Background: Group VIA Phospholipase A2 (iPLA2β) is activated in β-cell signaling.
Results: β-Cell p38 MAPK is activated by an iPLA2β-dependent mechanism and participates in insulin secretion and apoptosis.
Conclusion: p38 MAPK is a downstream effector of iPLA2β activation in β-cells.
Significance: Understanding how insulin secretion is impaired and β-cell apoptosis occurs could suggest therapeutic strategies for type 2 diabetes mellitus.
Keywords: Apoptosis, β-Cell, Ceramide, Endoplasmic Reticulum Stress, Insulin Secretion, MAP Kinases (MAPKs), Mass Spectrometry (MS), Pancreatic Islets, Phospholipase A
Abstract
Group VIA phospholipase A2 (iPLA2β) in pancreatic islet β-cells participates in glucose-stimulated insulin secretion and sarco(endo)plasmic reticulum ATPase (SERCA) inhibitor-induced apoptosis, and both are attenuated by pharmacologic or genetic reductions in iPLA2β activity and amplified by iPLA2β overexpression. While exploring signaling events that occur downstream of iPLA2β activation, we found that p38 MAPK is activated by phosphorylation in INS-1 insulinoma cells and mouse pancreatic islets, that this increases with iPLA2β expression level, and that it is stimulated by the iPLA2β reaction product arachidonic acid. The insulin secretagogue d-glucose also stimulates β-cell p38 MAPK phosphorylation, and this is prevented by the iPLA2β inhibitor bromoenol lactone. Insulin secretion induced by d-glucose and forskolin is amplified by overexpressing iPLA2β in INS-1 cells and in mouse islets, and the p38 MAPK inhibitor PD169316 prevents both responses. The SERCA inhibitor thapsigargin also stimulates phosphorylation of both β-cell MAPK kinase isoforms and p38 MAPK, and bromoenol lactone prevents both events. Others have reported that iPLA2β products activate Rho family G-proteins that promote MAPK kinase activation via a mechanism inhibited by Clostridium difficile toxin B, which we find to inhibit thapsigargin-induced β-cell p38 MAPK phosphorylation. Thapsigargin-induced β-cell apoptosis and ceramide generation are also prevented by the p38 MAPK inhibitor PD169316. These observations indicate that p38 MAPK is activated downstream of iPLA2β in β-cells incubated with insulin secretagogues or thapsigargin, that this requires prior iPLA2β activation, and that p38 MAPK is involved in the β-cell functional responses of insulin secretion and apoptosis in which iPLA2β participates.
Introduction
Type 2 diabetes mellitus (T2DM)2 is increasingly prevalent and is associated with obesity and cardiovascular disease (1–5). Overt T2DM is preceded by a period of insulin resistance during which euglycemia is maintained by compensatory hypersecretion of insulin by pancreatic islet β-cells (6, 7). Hyperglycemia ensues when β-cells can no longer sustain such hypersecretion (7–10) because of defective glucose-induced insulin secretion by residual β-cells and loss of ∼50% of β-cell mass at the onset of T2DM (10–14).
β-Cells sense the extracellular glucose concentration and modulate insulin secretion accordingly by processes that involve import of extracellular d-glucose by GLUT2 transporters and conversion to glucose 6-phosphate by glucokinase (15–18). Kinetic properties of these proteins cause the rate of d-glucose entry into glycolysis to be proportional to its extracellular concentration (17, 18).
Subsequent glycolytic and mitochondrial metabolism generate metabolic signals, including a rise in [ATP] relative to free [ADP] that causes closure of β-cell plasma membrane KATP channels (19–21). The resultant rise in membrane potential activates voltage-operated Ca2+ channels that mediate extracellular Ca2+ entry into β-cells (22, 23). This increases intracellular [Ca2+], which activates Ca2+-sensitive effectors (e.g. Ca2+/calmodulin-dependent protein kinase II) (24) that trigger insulin exocytosis.
These events are modulated by many other processes that include operation of other ion channels and pumps that affect membrane potential (25–27), mitochondrial reducing equivalent shuttles (28–32), and KATP-independent processes activated by glucose (33–34). Elevating β-cell cAMP also amplifies secretion (35–36), and β-cell signaling involves phospholipid hydrolysis and accumulation of phospholipid-derived mediators (36–44).
Stimulating islets with d-glucose induces hydrolysis of phospholipids and accumulation of nonesterified arachidonic acid (45, 46) by processes that require glucose metabolism (47) but not Ca2+ influx (45–47) and that amplify the glucose-induced rise in β-cell [Ca2+] (48, 49). Among participants in these events is a phospholipase A2 (PLA2) that does not require Ca2+ for catalytic activity, is activated by a rise in [ATP]/[ADP], and is inhibited by a bromoenol lactone (BEL) suicide substrate that also blocks glucose-induced arachidonate release by β-cells and blunts rises in β-cell [Ca2+] and insulin secretion (49–51).
This PLA2 activity is expressed by pancreatic islets and insulinoma cells from several species (50–55). When the cDNA for this PLA2 was cloned from islet libraries (53, 54), it was found to represent an enzyme now designated Group VIA PLA2 (iPLA2β) (56) that was the first recognized member of a potatin-like phospholipase (PNPLA) lipase family with important metabolic functions (57). Participation of iPLA2β in glucose-induced insulin secretion is reflected by the fact that secretion is impaired by its genetic deletion, by suppressing its expression, or by its pharmacologic inhibition (58–62). Moreover, overexpressing iPLA2β in insulinoma cells and mice amplifies insulin secretion (58, 62), and genetic iPLA2β deficiency in mice increases sensitivity to diet-induced diabetes by impairing compensatory insulin hypersecretion (61).
