Background: SLK promotes apoptosis and may control cell survival during renal injury or repair.
Results: Mutations in serine and threonine residues in the activation segment of SLK reduced kinase activity.
Conclusion: Phosphorylation of SLK plays a key role in activation and signaling.
Significance: Understanding the regulation of SLK activity is essential for developing novel therapeutic approaches to renal injury.
Keywords: Apoptosis, Kidney, Mutagenesis, Protein Kinases, Signal Transduction
Abstract
Expression and activation of the Ste20-like kinase, SLK, is increased during kidney development and recovery from ischemic acute kidney injury. SLK promotes apoptosis, and it may regulate cell survival during injury or repair. This study addresses the role of phosphorylation in the regulation of kinase activity. We mutated serine and threonine residues in the putative activation segment of the SLK catalytic domain and expressed wild type (WT) and mutant proteins in COS-1 or glomerular epithelial cells. Compared with SLK WT, the T183A, S189A, and T183A/S189A mutants showed reduced in vitro kinase activity. SLK WT, but not mutants, increased activation-specific phosphorylation of c-Jun N-terminal kinase (JNK) and p38 kinase. Similarly, SLK WT stimulated activator protein-1 reporter activity, but activation of activator protein-1 by the three SLK mutants was ineffective. To test if homodimerization of SLK affects phosphorylation, the cDNA encoding SLK amino acids 1–373 (which include the catalytic domain) was fused with a cDNA for a modified FK506-binding protein, Fv (Fv-SLK 1–373). After transfection, the addition of AP20187 (an FK506 analog) induced regulated dimerization of Fv-SLK 1–373. AP20187-stimulated dimerization enhanced the kinase activity of Fv-SLK 1–373 WT. In contrast, kinase activity of Fv-SLK 1–373 T183A/S189A was weak and was not enhanced after dimerization. Finally, apoptosis was increased after expression of Fv-SLK 1–373 WT but not T183A/S189A. Thus, phosphorylation of Thr-183 and Ser-189 plays a key role in the activation and signaling of SLK and could represent a target for novel therapeutic approaches to renal injury.
Introduction
Protein kinases form a diverse and important gene family in eukaryotes (1). They are involved in the regulation of many cellular processes, and their dysregulation can result in diseases (2, 3). The catalytic activity of protein kinases is tightly regulated, often via the activation segment, which may be phosphorylated by other kinases to facilitate catalytic activity (4). Typically, a kinase will phosphorylate the activation segment of a downstream kinase, allowing the downstream kinase to further propagate a signal (4). When unphosphorylated, the activation segment is largely unstructured. Phosphorylation of the activation segment at the primary phosphorylation site stabilizes the kinase in a conformation suitable for substrate binding. Kinases may also have secondary phosphorylation sites in their activation segment, which may enhance activity. Secondary phosphorylation may also aid in the recruitment of substrate by changing the conformation of a kinase to facilitate substrate binding. Once a kinase is stabilized and activated, the catalytic domain identifies a specific substrate and phosphorylates the substrate via its active site. This process can be facilitated by increasing the local concentration of a kinase relative to its substrate and may include dimerization of the kinase (4).
A subset of kinases can also autoactivate via self-phosphorylation of their activation segment by their catalytic domains (5). This form of activation requires that the activation segment contain a consensus sequence that can be recognized by the catalytic domain. In some kinases activation segment dimerization is a mechanism for kinase autophosphorylation of non-consensus sites (5). This allows the catalytic domain of one dimerization partner to phosphorylate the activation domain of the other partner even if the activation domain does not correspond to the substrate consensus sequence of the catalytic domain. Activation segment domain exchange is a mechanism proposed for the in trans activation of kinases that do not require a consensus sequence to be present in the activation segment of their binding partner. In this model dimerization is induced in an adjacent homodimerization domain. As such, the kinases are ideally positioned, so that a transient activation of one catalytic site leads to the phosphorylation of the activation segment of the binding partner. This in turn leads to the activation of the binding partner and the phosphorylation of the original kinase in its activation segment; the final result is the activation of two kinases, which can then phosphorylate downstream targets with or without prior dissociation (5).
SLK is a serine/threonine protein kinase related to yeast Ste20 (6, 7) and is classified as a group V germinal center kinase (6, 8). SLK is expressed ubiquitously in various tissues (9). In the kidney SLK is found in tubular and glomerular epithelial cells (GECs; podocytes)2 (10). Subcellularly, SLK is localized in the cytosol, nucleus, microtubules, and centrosomes (11, 12). The regulation and functions of SLK have received considerable attention in recent years. Downstream signaling by SLK involves mitogen-activated protein kinase pathways. We demonstrated that in kidney epithelial cells, overexpression of SLK activated the p38 mitogen-activated protein kinase pathway. SLK can also activate the c-Jun N-terminal kinase (JNK) and increase the transactivation of p53 (13, 14). Exposure of cultured kidney epithelial cells to chemical anoxia and re-exposure to glucose (which recapitulates ischemia-reperfusion in vivo) stimulated SLK activity (10). Overexpression of SLK in cultured GECs (which we have used to model the increased expression observed in the in vivo circumstances) resulted in a modest proapoptotic effect, and in the setting of ischemia reperfusion, SLK overexpression markedly exacerbated cell death (10, 13, 14). These effects were mediated via p38, p53, and caspases. Overexpression of SLK also induced apoptosis in other cell lines (15, 16). In addition, SLK may play a role in cell cycle progression (17). Together, the studies suggest that SLK may regulate cell survival during development, injury, or repair. In fibroblasts, SLK regulates cytoskeletal remodeling. SLK was found to be associated with the microtubular network, and activation of SLK via focal adhesion kinase and extracellular signal-regulated kinase pathways destabilized the actin network. This process affected focal adhesion turnover, cell adhesion, spreading, and motility. SLK can also increase stress fiber disassembly (11, 12), whereas SLK depletion disrupts radial microtubule arrays in a variety of cell types (18).
The SLK protein consists of ∼1204 or 1235 amino acids and contains an N-terminal catalytic domain (amino acids 34–292) and an extensive C-terminal “regulatory” domain that contains coiled-coils (15, 19, 20). The regulation of SLK activity is complex and multifactorial. Activity may be regulated by changes in expression (10). SLK mRNA is unstable and contains adenine- and uridine-rich elements in the 3′-untranslated region that appear to regulate mRNA stability (21). The C-terminal region containing coiled-coil domains was recently shown to facilitate dimerization of the protein (22). Activation may also be regulated by phosphorylation, and phosphorylation or dephosphorylation of SLK were associated with changes in SLK activity in some but not all earlier studies (14–16, 23–25). The activation loop of SLK contains at least two potential phosphorylation sites, Thr-183 and Ser-189, and based on the crystal structure of the SLK catalytic domain, it was proposed that activation may involve homodimerization of SLK catalytic domains and phosphorylation of Thr-183 and Ser-189 (25). Phosphorylation would occur as one SLK molecule enters into close contact with the catalytic domain of the other and vice versa. It should, however, be noted that the kinase domain of SLK recognizes an amino acid sequence that is not in the activation segment of SLK (25). Nevertheless, the SLK catalytic domain is able to form dimers in vitro and to phosphorylate its partner activation segment at Thr-183 and Ser-189. Phosphorylation may facilitate the formation of a hydrogen bond network, and a bond between Lys-63 and Glu-79 might be sufficient to lock an SLK monomer in an active conformation, suitable for substrate binding (25). It has also been noted that Thr-193 in SLK is highly conserved in the evolutionarily related kinases, DAPK3 (death-associated protein kinase-3) and CHK2 (checkpoint kinase-2), where it is a known phosphorylation site, possibly playing a regulatory role (25).
We selected amino acids Lys-63, Glu-79, Thr-183, Ser-189, and Thr-193 in SLK for further study. These amino acids were mutated to test their functional role in the activation of SLK. We demonstrate that mutations in amino acids in the activation segment (Thr-183, Ser-189, and Thr-193) reduce activity; moreover, mutations in amino acids that may form a bond in the catalytic domain, which sustains catalytic activity (Lys-63, Glu-E79), also result in a kinase with reduced activity.
EXPERIMENTAL PROCEDURES
Plasmid Construction
Missense mutations in full-length, wild type, hemagglutinin antigen epitope-tagged (HA)-SLK (SLK WT) (14) were generated using polymerase chain reaction (PCR)-based mutagenesis. All PCR primers are listed in Table 1. For the T183A mutation, the PCR reactions included full-length SLK forward and T183A reverse primers (T183A Reaction 1) and T183A forward/full-length SLK reverse primers (T183A Reaction 2). Full-length HA-SLK WT in pcDNA 3.1 (Invitrogen) was used as the template. Products of T183A reactions 1 and 2 were combined in a third PCR reaction using primers full-length SLK forward and full-length SLK reverse (Reaction 3) to generate a 1674-base pair SLK T183A cDNA. The PCR reaction included Pwo polymerase, 10 mm dNTP, PCR primers, and template DNA. Incubation was for 4 min at 94 °C; 1 min at 94 °C, 1 min at 60 °C, 2 min at 72 °C (35 cycles); final elongation for 10 min at 72 °C. By analogy, the full-length HA-SLK S189A mutant was generated using the primers full-length SLK forward/S189A reverse (S189A Reaction 1) and S189A forward/full-length SLK reverse (S189A Reaction 2). Products of S189A Reaction 1 and 2 then underwent PCR using Reaction 3 primers to generate the complete S189A cDNA. To generate the full-length HA-SLK T183A/S189A mutant, a cDNA containing the S189A mutation was employed as template for PCR reactions using the T183A Reaction 1 and 2 primers. These products were then incubated with the Reaction 3 primers to form the complete T183A/S189A cDNA.
TABLE 1.
