2). This finding is also supported by the profile of size exclusion chromatography, which demonstrates that the SUN domain can exist both as monomer and trimers in solution (Fig. 2, C and D). The size exclusion chromatography profile of the SUN domain shows two peaks that are consistent with what would be expected of a trimer and monomer, respectively. This is confirmed by SDS-PAGE and Western blot analysis using a polyclonal antibody specific for SUN2 (49). Of note, the trimers were not completely disrupted in the conditions under which the SDS-PAGE was carried out (Fig. 2D).
FIGURE 2.
Trimerization of the SUN domain. The SUN domain trimer resembles a cloverleaf sitting on a stem. A and B, views from perpendicular directions that mainly illustrate the interfaces related to the cloverleaf and the stem, respectively. The right panels are zoomed in pictures showing details of the interfaces. C, size exclusion chromatography profile of the SUN2 SUN domain. The trimer (Tr) and monomer (Mo) peaks are indicated. D, SDS-PAGE and Western blot analysis of protein fractions of the trimer and monomer peaks, as shown in C. Left panel, Western blot analysis using polyclonal anti-SUN2 antibody. Right panel, silver staining of SDS-PAGE gel. Tr, trimer; Mo, monomer; Ct, control (irrelevant protein); M, molecular mass marker. Note that the trimers are not completely disrupted in the SDS-PAGE experiment condition and can be better recognized by the polyclonal anti-SUN2 antibody than the monomer.
The SUN domain trimer exhibits a perfect 3-fold symmetry and resembles a cloverleaf (∼65 Å of diameter) sitting on a short fragment of stem (∼30 Å of length) (Fig. 2). A three-lobe cloverleaf is formed by bringing the three leaf subdomains (β-sandwiches) from the three protomers to a close vicinity of each other where they can intimately pack together using numerous loops as cushions. Three stalk subdomains (α-helices) congregate to form a three-helix bundle stem.
Interactions contributing to the assembly of the SUN domain trimer fall into three types: those between the β-sandwich subdomains that are used to form the cloverleaf; those between the α-helix subdomains that are used to form the stem; and those between the β-sandwich and the α-helix subdomains that help to maintain the overall architecture. The first set of interactions, which are located on the interface between neighboring β-sandwich subdomains, are mostly mediated by variable loops on both ends of each β-sandwich. Specifically, the A′B loop, the DE loop, and the C-terminal part of the AA′ loop from one protomer interact with the A′B loop and the N-terminal part of the AA′ loop from the adjacent protomer.
These flexible loops embed one onto the other, resulting in discernible complementarity among the β-sandwich subdomains of the three protomers (Fig. 2A and supplemental Fig. S2A). In detail, the cloverleaf conformation is stabilized by a network of hydrophobic packing, van der Waals contacts, electrostatic interactions, and hydrogen bonds, which involve the residues Pro579, Trp581, His596, Pro597, Asn599, Pro629, Ala631, Leu632, Pro634, and Asn635 from one protomer and the residues Asp541, Ala546, Tyr548, Leu550, Glu551, Ser552, Ala555, Arg588, Pro593, Arg613, Pro714, and His716 from another. In particular, the side chain of Arg588 not only forms the hydrogen bond with the side chain of Glu551 from the same protomer, but also pairs with the side chain of Trp581 on AA′ loop from the adjacent protomer through cation-pi interaction. All of these interactions contribute to maintaining the extended conformation of the AA′ loop as part of the potential binding site for other proteins.
The second set of interactions lock the α-helix subdomains of the three protomers together. The resultant three-helix bundle resembles a stem that holds the cloverleaf. The core of this helix bundle is composed of four layers of hydrophobic packing corresponding to the central residues Val527, Val531, Leu535, and Tyr538, respectively (Fig. 2B and supplemental Fig. S2, B and C). However, the distribution of the hydrophobic and polar residues along the helices is irregular when compared with standard heptad repeats seen in coiled coil motifs. As reflected in the primary sequence (VHHIVKQALQRY), the hydrophobic profile of SUN domain α-helix subdomain is much wider than that of other helices seen in coiled coils. For example, residue His528 extends the central hydrophobic interlock by flanking the interface between adjacent helices. Finally, two additional features further strengthen the SUN domain helical stem. Residue Val522 tightens the N terminus of the helix bundle by pointing its side chain toward the hydrophobic core, forming close contacts. Meanwhile residue Arg542, which partly pairs with Tyr538 from the same helix, forms a hydrogen bond with Ser539 from the adjacent helix, stabilizing the C-terminal end of the helix bundle.