Apoptosis is now considered an important contributor to β-cell loss in T2DM and can be induced by ER stress (13, 14), which results in iPLA2β activation in many cells (63–68). Reported observations suggest that iPLA2β may either protect β-cells from or sensitize β-cells to apoptosis (65, 66, 69–72), and this may reflect the fact that ER stress responses (e.g. the unfolded protein response) initially mitigate stress by triggering compensatory repair mechanisms (73–76). These pathways result in apoptosis only when stress is overwhelming and/or prolonged (76). iPLA2β may play similar dual roles in β-cell stress responses and is reported to protect against staurosporine-induced β-cell apoptosis (69, 70). In contrast, iPLA2β promotes β-cell apoptosis induced by the sarco(endo)plasmic reticulum (SERCA) inhibitor thapsigargin, which causes ER stress by reducing ER Ca2+ content (65, 66, 71, 72). Under these conditions, iPLA2β has clearly been shown to be activated (63–68).
Both glucose-induced insulin secretion and ER stress-induced apoptosis are important β-cell stimulus response events that are affected during the evolution of T2DM. Because iPLA2β participates in both processes, understanding its position in β-cell signaling pathways could suggest strategies to preserve β-cell secretion and to prolong β-cell survival and thereby to prevent or retard development of T2DM. Little is known about downstream events affected by iPLA2β activation, but mitogen-activated protein kinases (MAPKs) often mediate cellular responses to extracellular stimuli or stressors (77). We report here that p38 MAPK is activated in an iPLA2β-dependent manner in β-cells during both glucose-induced insulin secretion and ER stress-induced apoptosis.
EXPERIMENTAL PROCEDURES
Materials
Rainbow molecular mass standards, PVDF membrane, and Triton X-100 were obtained from Bio-Rad; SuperSignal West Femto Substrate was from Thermo Fisher; Coomassie reagent and SDS-PAGE supplies were from Invitrogen; collagenase, protease inhibitor mixture, thapsigargin, common reagents, and salts were from Sigma; bovine serum albumin (BSA; fatty acid-free, fraction V) was from MP Biomedicals (Solon, OH); forskolin was from Calbiochem; synthetic phospholipids used as internal standards in mass spectrometric analyses were from Avanti Polar Lipids (Alabaster, AL); racemic BEL and BEL enantiomers were from Cayman Chemical (Ann Arbor, MI); arachidonic acid was from NuChek Prep (Elysian, MN); p38 MAPK inhibitors PD169316 and SB203580 were from BIOMOL (Plymouth Meeting, PA); and solvents for sample preparation and mass spectrometric analyses were from Fisher. Krebs-Ringer bicarbonate buffer (KRB) contained 115 mm NaCl, 24 mm NaHCO3, 5 mm KCl, 1 mm MgCl2, 2.5 mm CaCl2, and 25 mm HEPES (pH 7.4).
Cell Culture
INS-1 rat insulinoma cells that had been stably transfected to overexpress iPLA2β and mock-transfected INS-1 cells were generated as described (58) and cultured in RPMI 1640 medium containing 11 mm glucose, 10% fetal calf serum, 10 mm Hepes buffer, 2 mm glutamine, 1 mm sodium pyruvate, 50 mm β-mercaptoethanol, 100 units/ml penicillin, and 100 μg/ml streptomycin. The medium was exchanged every 2 days, and the cell cultures were split once a week. Cells were grown to 80% confluence and harvested after treatment as indicated in the figure legends or under “Results.” All incubations were performed at 37 °C under an atmosphere of 95% air, 5% CO2.
Immunoblotting Analyses
Cells were harvested and sonicated, and an aliquot (30 μg) of lysate protein was analyzed by SDS-PAGE (4–20% Tris-glycine gel, Invitrogen), transferred onto Immobilon-P polyvinylidene difluoride membranes (Bio-Rad), and processed for immunoblotting analyses as described (60). The targeted factors and the primary antibody (from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) unless otherwise indicated) concentrations were as follows: phosphorylated p38 (p-p38) (1:500), p38 (1:500), iPLA2β (T-14) (1:2000), pMKK3/6 (1:1000), β-actin (1:10,000; Sigma). The secondary antibody concentration was 1:10,000. Immunoreactive bands were visualized by enhanced chemiluminescence (ECL).
Assessment of Apoptosis by Flow Cytometry
INS-1 cell apoptosis was determined essentially as described (69), using an Annexin-V-FLUOS staining kit (Roche Applied Science) according to the manufacturer's instructions. Briefly, harvested cells were washed with PBS and resuspended in Annexin-VFLUOS labeling solution (100 μl). After incubation (10–15 min, 15–25 °C), cells were transferred to fluorescence-activated cell sorting (FACS) tubes and diluted 1:5 with buffer provided in the kit. Fluorescence in cells was analyzed with a FACScan flow cytometer (BD Biosciences) at an excitation wavelength of 488 nm, and data were processed with WinMDI 2.9 software.