PCR primers
| Full-length SLK forward, CGACGGAGCCTTTGGGAAA |
| Full-length SLK reverse, TCCTTAGTACCACCAGCCTCAGGAC |
| T183A forward, AAACACGAGGGCAATTCAAAGAAGAGAT |
| T183A reverse, ATCTCTTCTTTGAATTGCCCTCGTGTTT |
| S189A forward, AGATGCCTTTATTGGTACACCATATTGGAT |
| S189A reverse, GTGTACCAATAAAGGCATCTCTTCTTTGAATT |
| T183E forward, AAACACGAGGAAAATTCAAAGAAGAGAT |
| T183E reverse, ATCTCTTCTTTGAATTTCCCTCGTGTTT |
| T183D forward, AAACACGAGGATAATTCAAAGAAGAGAT |
| T183D reverse, ATCTCTTCTTTGAATATCCCTCGTGTTT |
| S189E forward, AGATGAATTTATTGGTACACCATATTGGAT |
| S189E reverse, GTGTACCAATAAATTCATCTCTTCTTTGAATT |
| S189D forward, AGATGACTTTATTGGTACACCATATTGGAT |
| S189D reverse, GTGTACCAATAAAGTCATCTCTTCTTTGAATT |
| T193A forward, CTTTATTGGTGCACCATATTGGATGG |
| T193A reverse, AATATGGTGCACCAATAAAGGAATCTC |
| K63R forward, CTGCTGCAAGAGTGATTGACAC |
| K63R reverse, CAATCACTCTTGCAGCAGCTAAA |
| E79A forward, AGATTACATGGTAGCGATTGACATATTAGC |
| E79A reverse, GCTAATATGTCAATCGCTACCATGTAATCT |
| Primer 1, CCGGAATTCGCCGCCGCCATGTCCTTCTTCAATTTCCGTAAGA |
| -XbaI Primer 1, TAGAAGGCATCCAGAAGCTTGACTATAT |
| -XbaI Primer 2, TAGTCAAGCTTCTGGATGCCTTCTAT |
| Primer 2, CTAGCTAGTCTAGAGAGTTTATCTTCAGAGTTACTACGTTCTG |
PCR products were blunt end-ligated into the pPCR-Script Amp SK(+) cloning vector (PCR-Script Amp Cloning kit, Stratagene, La Jolla, CA). The vector containing the cDNA insert was digested with SalI and Bsp1407I restriction enzymes, and the SalI/Bsp14071 fragment was ligated into the pcDNA 3.1-HA-SLK, replacing the analogous WT fragment. All PCR products were verified by DNA sequencing.
For studies of homodimerization, the N-terminal domains of SLK WT and SLK mutants (amino acids 1–373) were fused with two modified FK506-binding protein (FKBP) domains (Fv-SLK 1–373) (22, 26–28). A silent mutation that removed an internal XbaI site in the catalytic domain of SLK and the SLK T183A/S189A mutant was created by PCR using Primer 1/-XbaI Primer 1 (-XbaI Reaction 1) and Primer 2/-XbaI Primer 2 (-XbaI Reaction 2). The SLK(-XbaI) and T183A/S189A(-XbaI) SLK cDNA was produced by PCR by combining the products from -XbaI reaction 1 and 2 and using Primer 1 and Primer 2. To create Fv-SLK 1–373 T193A, SLK(-XbaI) was used as template for PCRs with Primer 1 and T193A reverse (T193A Reaction 1) and T193A forward and Primer 2 (T193A Reaction 2) before being combined into the complete catalytic domain with Primer 1 and Primer 2 (T193A Reaction 3). Similarly, SLK mutants T183D, T183E, S189D, S189E, K63R, and E79A were produced using three reactions (Table 1). PCR products were blunt end-ligated into pPCR-Script Amp SK(+). The vector was digested with EcoRI and XbaI, and the WT or mutant cDNAs encoding the SLK kinase domains were subcloned into pC4M-Fv2E upstream of two modified FKBP domains and an HA tag (Ariad Pharmaceuticals, Cambridge, MA) (the myristoylation sequence was deleted from the pC4M-Fv2E vector). All PCR products were verified by DNA sequencing.
For studies of heterodimerization, SLK 1–373 WT and T183A/S189A cDNAs (described above) were subcloned into pC4M-F2E (without the myristoylation sequence) upstream of two unmodified FKBP domains and an HA tag (Ariad Pharmaceuticals). Also, SLK 1–373 WT, K63R and T183A/S189A cDNAs were subcloned into pC4M-RHE, upstream of a single FRB and an HA tag (Ariad Pharmaceuticals). FRB is a 93-amino acid portion of the FKBP-12-rapamycin-associated protein (FRAP or mTOR), which is sufficient for binding the complex of FKBP-rapamycin (29).
Cell Culture and Transfection
COS-1 monkey kidney cells and A431 human epidermoid carcinoma cells (which overexpress the epidermal growth factor receptor) were cultured in DMEM supplemented with 10% fetal bovine serum (Wisent, St. Bruno, QC, Canada). Rat GECs were characterized previously and were cultured in K1 medium (30). Experiments were conducted between passages 30 and 55. For transfection, cells were trypsinized and counted using a hemocytometer. 7.5 × 105 cells were passaged into 100-mm plates and incubated for 24 h. Cells were transiently transfected with 4.5 μl of Lipofectamine 2000 (Invitrogen) and 1.5 μg of plasmid DNA according to the procedure provided by the manufacturer. Transfected cells were rinsed with PBS and lysed with 150 μl of lysis buffer containing 1% Triton X-100, 125 mm NaCl, 10 mm Tris, pH 7.5, 1 mm EGTA, 2 mm Na3VO4, 5 mm Na4P3O7, 25 mm NaF, 20 μm leupeptin, 10 μm pepstatin, 50 μm bestatin, 15 μm E64, 0.8 μm aprotinin, 1 mm 4-(2-aminoethyl)benzenesulfonyl fluoride. Based on transfection of green fluorescent protein, the transfection efficiency in COS-1 cells was >60%, whereas transfection efficiency in GECs was ∼10% (22).
Immunoblotting
After the addition of Laemmli buffer to samples, proteins were separated by SDS-PAGE and were then electrophoretically transferred to a nitrocellulose membrane. Membranes were blocked with 5% BSA and incubated with primary antibody followed by horseradish peroxidase-conjugated secondary antibody. Then membranes were developed with ECL (GE Healthcare). Primary antibodies included mouse anti-HA, mouse anti-phosphotyrosine (PY20) (Santa Cruz Biotechnology, Santa Cruz, CA) and rabbit anti-p38 (Sigma) as well as rabbit anti-phospho-p38 (Thr-180/Tyr-182), rabbit anti-JNK, rabbit anti-phospho-JNK (Thr-183/Tyr-185), and rabbit anti-phosphoprotein kinase A (PKA) substrate, which recognizes the sequence RRXpS/T (all from Cell Signaling Technology, Danvers, MA). Secondary antibodies included sheep anti-mouse IgG and goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA). The ECL signals were quantified by scanning densitometry using NIH ImageJ software (10). Results were normalized for loading controls.
Immune Complex Kinase Assay
Cell lysates were immunoprecipitated with anti-HA antibody overnight at 4 °C or non-immune mouse IgG (control) and were then incubated with protein A-agarose for 1 h at 4 °C. After washing 3 times with lysis buffer and 4 times with kinase buffer (20 mm Hepes, pH 7.2, 20 mm β-glycerophosphate, 10 mm MgCl2, 1 mm dithiothreitol, 0.5 mm Na3VO4), samples were incubated with 0.5 mg/ml bovine brain myelin basic protein (MBP; Sigma) and 20 μm [γ-32P]ATP (2.5 μCi) for 5 min at 30 °C. After the addition of Laemmli buffer, samples were subjected to SDS-PAGE and autoradiography. Bands were quantified by scanning densitometry using NIH ImageJ software (10).
Dual Luciferase Reporter Assays
GECs were plated and co-transfected after 24 h with the cDNA of interest, pRL-TK (renilla luciferase), and activator protein-1 (AP-1) firefly luciferase reporter (31). pRL-TK serves as an internal control that quantifies transfection efficiency, whereas firefly luciferase serves as the principal reporter (13). Cell lysates were assayed using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI). Luciferase activity was measured in a Berthold Lumat LB 9507 luminometer.
Measurement of Apoptosis
Cells were stained with Hoechst H33342 dye (1 μg/ml) for 10 min at 37 °C without fixation. After washing with PBS, cells were stained with propidium iodide (5 μg/ml). Images were acquired using a Zeiss AxioObserver fluorescence microscope with visual output connected to an AxioCam digital camera, and the cells were then counted manually. Cell nuclei, which showed chromosomal condensation but which had an intact plasma membrane impermeable to propidium iodide, were counted separately from propidium iodide-stained cells (10, 13). In this assay, nuclei of apoptotic cells (which show chromatin condensation and/or fragmentation) stain brightly with H33342, but these cells do not stain with propidium iodide because apoptotic cells usually possess intact plasma membranes. However, in cell culture propidium iodide-positive cells are generally “late apoptotic,” as apoptotic cells are not phagocytosed and may proceed to necrosis. All other Hoechst H33342-stained cells were designated as “normal.”
Statistics
Densitometric quantification of immunoblots and autoradiograms was normalized to SLK WT, which was considered 1 arbitrary unit. One-way analysis of variance was used to determine significant differences among groups. Where significant differences were found, individual comparisons were made between groups using the t statistic and adjusting the critical value according to the Bonferroni method. Results are presented as the mean ± S.E.
