Background: The neutrophil protease cathepsin G induces myocyte anoikis.
Results: Cathepsin G promotes c-Cbl activation and interaction with focal adhesion proteins that leads to focal adhesion and myofibril protein degradation and myocyte anoikis.
Conclusion: c-Cbl is a key ligase required during cathepsin G-induced focal adhesion and myofibrillar protein degradation.
Significance: This is a novel mechanism to regulate focal adhesion and myofibril stability and turnover.
Keywords: Apoptosis, Cardiac Muscle, E3 Ubiquitin Ligase, Focal Adhesion Kinase, Neutrophil, Apoptosis, Cardiomyocytes, Cathepsin G, Focal Adhesion, c-Cbl
Abstract
The neutrophil-derived serine protease, cathepsin G (Cat.G), has been shown to induce myocyte detachment and apoptosis by anoikis through down-regulation of focal adhesion (FA) signaling. However, the mechanisms that control FA protein stability and turnover in myocytes are not well understood. Here, we have shown that the Casitas b-lineage lymphoma (c-Cbl), adaptor protein with an intrinsic E3 ubiquitin ligase activity, is involved in FA and myofibrillar protein stability and turnover in myocytes. Cat.G treatment induced c-Cbl activation and its interaction with FA proteins. Deletion of c-Cbl using c-Cbl knock-out derived myocytes or inhibition of c-Cbl ligase activity significantly reduced FA protein degradation, myofibrillar degeneration, and myocyte apoptosis induced by Cat.G. We also found that inhibition of the proteasome activity, but not the lysosome or the calpain activity, markedly attenuated FA and myofibrillar protein degradation induced by Cat.G. Interestingly, c-Cbl activation induced by Cat.G was mediated through epidermal growth factor receptor (EGFR) transactivation as inhibition of EGFR kinase activity markedly attenuated c-Cbl phosphorylation and FA protein degradation induced by Cat.G. These findings support a model in which neutrophil protease Cat.G promotes c-Cbl interaction with FA proteins, resulting in enhanced c-Cbl-mediated FA protein ubiquitination and degradation, myofibril degradation, and subsequent down-regulation of myocyte survival signaling.
Introduction
Inflammation is thought to induce myocardial remodeling through the release of reactive oxygen species, antimicrobial peptides, and proteases (1, 2). Although beneficial at early stages after myocardial injury, excessive activation of inflammatory cells detrimentally leads to cardiomyocyte death and tissue damage that ultimately leads to heart failure (1, 2). Neutrophil-derived serine protease, cathepsin G (Cat.G),3 has been shown to play an important role in tissue remodeling at sites of tissue injury through hydrolysis of a host of protein substrates including chemoattractants, extracellular matrix (ECM), and hormonal factors (3–5). In addition, Cat.G may cleave and activate G protein-coupled protease-activated receptors (PARs) as a mechanism to modulate coagulation and tissue remodeling (6). Pathophysiological concentration of Cat.G has been shown to induce endothelial cell and myocyte detachment and apoptosis by anoikis (7, 8). These effects of Cat.G on myocytes do not require PARs but involve MMP activation and epidermal growth factor receptor (EGFR) transactivation (8). Further studies have shown the role of focal adhesion (FA) signaling down-regulation as a mechanism whereby Cat.G modulates normal cell-cell or cell-ECM interactions necessary for myocyte survival (9). However, the mechanisms involved in FA signaling down-regulation are still poorly understood.
Precise control of protein synthesis, processing and degradation plays a fundamental role in regulating cardiac structure and function (10). Abnormal regulation of these steps can result in cardiomyopathies because of myofibrillar degeneration with contractile failure (10). Recent evidence indicates that the ubiquitin proteasome system (UPS), a system involved in removing misfolded and damaged proteins, is also responsible for the regulation and turnover of proteins involved in inflammatory process, cell cycle regulation, and hypertrophic gene expression, thereby regulating cardiac hypertrophy and remodeling (10). The formation of ubiquitin-protein conjugates involves three components that participate in a cascade of ubiquitin transfer reactions: a ubiquitin-activation enzyme (E1), a ubiquitin-conjugating enzyme (E2) and a ubiquitin ligase (E3) that acts at the last step of the cascade and has the greatest tissue/substrate specificity (11, 12). Once a protein has been marked with polyubiquitin conjugates, it is destined for degradation by the multicatalytic protease, the 26 S proteasome complex (13).
The 120-kDa proto-oncogene c-Cbl contains multiple protein interaction motifs, including tyrosine residues whose phosphorylation promotes the association of c-Cbl with numerous cytoplasmic signaling proteins, a phosphotyrosine-binding domain (PTB) that encompasses a Src homology domain 2 (SH2) (14), and a proline-rich motif. These domains allow interaction of c-Cbl with multiple signal transducers, including the p85 subunit of PI3-kinase, Grb2, and Src family kinases suggesting that c-Cbl acts as a docking protein to integrate signaling pathways (15). c-Cbl also contains a RING finger motif and a putative leucine zipper that acts as a RING-type ubiquitin ligase (E3) and negatively regulates receptor and non-receptor tyrosine kinases and other classes of receptors by promoting their ubiquitination and lysosomal/proteasomal degradation (15). Although c-Cbl phosphorylation in response to a variety of cell surface receptors has been documented, the precise biological function of c-Cbl is unclear as both negative and positive roles for c-Cbl have been proposed (15). In the heart, c-Cbl expression has been shown to be increased in response to pressure overload stimuli (16) and a recent study linked c-Cbl to human Noonan syndrome, one of the most common genetic syndromes associated with congenital heart disease (17). However, specific functions of c-Cbl in myocyte growth and cardiac function are still not well understood.
In the present study, we show that neutrophil-derived protease Cat.G coordinates signaling by growth factor receptors and FA proteins through activation of c-Cbl. We also found that c-Cbl ubiquitin ligase activity mediates myofibrillar and FA protein degradation induced by Cat.G. We conclude that c-Cbl controls the turnover of FA and myofibrillar proteins in response to neutrophil proteases that play a key role in the maintenance of ventricular myocyte survival after cardiac injury.
EXPERIMENTAL PROCEDURES
Materials
Cat.G, MG132, and lactacystin were obtained from Calbiochem. Monoclonal antibodies to c-Cbl, FAK, ubiquitin, and paxillin were obtained from BD Biosciences, and polyclonal antibody for FAK (used to detect FAK degradation products) and Cbl-b were from Santa Cruz Biotechnology. Phospho-c-Cbl Y774 and c-Cbl Y731, Bcl2, phospho-Bad-S112, Bad, c-IAP1, and XIAP were from Cell Signaling. Monoclonal antibody for phosphotyrosine was from Upstate Biotechnology and anti-troponin I was from Sigma. All other chemicals were from standard suppliers.
Neonatal Cardiomyocyte Isolation
Myocytes were isolated from the ventricles of neonatal Sprague-Dawley rats, wild-type (WT) or c-Cbl KO mice by collagenase digestion as previously described (9). After 30 min of preplating (to eliminate non-myocyte cell contamination), myocytes were plated in collagen precoated dishes or in fibronectin (BD Biosciences)-precoated glass coverslips at a density of 160,000/cm2 in 10% fetal bovine serum DMEM supplemented with 1 mmol/liter l-glutamine, antibiotic/antimycotic solution, and 100 μmol/liter 5-bromo-2-deoxyuridine (BrdU). Under these high density conditions, the myocytes form cell-cell contacts and display spontaneous contractile activity within 24 h of plating.