Ceramide Analyses by Electrospray Ionization (ESI) MS/MS
Lipids were extracted from INS-1 cells under acidic conditions, as described (78, 79). Briefly, cells were harvested and collected by centrifugation, and extraction buffer (2:2:1.8 (v/v/v) chloroform, methanol, 20 mm LiCl) containing 500 ng of C8-ceramide ([M + Li]+ m/z 432) internal standard was added to the cell pellet. After vortex-mixing and centrifugation (800 × g), the organic (lower) layer was collected, concentrated to dryness under nitrogen, and reconstituted in a chloroform/methanol mixture (1:1) containing 0.6% LiCl. The abundances of individual ceramide species relative to the C8-ceramide internal standard were measured by ESI/MS/MS scanning for constant neutral loss of 48, which reflects elimination of water and formaldehyde from [M + Li]+, a loss characteristic of ceramide-Li+ adducts upon low energy collisionally activated dissociation ESI/MS/MS (79). The measured quantities of individual ceramide molecular species were normalized to lipid phosphorus content, which was determined as described (66).
Insulin Secretion by Isolated Pancreatic Islets or INS-1 Cells
Genetically modified mice with disrupted iPLA2β genes (iPLA2β-null) and their wild type littermates were generated as described (61), as were transgenic mice that overexpress iPLA2β in β-cells under control of the rat insulin-1 promoter (62). All animal procedures were performed according to protocols approved by the Washington University School of Medicine Animal Studies Committee. Male mice (4–5 weeks of age) were anesthetized (ketamine/xylazine, 0.75 μl/g body weight) before euthanasia by cervical dislocation. The abdomen was incised, and the pancreas was harvested and used to prepare isolated pancreatic islets as described (80). Mouse islets or INS-1 cells were counted and used in experiments (30 islets or 0.2 million INS-1 cells/incubation) to measure insulin secretion ex vivo. Islets or cells were rinsed with KRB medium containing 0.1% BSA without glucose and placed in silanized glass tubes (12 × 75 mm) in that buffer, through which 95% air, 5% CO2 was bubbled before incubations. Tubes were capped and incubated (37 °C, 30 min, shaking water bath). Buffer was then replaced with KRB medium containing either 0 or 20 mm glucose and 0.1% BSA with or without forskolin (2.5 μm), and samples were incubated for 30 min. Secreted insulin was measured by radioimmunoassay.
Statistical Analyses
Results are presented as mean ± S.E. Data were evaluated by unpaired, two-tailed Student's t test for differences between two conditions or by analysis of variance with appropriate post hoc tests for larger sets (66). Significance levels are described in the figure legends, and a p value of <0.05 was considered statistically significant.
RESULTS
Level of p38 MAPK Phosphorylation Increases with iPLA2β Expression Level in INS-1 Insulinoma Cells and Isolated Pancreatic Islets
The intensity of a band recognized by an antibody directed at the doubly phosphorylated, activated form of p38 MAPK (81) relative to that of a band representing total p38 MAPK on Western blotting analyses was found to be greater in INS-1 cells that had been stably transfected to overexpress iPLA2β compared with cells transfected with empty vector (Fig. 1A and B), and this is also true of islets from transgenic mice that overexpress iPLA2β in β-cells compared with islets from wild-type mice (Fig. 1C). These observations indicate that increased expression of iPLA2β is associated with increased p38 MAPK phosphorylation in β-cells, and one possibility is that this might reflect an effect of iPLA2β reaction products, such as arachidonic acid, to stimulate p38 MAPK phosphorylation.
FIGURE 1.
Level of p38 MAPK phosphorylation increases in INS-1 cells and islets with iPLA2β expression level. Lysates from INS-1 cells or isolated pancreatic islets were analyzed by SDS-PAGE, transferred to PVDF membranes, and probed with antibody directed at phosphorylated p38 MAPK (81) (A, upper bands) or total p38 MAPK (A, lower bands). In B, the densitometric ratios of the bands for phospho-p38 and total p38 are plotted for stably transfected INS-1 cells that overexpress (OE) iPLA2β or for INS-1 cells transfected with vector only (Vector). In C, the densitometric ratios of the bands for phospho-p38 and total p38 are plotted for pancreatic islets isolated from transgenic (TG) mice that overexpress iPLA2β in β-cells or from wild type (WT) mice. Mean values (n = 3) are plotted in B and C, and S.E. values (error bars) are indicated. The p value for the comparison in B is 0.0001, and that for the comparison in C is 0.039.
Arachidonic Acid induces p38 MAPK Phosphorylation in INS-1 Cells
As illustrated in supplemental Fig. S1, when INS-1 insulinoma cells are incubated with exogenous arachidonic acid, a material recognized by an antibody directed at activated, doubly phosphorylated p38 MAPK accumulates in a time- and arachidonate concentration-dependent manner, although there is no observable change in signal for total p38 MAPK under these conditions. This indicates that arachidonic acid can induce phosphorylation of p38 MAPK.