The third set of interactions stabilizes the SUN domain trimer by linking the α-helix subdomain from one protomer to the β-sandwich subdomain from the adjacent protomer. For example, residues Lys532 and Gln536 on the helix of protomer A form hydrogen bond and electrostatic interaction with Glu671 on the β-sandwich of protomer B (Fig. 2 and supplemental Fig. S2C). Similar interactions also exist in the juncture connecting the cloverleaf with the stem.
Notably, the potential KASH domain-binding sites are located symmetrically on the three lobes of the cloverleaf upon trimerization of the SUN domain (supplemental Fig. S3A). Meanwhile, the AA′ loop located on the top the cloverleaf, as part of the active site, forms high density crystal contacts mainly involving residues Ile558, Cys562, Thr565, Thr570, Ile574, Leu580, and His583 (Fig. 1B and supplemental Fig. S4).
SUN Domain Binds to KASH Domain
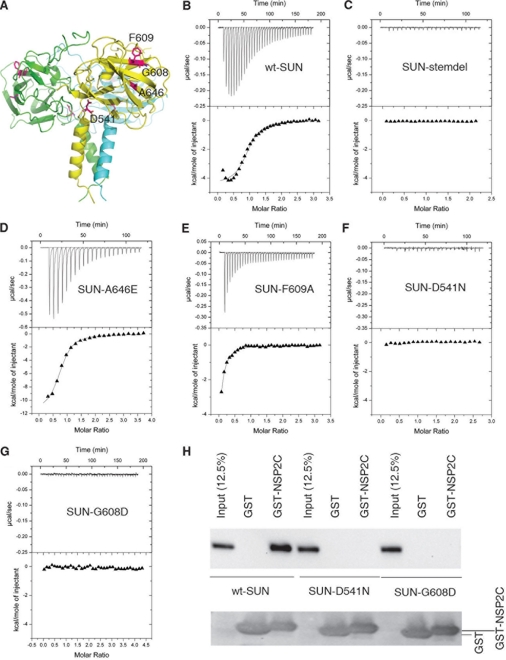
Previous studies have shown that the luminal domain of the SUN proteins may be involved in association with the luminal part of the KASH domain (14, 30, 42, 44, 51). Immunoprecipitation studies confirmed that an intact SUN domain of SUN2 is required for binding to the luminal region of the nesprin 2 KASH domain (supplemental Fig. S5). Using molecular modeling, we predict that an extended patch in the β-sandwich subdomain functions as a binding site for the KASH domain. To test this hypothesis, we performed ITC analysis using the purified recombinant SUN domain protein of human SUN2 and the synthesized KASH domain peptides (Fig. 3 and supplemental Fig. S6A).
FIGURE 3.
Interaction between the SUN domain and the KASH peptide. A, the trimeric unit is shown in ribbon representation, and the mutated residues are indicated. B–G, ITC was used to measure the binding affinity between the SUN domain and the KASH peptide. Purified recombinant wild type or mutant SUN domain proteins were analyzed in parallel and compared against each other. Deletion of the stem (SUN-stemdel) or D541N or G608D mutations abolished KASH binding. The calculated binding constants (Ka) for wild type (wt-SUN) and mutants (point mutations: SUN-A646E and SUN-F609A) are 6.59E5, 9.17E5, and 1.93E5 m−1, respectively. The calculated stoichiometry of SUN-NSP2S is n = 1.15 ± 0.02. H, in vitro pull-down analysis of SUN2-nesprin interaction. FLAG-tagged full-length wild-type SUN2 and D541N or G608D mutants were expression in 293T cells. The cell lysate was incubated with glutathione-Sepharose resin loaded with GST or GST-Nsp2C (C-terminal nesprin 2). The proteins associated with the resin were examined by Western blot using anti-FLAG antibody.
Calorimetry studies revealed that the SUN domain is sufficient to bind either the complete luminal part of human nesprin 2 KASH domain (NSP2C, SSEEDYSCTQANNFARSFYPMLRYTNGPPPT) or a shorter C-terminal fragment (NSP2S, NNFARSFEPMLRYTNGPPPT). ITC measurements reveal that the NSP2C peptide can bind to the SUN domain with a Kd of ∼1.8 μm (n = 1.15 ± 0.02). The affinity of NSP2C is consistent with the proposal that the SUN-KASH interaction may be dynamically regulated to adjust the mechanical properties of the network during active biological processes such as cell division.
Mutagenesis studies were performed to identify the structural features involved in binding to the KASH peptides. In particular we wanted to examine whether trimerization is required for the interaction with the KASH domain. Based on the SUN2 structure, we predict that the helical stem region of the SUN domain is essential for the formation of the trimer. Removal of the stem may not lead to the collapse of the entire structure, because the leaf and the stem subdomains appear to be folded independently. Alterations may, however, deform the cloverleaf structure and change the conformation of the extended AA′ loop that is part of the potential binding site for the KASH domain. Indeed, we found that deletion of the three-helix bundle stem of the SUN domain disrupted the trimer and abolished the binding to NS2PS (Fig. 3).