Expression of Adenoviral Vectors
Production of recombinant adenovirus carrying wild-type human EGFR with the CAG (WT-EGFR) and dominant negative EGFR (EGFR-CD533, a mutant that lacks the kinase domain of EGFR) was described elsewhere (9). Wild-type c-Cbl (WT-Cbl) was provided by Dr. A. Sanjay, Temple University and ligase-deficient c-Cbl (C381A-Cbl), a mutant that lacks RING finger domain necessary for ubiquitin ligase activity, was provided by Dr. A. Y. Tsayganov, Temple University. Adenoviral vectors were purified using a kit from Virapur and titrated using BD Adeno-X rapid titer kit (BD Bioscience). After 24 h of plating, neonatal rat cardiomyocytes (NRCMs) were infected at the indicated plaque-forming units (pfu)/cell in DMEM for 2 h, then 5% fetal bovine serum DMEM was added, and cells were incubated for an additional 48 h. Serum-free DMEM/F-12 medium was changed 1 h before the start of the experiments.
Immunoprecipitation and Immunoblot Analysis
Extraction of proteins from cultured cells was performed as described previously (9). Briefly, lysates were cleared by centrifugation at 12,000 rpm and the supernatants (800 μg of protein/ml) were subjected to immunoprecipitation with corresponding antibodies. After overnight incubation at 4 °C, protein A- or G-agarose beads were added and left for an additional 3 h. Immunocomplexes were then subjected to SDS-PAGE followed by Western blot analysis according to methods published previously or to the manufacturer's instructions (9). Each panel in each figure represents results from a single gel exposed for a uniform duration, with bands detected by enhanced chemiluminescence and quantified by laser scanning densitometry.
Immunofluorescence
NRCMs were grown on fibronectin-coated glass coverslips, fixed with 4% paraformaldehyde, permeabilized in phosphate-buffered saline containing 0.2% Triton X-100, and blocked with 1% bovine serum albumin. Cells were incubated with anti-c-Cbl polyclonal overnight at 4 °C. After washes, cells were incubated with anti-FAK monoclonal antibodies followed by incubation with Alexa-conjugated anti-mouse (-rabbit) Ig antibodies. After final washes and mounting, cells were examined using a epifluorescence microscope (Nikon).
c-Cbl Auto-ubiquitination Assay
c-Cbl ubiquitin ligase activity was performed using auto-ubiquitination assay kit from Enzo Life Sciences with minor modification. Briefly, c-Cbl immunoprecipitates were washed twice with lysis buffer, once with ubiquitination buffer and then were incubated with 30 μl of ligase reaction buffer mixture containing ubiquitination buffer, 1 mm DTT, 20 units/ml inorganic pyrophosphatase, 5 mm Mg-ATP, 2.5 μm ubiquitin, 100 nm E1, and 2.5 μm at 37 °C for 1 h. The reaction was quenched by adding 30 μl of 2× nonreducing gel loading buffer and heating at 95 °C for 5 min. Finally, the samples were assessed for immunoblotting with anti-ubiquitin for detecting ubiquitinated c-Cbl and for c-Cbl as a loading control.
Caspase-3 Assay
Caspase-3 activity was measured with the CaspACE assay system (Promega, Madison, WI). In brief, myocyte lysates were prepared by dounce homogenization in lysis buffer provided with the kit. The lysates were centrifuged at 15,000 × g for 20 min at 4 °C, and the supernatants containing 100 μg of protein were used for caspase-3 assay. Caspase-3 activity was examined by measurement of the rate of cleavage of fluorogenic-conjugated substrate (7-methoxycoumarin-4-yl)acetyl-Val-Asp-Gln-Met-Asp-Gly-Trp-Lys-(2,4-dinitrophenyl)-NH2. The specificity of the assay was confirmed by addition of the specific caspase-3 inhibitor Z-DEVD-FMK in the reaction mixture at a concentration of 50 μm during the incubation.
Apoptotic Cell Death ELISA
A cell death detection ELISA kit (Roche Applied Science) was used to quantitatively determine the apoptotic DNA fragmentation by measuring the cytosolic histone-associated mono- and oligo-nucleosomes fragments associated with apoptotic cell death.
Data Analysis
Data reported are mean ± S.E. Statistical significance was evaluated using one-way analysis of variance post-hoc test. A p value less than 0.05 was considered significant. All in vitro experiments were performed at least three times from three different cultures.
RESULTS
Cat.G Induces Proteasomal Degradation of FA Proteins
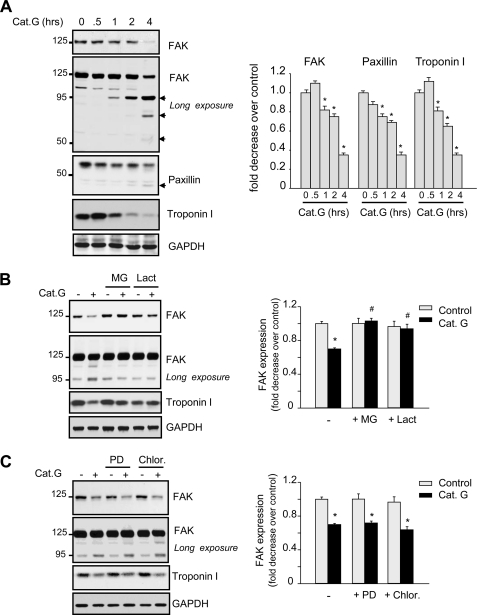
We showed previously that Cat.G treatment induced FA signaling down-regulation that was followed by their dissociation from FA sites (9). Here, we assessed the potential implication of the UPS in FA signaling down-regulation induced by Cat.G. Fig. 1A shows that treatment of NRCMs with Cat.G increased FAK degradation with detection of FAK degradation products at ∼95 kDa at 1 h after Cat.G treatment. Further degradation products of FAK were observed at ∼75 kDa and ∼55 kDa after Cat.G treatment for 4 h. Cat.G treatment also increased paxillin and troponin I degradation and induced loss of myofibril organization as assessed by troponin I and actin staining (supplemental Fig. S1). Pretreatment of NRCMs with specific proteasome inhibitors (MG132 or lactacystin), but not with calpain inhibitor (PD150606) or lysosome inhibitor (chloroquine), significantly protected FAK and troponin I from degradation induced by Cat.G treatment for 2 h (Fig. 1, B and C). These data together show that the UPS, and not calpains or lysosomes, is involved in FA and myofibril degradation induced by Cat.G.
FIGURE 1.
Cat.G induces proteasomal degradation of FA and myofibrillar proteins. A, representative immunoblots showing accumulation of FAK, paxillin, and troponin I in NRCM lysates from untreated or treated with 400 nmol/liter Cat.G for the indicated time. A longer exposure is provided for optimal visualization of the FAK cleavage products. B and C, NRCMs were pretreated with MG132 (MG, 5 μmol/liter) or lactacystin (Lac, 10 μmol/liter) (B), PD150606 (PD, 10 μmol/liter), or chloroquine (Chlor, 5 μmol/liter) (C) for 45 min and then treated with Cat.G for 2 h and immunoblotted with anti-FAK, -paxillin, or -troponin I antibodies. GAPDH was used as a loading control. Left: representative Western blots. Right: quantification of experiments expressed as mean ± S.E. from three separate cultures. *, p < 0.05 versus control, #, p < 0.05 versus Cat.G-treated cells.