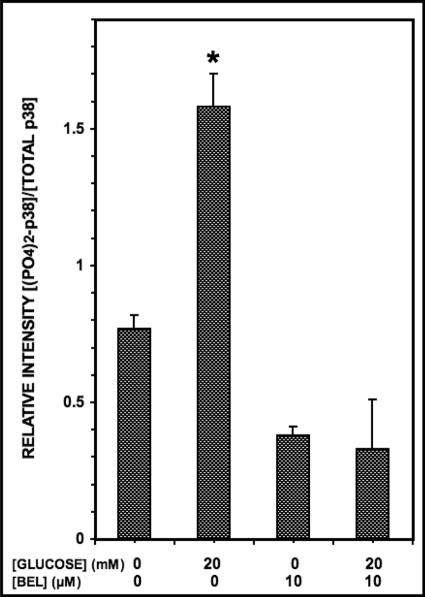
Stimulation of INS-1-OE Cells with d-Glucose Increases p38 MAPK Phosphorylation, and This Is Prevented by iPLA2β Inhibitor BEL
Stimulating INS-1 cells or pancreatic islet β-cells with d-glucose is known to result in iPLA2β activation as reflected by product release and subcellular redistribution of the enzyme (55, 58–62), and the compound BEL is an effective inhibitor of iPLA2β activity (82–84). To determine whether p38 MAPK phosphorylation is affected under these conditions, INS-1-OE cells were stimulated with 20 mm d-glucose (Fig. 2) in the presence or absence of BEL, and p38 MAPK phosphorylation was assessed in Western blotting experiments like those in Fig. 1 and supplemental Fig. S1 in which the intensity of the band recognized by an antibody directed against doubly phosphorylated p38 MAPK is compared with that of the band representing total immunoreactive p38 MAPK. These experiments revealed that 20 mm d-glucose increases p38 MAPK phosphorylation in INS-1-OE cells and that this is prevented by the iPLA2β inhibitor BEL (Fig. 2). In the context of the findings in Fig. 1 and supplemental Fig. S1, observations illustrated in Fig. 2 suggest that iPLA2β activation that occurs in INS-1 cells stimulated with d-glucose results in p38 MAPK phosphorylation and activation and raise the question of whether this is of functional significance in the insulin secretory response to glucose in which iPLA2β is known to be involved (58–62).
FIGURE 2.
Glucose stimulates p38 MAPK phosphorylation in INS-1-OE cells, and this is prevented by the iPLA2β inhibitor BEL. Stably transfected INS-1 cells that overexpress iPLA2β were preincubated (1 h, 37 °C) with BEL (10 μm) or DMSO vehicle alone. The medium was then replaced by fresh medium containing 20 mm or no d-glucose without or with BEL (10 μm), and the cells were again incubated (30 min, 37 °C). At the end of that incubation interval, cell lysates were prepared and analyzed by SDS-PAGE and immunoblotting with antibody directed at phosphorylated p38 MAPK or total p38 MAPK as in Fig. 1. The figure displays mean values (n = 3) for the densitometric ratios of the bands for phospho-p38 and total p38, and S.E. values (error bars) are indicated. *, p < 0.05 for the value at the indicated condition versus that for 20 mm glucose with BEL.
Insulin Secretion Is Amplified by Overexpression of iPLA2β in INS-1 Insulinoma Cells and in Isolated Pancreatic Islets, and This Is Prevented by Pharmacologic Inhibition of p38 MAPK
Glucose-induced insulin secretion from isolated pancreatic islets and INS-1 cells is associated with iPLA2β activation, is suppressed by pharmacologic inhibition or genetic ablation of iPLA2β activity, and is amplified by overexpression of iPLA2β, particularly in the presence of agents that increase β-cell levels of cAMP (55, 58–62), as illustrated in Fig. 3. Fig. 3A demonstrates that pancreatic islets from wild-type mice exhibit robust insulin secretion when stimulated with 20 mm d-glucose and the adenylyl cyclase activator forskolin, and this is also true for islets of transgenic mice that overexpress iPLA2β in β-cells. In addition, the latter exhibit an amplified insulin secretory response compared with the former, as reported previously (62), and this is also true for the INS-1 cells in Fig. 3B.
FIGURE 3.
Secretagogue-induced insulin secretion from INS-1 insulinoma cells and pancreatic islets increases with iPLA2β expression level and is suppressed by pharmacologic inhibition of p38 MAPK. In A, pancreatic islets isolated from wild-type mice (WT; blue or yellow bars) or from transgenic mice (TG; red or green bars) that overexpress iPLA2β in islet β-cells were preincubated (1 h, 37 °C) with the p38 MAPK inhibitor (85) PD169316 (20 μm) (yellow or green bars) or vehicle (blue or red bars) and then placed in fresh KRB medium containing 0 or 20 mm d-glucose without or with 2.5 μm forskolin and incubated (30 min, 37 °C). In B, similar experiments were performed with INS-1 insulinoma cells stably transfected with empty vector (V; blue or yellow bars) or with a construct that causes overexpression of iPLA2β (OE; red or green bars) that were incubated without (blue or red bars) or with (yellow or green bars) PD169316 and with 0 or 20 mm d-glucose and 0 or 2.5 μm forskolin, as in A. At the end of the incubation period, aliquots of medium were removed for measurement of insulin content as described under “Experimental Procedures.” Mean values are displayed, and S.E. values (error bars) are indicated (n = 3). *, p < 0.05 for the comparison of the indicated condition and the analogous condition in which incubation was performed in the presence of PD169316.
We have reported previously that INS-1 cells transfected with empty vector exhibit poor insulin secretory responses to glucose and that glucose responsiveness is greatly amplified by overexpressing iPLA2β so that the responses of the insulinoma cells more closely resemble that of native islets (58). Similarly, in the experiments illustrated in Fig. 3B, the secretory responses of iPLA2β-overexpressing INS-1 cells also more closely resemble those of native wild type islets (see Fig. 3A) than do responses of INS-1 cells transfected with empty vector.