RESULTS
Activation Segment Mutations Inhibit SLK Kinase Activity
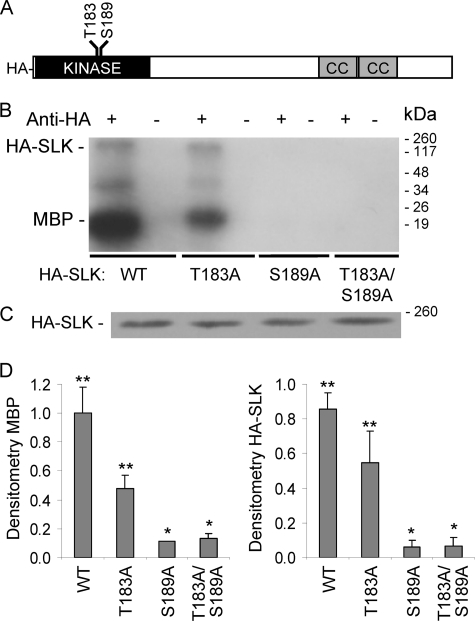
To understand the role of the activation segment of SLK in regulating catalytic activity, we focused on the role of serine and threonine residues that are highly conserved among SLK and other protein kinase catalytic domains. Such serines and threonines can serve as substrates for other kinases, activating the kinase catalytic site upon being phosphorylated. Mutations to alanine permit the silencing of these sites without disturbing the three-dimensional architecture of the protein. In an in vitro model, which employed isolated catalytic domains of SLK, Thr-183 and Ser-189 were identified as phosphorylation sites (25). Mutations, including T183A, S189A, and T183A/S189A, were introduced into the HA-tagged full-length cDNA of SLK via PCR (Fig. 1A). COS-1 cells were transiently transfected with the full-length SLK WT or mutant plasmids, and expression of SLK was confirmed with SDS-PAGE and immunoblotting of cell lysates (Fig. 1C). To determine the effect of the T183A, S189A, and T183A/S189A mutations on the kinase activity of SLK, lysates of cells expressing HA-SLK WT or mutants were immunoprecipitated with anti-HA antibody, and the immune complexes were incubated with [γ-32P]ATP and MBP. Compared with SLK WT, SLK T183A tended to reduce MBP phosphorylation, whereas the S189A mutation as well as the double mutation appeared to be more severe, completely abolishing catalytic activity (Fig. 1B and D). In parallel, the phosphorylation of SLK was also reduced by the mutations (Fig. 1, B and D).
FIGURE 1.
T183A, S189A, and T183A/S189A mutations in the SLK activation domain inhibit catalytic activity and SLK phosphorylation. A, full-length HA-SLK (1204 amino acids) is shown. The kinase domain (amino acids 34–292), position of mutations, and coiled-coil (CC) domain are indicated. B, COS-1 cells were transiently transfected with WT or mutant full-length HA-SLK. After 48 h lysates were immunoprecipitated with anti-HA antibody (+) or nonimmune IgG (control; −). Kinase activity was monitored by the incorporation of 32P into MBP (representative autoradiogram). C, lysates immunoblotted with anti-HA antibody show equal protein expression. D, normalized densitometry of MBP (left) and HA-SLK phosphorylation (right). *, p < 0.0006 versus SLK WT; **, p < 0.0003 versus control immunoprecipitation with nonimmune IgG, n = 5. In both graphs, the densitometry of SLK WT was set to 1.0 arbitrary unit.
Expression of Activation Segment SLK Mutants Affects Activation of JNK, p38, and AP-1
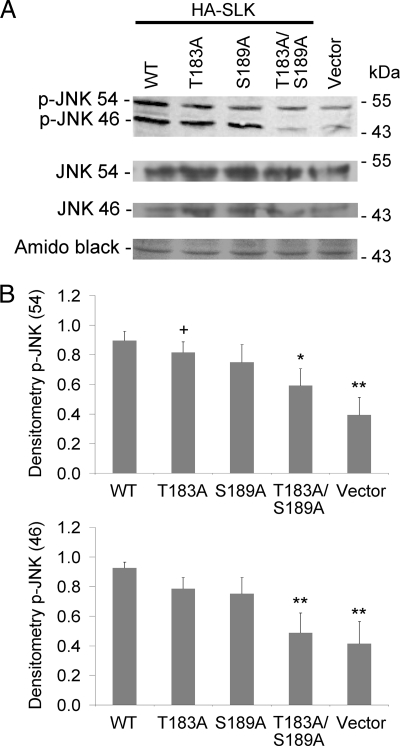
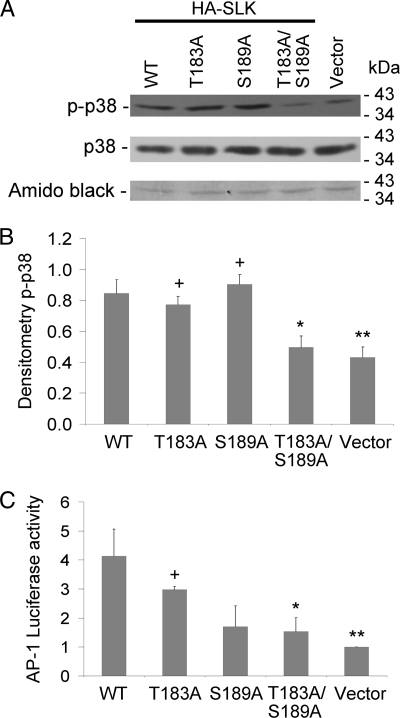
Having demonstrated that mutations in Thr-183 and Ser-189 impaired kinase activity, the next step was to examine the effect of these mutations on downstream signaling by SLK. Overexpression of SLK has previously been shown to activate stress kinase pathways, including apoptosis signal-regulating kinase-1 and p38 (14) as well as JNK (13). These kinase pathways are typically anti-proliferative and proapoptotic. To determine whether the activation segment point mutations affected downstream signaling, SLK WT, T183A, S189A, and T183A/S189A mutants were overexpressed in GECs, a well differentiated kidney epithelial cell line. Phospho-specific antibodies were used to monitor activation-specific changes in JNK (Fig. 2) and p38 (Fig. 3). SLK WT significantly increased phospho-JNK compared with control. In contrast, SLK activation domain mutants T183A, S189A, and in particular T183A/S189A showed a reduced ability to stimulate JNK phosphorylation (Fig. 2, A and B). Similarly, SLK WT significantly increased p38 phosphorylation compared with control, but the T183A/S189A double mutant failed to increase p38 phosphorylation above base line (Fig. 3, A and B). SLK T183A and SLK S189A were able to stimulate p38 phosphorylation. In these experiments the expression of JNK and p38 remained constant (Figs. 2A and 3A, respectively).
FIGURE 2.
Mutations in the SLK activation domain reduce JNK phosphorylation. GECs were transiently transfected with full-length HA-SLK WT, activation domain mutants (T183A, S189A, and T183A/S189A), or control vector. Lysates were immunoblotted with anti-phospho-JNK (p-JNK) or anti-JNK antibodies after 48 h. (A, representative immunoblot; B, normalized densitometry of 54- and 46-kDa p-JNK). JNK protein expression was unaffected by SLK mutations. The Amido Black stain demonstrates protein loading. *, p < 0.05; **, p < 0.005 versus SLK WT; +, < 0.05 versus vector, n = 6.
FIGURE 3.
Mutations in the SLK activation domain reduce p38 phosphorylation and AP-1 luciferase activity. GECs were transiently transfected with full-length HA-SLK WT, activation domain mutants (T183A, S189A, and T183A/S189A), or control vector. Lysates were immunoblotted with anti-phospho-p38 (p-p38) or anti-p38 antibodies after 48 h. (A, representative immunoblot; B, normalized densitometry of p-p38). p38 protein expression was unaffected by SLK mutations. The Amido Black stain demonstrates protein loading. *, p < 0.02; **, p < 0.003 versus SLK WT; +, p < 0.007 versus vector, n = 6. C, normalized AP-1 luciferase reporter assay is shown. GECs were transfected with full-length HA-SLK WT, activation domain mutants, or control vector plus an AP-1-luciferase reporter. Luciferase activity was measured after 48 h. *, p < 0.006; **, p < 0.002 versus SLK WT; +, p < 0.04 versus vector, n = 4.
To further confirm the functional role of Thr-183 and Ser-189, we examined the effect of SLK on the activation of AP-1. AP-1 is a transcription factor that lies downstream of pathways, including JNK and p38, and controls a number of cellular processes, such as differentiation, proliferation, and apoptosis (32). AP-1 activity was monitored using an AP-1-luciferase reporter. SLK WT and to a lesser extent SLK T183A stimulated AP-1 reporter activity, whereas S189A and T183A/S189A were ineffective (Fig. 3C). Together these results confirm that SLK signals via JNK and p38 and demonstrate a critical role for activation segment phosphorylation.