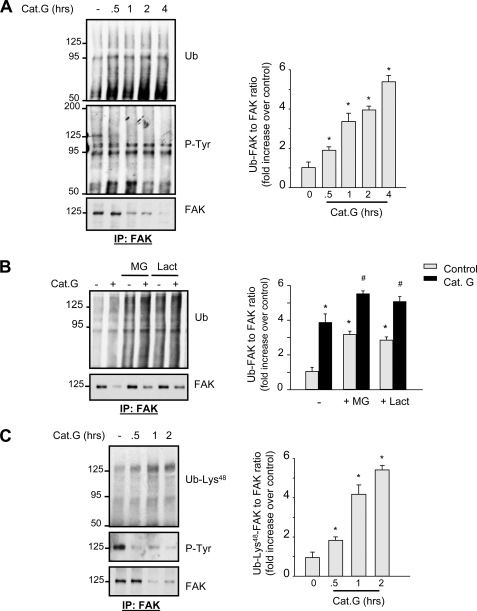
As proteins targeted for proteasomal degradation need to be ubiquitinated by ubiquitin ligases, we next assessed whether Cat.G treatment induces FA protein ubiquitination by immunoblot analysis. Cat.G treatment increased FAK ubiquitination that had slow kinetics and was detected as smear bands at ∼125 kDa by immunoblot (Fig. 2A). Other FAK immunoprecipitates at ∼95, ∼75, and ∼55 kDa also showed high levels of ubiquitination in response to Cat.G treatment. These bands were not detected in anti-rabbit IgG immunoprecipitates and had an apparent molecular weight that corresponded to FAK degradation products (supplemental Fig. S2). Interestingly, FAK tyrosine phosphorylation and accumulation at ∼125 kDa decreased significantly after Cat.G treatment. These data together suggest that FAK inactivation, ubiquitination, and degradation are associated events.
FIGURE 2.
Cat.G induces FAK ubiquitination. A–C, NRCMs were pretreated with MG132, lactacystin or their vehicle and treated with Cat.G for the indicated time (A and C) or for 2 h (B). Left: lysates were immunoprecipitated with anti-FAK antibodies and blotted with anti-ubiquitin (Ub), -phosphotyrosine (P-Tyr), -ubiquitin-Lys48 (Ub-Lys48), or -FAK antibodies. Right: Quantification of experiments expressed as mean ± S.E. from three separate cultures. *, p < 0.05 versus control, #,p < 0.05 versus Cat.G-treated cells.
To study the mechanism of FAK ubiquitination, NRCMs were pre-treated with MG132 or lactacystin prior to stimulation with Cat.G for 1 h. Immunoprecipitation analysis showed that pretreatment with MG132 or lactacystin resulted in marked accumulation of basal ubiquitinated FAK, indicating that the treatment did interfere with proteasomal activity in NRCMs (Fig. 2B), and treatment with Cat.G resulted in a relatively limited accumulation of ubiquitinated FAK when compared with Cat.G-treated control cells.
To assess whether the ubiquitin chain attached to FAK is linked through Lys48 in ubiquitin, a ubiquitin chain structure that is usually required for substrate targeting to the 26 S proteasome (18), FAK immunoprecipitates were immunoblotted with antibodies that recognize ubiquitin linked at Lys48. As shown in Fig. 2C, Cat.G treatment time dependently increased FAK ubiquitination at Lys48 that was associated with decreased tyrosine phosphorylation and accumulation of FAK. Increased ubiquitination at Lys48 was also observed at ∼95 kDa suggesting that Cat.G treatment induces FAK ubiquitination that leads to its degradation by the 26 S proteasome.
Cat.G Induces E3 Ubiquitin Ligase c-Cbl Activation
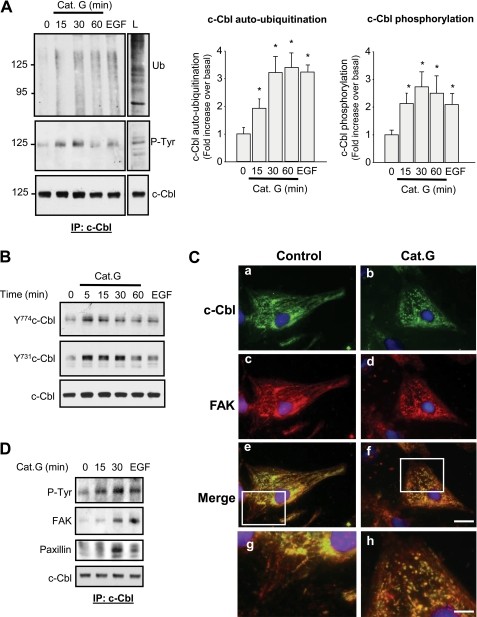
Increased FA protein ubiquitination in response to Cat.G suggests that activation of an E3 ubiquitin ligase is involved in transferring ubiquitin residue to FA proteins. We next assessed whether c-Cbl, a E3 ubiquitin ligase known to interact with several molecules of FA complex (19), mediates Cat.G-induced FAK ubiquitination and degradation. As shown in Fig. 3A, immunoprecipitation analysis coupled with in vitro ubiquitination assay revealed that c-Cbl ubiquitin ligase activity was increased in response to Cat.G. This increase was sustained for over 60 min after Cat.G treatment and was similar to that seen when cells were treated with EGF for 5 min. Along with this increase in c-Cbl auto-ubiquitination, Cat.G treatment caused c-Cbl tyrosine phosphorylation that occurred at Tyr774 and Tyr731 residues, two sites involved in c-Cbl activation and interaction with PI3-kinase, respectively (Fig. 3, A and B) (15). Consistent with c-Cbl activation, a reorganization of c-Cbl labeling was observed 15 min after Cat.G treatment (Fig. 3C). In control NRCMs, c-Cbl staining consisted of fibrillar-like structure throughout the cytoplasm and at the FA sites with some dense staining concentrated at the perinuclear region (Fig. 3, Ca). After Cat.G exposure, c-Cbl localization at fibrillar-like structures and FA sites significantly decreased and c-Cbl immunostaining was present in globule-shaped structures that were present throughout the cytoplasm and in the perinuclear region (Fig. 3, Cb). Similar c-Cbl immunostaining patterns were also observed when Cat.G-treated cells were labeled with anti-ubiquitin antibodies (supplemental Fig. S3), suggesting that these globules may represent domains of ubiquitin-dependent protein degradation within cardiomyocytes.
FIGURE 3.
Cat.G induces c-Cbl activation and organization. A and B, NRCMs were treated with 400 nmol/liter Cat.G for the indicated time or with 100 ng/ml EGF for 5 min. A, left: c-Cbl immunoprecipitates were assayed for auto-ubiquitination assay and immunoblotted for ubiquitin (Ub), phosphotyrosine (P-Tyr) or c-Cbl. L, lysate from NRCMs treated with EGF for 5 min was taken as control. Right: quantification of experiments expressed as mean ± S.E. from three separate cultures. *, p < 0.05 versus control. B, representative immunoblots showing phospho-c-Cbl-Y774 and -Y731 and c-Cbl accumulation. C, NRCMs untreated (a, c, e, g) or treated with Cat.G (b, d, f, h) for 15 min were fixed and stained for c-Cbl (green) or FAK (red) and counterstained with DAPI (blue). (Bar, 20 μm) Higher magnification of e and f was illustrated in g and h, respectively to show co-localization of c-Cbl and FAK. (Bar, 5 μm). D, c-Cbl immunoprecipitates from NRCMs treated with Cat.G for the indicated times or EGF for 5 min were resolved on SDS-PAGE and immunoblotted with anti-phosphotyrosine (P-Tyr), -FAK, -paxillin, or -c-Cbl antibodies.