To examine the possibility that p38 MAPK activation might be involved in the amplifying effect of iPLA2β overexpression on insulin secretion, we examined effects of pharmacologic inhibition of p38 MAPK activity with the compound PD169316 (85), and substantial inhibition of insulin secretion induced by 20 mm d-glucose and forskolin is observed with both islets (Fig. 3A) and INS-1 cells (Fig. 3B) incubated with PD169316. These observations are consistent with the possibility that p38 MAPK activation participates in the amplification of insulin secretion that occurs when iPLA2β is overexpressed in β-cells. In addition, the fact that Fig. 3A demonstrates that the p38 MAPK inhibitor PD169316 greatly attenuates insulin secretion induced by 20 mm d-glucose and forskolin from wild type islets indicates that p38 MAPK also participates in the signaling pathway activated by glucose and forskolin in β-cells with normal endogenous levels of iPLA2β expression that have not been genetically manipulated to overexpress iPLA2β.
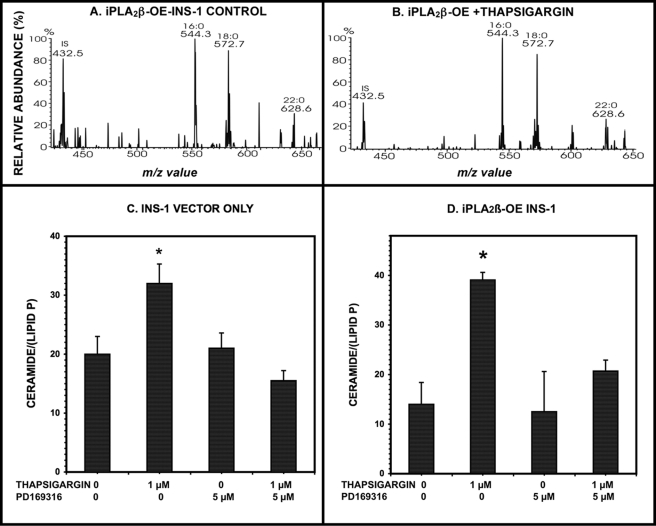
Pharmacologic Inhibition of p38 MAPK Results in Attenuation of Ceramide Accumulation in β-Cells in Which ER Stress Is Induced by Treatment with Thapsigargin
Another stimulus that is known to result in iPLA2β activation in a variety of cells, including β-cells, vascular smooth muscle cells, and macrophages, is induction of ER stress by depleting ER Ca2+ content with the SERCA inhibitor thapsigargin (63–68, 71, 72). This is associated with iPLA2β activation, as reflected by product generation and subcellular redistribution of the enzyme (63–68, 71, 72). Many cells, including β-cells and macrophages, inter alia, undergo apoptosis under these conditions (63–67, 71, 72). Thapsigargin-induced apoptosis of β-cells is associated with ceramide accumulation that occurs as a consequence of transcriptional up-regulation of neutral sphingomyelinase, and these events require iPLA2β activation and are blocked by pharmacologic inhibition or genetic ablation of iPLA2β and are amplified by iPLA2β overexpression (65, 71, 72).
If p38 MAPK activation is a general consequence of iPLA2β activation in β-cells, then it is possible that p38 MAPK participates in the induction of ceramide accumulation and subsequent apoptosis of β-cells induced by ER stress. To explore this possibility, we examined the effects of pharmacologic inhibition of p38 MAPK activity with PD169316 (85) on these events. Fig. 4 illustrates that thapsigargin does induce ceramide accumulation (Fig. 4, A and B) in parental INS-1 cells transfected with only vector (Fig. 4C), as demonstrated by ESI/MS/MS constant neutral loss scanning measurements with an internal standard (65, 79), and this effect is amplified by iPLA2β overexpression (Fig. 4D) and attenuated by PD169316 (Fig. 4, C and D). These observations are consistent with the possibility that iPLA2β-mediated p38 MAPK activation is involved in the chain of events triggered by induction of ER stress in β-cells that leads to accumulation of ceramide.
FIGURE 4.
Induction of ER stress with thapsigargin induces ceramide accumulation in INS-1 cells, and this is prevented by pharmacologic inhibition of p38 MAPK. INS-1 insulinoma cells stably transfected with a construct that causes overexpression of iPLA2β (OE) or with empty vector (V) were preincubated (1 h, 37 °C) with the p38 MAPK inhibitor PD169316 (5 μm) or with vehicle diluent alone and then placed in fresh KRB medium without or with thapsigargin (1 μm). At the end of the incubation period, cells were collected, homogenized, and extracted by a modified Bligh-Dyer method. The extract was admixed with internal standard C8-ceramide, concentrated, reconstituted, infused in a solution to which Li+ had been added, and analyzed by ESI/MS/MS scanning for constant neutral loss (CNL) of 48 from [M + Li]+ ions as described under “Experimental Procedures.” Quantitation of each ceramide species was achieved by dividing the ion current at the m/z value for that species by the ion current of the internal standard at m/z 432.5 and interpolating from a calibration curve. This value was then normalized to the measured lipid phosphorus content of the sample. A and B, representative CNL spectra from INS-1 cells incubated only with vehicle or with 1 μm thapsigargin, respectively. In C and D, mean values (n = 3) for the normalized total amount of ceramide species under the indicated conditions are displayed for INS-1 cells that are transfected with empty vector (C) or that stably overexpress iPLA2β (D), and S.E. values (error bars) are indicated. *, p < 0.05 for the comparison of the indicated condition versus the analogous condition in which incubation was performed in the presence of PD169316.