Point mutations of a number of certain amino acid residues in the putative KASH domain-binding site were created. The F609A mutation reduced the NS2PS binding activity, whereas A646E appears to slightly enhance the binding (Fig. 3). In addition, the R692A mutation modestly affected binding to the nesprin KASH domain (supplemental Fig. S6B).
We sought to establish the linkage between the SUN domain structural features and the biological functions. The effect of two point mutations, namely D541N and G608D, were studied. These two sites are conserved in SUN2 across species (supplemental Fig. S1). The D541N or G608D mutations have been shown to compromise nuclear migration in C. elegans (12, 27). We found that alteration of Gly608 to aspartic acid, which resides within the putative KASH domain-binding site, completely abolished binding to the KASH domain. Similarly, the D541N mutation, which is located in the junction connecting the stalk-like helix and the clover-like β-sandwiches, also resulted in loss of binding to nesprin. The mutation of G608D did not cause any significant changes in the global folding or trimerization of the protein, as shown by circular dichroism, gel filtration, and SDS-PAGE (supplemental Fig. S7). Similarly, despite the fact that the D541N mutation caused a modest change in the ratio of monomers and trimers, the alteration had little effect on folding, and the predominant effect of the mutation is loss of binding to nesprin (Fig. 3 and supplemental Fig. S7). Collectively, these results indicate that the trimeric form of the SUN domain associates with the KASH domain via the proposed binding site and that the interaction appears critical for the function of the LINC complex in nuclear migration.
DISCUSSION
Recent studies indicate that the SUN domain proteins interact with KASH domain proteins and form the evolutionarily conserved LINC complex, which spans the perinuclear space between the double nuclear membranes and couple the nuclear interior to the peripheral cytoskeleton (5, 14, 17, 30, 42). Here we provide the first crystallographic structure of the SUN domain, which reveals a homotrimer that resembles a cloverleaf sitting on a stem. Overall, the trimerization of the SUN domain buries ∼6200 Å2 of accessible molecular surface, suggesting a rather stable conformation. Intriguingly, the organization scheme of the SUN domain trimer is reminiscent of that of TRAF2 (tumor necrosis factor receptor-associated factor 2), a signaling adaptor protein that functions as a trimer and recruits the cytoplasmic domain of tumor necrosis factor receptors by forming a 3:3 complex (52, 53) (supplemental Fig. S3). Moreover, the potential KASH-binding sites of the SUN domain trimer are located on each lobe of the cloverleaf in a manner similar to that observed in the TRAF2 trimer, where the corresponding sites are used to complex with the tumor necrosis factor receptor family member (such as CD40) intracellular domain and mediate signaling. We have constructed a model depicting how the SUN domain trimer binds to the KASH domain.
Modeling of the SUN-KASH Complex
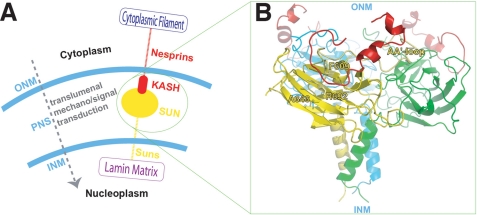
The KASH domain is highly conserved (22). McGee et al. (18) studied the interactions between UNC-83 and UNC-84 in C. elegans and suggested that the consensus sequence in KASH domain required for SUN domain association is FXPXXXYXNGPPPX. The structure of the KASH domain luminal part (KASH-LD) using the three-dimensional structures of the homologous fragments provided in the Protein Data Bank was modeled and revealed that the structure of KASH-LD consists of two mini helices followed by a C-shaped loop (C-loop) at the C terminus. We then modeled the SUN-KASH complex using the structurally identified potential KASH-binding site, i.e. the residue cluster located on the CHEF β-sheet and the AA′ loop as a docking site (Fig. 4). We find that the C-loop of KASH-LD fits into its potential binding site in the SUN domain, forming a 3:3 complex.
FIGURE 4.
Model of SUN-KASH protein complex that spans the nuclear envelope. A, a cartoon illustration showing the SUN-KASH as part of the LINC complex molecular chain connecting the cytoskeleton with the nucleoskeleton. B, the SUN-KASH complex model built upon the crystal structure of SUN domain and the modeled KASH domain luminal structure. The AA′ loop and residues of interest are labeled.