Homodimerization of SLK Increases Kinase Activity of SLK WT but Not SLK Mutants
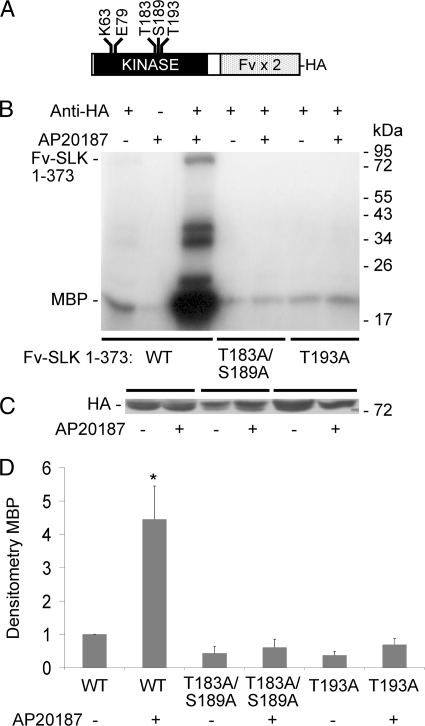
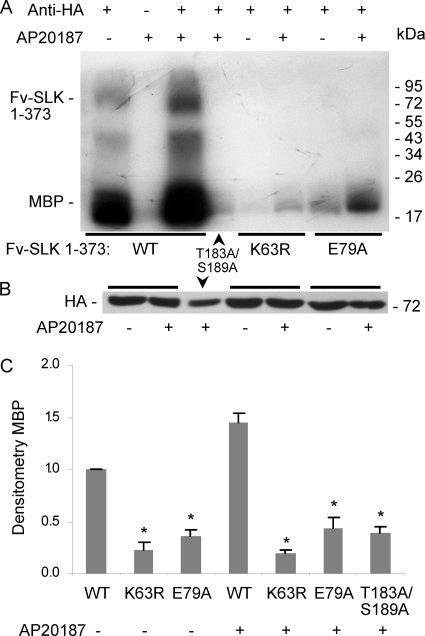
The Thr-183 and Ser-189 sites of SLK are important for catalytic activity, but it has also been suggested that the two phosphorylation sites play a role in SLK autoactivation in trans via activation segment domain exchange (25). Homodimerization of SLK could allow for the catalytic site of one SLK molecule to phosphorylate the activation segment of another SLK molecule, which in turn could phosphorylate the first SLK molecule, should it not be fully active already. To determine the effect of homodimerization on SLK activity, we produced cDNAs consisting of the 373 N-terminal amino acids of SLK (containing the catalytic domain) fused at the C terminus with two modified FKBP domains (Fv-SLK 1–373; Fig. 4A) (22). The addition of the FK506 analog, AP20187, induces regulated dimerization of the Fv domains; previously, we showed that in the absence of AP20187, Fv-SLK 1–373 is almost exclusively monomeric, whereas AP20187 results in dimerization of ∼35% of total (22). In addition to the T183A/S189A mutation, we also examined the role of Thr-193 phosphorylation, as Thr-193 in SLK is highly conserved among evolutionarily related protein kinases. After transfection of COS-1 cells, Fv-SLK 1–373 WT (not treated with AP20187) showed modest kinase activity (as monitored by incorporation of 32P into MBP), whereas treatment with AP20187 markedly increased kinase activity compared with untreated (Fig. 4, B–D). In contrast, Fv-SLK 1–373 T183A/S189A and Fv-SLK 1–373 T193A showed insignificant kinase activity in the presence or absence of AP20187 (Fig. 4, B–D). By analogy, phosphorylation of Fv-SLK 1–373 WT increased from 1.0 unit in untreated cells to 5.4 ± 1.4 units in AP20187-treated cells (p = 0.0002, n = 4). Phosphorylation of Fv-SLK 1–373 T183A/S189A and T193A (untreated or treated) was negligible.
FIGURE 4.
Homodimerization increases the kinase activity of Fv-SLK 1–373 WT but not SLK T183A/S189A and T193A mutants. A, HA-Fv-SLK 1–373 is shown. The positions of mutations in the kinase domain are indicated. B, COS-1 cells were transiently transfected with HA-Fv-SLK 1–373 WT or mutants. AP20187 (100 nm) was added as indicated at 45 h. Then after 3 h lysates were immunoprecipitated with anti-HA antibody (+), or nonimmune IgG (control; −). Kinase activity was monitored by the incorporation of 32P into MBP (representative autoradiogram). C, lysates immunoblotted with anti-HA antibody show equal protein expression. D, normalized densitometry of MBP is shown. **, p < 0.0001 AP20187 versus untreated (WT), n = 4.
T183A/S189A SLK Double Mutant Fails to Induce Apoptosis
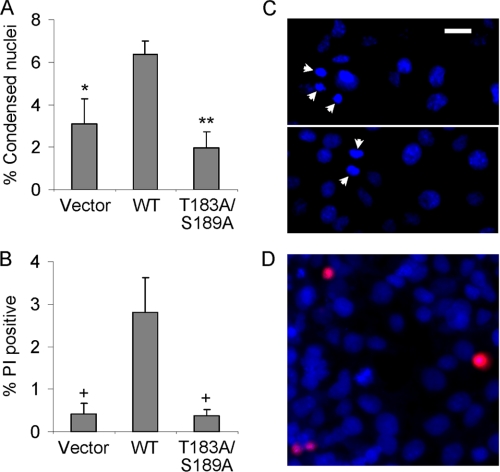
Expression of SLK can induce apoptosis and can exacerbate apoptosis during in vitro ischemia-reperfusion injury (10, 13, 14). To examine the effects of phosphorylation in the SLK activation segment on cell death, GECs were co-transfected with Fv-SLK 1–373 WT, Fv-SLK 1–373 T183A/S189A double mutant, or vector and were incubated with AP20187. Then cells were stained with Hoechst H33342 and propidium iodide (Fig. 5). Hoechst H33342 stains nuclei, and cells showing condensed nuclei were considered “apoptotic.” Propidium iodide is only able to enter the cytoplasm of cells with damaged plasma membranes, although in culture, propidium iodide-stained cells are most likely apoptotic (late apoptotic). Compared with control (vector), Fv-SLK 1–373 WT-transfected cells showed significantly enhanced apoptosis (Fig. 5, A and B). In contrast, cells transfected with Fv-SLK 1–373 T183A/S189A did not show significant increases in apoptosis compared with control (Fig. 5, A and B). These results are consistent with previous studies which showed that the overexpression of SLK WT stimulated proapoptotic pathways in cultured cells (10), and the results implicate a functionally important role for Thr-183 and Ser-189 phosphorylation.
FIGURE 5.
Fv-SLK 1–373 WT, but not the T183A/S189A double mutant, induces apoptosis. GECs were transiently transfected with Fv-SLK 1–373 WT, Fv-SLK T183A/S189A, or control vector. After 48 h cells were incubated with AP20187 (100 nm). Apoptotic cells, i.e. cells with condensed nuclei (Hoechst H33342 staining; A) and propidium iodide-positive cells (B) were measured after 3 h. A, *, p < 0.05; **, p < 0.005 versus Fv-SLK 1–373 WT, n = 4. B, +, p < 0.05 versus Fv-SLK 1–373 WT, n = 4. C and D, examples of cells transfected with Fv-SLK 1–373 WT show condensed nuclei (Hoechst H33342 staining; C, two panels, arrowheads) and propidium iodide-stained (red) cells (D). Bar = 15 μm.
Phosphomimetic Mutations in SLK Do Not Enhance Kinase Activity
In these experiments Thr-183 and Ser-189 were mutated to aspartic and glutamic acid (instead of alanine) to produce Fv-SLK 1–373 T183D, T183E, S189D, and S189E. Such mutations are believed to imitate the negative charge that would be present when a serine or a threonine is phosphorylated. COS-1 cells were transfected with SLK 1–373 WT or mutants and were then treated with or without AP20187 to induce homodimerization. By analogy to the result shown in Fig. 4B, in these experiments Fv-SLK WT displayed minor basal kinase activity, which was stimulated substantially by AP20187 (Table 2). Surprisingly, instead of increasing kinase activity, the Fv-SLK 1–373 T183D, T183E, S189D, and S189E mutants showed negligible kinase activity, and there was no significant stimulatory effect of AP20187 (Table 2). Because the phosphomimetic mutations demonstrated the same effects as T183A/S189A and T193A mutants (Fig. 4B), the results indicate that phosphorylation, but not a negative charge-induced conformational change, is required for kinase activity. Alternatively, the negative charges at Ser-189 and Thr-183 may be necessary for kinase activity, but they may not be sufficient.
TABLE 2.
Kinase activity of Fv-SLK 1–373 T183D, T183E, S189D, and S189E
The experimental protocol was analogous to the one described in the legend to Fig. 4. Kinase activity was monitored by the incorporation of 32P into MBP. The densitometry of MBP is presented.
| Untreated | AP20187 | |
|---|---|---|
| WT | 1.00 | 2.72 ± 0.81a |
| T183D | 0.10 ± 0.05 | 0.16 ± 0.09 |
| T183E | 0.08 ± 0.03 | 0.12 ± 0.04 |
| WT | 1.00 | 6.79 ± 3.39b |
| S189D | 0.33 ± 0.14 | 1.60 ± 0.84 |
| S189E | 0.25 ± 0.12 | 1.38 ± 0.49 |
a p < 0.002, AP20187 versus untreated (WT), n = 4.
b p < 0.01 AP20187, versus untreated (WT), n = 4.
Analysis of Phosphorylation at Ser-189
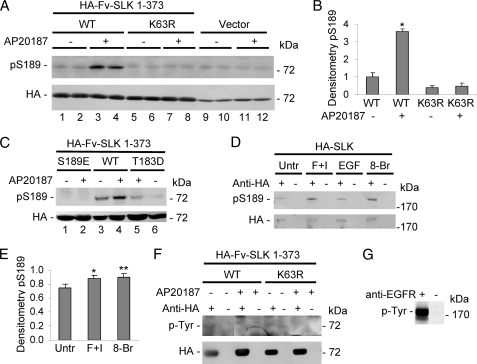
The amino acid sequence around Ser-189 in SLK (RRDSF) is a putative phosphorylation motif for PKA (33). The same amino acid sequence is not present anywhere else in SLK. Using an antibody that recognizes the sequence RRXpS/T (“anti-phospho-PKA substrate antibody”), phosphorylation of Fv-SLK 1–373 WT was demonstrated (Fig. 6, A–C). Compared with cells that were transfected with vector (Fig. 6A, lanes 9–12), phosphorylation was weak under basal conditions (i.e. in monomeric Fv-SLK 1–373) and increased after dimerization with AP20187 (Fig. 6, A, lanes 1–4, B, and C, lanes 3 and 4). Lys-63 is the ATP binding site in SLK, and the K63R mutation abolishes kinase activity (see below) (15). There was no detectable phosphorylation of Ser-189 after transfection with Fv-SLK 1–373 K63R (Fig. 6A, lanes 5–8, and B). We also examined the effect of the T183D and S189E mutations on Ser-189 phosphorylation. In the Fv-SLK 1–373 T183D mutant, there was slight basal phosphorylation of the Fv-SLK 1–373 monomer, and there was a small increase in Ser-189 phosphorylation after the addition of AP20187 to induce dimerization (Fig. 6C, lanes 5 and 6). As expected, phosphorylation of Ser-189 was not detectable in the Fv-SLK 1–373 S189E mutant (Fig. 6C, lanes 1 and 2). These results demonstrate that Ser-189 phosphorylation is associated with dimerization of Fv-SLK 1–373 WT, and the ∼4-fold increase in Ser-189 phosphorylation (Fig. 6B) correlates with kinase activity, as monitored by 32P incorporation into MBP (Fig. 4D).