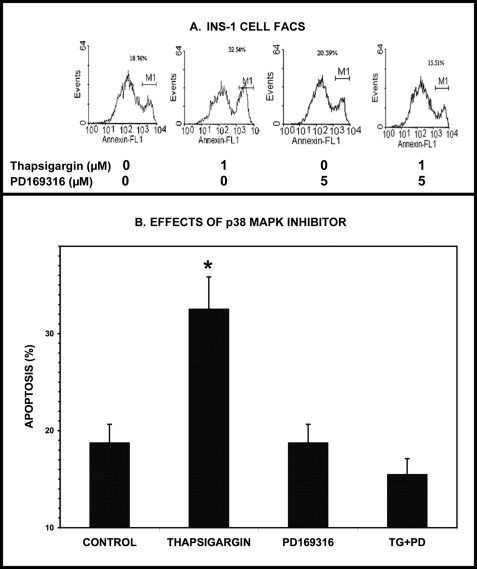
Thapsigargin-induced Apoptosis of INS-1-OE Cells Is Attenuated by p38 MAPK Inhibitor PD169316
As indicated above, inducing ER stress in INS-1 cells is associated with iPLA2β activation, ceramide accumulation, and apoptosis. This apoptotic response is amplified by overexpression of iPLA2β and attenuated by pharmacologic inhibition or genetic ablation of iPLA2β activity (63, 65–67, 71, 72). Because p38 MAPK activation appears to occur as a consequence of iPLA2β activation and to participate in thapsigargin-induced ceramide accumulation in β-cells, it is possible that p38 MAPK participates in the induction of apoptosis by ER stress. To examine this possibility, we determined the effects of pharmacologic inhibition of p38 MAPK activity with PD169316 (85) on thapsigargin-induced apoptosis of INS-1-OE cells, as reflected by phosphatidylserine externalization visualized with Annexin IV binding and FACS (69). As illustrated in Fig. 5, thapsigargin-induced INS-1-OE cell apoptosis is attenuated by PD169316.
FIGURE 5.
Induction of ER stress with thapsigargin induces apoptosis of INS-1-OE cells, and this is prevented by pharmacologic inhibition of p38 MAPK. In A, stably transfected INS-1 insulinoma cells that overexpress iPLA2β were preincubated (1 h, 37 °C) with the p38 MAPK inhibitor PD169316 (5 μm) or with diluent vehicle alone and then placed in fresh KRB medium without or with thapsigargin (1 μm). At the end of the incubation period, cells were collected, stained with Annexin-V, and analyzed by FACS, as described under “Experimental Procedures.” In B, mean values for the percentage of apoptotic cells for each condition are displayed (n = 3), and S.E. values (error bars) are indicated. *, p < 0.05 for the indicated condition versus the analogous condition in which incubation was performed in the presence of PD169316.
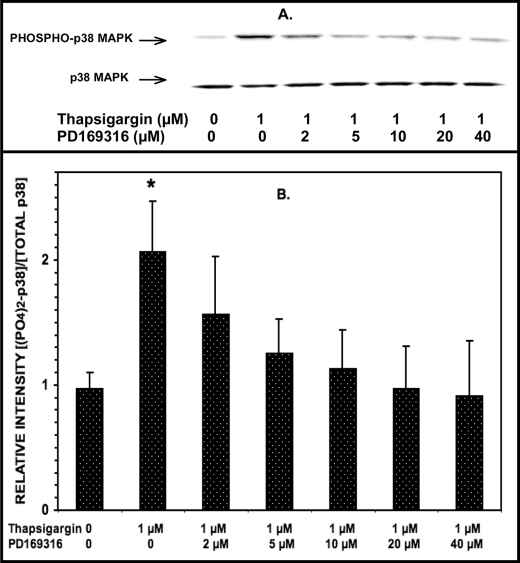
Induction of ER Stress in INS-1-OE Cells with Thapsigargin Increases p38 MAPK Phosphorylation, and This Is Prevented by p38 MAPK Inhibitor PD169316 in a Concentration-dependent Manner
The findings in Figs. 4 and 5 indicate that the p38 MAPK inhibitor PD169316 attenuates thapsigargin-induced ceramide accumulation in and apoptosis of INS-1 cells. This implies that thapsigargin should induce activation of p38 MAPK in INS-1 cells, as reflected by its increased phosphorylation, and that this should be attenuated by PD169316 within the concentration range shown to attenuate thapsigargin-induced ceramide accumulation and apoptosis.
To examine this possibility, INS-1-OE cells were treated with thapsigargin in the presence of various concentrations of PD169316, and p38 MAPK phosphorylation was assessed in Western blotting experiments as in Fig. 3. These experiments revealed that incubating INS-1-OE cells with thapsigargin increases p38 MAPK phosphorylation and that this effect is attenuated in a concentration-dependent manner by PD169316 (Fig. 6). Substantial and significant inhibition of thapsigargin-induced p38 MAPK phosphorylation was observed at a PD169316 concentration of 5 μm, and essentially complete inhibition was observed at a concentration of 20 μm.