To explore whether FAK ubiquitination observed in response to Cat.G is a direct consequence of c-Cbl activation, we analyzed c-Cbl association to FAK and paxillin. Immunoprecipitation studies showed that increased c-Cbl tyrosine phosphorylation observed in response to Cat.G or EGF treatment was associated with increased interaction of c-Cbl and FAK or paxillin (Fig. 3D). Consistent with c-Cbl interaction with FA proteins, a reorganization of c-Cbl and FAK immunolabeling was observed after Cat.G treatment. FAK immunostaining which was observed at FA sites and surrounding the nucleus in control NRCMs (Fig. 3, Cc) showed co-localization with c-Cbl staining at the globule-shaped structures after Cat.G treatment that were present throughout the cytoplasm and in the perinuclear region (Fig. 3, Cf). Together, these data indicate that Cat.G treatment triggers c-Cbl activation and its association with FAK in cardiomyocytes.
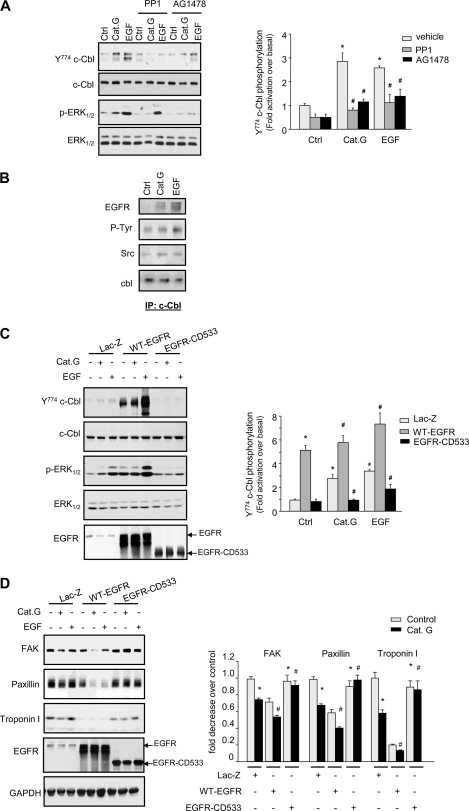
Cat.G Induces c-Cbl Phosphorylation through EGFR Transactivation
To explore the mechanisms involved in c-Cbl phosphorylation induced by Cat.G, cells were pretreated with EGFR kinase inhibitor (AG1478) or Src kinase inhibitor (PP1) prior to stimulation with Cat.G. c-Cbl phosphorylation induced by Cat.G or EGF (taken as a positive control) was markedly reduced by AG1478 and PP1 treatment (Fig. 4A), suggesting a role for EGFR and Src kinase activity in c-Cbl phosphorylation induced by Cat.G. Similar effect of these inhibitors was observed on the activation of EGFR downstream signaling as evidenced by ERK1/2 phosphorylation. Consistent with the role of EGFR and Src kinase activity in c-Cbl phosphorylation, Cat.G treatment increased c-Cbl interaction with EGFR and Src that was similar to that of cells treated with EGF (Fig. 4B). To gain insight into the role of EGFR in Cat.G signaling, we conducted gain- or loss-of-function experiments in which we transduced NRCMs with adenoviruses carrying wild-type EGFR (WT-EGFR) or a mutated construct expressing catalytically inactive EGFR (EGFR-CD533, EGFR lacking the C-terminal 533 amino acids and acts as a potent inhibitor of EGFR). EGFR-CD533 overexpression significantly reduced c-Cbl and ERK1/2 phosphorylation induced by Cat.G or EGF for 5 min with no detectable effect on c-Cbl or ERK1/2 expression levels (Fig. 4C). EGFR-CD533 overexpression also markedly reduced FAK, paxillin, and troponin I degradation induced by Cat.G for 2 h (Fig. 4D). In contrast, myocytes infected with WT-EGFR viruses showed basal increase in c-Cbl and ERK1/2 phosphorylation and FAK and troponin I degradation compared with Lac-Z controls and treatment with Cat.G further enhanced c-Cbl and ERK1/2 phosphorylation and subsequent FAK, paxillin and troponin I degradation (Fig. 4, C and D).
FIGURE 4.
Cat.G mediates c-Cbl activation through EGFR transactivation. A, NRCMs were pretreated with PP1 (10 μmol/liter) or AG1478 (10 μmol/liter) prior to treatment with Cat.G or EGF for 5 min. Cell lysates were assayed for phospho-c-Cbl-Y774, phospho-ERK1/2, c-Cbl and ERK1/2 immunoblot. Left: representative immunoblots. Right: quantification of experiments expressed as mean ± S.E. from three separate cultures. *, p < 0.05 versus control; #, p < 0.05 versus Cat.G or EGF-treated cells. B, c-Cbl immunoprecipitates from NRCMs treated with Cat.G or EGF for 5 min were immunoblotted with anti-EGFR, -phosphotyrosine (P-Tyr), -Src, or -c-Cbl antibodies. C and D, NRCMs were infected with Lac-Z, WT-EGFR, or EGFR-CD533 adenoviruses and then treated with Cat.G or EGF for 5 min (C) or 2 h (D). Left: representative immunoblots with GAPDH taken as loading control. Right: quantification of experiments expressed as mean ± S.E. from three separate cultures. *, p < 0.05 versus Lac-Z control, #, p < 0.05 versus Cat.G-treated cells.
Inhibition of c-Cbl Ligase Activity Prevents FA and Myofibril Protein Degradation Induced by Cat.G
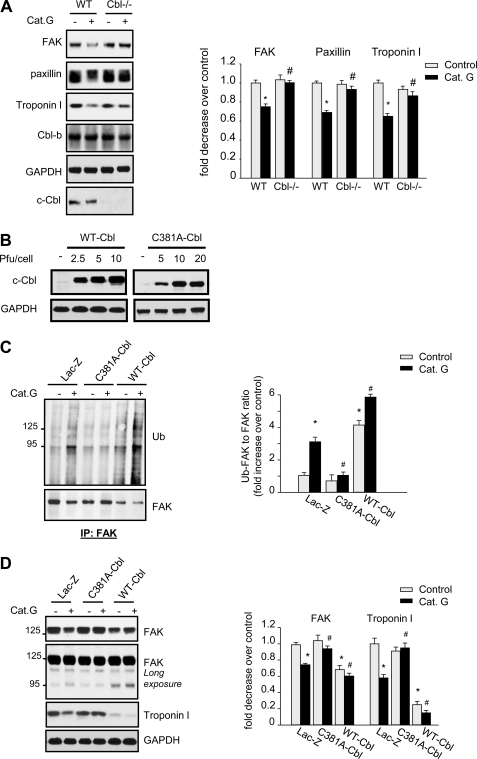
We next assessed the role of c-Cbl in mediating FA and myofibrillar proteins degradation induced by Cat.G using neonatal myocytes isolated from WT or c-Cbl knock-out (KO) mice. As expected, treatment of WT derived myocytes with Cat.G for 2 h induced FAK, paxillin and troponin I degradation (Fig. 5A). Interestingly, degradation of these proteins was markedly attenuated in c-Cbl KO-derived myocytes. To gain insight into the role of c-Cbl ubiquitin ligase activity in mediating FAK ubiquitination and degradation induced by Cat.G, we infected NRCMs with recombinant adenoviral vectors carrying cDNAs encoding Lac-Z, wild type c-Cbl (WT-Cbl) or c-Cbl point mutant (C381A-Cbl), in which a single amino acid change abolishes the ligase activity resulting in a dominant-negative isoform of c-Cbl (20). Myocytes infected with WT-Cbl at 2.5, 5, and 10 pfu/cell showed an increased level of c-Cbl by 4-, 6-, and 8-fold, respectively over Lac.Z control (Fig. 5B). WT-Cbl overexpression significantly increased basal FAK ubiquitination compared with Lac-Z untreated controls and treatment with Cat.G slightly increased the level of ubiquitinated FAK compared with Cat.G-treated Lac-Z-infected cells (Fig. 5C). Myocytes infected with C381A-Cbl viruses at 5, 10, and 20 pfu/cell showed increased levels of c-Cbl mutant by 3-, 5-, and 8-fold, respectively over Lac-Z control (Fig. 5B). C381A-Cbl mutant overexpression markedly attenuated Cat.G-induced FAK ubiquitination (Fig. 5C). This effect of c-Cbl on FAK ubiquitination was translated on its protein accumulation levels. Overexpression of WT-Cbl increased basal degradation of FAK and troponin I (−26 ± 3% and −35 ± 5% compared with Lac-Z control, respectively) and treatment with Cat.G slightly enhanced this FAK and troponin I degradation compared with Cat.G-treated controls (−38 ± 4% and −65 ± 4% compared with Cat.G-treated Lac-Z, respectively) (Fig. 5D). In contrast, C381A-Cbl mutant expression had no effect on basal FAK and troponin I level accumulation, but markedly reduced their degradation induced by Cat.G (−10 ± 2% and −5 ± 3% compared with Cat.G-treated Lac-Z, respectively). Interestingly, overexpression of Y731F-Cbl, a c-Cbl mutant lacking the PI3 kinase-interacting domain, showed enhanced FAK and troponin I degradation both in Lac.Z-infected controls and in cells treated with Cat.G (supplemental Fig. S4). These data together indicate that the ubiquitin ligase activity of c-Cbl is involved in the ubiquitination and proteasomal degradation of FA and myofibrillar proteins induced by Cat.G, while its PI3-kinase-interacting domain confers a protective effect.