FIGURE 6.
Homodimerization of Fv-SLK 1–373 WT and activation of PKA increase phosphorylation of Ser-189. A–C, COS-1 cells were transiently transfected with HA-Fv-SLK 1–373 WT, K63R, S189E, or T183D, or vector (control). AP20187 (100 nm) was added as indicated at 45 h. Then, after 3 h lysates were immunoblotted with anti-RRXpS/T (phospho-PKA substrate) or anti-HA antibodies. Ser-189 phosphorylation was increased after treatment of Fv-SLK 1–373 WT-expressing cells with AP20187. Phosphorylation was trivial or absent in the Fv-SLK 1–373 K63R and S189E mutant (with and without AP20187). The Fv-SLK 1–373 T183D mutant shows low basal Ser-189 phosphorylation, which was increased modestly with AP20187. In panel A, the anti-HA antibody identifies a nonspecific band in vector-transfected cells at ∼72 kDa. B, densitometry of Ser(P)-189 (pS189). *, p < 0.0001 versus WT −, untreated and p < 0.0001 versus K63R, n = 3. D and E, COS-1 cells were transfected with full-length HA-SLK WT and then untreated or incubated with forskolin (F; 100 μm) + 3-isobutyl-1-methylxanthine (I; 1 mm), 8-bromo-cAMP (8-Br; 1 mm), or epidermal growth factor (EGF, 100 ng/ml) for 30 min. Lysates were immunoprecipitated with anti-HA antibody (+) or nonimmune IgG (-) and were immunoblotted with anti-RRXpS/T or anti-HA antibodies. Treatment with forskolin + 3-isobutyl-1-methylxanthine or 8-Br increased Ser-189 phosphorylation compared with untreated or EGF-treated cells. E, densitometry of Ser(P)-189 is shown. *, p < 0.035; **, p < 0.02 versus untreated, n = 5. F, tyrosine phosphorylation of SLK was not detected. COS-1 cells were transfected with HA-SLK 1–373 WT or K63R and were then incubated with or without AP20187. Lysates were immunoprecipitated with anti-HA antibody (+) or nonimmune IgG (−) and were then immunoblotted with anti-phosphotyrosine (p-Tyr) or anti-HA antibodies. G, to verify the reactivity of the anti-phosphotyrosine antibody, lysates of A431 cells that had been stimulated with EGF were immunoprecipitated with anti-EGF receptor (EGFR) antibody (+) or nonimmune serum in control (−) and were then immunoblotted with anti-phosphotyrosine antibody.
Because Ser-189 is within a putative PKA phosphorylation site, we also examined if activation of PKA could result in Ser-189 phosphorylation. In these experiments cells were transfected with full-length HA-SLK WT and were then untreated or incubated with either forskolin+3-isobutyl-1-methylxanthine or 8-bromo-cAMP (a cell-permeant analog of cAMP). Treatment with forskolin + 3-isobutyl-1-methylxanthine or 8-bromo-cAMP increased Ser-189 phosphorylation by ∼20% compared with untreated (Fig. 6, D and E). These experiments indicate that Ser-189 can be phosphorylated by PKA, but the effect is small. In contrast to PKA, treatment of cells with epidermal growth factor (negative control) (15) did not result in Ser-189 phosphorylation (Fig. 6D). However, the same concentration of epidermal growth factor stimulated tyrosine phosphorylation of the epidermal growth factor receptor (Fig. 6G) as well as phosphorylation of extracellular signal-regulated kinase (data not shown).
Analysis of Tyrosine Phosphorylation
The above experiments focused on the role of serine phosphorylation in the activation of SLK. In the following set of experiments we examined if activation of SLK may be associated with tyrosine phosphorylation in the catalytic domain, e.g. Tyr-195. Cells were transfected with HA-SLK 1–373 WT or K63R and were then incubated with or without AP20187. Lysates were immunoprecipitated with anti-HA antibody and were then immunoblotted with anti-phosphotyrosine antibody. Tyrosine phosphorylation of the SLK catalytic domain was not detected (Fig. 6F).
K63R and E79A Mutations Result in Reduced Kinase Activity
The studies described above addressed the role of various phosphorylation sites in the regulation of the kinase activity of SLK. However, additional amino acids may also be required for sustaining kinase activity together with phosphorylation. Based on the crystal structure of the SLK catalytic domain, Lys-63 may form a salt bridge with Glu-79, which may lock SLK in an active conformation after phosphorylation of SLK at Thr-183 and Ser-189 (25). The Fv-SLK 1–373 E79A mutant (Fig. 4A) was generated to test if Glu-79 is functionally important. Fv-SLK 1–373 WT as well as E79A, K63R, or T183A/S189A mutants were transfected in COS-1 cells, and the cells were then treated with or without AP20187. As expected, the K63R mutation abolished kinase activity (with or without AP20187) as monitored by incorporation of 32P into MBP (Fig. 7, A–C). Kinase activity (with or without AP20187) was markedly attenuated in the Fv-SLK 1–373 E79A mutant, demonstrating the functional importance of Glu-79 (Fig. 7, A–C). Compared with Fv-SLK 1–373 WT (+AP20187), the marked reduction in kinase activity by the E79A mutation was similar to the reduction by the T183A/S189A double mutation (Fig. 7, A–C).
FIGURE 7.
The Fv-SLK 1–373 E79A mutant shows reduced kinase activity, whereas K63R shows trivial activity. The protocol is analogous to the one in Fig. 6. The Fv-SLK 1–373 WT lanes in the autoradiogram are overexposed to allow comparison with E79A, K63R. *, p < 0.0001 versus Fv-SLK 1–373 WT, n = 6.
Interaction of SLK WT with T183A/S189A Double Mutant
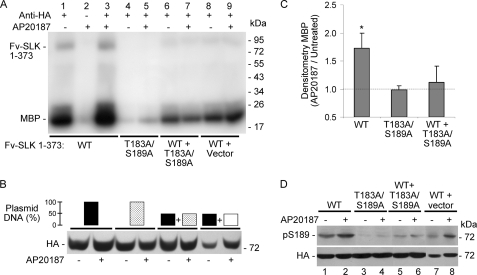
To further examine potential interactions of the SLK activation segments, we evaluated kinase activity when both WT and T183A/S189A double mutant SLK catalytic domains were expressed together. COS-1 cells were transfected with Fv-SLK 1–373 WT alone (100% amount of plasmid DNA), Fv-SLK 1–373 T183A/S189A alone (100% amount of plasmid DNA), Fv-SLK 1–373 WT (50% amount of plasmid DNA) together with Fv-SLK 1–373 T183A/S189A (50% amount of plasmid DNA), and Fv-SLK 1–373 WT (50% amount of plasmid DNA) together with control vector (50% amount of plasmid DNA) (Fig. 8B). Cells were then treated with or without AP20187, and kinase activity in the cell lysates was monitored by incorporation of 32P into MBP. Similar to the results shown in Fig. 4, AP20187-induced dimerization enhanced the activity of Fv-SLK 1–373 WT compared with untreated (Fig. 8, A, lane 1 versus 3, and C, left-most column). As in Fig. 4, compared with Fv-SLK 1–373 WT (Fig. 8A, lanes 1 and 3), a significant reduction in activity was noted in the monomeric Fv-SLK 1–373 double mutant, and there was no stimulatory effect of AP20187 (Fig. 8, A, lanes 4 and 5, and C, center column). In cells co-transfected with Fv-SLK 1–373 WT and Fv-SLK 1–373 T183A/S189A, one would predict the presence of both monomers, and after the addition of AP20187, one would predict formation of WT homodimers (25%), mutant homodimers (25%), and WT and mutant heterodimers (50%). Compared with the T183A/S189A double mutant alone (Fig. 8A, lanes 4 and 5), slightly greater MBP phosphorylation was noted when Fv-SLK 1–373 WT was co-expressed with the double mutant, but the stimulatory effect of AP20187 on MBP phosphorylation in these cells was not statistically significant (Fig. 8, A, lanes 6 and 7, and C, right column). Actually, MBP phosphorylation in the WT+T183A/S189A mixture (Fig. 8A, lane 6) was comparable with WT alone when the reduced amount of Fv-SLK 1–373 WT was transfected (Fig. 8A, lane 8), suggesting that the MBP phosphorylation in the mixture was solely due to the activity of WT. By analogy, immunoblotting with anti-RRXpS/T antibody showed that AP20187 stimulated SLK Ser-189 phosphorylation in cells transfected with Fv-SLK 1–373 WT or WT + vector (Fig. 8D, lanes 1 and 2 and lanes 7 and 8). As expected, Ser-189 phosphorylation was absent in cells transfected with Fv-SLK 1–373 T183A/S189A (Fig. 8D, lanes 3 and 4) and was slight in the WT+T183A/S189A mixture (Fig. 8D, lanes 5 and 6). Therefore, Fv-SLK 1–373 WT did not appear to “rescue” the double mutant upon dimerization.
FIGURE 8.
Interaction of Fv-SLK 1–373 WT with Fv-SLK 1–373 T183A/S189A. A–C, kinase activity is shown. COS-1 cells were transfected with Fv-SLK 1–373 WT alone (plasmid DNA 100%), Fv-SLK 1–373 T183A/S189A alone (100%), Fv-SLK 1–373 WT (50%) together with Fv-SLK 1–373 T183A/S189A (50%), and Fv-SLK 1–373 WT (50%) together with control vector (50%) (B). After 45 h cells were treated with (+) or without (−) AP20187 (100 nm). Then, after 3 h lysates were immunoprecipitated with anti-HA antibody (+) or nonimmune IgG (control; −). Kinase activity was monitored by the incorporation of 32P into MBP (A, representative autoradiogram). B, lysates were immunoblotted with anti-HA antibody to demonstrate protein expression. C, normalized densitometry of MBP shows the -fold increase in phosphorylated MBP in AP20187-treated cells compared with untreated. *, p < 0.005 AP20187 versus untreated, n = 8. D, Ser-189 phosphorylation (pS189) is shown. COS-1 cells were transfected with plasmid DNAs, as shown in panel B (top). Then cells were treated with (+) or without (−) AP20187, and lysates were immunoblotted with anti-RRXpS/T or anti-HA antibodies.