Because the three-helix bundle stem is critical for SUN domain trimer formation, removal of this stem would deform the cloverleaf structure and alter the conformation of the extended AA′ loop that is part of the potential binding site for KASH domain. Consistent with this model, our ITC data show that deletion of SUN domain stem abolished its KASH binding. The D541N mutation, located on the loop connecting the “stem” and the “leaf,” also disrupted binding to KASH. Asp541 forms interactions with Arg707 and Arg709, maintaining the relative orientation between the stem and the leaf. Our results support the notion that changing the configuration of the stemlike region may modulate the SUN-KASH interaction.
The mutagenesis studies of the surface residues such as Gly608, Phe609, Ala646, and Arg692, which are located in or adjacent to the putative KASH binding site, have indicated that the binding between SUN and KASH is most likely mediated through the local interactions that are dependent on the specific side chain conformations of these residues. For example, the G608D mutation completely abolished binding to KASH (Fig. 3). Gly608 is adjacent to a network of interactions mediated by the hydrogen bonding between Arg561 and Gln607/Gln604 and the cation-pi paring with F609/F603. In the G608D mutation, the introduced side chain would block the side chain of Arg561 and disrupt the Arg561-mediated interactions. As a result, the conformation of the predicted KASH-interacting patch would be altered.
Replacement of Phe609 with an alanine may disrupt hydrophobic interactions with nonpolar residues (possibly the terminal proline residues) in the C-loop of KASH domain, leading to a decreased SUN-KASH affinity (Fig. 3). Altering the size and polarity of residues Ala646 and Arg692 would also affect the side chain conformations of the surrounded hydrophobic residues in the active site.
The shorter version of the KASH peptide used in this study corresponds to the consensus sequence of KASH domain and thus may represent the minimal region required for binding to the SUN domain. The KASH domain sequence would form a mini helix followed by a terminal loop with three proline residues in a row on the tip. The proline-rich tip is critical for SUN-KASH interaction because deletion of the proline residues (17) or modifying its conformation by adding additional terminal residues disrupts the complex formation (42).
Molecular Network Formed by SUN-KASH Complexes in Nuclear Envelope
The current crystallographic and modeling studies identify a 3:3 complex formed by the SUN domain and KASH domain. The dimension of the complex along the direction perpendicular to the nuclear double membranes is ∼60 Å.
Previous studies showed that SUN1 and SUN2 can form homo- or hetero-oligomers via two predicated coiled coil motifs located in the luminal region near the N-terminal end of the SUN domain (14, 30, 42, 45, 49, 50). Oligomerization analysis of the potential coiled coil motifs within the luminal domain predicts that although the first coiled coil is highly likely to be dimeric, the second one has a modest probability to be trimeric (58) (supplemental Fig. S7). Thus, these two coiled coil motifs, together with the SUN domain, may respectively guide dimerization and trimerization at distinct regions of the SUN protein luminal region (supplemental Fig. S9).
It is conceivable that the SUN2 proteins form an extended network within the PNS through the SUN domain and the coiled coils (Fig. 4C). Alternatively, through the dimeric coiled coil motif, the SUN2 trimer may associate with SUN1 or other yet to be identified proteins. Interestingly, a recent study revealed that intermolecular disulfide bonds can form among the SUN1 molecules (50), suggesting that additional regions of SUN protein may be involved in the assembly of the network.
We have demonstrated that the SUN domain alone is sufficient for KASH binding. Interestingly, our results, as well as a number of previous studies (14, 17, 42, 49), also indicate that a deletion mutant containing the second coiled coil motif and the intact SUN domain of human SUN2 was unable to bind to the KASH domain of nesprin 2G (Fig. 3 and supplemental Fig. S5). It is possible that, in the deleted form, the coiled coil region adjacent to the SUN domain may fold back and impede the active KASH domain-binding site. Notably, a fragment that contains both coiled coil regions, and the SUN domain retains the ability to bind nesprin (supplemental Fig. S5). We speculate that the regions N-terminal to the SUN domain may either participate in trimerization or mediate a regulatory role in the SUN-KASH interactions.
Biological Significance of SUN-KASH Interaction
The nuclear envelope protein ensembles formed by SUN1, SUN2, and their associated proteins are involved in multiple cellular and developmental processes including cell division, apoptosis, migration, and establishment of polarity (30, 54). For example, SUN1 has been implicated to play a critical role for the distribution of nuclear pore complex across the nuclear surface (55), whereas SUN2 is involved in tethering mammalian meiotic telomeres to the nuclear envelope (38). The SUN domain of SUN2 contributes to localization to the inner membrane of the nuclear envelope (56), probably through association with nesprin.