FIGURE 5.
c-Cbl mediates FAK ubiquitination and degradation induced by Cat.G. A, neonatal myocytes derived from WT or c-Cbl KO mice were treated with Cat.G for 2 h and were assessed for immunoblot analysis. B, NRCMs were infected with WT-Cbl or C381A-Cbl at the indicated pfu/cell and were assessed for c-Cbl immunoblot. C and D, NRCMs infected with adenoviruses expressing Lac-Z (10 pfu/cell), C381A-Cbl (5 pfu/cell) or WT-Cbl (10 pfu/cell) were treated with Cat.G for 2 h and assessed for immunoprecipitation assay or immunoblot analysis. Left: representative immunoblots with GAPDH taken as loading control. Right: quantification of experiments expressed as mean ± S.E. from three separate cultures. *, p < 0.05 versus control, #, p < 0.05 versus Cat.G-treated cells.
Inhibition of c-Cbl Ubiquitin Ligase Activity Protects Myocytes from Apoptosis Induced by Cat.G
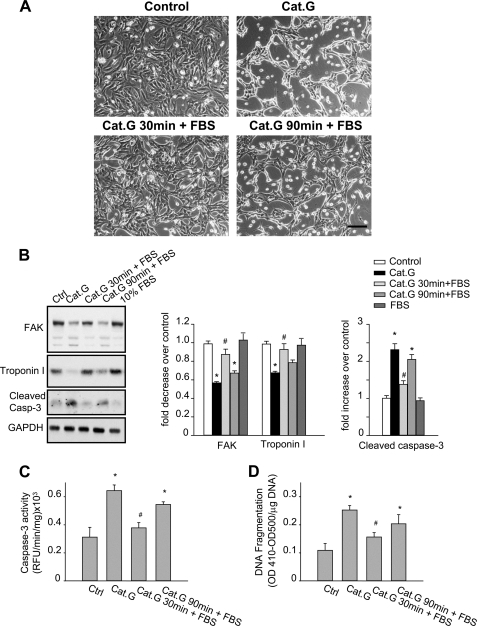
We have previously shown that NRCMs undergo retractile morphological changes and detachment from the ECM after Cat.G treatment, thus leading to myocyte death by anoikis (8, 9). Interestingly, we found that myocytes can be resuscitated from detachment if Cat.G was removed from the media before FAK degradation for up to 1 h, suggesting that FAK inactivation observed in response to Cat.G was reversible and was dispensable for myocyte survival (Fig. 6, A and B). However, the ability to rescue myocytes from Cat.G-induced myocyte morphology changes was abolished once FAK degradation started (1 h after Cat.G treatment). Following the same protocol, we found a close correlation between FAK degradation and troponin I degradation, caspase-3 cleavage/activation and DNA fragmentation in response to Cat.G (Fig. 6, B–D). These data demonstrate that prolonged FAK tyrosine dephosphorylation and inactivation triggers its degradation, thus leading to myocyte apoptosis.
FIGURE 6.
Cat.G-induced myocyte anoikis is temporally associated with FA protein degradation. A–D, NRCMs were untreated, treated with Cat.G for 4 h, treated with Cat.G for 30 min followed by a washout, treated with Cat.G for 90 min followed by a washout, or treated with 10% FBS. A, phase contrast photomicrographs (Bar, 100 μm). B, representative immunoblot of FAK, troponin I and cleaved caspase-3. GAPDH was used as a loading control. Right: quantification of experiments expressed as mean ± S.E. from three separate cultures. *, p < 0.05 versus control, #, p < 0.05 versus Cat.G-treated cells. C and D, NRCMs were treated as indicated and assayed for caspase-3 activity using specific fluorogenic substrate or DNA fragmentation assay using anti-histone antibody ELISA. Results are expressed as relative fluorescence unit (RFU)/min/mg of proteins (C) or as (A410-A500)/mg DNA (D) for triplicate determinations from a single experiment (mean ± S.E.). *, p < 0.05 versus untreated control, #, p < 0.05 versus Cat.G-treated cells.
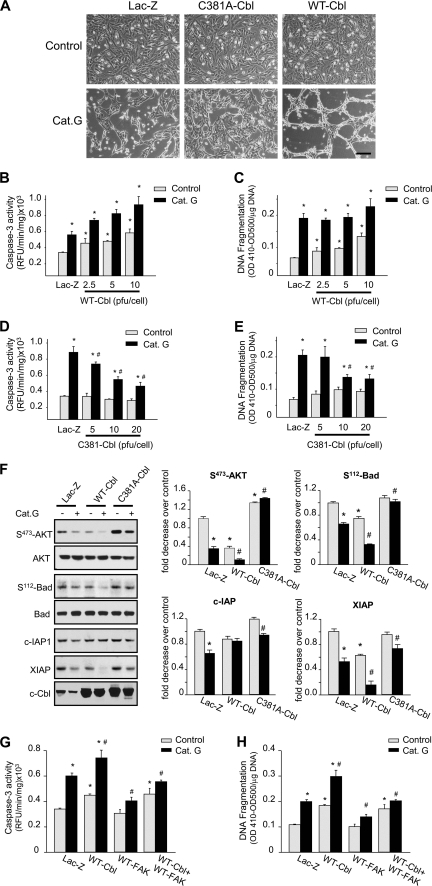
To determine whether c-Cbl activation is involved in Cat.G-induced myocyte apoptosis by anoikis, myocytes infected with recombinant adenovirus expressing WT-Cbl, C381A-Cbl, or Lac-Z were evaluated for apoptotic signaling. As shown in Fig. 7A, overexpression of C381A-Cbl mutant significantly delayed, but did not completely block, myocyte retractile morphological changes and cell detachment induced by Cat.G for 4 h. In contrast, overexpression of WT-Cbl increased more myocyte detachment induced by Cat.G. Along with cell detachment, myocytes undergo apoptosis when treated with Cat.G for 8 h as evidenced by an increase in DNA fragmentation and caspase-3 activity. WT-Cbl overexpression dose dependently promoted basal cardiomyocyte apoptosis compared with Lac-Z-infected controls and additional treatment with Cat.G further enhanced myocyte apoptosis (Fig. 7, B and C). In contrast, C381A-Cbl mutant overexpression dose dependently reduced caspase-3 activation and DNA fragmentation induced by Cat.G (Fig. 7, D and E). Concomitant with the effect of C381A-Cbl on reducing myocyte apoptosis induced by Cat.G, overexpression of this c-Cbl mutant prevented the decrease in AKT and Bad phosphorylation and c-IAP and XIAP expression induced by Cat.G (Fig. 7F). In contrast, WT-Cbl overexpression decreased basal XIAP expression and AKT and Bad phosphorylation as compared with the Lac-Z-infected myocytes and treatment with Cat.G further enhanced this decrease.