Heterodimerization of SLK 1–373 WT with T183A/S189A and K63R Mutants
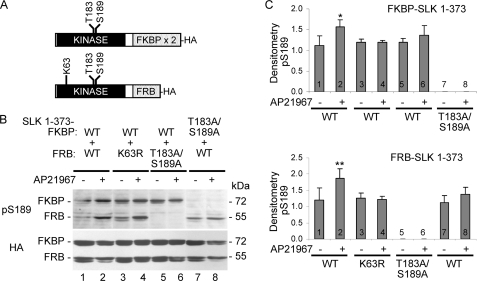
These experiments examined the phosphorylation of Ser-189 after heterodimerization. cDNAs consisting of the N-terminal domains of WT or mutant SLK (amino acids 1–373) fused at the C terminus with two FKBP domains (FKBP-SLK 1–373) or a single FRB domain (FRB-SLK 1–373; Fig. 9A) were produced. The addition of the non-immunosuppressive rapamycin analog, AP21967, induces regulated heterodimerization of a single or double FKBP domain with the FRB domain (29). The WT and mutant constructs were transfected in COS-1 cells, and after treatment with or without AP21967, cell lysates were immunoblotted with anti-RRXpS/T antibody. It should be noted that the FKBP fusion proteins are larger than the FRB fusion proteins (Fig. 9A), allowing discrimination by SDS-PAGE.
FIGURE 9.
Effects of SLK 1–373 WT dimerization with T183A/S189A and K63R mutants on the phosphorylation of Ser-189). COS-1 cells were transiently transfected with HA-FKBP-SLK 1–373 WT + HA-FRB-SLK 1–373 WT (lanes 1 and 2), HA-FKBP-SLK 1–373 WT + HA-FRB-SLK 1–373 K63R (lanes 3 and 4), HA-FKBP-SLK 1–373 WT + HA-FRB-SLK 1–373 T183A/S189E (lanes 5 and 6), or HA-FKBP-SLK 1–373 T183A/S189A + HA-FRB-SLK 1–373 WT (lanes 7 and 8). AP21967 (100 nm) was added as indicated at 24 h to induce heterodimerization of FKBP- with FRB-SLK proteins. Then, after 24 h lysates were immunoblotted with anti-RRXpS/T or anti-HA antibodies (A). Ser-189 phosphorylation (pS189) was increased after heterodimerization of HA-FKBP-SLK 1–373 WT and HA-FRB-SLK 1–373 WT induced by AP21967. C, *, p < 0.02; **, p < 0.015 AP20187 versus untreated, n = 4. In all other heterodimerizations there were no significant changes in Ser-189 phosphorylation.
All fusion proteins were expressed in COS-1 cells (Fig. 9B, lower panel). Although there was some variability in the level of basal Ser-189 phosphorylation among experiments, basal phospho-Ser-189 (i.e. without AP21967) was detected in FKBP-SLK 1–373 WT (Fig. 9B, upper panel, lanes 1, 3, and 5), FRB-SLK 1–373 WT (Fig. 9B, lanes 1 and 7), and FRB-SLK 1–373 K63R (Fig. 9B, lane 3). As expected, there was a complete absence of basal Ser-189 phosphorylation in the FKBP-SLK 1–373 T183A/S189A (Fig. 9B, lane 7) and FRB-SLK 1–373 T183A/S189A mutants (Fig. 9B, lane 5). Phosphorylation of Ser-189 increased consistently in both FKBP-SLK 1–373 WT and FRB-SLK 1–373 WT after induction of dimerization by AP21967 (Fig. 9, B, lane 1 versus 2, C, columns 1 versus 2). In contrast, increases in Ser-189 phosphorylation in SLK WT and K63R after heterodimerization of WT with the K63R mutant were not consistent, and the mean differences between AP21967-treated and untreated were not significant (Fig. 9, B, lane 3 versus 4, and C, column 3 versus 4). Thus, in the WT-K63R heterodimer, neither partner underwent Ser-189 phosphorylation. The K63R mutation, besides blocking kinase activity, may potentially affect the conformation of the activation segment (25) and prevent its phosphorylation. There was a tendency toward an increase in Ser-189 phosphorylation in SLK WT after heterodimerization of WT with the T183A/S189A mutant, but the increases were not statistically significant (Fig. 9, B, lane 5 versus 6, and C, column 5 versus 6 as well as Fig. 9, B, lane 7 versus 8, and C, column 7 versus 8). It is conceivable that phosphorylation at the Thr-193 site in the T183A/S189A mutant may have accounted for some inefficient Ser-189 phosphorylation in SLK WT.
In these experiments the increase in Ser-189 phosphorylation above the basal level after dimerization of FKBP-SLK 1–373 WT with FRB-SLK 1–373 WT (Fig. 9C, columns 1 and 2) was clearly significant but not as pronounced as in homodimerized Fv-SLK 1–373 WT (Fig. 6B, first two columns). Therefore, heterodimerization of FKBP with FRB was probably not as efficient as homodimerization of the two Fv domains. In this assay basal phosphorylation may be influenced by the type of dimerization domain, whereas the efficiency of dimerization is dependent on certain physical factors of the fusion protein such as proximity of the domains, their relative orientation, and conformational flexibility/rigidity as well as physical factors of the dimerizing drug (26, 29).
DISCUSSION
The present study addressed the activation of SLK using full-length SLK as well as the catalytic domain of SLK coupled to regulatable dimerization domains. Mutations in Thr-183, Ser-189, and Thr-193 resulted in significantly reduced catalytic activity (32P incorporation into MBP) and/or phosphorylation as monitored by 32P incorporation into SLK and by anti-RRXpS/T antibody, which recognizes phospho-Ser-189 (Figs. 1, 4, and 6). Phosphorylation of SLK paralleled exogenous kinase activity and was most likely due to homodimerization and autophosphorylation. The decreased catalytic activity of the full-length SLK T183A/S189A mutant (Fig. 1) was also reflected in impaired JNK and p38 phosphorylation (Figs. 2 and 3). Single mutations (T183A and S189A) were not as effective in impairing the ability of SLK to induce JNK or p38 phosphorylation (Figs. 2 and 3), which was distinct from the more substantial loss of catalytic activity observed in the in vitro kinase assays (Fig. 1). This discrepancy may be related to the different cell lines employed in the assays and transfection efficiency.
Activated JNK and p38 phosphorylate downstream targets in the cytosol or the nucleus, including, AP-1, c-Jun, Bax, p53, and others (34, 35). AP-1 may control cell differentiation, proliferation, or apoptosis (32). By analogy to JNK and p38, expression of full-length SLK stimulated AP-1 reporter activity, and stimulation was weaker after expression of SLK T183A, whereas the S189A and T183A/S189A mutants were ineffective (Fig. 3). Previous studies demonstrated that overexpression of SLK induced proapoptotic mediators and exacerbated cell death (10, 13, 14). The present study is in keeping with these earlier results, as it demonstrates that expression of the WT SLK catalytic domain induced apoptosis, whereas the T183A/S189A double mutant was inactive (Fig. 5). Thus, activation segment phosphorylation is important for induction of a functional effect.
The SLK amino acid sequence shows α-helical coiled-coil structures in the C-terminal domain (19). Proteins with coiled-coils may dimerize/oligomerize via this structure, and we demonstrated that SLK C-terminal domains can dimerize and that in cytosolic fractions of cells, full-length SLK exists as a high molecular mass complex, consistent with a constitutive homodimer or oligomer (22). Such homodimerization or oligomerization may facilitate activation of SLK by increasing the local concentration of catalytic domains. To confirm this proposition, we examined if dimerization facilitates autophosphorylation and activation by employing Fv-SLK 1–373, which allows for controlled dimerization upon the addition of AP20187. Monomeric Fv-SLK 1–373 WT displayed minor catalytic activity and phosphorylation in an in vitro kinase assay (Figs. 4 and 7). Overexpression of Fv-SLK 1–373 WT may have provided a sufficient concentration of the protein to allow transient dimerization of SLK catalytic domains leading to minor autoactivation. Nevertheless, the addition of AP20187 greatly enhanced both autoactivation and catalytic activity of SLK WT, and the effect was abolished by T183A/S189A and T193A mutations (Figs. 4, 6, and 7).
To further examine the dimerization and phosphorylation of the SLK activation segments, we compared the kinase activity and Ser-189 phosphorylation of SLK 1–373 WT-WT dimers with WT-T183A/S189A dimers using Fv-Fv homodimerization and FKBP-FRB heterodimerization systems. Kinase activity and phosphorylation of Ser-189 were impaired in the WT-T183A/S189A dimers, compared with WT-WT (Fig. 8 and 9). In addition, homodimers of SLK 1–373 T183D, T183E, S189D, and S189E were catalytically inactive (Table 2), showing that the addition of negative charge alone was not sufficient to mimic serine/threonine phosphorylation and enhance kinase activity, in keeping with other reports where phosphomimetic mutations did not mimic the effects of phosphorylation (36, 37). Based on crystallography data, it was proposed that phosphorylation leads to the ordering of the activation segments (25). Thr-183 phosphorylation could stabilize a hydrogen bond network between activation segment dimers and could promote Ser-189 phosphorylation (25). In the present study the T183A mutant appeared to be more active than S189A in certain assays (Figs. 1 and 3C), suggesting that Ser-189 can be phosphorylated independently of Thr-183, but in the Fv-SLK 1–373 dimerization experiments phosphomimetic mutations at either site abolished activity.