FIGURE 6.
Induction of ER stress with thapsigargin stimulates p38 MAPK phosphorylation in INS-1-OE cells, and this is prevented by the p38 MAPK inhibitor PD169316 in a concentration-dependent manner. Stably transfected INS-1 insulinoma cells that overexpress iPLA2β were preincubated (1 h, 37 °C) without or with varied concentrations of the p38 MAPK inhibitor PD169316 (0, 2, 5, 10, 20, or 40 μm). Then the medium was replaced by fresh medium containing 1 μm or no thapsigargin without or with varied concentrations of PD169319 (the same as used in the preincubation) and incubated (30 min, 37 °C). At the end of the incubation interval, cell lysates were prepared and analyzed by SDS-PAGE and immunoblotting with antibody directed at phosphorylated p38 MAPK or total p38 MAPK (A) as in Fig. 1. The immunoblots were analyzed by densitometry, and B displays mean values (n = 3) for the densitometric ratios of the bands for phospho-p38 and total p38. S.E. values (error bars) are indicated. *, p < 0.05 for the value at the indicated condition versus the analogous condition in which incubation was performed in the presence of 40 μm PD169316.
This corresponds to the range in which functional effects of PD169316 were observed on insulin secretion (Fig. 3), ceramide accumulation (Fig. 4), and apoptosis (Fig. 5), respectively. A second p38 MAPK inhibitor (SB203580) also inhibited thapsigargin-induced p38 MAPK phosphorylation (supplemental Fig. S2) but was less potent than PD169316, and at the concentrations required to achieve adequate inhibition, SB203580 exerted cytotoxic effects that precluded its use in experiments examining apoptosis, as also reported by others (85).
Thapsigargin also induced an increase in p38 MAPK phosphorylation in INS-1 cells transfected with empty vector that do not overexpress iPLA2β, and this was also suppressed by PD169316 (supplemental Fig. S3A). Thapsigargin also increased apoptosis in these cells, and that response too was suppressed by PD169316 (supplemental Fig. S3B), as was thapsigargin-induced ceramide accumulation (Fig. 4). The findings in supplemental Fig. S3 and Fig. 4 thus indicate that p38 MAPK participates in the signaling pathway activated by thapsigargin in β-cells with normal endogenous levels of iPLA2β that have not been genetically manipulated to overexpress iPLA2β, although the magnitudes of these responses are larger in cells that do overexpress iPLA2β.
The Thapsigargin-induced Increase in p38 MAPK Phosphorylation in INS-1 Cells Is Prevented by iPLA2β Inhibitor BEL, and This Is Reversed by Adding Exogenous Arachidonic Acid
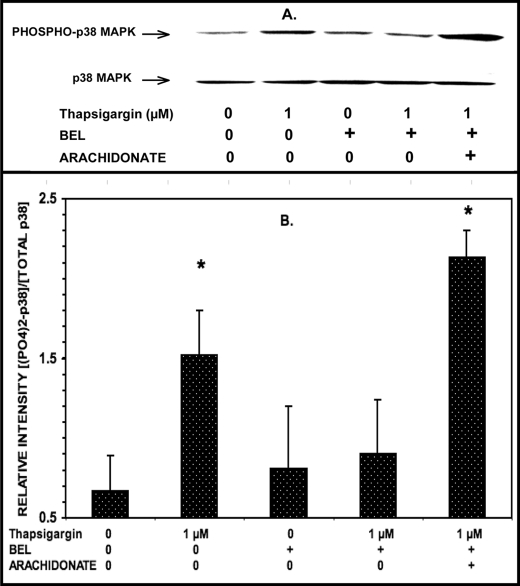
Inducing ER stress in INS-1 cells induces ceramide accumulation and apoptosis, and both effects are prevented by the iPLA2β inhibitor BEL (65, 71, 72) and the p38 MAPK inhibitor PD169316 (Figs. 4 and 5). Glucose-induced p38 MAPK phosphorylation in β-cells is suppressed by the iPLA2β inhibitor BEL (Fig. 2), and β-cell p38 MAPK phosphorylation is stimulated by the iPLA2β reaction product arachidonic acid (supplemental Fig. S2) or by overexpression of iPLA2β (Fig. 1). These findings suggest that activation of iPLA2β in β-cells results in subsequent activation of p38 MAPK that is dependent on iPLA2β activity.
To test this possibility, the effects of the iPLA2β inhibitor BEL on thapsigargin-induced p38 MAPK activation and phosphorylation in β-cells were examined. As illustrated in Fig. 7, BEL suppressed thapsigargin-induced p38 MAPK phosphorylation in INS-1-OE cells. In addition, inhibition of p38 MAPK phosphorylation by BEL was reversed by the addition of the iPLA2β reaction product arachidonic acid (Fig. 7). Examination of the effects of BEL enantiomers separately indicated that (S)-BEL but not (R)-BEL inhibited thapsigargin-induced p38 MAPK phosphorylation (supplemental Fig. S4). Because (S)-BEL preferentially inhibits iPLA2β, whereas (R)-BEL preferentially inhibits iPLA2γ (86), the findings in supplemental Fig. S4 are consistent with the hypothesis that β-cell iPLA2β is the BEL-sensitive enzyme involved in signaling pathways that result in p38 MAPK phosphorylation.
FIGURE 7.