FIGURE 7.
c-Cbl mediates Cat.G-induced myocyte anoikis. A, phase contrast photomicrographs of NRCMs infected with Lac-Z (10 pfu/cell), C381A-Cbl (5 pfu/cell), or WT-Cbl adenoviruses (10 pfu/cell) for 48 h and untreated or treated with Cat.G for 4 h. (Bar, 100 μm). B–E, NRCMs were infected with Lac-Z, C381A-Cbl or WT-Cbl adenoviruses at the indicated titration and processed for caspase-3 activity (B and D) or DNA fragmentation assay (C and E). Results are expressed as relative fluorescence unit (RFU)/min/mg of proteins (B and D) or as (A410-A500)/mg DNA (C and E) for triplicate determinations from a single experiment (mean ± S.E.). *, p < 0.05 versus Lac-Z control; #, p < 0.05 versus Cat.G-treated Lac-Z-infected myocytes. F, left: representative immunoblots showing accumulation of phospho-AKT(S473), phospho-Bad(S112), AKT, Bad, XIAP, c-IAP-1, and c-Cbl. Right: quantification of experiments expressed as mean ± S.E. from three separate cultures. G and H, NRCMs were infected with Lac-Z (10 pfu/cell), WT-FAK (10 pfu/cell), WT-Cbl (10 pfu/cell), or WT-FAK + WT-Cbl in presence or absence of Cat.G and were assessed for caspase-3 activity (G) or DNA fragmentation assay (H). *, p < 0.05 versus Lac-Z control; #, p < 0.05 versus Cat.G-treated Lac-Z-infected myocytes.
To delineate whether Cat.G-induced c-Cbl activation mediates myocyte apoptosis through FA protein down-regulation, NRCMs were infected with WT-FAK, WT-Cbl, or Lac-Z and assessed for apoptosis assays. FAK overexpression markedly attenuated caspase-3 activity and DNA fragmentation induced by Cat.G both in Lac-Z and WT-infected cells (Fig. 7, G and H). However, FAK overexpression had no detectable effect on basal caspase-3 activity and DNA fragmentation induced in WT-Cbl infected controls. These data together show that while FAK degradation is required for myocyte apoptosis induced by Cat.G, it is not sufficient to mediate c-Cbl-induced myocyte apoptosis.
DISCUSSION
In this study, we identified a novel role for the E3 ubiquitin ligase and adaptor molecule c-Cbl in mediating myocyte apoptosis induced by neutrophil-derived serine protease Cat.G. c-Cbl phosphorylation was induced in response to Cat.G along with its interaction with FA proteins. Furthermore, deletion of c-Cbl or inhibition of its ligase activity provided endogenous protection against Cat.G-induced proteasomal degradation of FA and myofibrillar proteins. These data highlight c-Cbl as one of the few identified signaling molecules that is specifically involved in FA and myofibrillar protein turnover in myocytes.
The dynamic formation and disassembly of myofibrillar proteins and their organization in the sarcomere are critically important for normal myocyte growth and function. Because myofibrillar proteins are much more stable when associated with each other in the actomyosin complex or intact myofibrils, studies have shown that calpains (21) or caspase-3 (22) activity is required for the dissociation of sarcomeric proteins from the assembled myofibril before their degradation by the UPS (23). However, our study presents new evidence about dynamic turnover of FA proteins, which plays an important role in costamere formation and myofibril organization (24), and may constitute an important mechanism that regulates sarcomere homeostasis. These findings raise the possibility that FA protein ubiquitination and subsequent degradation by the UPS in response to neutrophil derived proteases could trigger disassembly of intact myofibrils therefore allowing their degradation. Also interesting were the findings that these components of the FA complex were degraded before the earliest detection of caspase-3 activation and independently of calpain or lysosome activation, ruling out any involvement of caspase-3, calpains, or lysosomes in early FA and myofibril protein degradation induced by Cat.G (Fig. 1C). Previous studies showed the role of preserving intact FA signaling in myofibril organization and myocyte protection in response to several factors including Cat.G (Fig. 7 and Ref. 9). Moreover, specific deletion of FA proteins in cardiomyocytes leads to cardiomyopathy (25, 26) and/or exacerbate myocyte loss and cardiac contractile dysfunction induced after ischemia reperfusion injury (27). These findings in mice were also observed in human failing hearts where FA protein degradation was observed (28). These data together suggest that coordinated control of FA protein turnover regulates myofibril stability and myocyte survival in response to injury.
Because FA protein ubiquitination controls their proteasomal degradation, it is potentially an important mechanism for the regulation of their functional state and for the activation of their downstream signaling pathways. In this study, we showed that Cat.G treatment induced an increase in FAK ubiquitination that occurred concomitantly with its degradation. To investigate which E3 ubiquitin ligases are likely to mediate Cat.G-induced FAK ubiquitination, we examined c-Cbl activation, a ubiquitin ligase and multifunctional adaptor protein that is known to associate with several regulators of the cytoskeleton (15, 29). We found that treatment with Cat.G increased ubiquitin ligase activity of c-Cbl associated with an increase in its tyrosine phosphorylation and its interaction with FA proteins, FAK and paxillin. This interaction seems to be localized at small globule-shaped structures in the cytoplasm and in the perinuclear region, suggesting that it may serve as nucleation sites for the degradation of FA and cytoskeletal proteins. Interestingly, c-Cbl deletion or expression of ligase-deficient mutants of Cbl (C381A-Cbl) reduced FAK/paxillin ubiquitination and attenuated both FA and myofibrillar protein degradation while overexpression of WT-Cbl promoted myocyte apoptosis and enhanced myocyte responses to Cat.G. This implies that FAK/paxillin is a downstream effector of c-Cbl in response to Cat.G that leads to myofibrillar proteins degradation and myocyte apoptosis. Consistent with these findings, FAK expression rescues myocyte apoptosis induced by Cat.G treatment, but not, by WT-Cbl expression, suggesting that myocyte apoptosis induced by c-Cbl overexpression involved c-Cbl downstream signaling molecules other than FAK. In this regard, activation of c-Cbl has been shown to target several activated receptor and non-receptor tyrosine kinases and mediate their down-regulation, thus providing a means by which signaling processes can be negatively regulated (15, 29). Consequently, inactivation of ubiquitin ligase activity of c-Cbl may lead to enhanced and prolonged signaling, a function, which can explain the increased activation of AKT in myocytes expressing ligase-deficient mutant of c-Cbl (Fig. 7). Indeed, deletion of the RING finger domains of c-Cbl has been shown to induce its tyrosine phosphorylation and to activate its tumorigenic potential (15, 29). Interestingly, we found that mutation of c-Cbl at the PI3 kinase interacting domain enhanced FA and myofibril degradation and myocyte apoptosis compared with WT-Cbl. These data together show the dual function of c-Cbl domains in regulating myocyte apoptosis.