Protein kinases can be activated through various mechanisms. Receptor-tyrosine kinases generally undergo ligand-dependent dimerization and trans-phosphorylation (38). An upstream kinase can potentially recognize the activation segment of a kinase as substrate and activate catalytic activity by phosphorylating the activation segment (25). The addition of a phosphate group at the activation segment may be coupled to a conformational change that allows sustained catalytic activity (25). By crystallography, the catalytic domain of SLK is structurally related to the protein kinase, CHK2 (25). A kinase autoactivation mechanism involving trans-autophosphorylation was proposed in part based on the crystallography structure of CHK2 (5, 39). Thus, the activation segment of one kinase molecule transiently adopts an active conformation, allowing the activation segment from a second molecule to bind and be phosphorylated. Dimerization compensates for the instability of the non-phosphorylated activation segment by increasing the local concentrations of the kinase domains. Once reciprocally phosphorylated, the dimeric kinase continues to activate downstream targets. Other kinases that may undergo similar activation include DAPK3 and oxidative stress-responsive-1 (OSR1), a regulator of Na+/K+/2Cl− cotransporters (40). Models developed from crystallography findings, however, have some limitations, as they are based on the use of isolated catalytic domains studied in vitro. Catalytic domain oligomers show low affinity interactions, and whereas SLK and DAPK3 formed dimers in solution (to a minor extent), OSR1 did not (25, 40, 41). CHK2 and OSR1 also require the actions of other protein kinases and sequences outside of their kinase domains for activity and activation (5, 40). Alternatively, in intact cells dimerization of the SLK catalytic domain is likely constitutive and dependent on the C-terminal coiled-coil regions (14, 22). In the developing kidney and renal ischemia-reperfusion injury in vivo, endogenous SLK expression was increased (10), probably related to decreased SLK mRNA degradation (21). SLK expression also increased in neuronal cells during brain development (9). Up-regulated expression would augment the concentration of SLK protein in the cell and may thereby increase the likelihood of trans-autophosphorylation.
As expected, the Fv-SLK 1–373 K63R mutant did not possess kinase activity, and in addition activity of SLK was markedly reduced by the E79A mutation (Fig. 7). Based on the crystal structure of SLK catalytic domains in vitro, it was proposed that the activation segments are in an active conformation after dimerization and activation segment domain exchange (25). A salt bridge could form between the αC helix glutamate (Glu-79) and the active site lysine (Lys-63), correctly positioning the αC helix for catalytic activity and stabilizing the monomer in an active confirmation. Disruption of this salt bridge would thus prevent sustained catalytic activity after activation segment domain exchange. This prediction is supported by our study. Taken together, our results show that in cells, dimerization of the activation segment and phosphorylation at residues Thr-183, Ser-189, and Thr-193 plays a key role in the regulation of the catalytic activity of SLK. Furthermore, residues Lys-63 and Glu-79 may be playing a role in sustained catalytic activity by forming a bond and locking SLK in an active conformation.
Phosphoproteomic analyses of mouse tissues and cell lines identified constitutive Ser-189 phosphorylation in endogenous SLK in multiple tissues/cells, whereas phosphorylation of Thr-183 and Thr-193 was present but was less prevalent (42–45).3 Other serine/threonine phosphorylation sites outside of the catalytic domain were also noted. Ser-189 in SLK is within a putative phosphorylation motif for PKA (33), and stimulation of PKA resulted in weak Ser-189 phosphorylation in the present study (Fig. 6). In phosphoproteomic analyses of renal collecting duct cells (43) and synovial fibroblasts,3 agonists that activated PKA (vasopressin and prostaglandin E2, respectively) either had no effect or enhanced phosphorylation of Thr-183 and Ser-189 in endogenous SLK by ∼10–20%. Whether PKA contributes to basal or stimulated SLK phosphorylation under pathophysiological circumstances will require further investigation. Stimulation of macrophages with lipopolysaccharide did not enhance Thr-183 and Ser-189 phosphorylation (44). A study in HeLa cells showed a ∼2-fold variation in Thr-183 and Ser-189 phosphorylation during the cell cycle (45), in keeping with variations in SLK activity, reported earlier (23). Although casein kinase II phosphorylation of positions 347 and 348 of SLK was shown to negatively regulate kinase activity (24), the functional relevance of the other putative SLK phosphorylation sites will require further study.
Defining the regulation of SLK activity is important for obtaining a better understanding of its functional role in pathophysiology. Increased endogenous SLK activity has been demonstrated in the developing kidney as well as renal ischemia-reperfusion injury in vivo and in a cell culture model (10). Endogenous SLK was also shown to be phosphorylated in the developing kidney (10) and in renal ischemia-reperfusion injury models.4 Podocyte-specific overexpression of SLK in mice resulted in albuminuria and podocyte injury, consistent with apoptosis (46). These results suggest that SLK may exacerbate cell damage in glomerular disease and acute kidney injury. SLK was among several proapoptotic genes up-regulated in the lung after renal ischemia-reperfusion injury, suggesting a role for SLK in mediating the adverse distant organ effects of acute kidney injury (47). SLK may modulate vasodilation via RhoA and the angiotensin II type 2 receptor (48). A potential role for SLK in cancer cell motility and invasiveness via interaction with v-Src (24) and ErbB2 (49) as well as cytoskeletal proteins has been demonstrated. Improved understanding of how SLK is regulated could unravel the apparent contradiction between SLK as a protein that may induce apoptosis in normal cell lines but leads to increased metastasis and a worse outcome in cancer cells. The delineation of key phosphorylation sites and dimer interactions also provides potential novel targets for design of compounds, which may be useful in the treatment of acute kidney injury, cancer, and other diseases.
Acknowledgments
We thank Dr. Louise Larose and Dr. John Di Battista (McGill University) for helpful discussion and Ariad Pharmaceuticals for providing the homodimerization kit.
This work was supported by Canadian Institutes of Health Research Grants MOP-53264 and MOP-84213 and the Kidney Foundation of Canada.
J. A. Di Battista, personal communication.
A. Cybulsky, unpublished observations.
- GEC
- glomerular epithelial cell
- AP-1
- activator protein-1
- FKBP
- FK506-binding protein
- MBP
- myelin basic protein
- OSR1
- oxidative stress-responsive-1
- DAPK3
- death-associated protein kinase-3
- CHK2
- checkpoint kinase-2.
REFERENCES
- 1. Scheeff E. D., Bourne P. E. (2005) Structural evolution of the protein kinase-like superfamily. PLoS Comput. Biol. 1, e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ali A. S., Ali S., El-Rayes B. F., Philip P. A., Sarkar F. H. (2009) Exploitation of protein kinase C. A useful target for cancer therapy. Cancer Treat. Rev. 35, 1–8 [DOI] [PubMed] [Google Scholar]
- 3. Cowley B. D., Jr. (2008) Calcium, cyclic AMP, and MAP kinases. Dysregulation in polycystic kidney disease. Kidney Int. 73, 251–253 [DOI] [PubMed] [Google Scholar]
- 4. Nolen B., Taylor S., Ghosh G. (2004) Regulation of protein kinases. Controlling activity through activation segment conformation. Mol. Cell 15, 661–675 [DOI] [PubMed] [Google Scholar]
- 5. Oliver A. W., Knapp S., Pearl L. H. (2007) Activation segment exchange. A common mechanism of kinase autophosphorylation? Trends Biochem. Sci. 32, 351–356 [DOI] [PubMed] [Google Scholar]
- 6. Dan I., Watanabe N. M., Kusumi A. (2001) The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol. 11, 220–230 [DOI] [PubMed] [Google Scholar]
- 7. Zhao Z. S., Leung T., Manser E., Lim L. (1995) Pheromone signaling in Saccharomyces cerevisiae requires the small GTP-binding protein Cdc42p and its activator CDC24. Mol. Cell. Biol. 15, 5246–5257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Delpire E. (2009) The mammalian family of sterile 20p-like protein kinases. Pflugers Arch. 458, 953–967 [DOI] [PubMed] [Google Scholar]
- 9. Zhang Y. H., Hume K., Cadonic R., Thompson C., Hakim A., Staines W., Sabourin L. A. (2002) Expression of the Ste20-like kinase SLK during embryonic development and in the murine adult central nervous system. Brain Res. Dev. Brain Res. 139, 205–215 [DOI] [PubMed] [Google Scholar]
- 10. Cybulsky A. V., Takano T., Papillon J., Khadir A., Bijian K., Chien C. C., Alpers C. E., Rabb H. (2004) Renal expression and activity of the germinal center kinase SK2. Am. J. Physiol. Renal Physiol. 286, F16–F25 [DOI] [PubMed] [Google Scholar]
- 11. Wagner S., Flood T. A., O'Reilly P., Hume K., Sabourin L. A. (2002) Association of the Ste20-like kinase (SLK) with the microtubule. Role in Rac1-mediated regulation of actin dynamics during cell adhesion and spreading. J. Biol. Chem. 277, 37685–37692 [DOI] [PubMed] [Google Scholar]