In particular, SUN2 and nesprin are involved in nuclear migration, a process important for cell mobility as well as organogenesis (34, 35). In this regard, we have identified the structural features (i.e. the regions adjacent to Asp541 and Gly608) that are critical for SUN-KASH binding. In C. elegans, the D541N and G608D mutants showed defects in nuclear migration (12, 27). Both Asn541 and Gly608 are conserved in the SUN proteins, suggesting that these sites play a role in maintaining structure and function. Our results also raised the possibility that the stem region, as well as the coiled coils N-terminal to the SUN domain, may constitute a regulatory element, through which the function of the LINC complex can be modulated.
In a recent study, the SUN2 SUN domain mutation of G670S has been linked to non-Hodgkin lymphomas (57). Gly670 is conserved and is immediately adjacent to Glu671, which forms hydrogen bond and electrostatic interactions with Lys532 and Gln536 from the helical stem region of the neighboring protomer. Mutation of G670S may interfere with these interactions and alter the trimeric conformation that is required for KASH association.
In summary, our work provides a structural model that helps to understand the LINC complex, where the luminal regions of SUN2 protein interact with each other and form a superamolecular network, whereas the C-terminal SUN domain binds with the KASH domain and forms a 3:3 complex. As a physical link across the nuclear envelope, the SUN-KASH network not only contributes to the structural integrity of the cytoplasmic and nuclear compartments but also helps to maintain appropriate mechanical properties of normal cells as recently reported (42, 43).
Supplementary Material
Acknowledgments
We greatly appreciate Dr. Catherine M. Shanahan and Qiuping Zhang (University of Cambridge) for providing Nesprin-GFP construct as a generous gift. This work was carried out in part at National Synchrotron Light Source X6A at the Brookhaven National Laboratory and Shanghai Synchrotron Radiation Facility 17U. We also thank the Dr. Walter Englander Lab at the University of Pennsylvania for the help with the circular dichroism experiments.
This work was supported by the National Cancer Institute, the Abramson Family Cancer Research Institute at the University of Pennsylvania, the Women's Cancer Program at the Samuel Oschin Comprehensive Cancer Institute, the Women's Guild Lung Institute of the Cedars-Sinai Medical Center, and Grants 30970566 and 10979005 from the National Natural Science Foundation of China.

This article contains supplemental text and Figs. S1–S9.
The atomic coordinates and structure factors (code 3UNP) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
- NE
- nuclear envelope
- INM
- inner nuclear membrane
- ONM
- outer nuclear membrane
- PNS
- perinuclear space
- SUN
- Sad1-UNC84 homology
- KASH
- Klarsicht, ANC-1 and Syne homology.
REFERENCES
- 1. Burke B., Ellenberg J. (2002) Remodelling the walls of the nucleus. Nat. Rev. Mol. Cell Biol. 3, 487–497 [DOI] [PubMed] [Google Scholar]
- 2. Burke B., Stewart C. L. (2002) Life at the edge. The nuclear envelope and human disease. Nat. Rev. Mol. Cell Biol. 3, 575–585 [DOI] [PubMed] [Google Scholar]
- 3. Burke B., Stewart C. L. (2006) The laminopathies. The functional architecture of the nucleus and its contribution to disease. Annu. Rev. Genomics Hum Genet. 7, 369–405 [DOI] [PubMed] [Google Scholar]
- 4. Roux K. J., Burke B. (2007) Nuclear envelope defects in muscular dystrophy. Biochim. Biophys. Acta 1772, 118–127 [DOI] [PubMed] [Google Scholar]
- 5. Stewart C. L., Roux K. J., Burke B. (2007) Blurring the boundary. The nuclear envelope extends its reach. Science 318, 1408–1412 [DOI] [PubMed] [Google Scholar]
- 6. Puckelwartz M. J., Kessler E., Zhang Y., Hodzic D., Randles K. N., Morris G., Earley J. U., Hadhazy M., Holaska J. M., Mewborn S. K., Pytel P., McNally E. (2008) Disruption of nesprin-1 produces an Emery Dreifuss Muscular Dystrophy-like phenotype in mice. Hum. Mol. Genet. 18, 607–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hung L. Y., Tseng J. T., Lee Y. C., Xia W., Wang Y. N., Wu M. L., Chuang Y. H., Lai C. H., Chang W. C. (2008) Nuclear epidermal growth factor receptor (EGFR) interacts with signal transducer and activator of transcription 5 (STAT5) in activating Aurora-A gene expression. Nucleic Acids Res. 36, 4337–4351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lin S. Y., Makino K., Xia W., Matin A., Wen Y., Kwong K. Y., Bourguignon L., Hung M. C. (2001) Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat. Cell Biol. 3, 802–808 [DOI] [PubMed] [Google Scholar]