The role of ubiquitination in FA protein turnover and cell growth is not well understood. Previous studies showed FAK ubiquitination and proteasomal degradation in response to chemokines or growth factor stimulation (30). However, in these studies FAK ubiquitination has been shown to be dependent on FAK tyrosine phosphorylation and may play a role in cell adhesion/migration. Our study herein showed that FAK ubiquitination and degradation induced by Cat.G was tightly associated with FAK tyrosine dephosphorylation and was observed during cell detachment and apoptosis by anoikis. Whether tyrosine phosphorylation on FAK is necessary to its ubiquitination and degradation remains to be determined. However, previous studies from our group showed the role of FAK Tyr397 residue in mediating Cat.G-induced myofibrillar disorganization and myocyte apoptosis induced by Cat.G, suggesting a role of this phosphorylation site in FAK protein turnover (9). Interestingly, we found that both c-Cbl activation and FAK ubiquitination and degradation are mediated through EGFR transactivation. Inhibition of EGFR tyrosine kinase activity was efficient in attenuating c-Cbl phosphorylation and FA and myofibril degradation induced by Cat.G while overexpression of WT-EGFR enhanced c-Cbl phosphorylation and consequently exacerbated FAK and troponin I degradation induced by Cat.G. These data extend previous studies showing the role of MMP-dependent EGFR transactivation in Cat.G-induced FAK tyrosine dephosphorylation in NRCMs (9) and provide a possible explanation to the paradoxical effects of EGFR activation on cell detachment and FA signaling down-regulation in cells expressing high levels of EGFR (31, 32). It is noteworthy that EGF treatment of NRCMs also induced c-Cbl activation and FAK tyrosine dephosphorylation. However, this activation occurred early, was transient and was not associated with myocyte death. We speculate that sustained activation of c-Cbl in response to Cat.G leads to persistent tyrosine dephosphorylation of FAK, thereby inducing FAK ubiquitination and its translocation to the proteasome for degradation.
The role of muscle-specific ubiquitin protein ligases as molecules that affect the severity of cardiac diseases is complex and often ambiguous. Activation of E3 ligases CHIP and MDM2 protected myocardium from IR injury (33, 34), while activation of Atrogin1 and MuRF1 attenuated cardiac hypertrophy by interacting with calcineurin and PKCϵ, respectively (35, 36). The current study adds c-Cbl to the list of E3 ubiquitin ligases that play a role in myocyte protection in response to neutrophil-derived serine proteases. Because selective down-regulation of FA proteins appears to be critical in stabilizing the myofibrils, the reduced loss of FA proteins in c-Cbl-deficient myocyte might lead to reduced myocyte loss that may occur during certain cardiac pathologies that involve neutrophils infiltration and activation. The recent study linking c-Cbl gene mutation to Noonan syndrome in humans, a genetic disease associated with several developmental disorders including congenital heart defects and hypertrophic cardiomyopathy (17), together with our findings that c-Cbl expression and activation is involved in myocyte death strongly suggest that c-Cbl may constitute novel targets for therapy offering cardioprotection.
In summary, this study points toward a previously unappreciated role of c-Cbl in cardiomyocyte death. Our data support a model in which neutrophil derived serine proteases promote c-Cbl interaction with FA proteins, resulting in enhanced c-Cbl-mediated FA protein ubiquitination and degradation, myofibril degradation and subsequent down-regulation of myocyte survival signaling.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants HL360338 and HL 360343.

This article contains supplemental Figs. S1–S4.
- Cat.G
- cathepsin G
- ECM
- extracellular matrix
- FA
- focal adhesion
- NRCM
- neonatal rat cardiomyocytes
- PAR
- protease-activated receptor
- UPS
- ubiquitin proteasome system.
REFERENCES
- 1. Nian M., Lee P., Khaper N., Liu P. (2004) Inflammatory cytokines and postmyocardial infarction remodeling. Circ. Res. 94, 1543–1553 [DOI] [PubMed] [Google Scholar]
- 2. Frangogiannis N. G., Smith C. W., Entman M. L. (2002) The inflammatory response in myocardial infarction. Cardiovasc Res. 53, 31–47 [DOI] [PubMed] [Google Scholar]
- 3. Hansen P. R. (1995) Role of neutrophils in myocardial ischemia and reperfusion. Circulation 91, 1872–1885 [DOI] [PubMed] [Google Scholar]
- 4. Molino M., Blanchard N., Belmonte E., Tarver A. P., Abrams C., Hoxie J. A., Cerletti C., Brass L. F. (1995) Proteolysis of the human platelet and endothelial cell thrombin receptor by neutrophil-derived cathepsin G. J. Biol. Chem. 270, 11168–11175 [DOI] [PubMed] [Google Scholar]
- 5. Wiedow O., Meyer-Hoffert U. (2005) Neutrophil serine proteases: potential key regulators of cell signaling during inflammation. J. Inter. Med. 257, 319–328 [DOI] [PubMed] [Google Scholar]
- 6. Sambrano G. R., Huang W., Faruqi T., Mahrus S., Craik C., Coughlin S. R. (2000) Cathepsin G activates protease-activated receptor-4 in human platelets. J. Biol. Chem. 275, 6819–6823 [DOI] [PubMed] [Google Scholar]
- 7. Iacoviello L., Kolpakov V., Salvatore L., Amore C., Pintucci G., de Gaetano G., Donati M. B. (1995) Human endothelial cell damage by neutrophil-derived cathepsin G. Role of cytoskeleton rearrangement and matrix-bound plasminogen activator inhibitor-1. Arterioscler. Thromb. Vasc. Biol. 15, 2037–2046 [DOI] [PubMed] [Google Scholar]
- 8. Sabri A., Alcott S. G., Elouardighi H., Pak E., Derian C., Andrade-Gordon P., Kinnally K., Steinberg S. F. (2003) Neutrophil cathepsin G promotes detachment-induced cardiomyocyte apoptosis via a protease-activated receptor-independent mechanism. J. Biol. Chem. 278, 23944–23954 [DOI] [PubMed] [Google Scholar]
- 9. Rafiq K., Kolpakov M. A., Abdelfettah M., Streblow D. N., Hassid A., Dell'Italia L. J., Sabri A. (2006) Role of protein-tyrosine phosphatase SHP2 in focal adhesion kinase down-regulation during neutrophil cathepsin G-induced cardiomyocytes anoikis. J. Biol. Chem. 281, 19781–19792 [DOI] [PubMed] [Google Scholar]
- 10. Wang X., Robbins J. (2006) Heart failure and protein quality control. Circ. Res. 99, 1315–1328 [DOI] [PubMed] [Google Scholar]
- 11. Ciechanover A., Orian A., Schwartz A. L. (2000) The ubiquitin-mediated proteolytic pathway: mode of action and clinical implications. J. Cell Biochem. Suppl. 34, 40–51 [DOI] [PubMed] [Google Scholar]