- 12. Zinovkina L. A., Poltaraus A. B., Solovyanova O. B., Nadezhdina E. S. (1997) Chinese hamster protein homologous to human putative protein kinase KIAA0204 is associated with nuclei, microtubules, and centrosomes in CHO-K1 cells. FEBS Lett. 414, 135–139 [DOI] [PubMed] [Google Scholar]
- 13. Cybulsky A. V., Takano T., Guillemette J., Papillon J., Volpini R. A., Di Battista J. A. (2009) The Ste20-like kinase SLK promotes p53 transactivation and apoptosis. Am. J. Physiol. Renal Physiol. 297, F971–F980 [DOI] [PubMed] [Google Scholar]
- 14. Hao W., Takano T., Guillemette J., Papillon J., Ren G., Cybulsky A. V. (2006) Induction of apoptosis by the Ste20-like kinase SLK, a germinal center kinase that activates apoptosis signal-regulating kinase and p38. J. Biol. Chem. 281, 3075–3084 [DOI] [PubMed] [Google Scholar]
- 15. Sabourin L. A., Rudnicki M. A. (1999) Induction of apoptosis by SLK, a Ste20-related kinase. Oncogene 18, 7566–7575 [DOI] [PubMed] [Google Scholar]
- 16. Sabourin L. A., Tamai K., Seale P., Wagner J., Rudnicki M. A. (2000) Caspase 3 cleavage of the Ste20-related kinase SLK releases and activates an apoptosis-inducing kinase domain and an actin-disassembling region. Mol. Cell. Biol. 20, 684–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O'Reilly P. G., Wagner S., Franks D. J., Cailliau K., Browaeys E., Dissous C., Sabourin L. A. (2005) The Ste20-like kinase SLK is required for cell cycle progression through G2. J. Biol. Chem. 280, 42383–42390 [DOI] [PubMed] [Google Scholar]
- 18. Burakov A. V., Zhapparova O. N., Kovalenko O. V., Zinovkina L. A., Potekhina E. S., Shanina N. A., Weiss D. G., Kuznetsov S. A., Nadezhdina E. S. (2008) Ste20-related protein kinase LOSK (SLK) controls microtubule radial array in interphase. Mol. Biol. Cell 19, 1952–1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yamada E., Tsujikawa K., Itoh S., Kameda Y., Kohama Y., Yamamoto H. (2000) Molecular cloning and characterization of a novel human STE20-like kinase, hSLK. Biochim. Biophys. Acta 1495, 250–262 [DOI] [PubMed] [Google Scholar]
- 20. Lupas A., Van Dyke M., Stock J. (1991) Predicting coiled coils from protein sequences. Science 252, 1162–1164 [DOI] [PubMed] [Google Scholar]
- 21. Cybulsky A. V., Takano T., Papillon J., Hao W., Mancini A., Di Battista J. A., Cybulsky M. I. (2007) The 3′-untranslated region of the Ste20-like kinase SLK regulates SLK expression. Am. J. Physiol. Renal Physiol. 292, F845–F852 [DOI] [PubMed] [Google Scholar]
- 22. Delarosa S., Guillemette J., Papillon J., Han Y. S., Kristof A. S., Cybulsky A. V. (2011) Activity of the Ste20-like kinase, SLK, is enhanced by homodimerization. Am. J. Physiol. Renal Physiol. 301, F554–F564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ellinger-Ziegelbauer H., Karasuyama H., Yamada E., Tsujikawa K., Todokoro K., Nishida E. (2000) Ste20-like kinase (SLK), a regulatory kinase for polo-like kinase (Plk) during the G2/M transition in somatic cells. Genes Cells 5, 491–498 [DOI] [PubMed] [Google Scholar]
- 24. Chaar Z., O'reilly P., Gelman I., Sabourin L. A. (2006) v-Src-dependent down-regulation of the Ste20-like kinase SLK by casein kinase II. J. Biol. Chem. 281, 28193–28199 [DOI] [PubMed] [Google Scholar]
- 25. Pike A. C., Rellos P., Niesen F. H., Turnbull A., Oliver A. W., Parker S. A., Turk B. E., Pearl L. H., Knapp S. (2008) Activation segment dimerization. A mechanism for kinase autophosphorylation of non-consensus sites. EMBO J. 27, 704–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Amara J. F., Clackson T., Rivera V. M., Guo T., Keenan T., Natesan S., Pollock R., Yang W., Courage N. L., Holt D. A., Gilman M. (1997) A versatile synthetic dimerizer for the regulation of protein-protein interactions. Proc. Natl. Acad. Sci. U.S.A. 94, 10618–10623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chang D. W., Yang X. (2003) Activation of procaspases by FK506-binding protein-mediated oligomerization. Sci. STKE 2003, PL1. [DOI] [PubMed] [Google Scholar]
- 28. Crabtree G. R., Schreiber S. L. (1996) Three-part inventions. Intracellular signaling and induced proximity. Trends Biochem. Sci. 21, 418–422 [DOI] [PubMed] [Google Scholar]
- 29. Muthuswamy S. K., Gilman M., Brugge J. S. (1999) Controlled dimerization of ErbB receptors provides evidence for differential signaling by homo- and heterodimers. Mol. Cell. Biol. 19, 6845–6857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Coers W., Reivinen J., Miettinen A., Huitema S., Vos J. T., Salant D. J., Weening J. J. (1996) Characterization of a rat glomerular visceral epithelial cell line. Exp. Nephrol. 4, 184–192 [PubMed] [Google Scholar]
- 31. Huber T. B., Kottgen M., Schilling B., Walz G., Benzing T. (2001) Interaction with podocin facilitates nephrin signaling. J. Biol. Chem. 276, 41543–41546 [DOI] [PubMed] [Google Scholar]
- 32. Shaulian E., Karin M. (2001) AP-1 in cell proliferation and survival. Oncogene 20, 2390–2400 [DOI] [PubMed] [Google Scholar]
- 33. Kennelly P. J., Krebs E. G. (1991) Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J. Biol. Chem. 266, 15555–15558 [PubMed] [Google Scholar]
- 34. Davis R. J. (2000) Signal transduction by the JNK group of MAP kinases. Cell 103, 239–252 [DOI] [PubMed] [Google Scholar]
- 35. De Chiara G., Marcocci M. E., Torcia M., Lucibello M., Rosini P., Bonini P., Higashimoto Y., Damonte G., Armirotti A., Amodei S., Palamara A. T., Russo T., Garaci E., Cozzolino F. (2006) Bcl-2 Phosphorylation by p38 MAPK. Identification of target sites and biologic consequences. J. Biol. Chem. 281, 21353–21361 [DOI] [PubMed] [Google Scholar]
- 36. Corbit K. C., Trakul N., Eves E. M., Diaz B., Marshall M., Rosner M. R. (2003) Activation of Raf-1 signaling by protein kinase C through a mechanism involving Raf kinase inhibitory protein. J. Biol. Chem. 278, 13061–13068 [DOI] [PubMed] [Google Scholar]
- 37. Paleologou K. E., Schmid A. W., Rospigliosi C. C., Kim H. Y., Lamberto G. R., Fredenburg R. A., Lansbury P. T., Jr., Fernandez C. O., Eliezer D., Zweckstetter M., Lashuel H. A. (2008) Phosphorylation at Ser-129 but not the phosphomimics S129E/D inhibits the fibrillation of α-synuclein. J. Biol. Chem. 283, 16895–16905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lemmon M. A., Schlessinger J. (2010) Cell signaling by receptor-tyrosine kinases. Cell 141, 1117–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pirruccello M. (2006) A dimeric kinase assembly underlying autophosphorylation in the p21-activated kinases. J. Mol. Biol. 361, 312–326 [DOI] [PubMed] [Google Scholar]
- 40. Lee S. J., Cobb M. H., Goldsmith E. J. (2009) Crystal structure of domain-swapped STE20 OSR1 kinase domain. Protein Sci. 18, 304–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li Y., Palmer A. G. (2009) Domain swapping in the kinase superfamily: OSR1 joins the mix. Protein Sci. 18, 678–681 [Google Scholar]
- 42. Huttlin E. L., Jedrychowski M. P., Elias J. E., Goswami T., Rad R., Beausoleil S. A., Villén J., Haas W., Sowa M. E., Gygi S. P. (2010) A tissue-specific atlas of mouse protein phosphorylation and expression. Cell 143, 1174–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rinschen M. M., Yu M. J., Wang G., Boja E. S., Hoffert J. D., Pisitkun T., Knepper M. A. (2010) Quantitative phosphoproteomic analysis reveals vasopressin V2 receptor-dependent signaling pathways in renal collecting duct cells. Proc. Natl. Acad. Sci. U.S.A. 107, 3882–3887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weintz G., Olsen J. V., Frühauf K., Niedzielska M., Amit I., Jantsch J., Mages J., Frech C., Dölken L., Mann M., Lang R. (2010) The phosphoproteome of toll-like receptor-activated macrophages. Mol. Syst. Biol. 6, 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Olsen J. V., Vermeulen M., Santamaria A., Kumar C., Miller M. L., Jensen L. J., Gnad F., Cox J., Jensen T. S., Nigg E. A., Brunak S., Mann M. (2010) Quantitative phosphoproteomics reveals widespread full phosphorylation site occupancy during mitosis. Sci. Signal. 3, ra3. [DOI] [PubMed] [Google Scholar]
- 46. Cybulsky A. V., Takano T., Papillon J., Guillemette J., Herzenberg A. M., Kennedy C. R. (2010) Podocyte injury and albuminuria in mice with podocyte-specific overexpression of the Ste20-like kinase, SLK. Am. J. Pathol. 177, 2290–2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hassoun H. T., Lie M. L., Grigoryev D. N., Liu M., Tuder R. M., Rabb H. (2009) Kidney ischemia-reperfusion injury induces caspase-dependent pulmonary apoptosis. Am. J. Physiol. Renal Physiol. 297, F125–F137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Guilluy C., Rolli-Derkinderen M., Loufrani L., Bourgé A., Henrion D., Sabourin L., Loirand G., Pacaud P. (2008) Ste20-related kinase SLK phosphorylates Ser-188 of RhoA to induce vasodilation in response to angiotensin II type 2 receptor activation. Circ. Res. 102, 1265–1274 [DOI] [PubMed] [Google Scholar]
- 49. Roovers K., Wagner S., Storbeck C. J., O'Reilly P., Lo V., Northey J. J., Chmielecki J., Muller W. J., Siegel P. M., Sabourin L. A. (2009) The Ste20-like kinase SLK is required for ErbB2-driven breast cancer cell motility. Oncogene 28, 2839–2848 [DOI] [PubMed] [Google Scholar]