- 9. Oksvold M., Huitfeldt H., Stang E., Madshus I. (2002) Localizing the EGF receptor. Nat. Cell Biol. 4, E22–E23 [DOI] [PubMed] [Google Scholar]
- 10. Xie Y., Hung M. C. (1994) Nuclear localization of p185neu tyrosine kinase and its association with transcriptional transactivation. Biochem. Biophys. Res. Commun. 203, 1589–1598 [DOI] [PubMed] [Google Scholar]
- 11. Starr D. A., Han M. (2002) Role of ANC-1 in tethering nuclei to the actin cytoskeleton. Science 298, 406–409 [DOI] [PubMed] [Google Scholar]
- 12. Lee K. K., Starr D., Cohen M., Liu J., Han M., Wilson K. L., Gruenbaum Y. (2002) Lamin-dependent localization of UNC-84, a protein required for nuclear migration in Caenorhabditis elegans. Mol. Biol. Cell 13, 892–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Starr D. A., Han M. (2003) ANChors away. An actin based mechanism of nuclear positioning. J. Cell Sci. 116, 211–216 [DOI] [PubMed] [Google Scholar]
- 14. Crisp M., Liu Q., Roux K., Rattner J. B., Shanahan C., Burke B., Stahl P. D., Hodzic D. (2006) Coupling of the nucleus and cytoplasm. Role of the LINC complex. J. Cell Biol. 172, 41–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haque F., Lloyd D. J., Smallwood D. T., Dent C. L., Shanahan C. M., Fry A. M., Trembath R. C., Shackleton S. (2006) SUN1 interacts with nuclear lamin A and cytoplasmic nesprins to provide a physical connection between the nuclear lamina and the cytoskeleton. Mol. Cell. Biol. 26, 3738–3751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hodzic D. M., Yeater D. B., Bengtsson L., Otto H., Stahl P. D. (2004) Sun2 is a novel mammalian inner nuclear membrane protein. J. Biol. Chem. 279, 25805–25812 [DOI] [PubMed] [Google Scholar]
- 17. Padmakumar V. C., Libotte T., Lu W., Zaim H., Abraham S., Noegel A. A., Gotzmann J., Foisner R., Karakesisoglou I. (2005) The inner nuclear membrane protein Sun1 mediates the anchorage of Nesprin-2 to the nuclear envelope. J. Cell Sci. 118, 3419–3430 [DOI] [PubMed] [Google Scholar]
- 18. McGee M. D., Rillo R., Anderson A. S., Starr D. A. (2006) UNC-83 is a KASH protein required for nuclear migration and is recruited to the outer nuclear membrane by a physical interaction with the SUN protein UNC-84. Mol. Biol. Cell 17, 1790–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jaspersen S. L., Martin A. E., Glazko G., Giddings T. H., Jr., Morgan G., Mushegian A., Winey M. (2006) The Sad1-UNC-84 homology domain in Mps3 interacts with Mps2 to connect the spindle pole body with the nuclear envelope. J. Cell Biol. 174, 665–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ketema M., Wilhelmsen K., Kuikman I., Janssen H., Hodzic D., Sonnenberg A. (2007) Requirements for the localization of nesprin-3 at the nuclear envelope and its interaction with plectin. J. Cell Sci. 120, 3384–3394 [DOI] [PubMed] [Google Scholar]
- 21. Wilhelmsen K., Ketema M., Truong H., Sonnenberg A. (2006) KASH-domain proteins in nuclear migration, anchorage and other processes. J. Cell Sci. 119, 5021–5029 [DOI] [PubMed] [Google Scholar]
- 22. Starr D. A., Fischer J. A. (2005) KASH 'n Karry. The KASH domain family of cargo-specific cytoskeletal adaptor proteins. Bioessays 27, 1136–1146 [DOI] [PubMed] [Google Scholar]
- 23. Zhang Q., Skepper J. N., Yang F., Davies J. D., Hegyi L., Roberts R. G., Weissberg P. L., Ellis J. A., Shanahan C. M. (2001) Nesprins. A novel family of spectrin-repeat-containing proteins that localize to the nuclear membrane in multiple tissues. J. Cell Sci. 114, 4485–4498 [DOI] [PubMed] [Google Scholar]
- 24. Padmakumar V. C., Abraham S., Braune S., Noegel A. A., Tunggal B., Karakesisoglou I., Korenbaum E. (2004) Enaptin, a giant actin-binding protein, is an element of the nuclear membrane and the actin cytoskeleton. Exp. Cell Res. 295, 330–339 [DOI] [PubMed] [Google Scholar]