- 12. Friedman J., Xue D. (2004) To live or die by the sword: the regulation of apoptosis by the proteasome. Dev. Cell. 6, 460–461 [DOI] [PubMed] [Google Scholar]
- 13. Coux O., Tanaka K., Goldberg A. L. (1996) Structure and functions of the 20 S and 26 S proteasomes. Ann. Rev. Biochem. 65, 801–847 [DOI] [PubMed] [Google Scholar]
- 14. Meng W., Sawasdikosol S., Burakoff S. J., Eck M. J. (1999) Structure of the N-terminal domain of Cbl complexed to its binding site on ZAP-70 kinase. Nature 398, 84–90 [DOI] [PubMed] [Google Scholar]
- 15. Schmidt M. H., Dikic I. (2005) The Cbl interactome and its functions. Nat. Rev. Mol. Cell Biol. 6, 907–918 [DOI] [PubMed] [Google Scholar]
- 16. Balasubramanian S., Mani S., Shiraishi H., Johnston R. K., Yamane K., Willey C. D., Cooper G., 4th, Tuxworth W. J., Kuppuswamy D. (2006) Enhanced ubiquitination of cytoskeletal proteins in pressure overloaded myocardium is accompanied by changes in specific E3 ligases. J. Mol. Cell. Cardiol. 41, 669–679 [DOI] [PubMed] [Google Scholar]
- 17. Niemeyer C. M., Kang M. W., Shin D. H., Furlan I., Erlacher M., Bunin N. J., Bunda S., Finklestein J. Z., Sakamoto K. M., Gorr T. A., Mehta P., Schmid I., Kropshofer G., Corbacioglu S., Lang P. J., Klein C., Schlegel P. G., Heinzmann A., Schneider M., Starý J., van den Heuvel-Eibrink M. M., Hasle H., Locatelli F., Sakai D., Archambeault S., Chen L., Russell R. C., Sybingco S. S., Ohh M., Braun B. S., Flotho C., Loh M. L. (2010) Germline CBL mutations cause developmental abnormalities and predispose to juvenile myelomonocytic leukemia. Nat. Genet. 42, 794–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chau V., Tobias J. W., Bachmair A., Marriott D., Ecker D. J., Gonda D. K., Varshavsky A. (1989) A multiubiquitin chain is confined to specific lysine in a targeted short-lived protein. Science 243, 1576–1583 [DOI] [PubMed] [Google Scholar]
- 19. Sanjay A., Houghton A., Neff L., DiDomenico E., Bardelay C., Antoine E., Levy J., Gailit J., Bowtell D., Horne W. C., Baron R. (2001) Cbl associates with Pyk2 and Src to regulate Src kinase activity, α(v)β(3) integrin-mediated signaling, cell adhesion, and osteoclast motility. J. Cell Biol. 152, 181–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Joazeiro C. A., Wing S. S., Huang H., Leverson J. D., Hunter T., Liu Y. C. (1999) The tyrosine kinase negative regulator c-Cbl as a RING-type, E2-dependent ubiquitin-protein ligase. Science 286, 309–312 [DOI] [PubMed] [Google Scholar]
- 21. Tidball J. G., Spencer M. J. (2002) Expression of a calpastatin transgene slows muscle wasting and obviates changes in myosin isoform expression during murine muscle disuse. J. Physiol. 545, 819–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Du J., Wang X., Miereles C., Bailey J. L., Debigare R., Zheng B., Price S. R., Mitch W. E. (2004) Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J. Clin. Invest. 113, 115–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Solomon V., Goldberg A. (1996) Importance of the ATP-ubiquitin-proteasome pathway in the degradation of soluble and myofibrillar proteins in rabbit muscle extracts. J. Biol. Chem. 271, 26690–26697 [DOI] [PubMed] [Google Scholar]
- 24. Samarel A. M. (2005) Costameres, focal adhesions, and cardiomyocyte mechanotransduction. Am. J. Physiol. Heart. Circ. Physiol. 289, H2291–2301 [DOI] [PubMed] [Google Scholar]
- 25. Shai S. Y., Harpf A. E., Babbitt C. J., Jordan M. C., Fishbein M. C., Chen J., Omura M., Leil T. A., Becker K. D., Jiang M., Smith D. J., Cherry S. R., Loftus J. C., Ross R. S. (2002) Cardiac myocyte-specific excision of the β1 integrin gene results in myocardial fibrosis and cardiac failure. Circ. Res. 90, 458–464 [DOI] [PubMed] [Google Scholar]
- 26. White D. E., Coutu P., Shi Y.-F., Tardif J.-C., Nattel S., St. Arnaud R., Dedhar S., Muller W. J. (2006) Targeted ablation of ILK from the murine heart results in dilated cardiomyopathy and spontaneous heart failure. Genes Dev. 20, 2355–2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hakim Z. S., DiMichele L. A., Rojas M., Meredith D., Mack C. P., Taylor J. M. (2009) FAK regulates cardiomyocyte survival following ischemia/reperfusion. J. Mol. Cell Cardiol. 46, 241–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pfister R., Acksteiner C., Baumgarth J., Burst V., Geissler H. J., Margulies K. B., Houser S., Bloch W., Flesch M. (2007) Loss of β1D-integrin function in human ischemic cardiomyopathy. Basic. Res. Cardiol. 102, 257–264 [DOI] [PubMed] [Google Scholar]
- 29. Thien C. B., Langdon W. Y. (2001) Cbl: many adaptations to regulate protein tyrosine kinases. Nat. Rev. Mol. Cell Biol. 2, 294–307 [DOI] [PubMed] [Google Scholar]
- 30. Le Y., Zhu B. M., Harley B., Park S. Y., Kobayashi T., Manis J. P., Luo H. R., Yoshimura A., Hennighausen L., Silberstein L. E. (2007) SOCS3 protein developmentally regulates the chemokine receptor CXCR4-FAK signaling pathway during B lymphopoiesis. Immunity 27, 811–823 [DOI] [PubMed] [Google Scholar]
- 31. Lu Z., Jiang G., Blume-Jensen P., Hunter T. (2001) Epidermal growth factor-induced tumor cell invasion and metastasis initiated by dephosphorylation and downregulation of focal adhesion kinase. Mol. Cell Biol. 21, 4016–4031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vadlamudi R. K., Adam L., Nguyen D., Santos M., Kumar R. (2002) Differential regulation of components of the focal adhesion complex by heregulin: role of phosphatase SHP-2. J. Cell. Physiol. 190, 189–199 [DOI] [PubMed] [Google Scholar]
- 33. Zhang C., Xu Z., He X. R., Michael L. H., Patterson C. (2005) CHIP, a cochaperone/ubiquitin ligase that regulates protein quality control, is required for maximal cardioprotection after myocardial infarction in mice. Am. J. Physiol. Heart. Circ. Physiol. 288, H2836–H2842 [DOI] [PubMed] [Google Scholar]
- 34. Toth A., Nickson P., Qin L. L., Erhardt P. (2006) Differential regulation of cardiomyocyte survival and hypertrophy by MDM2, an E3 ubiquitin ligase. J. Biol. Chem. 281, 3679–3689 [DOI] [PubMed] [Google Scholar]
- 35. Arya R., Kedar V., Hwang J. R., McDonough H., Li H. H., Taylor J., Patterson C. (2004) Muscle ring finger protein-1 inhibits PKC{ϵ} activation and prevents cardiomyocyte hypertrophy. J. Cell Biol. 167, 1147–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li H. H., Kedar V., Zhang C., McDonough H., Arya R., Wang D. Z., Patterson C. (2004) Atrogin-1/muscle atrophy F-box inhibits calcineurin-dependent cardiac hypertrophy by participating in an SCF ubiquitin ligase complex. J. Clin. Invest. 114, 1058–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.