- 25. Zhang Q., Ragnauth C. D., Skepper J. N., Worth N. F., Warren D. T., Roberts R. G., Weissberg P. L., Ellis J. A., Shanahan C. M. (2005) Nesprin-2 is a multi-isomeric protein that binds lamin and emerin at the nuclear envelope and forms a subcellular network in skeletal muscle. J. Cell Sci. 118, 673–687 [DOI] [PubMed] [Google Scholar]
- 26. Hagan I., Yanagida M. (1995) The product of the spindle formation gene sad1+ associates with the fission yeast spindle pole body and is essential for viability. J. Cell Biol. 129, 1033–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Malone C. J., Fixsen W. D., Horvitz H. R., Han M. (1999) UNC-84 localizes to the nuclear envelope and is required for nuclear migration and anchoring during C. elegans development. Development 126, 3171–3181 [DOI] [PubMed] [Google Scholar]
- 28. Dreger M., Bengtsson L., Schöneberg T., Otto H., Hucho F. (2001) Nuclear envelope proteomics. Novel integral membrane proteins of the inner nuclear membrane. Proc. Natl. Acad. Sci. U.S.A. 98, 11943–11948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kennedy C., Sebire K., de Kretser D. M., O'Bryan M. K. (2004) Human sperm associated antigen 4 (SPAG4) is a potential cancer marker. Cell Tissue Res. 315, 279–283 [DOI] [PubMed] [Google Scholar]
- 30. Tzur Y. B., Wilson K. L., Gruenbaum Y. (2006) SUN-domain proteins. “Velcro” that links the nucleoskeleton to the cytoskeleton. Nat. Rev. Mol. Cell Biol. 7, 782–788 [DOI] [PubMed] [Google Scholar]
- 31. Starr D. A., Hermann G. J., Malone C. J., Fixsen W., Priess J. R., Horvitz H. R., Han M. (2001) unc-83 encodes a novel component of the nuclear envelope and is essential for proper nuclear migration. Development 128, 5039–5050 [DOI] [PubMed] [Google Scholar]
- 32. Horvitz H. R., Sulston J. E. (1980) Isolation and genetic characterization of cell-lineage mutants of the nematode Caenorhabditis elegans. Genetics 96, 435–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sulston J. E., Horvitz H. R. (1981) Abnormal cell lineages in mutants of the nematode Caenorhabditis elegans. Dev. Biol. 82, 41–55 [DOI] [PubMed] [Google Scholar]
- 34. Luxton G. W., Gomes E. R., Folker E. S., Vintinner E., Gundersen G. G. (2010) Linear arrays of nuclear envelope proteins harness retrograde actin flow for nuclear movement. Science 329, 956–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yu J., Lei K., Zhou M., Craft C. M., Xu G., Xu T., Zhuang Y., Xu R., Han M. (2011) KASH protein Syne-2/Nesprin-2 and SUN proteins SUN1/2 mediate nuclear migration during mammalian retinal development. Hum. Mol. Genet. 20, 1061–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fridkin A., Mills E., Margalit A., Neufeld E., Lee K. K., Feinstein N., Cohen M., Wilson K. L., Gruenbaum Y. (2004) Matefin, a Caenorhabditis elegans germ line-specific SUN-domain nuclear membrane protein, is essential for early embryonic and germ cell development. Proc. Natl. Acad. Sci. U.S.A. 101, 6987–6992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hoffenberg S., Liu X., Nikolova L., Hall H. S., Dai W., Baughn R. E., Dickey B. F., Barbieri M. A., Aballay A., Stahl P. D., Knoll B. J. (2000) A novel membrane-anchored Rab5 interacting protein required for homotypic endosome fusion. J. Biol. Chem. 275, 24661–24669 [DOI] [PubMed] [Google Scholar]
- 38. Schmitt J., Benavente R., Hodzic D., Höög C., Stewart C. L., Alsheimer M. (2007) Transmembrane protein Sun2 is involved in tethering mammalian meiotic telomeres to the nuclear envelope. Proc. Natl. Acad. Sci. U.S.A. 104, 7426–7431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chikashige Y., Tsutsumi C., Yamane M., Okamasa K., Haraguchi T., Hiraoka Y. (2006) Meiotic proteins Bqt1 and Bqt2 tether telomeres to form the bouquet arrangement of chromosomes. Cell 125, 59–69 [DOI] [PubMed] [Google Scholar]
- 40. Tomita K., Cooper J. P. (2006) The meiotic chromosomal bouquet. SUN collects flowers. Cell 125, 19–21 [DOI] [PubMed] [Google Scholar]
- 41. Schoggins J. W., Wilson S. J., Panis M., Murphy M. Y., Jones C. T., Bieniasz P., Rice C. M. (2011) A diverse range of gene products are effectors of the type I interferon antiviral response. Nature 472, 481–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stewart-Hutchinson P. J., Hale C. M., Wirtz D., Hodzic D. (2008) Structural requirements for the assembly of LINC complexes and their function in cellular mechanical stiffness. Exp. Cell Res. 314, 1892–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lombardi M. L., Jaalouk D. E., Shanahan C. M., Burke B., Roux K. J., Lammerding J. (2011) The interaction between nesprins and Sun proteins at the nuclear envelope is critical for force transmission between the nucleus and cytoskeleton. J. Biol. Chem. 286, 26743–26753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. King M. C., Drivas T. G., Blobel G. (2008) A network of nuclear envelope membrane proteins linking centromeres to microtubules. Cell 134, 427–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xiong H., Rivero F., Euteneuer U., Mondal S., Mana-Capelli S., Larochelle D., Vogel A., Gassen B., Noegel A. A. (2008) Dictyostelium Sun-1 connects the centrosome to chromatin and ensures genome stability. Traffic 9, 708–724 [DOI] [PubMed] [Google Scholar]
- 46. Penkner A., Tang L., Novatchkova M., Ladurner M., Fridkin A., Gruenbaum Y., Schweizer D., Loidl J., Jantsch V. (2007) The nuclear envelope protein Matefin/SUN-1 is required for homologous pairing in C. elegans meiosis. Dev. Cell 12, 873–885 [DOI] [PubMed] [Google Scholar]
- 47. Ding X., Xu R., Yu J., Xu T., Zhuang Y., Han M. (2007) SUN1 is required for telomere attachment to nuclear envelope and gametogenesis in mice. Dev. Cell 12, 863–872 [DOI] [PubMed] [Google Scholar]
- 48. Conrad M. N., Lee C. Y., Wilkerson J. L., Dresser M. E. (2007) MPS3 mediates meiotic bouquet formation in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U.S.A. 104, 8863–8868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang Q., Du X., Cai Z., Greene M. I. (2006) Characterization of the structures involved in localization of the SUN proteins to the nuclear envelope and the centrosome. DNA Cell Biol. 25, 554–562 [DOI] [PubMed] [Google Scholar]
- 50. Lu W., Gotzmann J., Sironi L., Jaeger V. M., Schneider M., Luke Y., Uhlen M., Szigyarto C. A., Brachner A., Ellenberg J., Foisner R., Noegel A. A., Karakesisoglou I. (2008) Sun1 forms immobile macromolecular assemblies at the nuclear envelope. Biochim. Biophys. Acta 1783, 2415–2426 [DOI] [PubMed] [Google Scholar]
- 51. Fridkin A., Penkner A., Jantsch V., Gruenbaum Y. (2008) SUN-domain and KASH-domain proteins during development, meiosis and disease. Cell Mol. Life Sci. 66, 1518–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ye H., Park Y. C., Kreishman M., Kieff E., Wu H. (1999) The structural basis for the recognition of diverse receptor sequences by TRAF2. Mol. Cell 4, 321–330 [DOI] [PubMed] [Google Scholar]
- 53. Wajant H., Scheurich P. (2001) Tumor necrosis factor receptor-associated factor (TRAF) 2 and its role in TNF signaling. Int. J. Biochem. Cell Biol. 33, 19–32 [DOI] [PubMed] [Google Scholar]
- 54. Tzur Y. B., Margalit A., Melamed-Book N., Gruenbaum Y. (2006) Matefin/SUN-1 is a nuclear envelope receptor for CED-4 during Caenorhabditis elegans apoptosis. Proc. Natl. Acad. Sci. U.S.A. 103, 13397–13402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Liu Q., Pante N., Misteli T., Elsagga M., Crisp M., Hodzic D., Burke B., Roux K. J. (2007) Functional association of Sun1 with nuclear pore complexes. J. Cell Biol. 178, 785–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Turgay Y., Ungricht R., Rothballer A., Kiss A., Csucs G., Horvath P., Kutay U. (2010) A classical NLS and the SUN domain contribute to the targeting of SUN2 to the inner nuclear membrane. EMBO J. 29, 2262–2275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cerhan J. R., Ansell S. M., Fredericksen Z. S., Kay N. E., Liebow M., Call T. G., Dogan A., Cunningham J. M., Wang A. H., Liu-Mares W., Macon W. R., Jelinek D., Witzig T. E., Habermann T. M., Slager S. L. (2007) Genetic variation in 1253 immune and inflammation genes and risk of non-Hodgkin lymphoma. Blood 110, 4455–4463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wolf E., Kim P. S., Berger B. (1997) MultiCoil. A program for predicting two- and three-stranded coiled coils. Protein Sci. 6, 1179–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.