The iPLA2β inhibitor BEL suppresses thapsigargin-induced p38 MAPK phosphorylation in INS-1-OE cells, and this is reversed by exogenous arachidonic acid. Stably transfected INS-1 cells that overexpress iPLA2β were preincubated (1 h, 37 °C) with BEL (10 μm) or DMSO vehicle alone. Then the medium was replaced by fresh medium containing 1 μm or no thapsigargin without or with BEL (10 μm) and without or with exogenous arachidonic acid (70 μm) and incubated (30 min, 37 °C). At the end of the incubation interval, cell lysates were prepared and analyzed by SDS-PAGE and immunoblotting with antibody directed at phosphorylated p38 MAPK or total p38 MAPK (A) as in Fig. 1. B displays mean values (n = 3) for the densitometric ratios of the bands for phospho-p38 and total p38. S.E. values (error bars) are indicated. *, p < 0.05 for the value at the indicated condition versus the condition in which incubation was performed in the presence of 1 μm thapsigargin, 10 μm BEL, and no exogenous arachidonic acid.
Together, the findings in Figs. 4–7 and supplemental Figs. S1–S4 indicate that iPLA2β activation that occurs in INS-1 cells treated with thapsigargin results in p38 MAPK phosphorylation and activation, and a mechanism whereby that could occur is via activation of an upstream kinase for which p38 MAPK is a substrate.
Upstream Protein Kinases and Small GTP-binding Proteins Are Involved in iPLA2β-mediated Activation of p38 MAPK in INS-1 Cells
Recent reports indicate that iPLA2β is involved in activation of the Rho family small G proteins Rac1 and Cdc42 in macrophages and vascular smooth muscle cells and that this requires conversion of arachidonic acid released by iPLA2β to 12/15-lipoxygenase products (87–89). Rac1 and Cdc42 are known to bind to the protein kinase myosin light-chain kinase-1 (MLK1), and this can result in p38 MAPK phosphorylation via the action of the protein kinase MEK3 (90–92). Alternatively, Rac1 and Cdc42 can promote p38 MAPK phosphorylation via interaction with p21-activated kinases family member protein kinases (93, 94). These observations raise the possibility that the signaling sequence that is activated in β-cells stimulated with thapsigargin might involve sequential iPLA2β activation, arachidonic acid release and oxygenation, and Rho family G-protein activation, followed by activation of MLK1 and MEK3 and then phosphorylation of p38 MAPK.
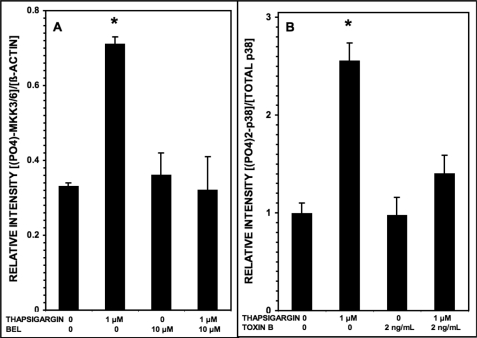
To examine these possibilities, we first determined whether treating INS-1 cells with thapsigargin results in MEK3 phosphorylation, and Fig. 8A illustrates that this is the case and that the iPLA2β inhibitor BEL prevents this effect, suggesting that iPLA2β participates in this signaling cascade. To examine the potential involvement of small G proteins in these processes, we determined effects of Clostridium difficile toxin B, which modifies and inhibits small G proteins of the Rho family via its glucosyltransferase activity (95). Toxin B alone increases phosphorylation of p38 MAPK in HaCaT cells (96), and this is also true for INS-1 cells, although toxin B also blocks the effect of thapsigargin to stimulate p38 MAPK phosphorylation in INS-1 cells (Fig. 8B). These findings indicate that in INS-1 cells treated with thapsigargin, the protein kinase MEK3 is activated, which is known to act as an upstream kinase in p38 MAPK phosphorylation in other cells (90–92). The observations in Figs. 7 and 8 thus indicate that inhibiting iPLA2β with BEL blocks thapsigargin-induced phosphorylation of both MEK3 and p38 MAPK in INS-1 cells, which suggests that sequential activation of iPLA2β, MEK3, and p38 MAPK occurs in INS-1 cells treated with thapsigargin.
FIGURE 8.
Phosphorylation of MAPK kinases 3/6 and of p38 MAPK induced in INS-1-OE cells by thapsigargin is suppressed by the iPLA2β inhibitor BEL and by the Rho family small G-protein inhibitor C. difficile toxin B, respectively. In A, stably transfected INS-1 cells that overexpress iPLA2β were preincubated (1 h, 37 °C) with BEL (10 μm) or DMSO vehicle alone. Then the medium was replaced by fresh medium containing 1 μm or no thapsigargin without or with BEL (10 μm), and the cells were again incubated (30 min, 37 °C). At the end of that incubation interval, cell lysates were prepared and analyzed by SDS-PAGE and immunoblotting with antibody directed at phosphorylated MAPK kinases 3/6 (MKK3/6) or against β-actin. Mean values (n = 3) are displayed for the densitometric ratios of the bands for phospho-MKK3/6 and β-actin, and S.E. values (error bars) are indicated. *, p < 0.05 for the value at the indicated condition versus the condition with 1 μm thapsigargin and 10 μm BEL. In B, stably transfected INS-1 cells that overexpress iPLA2β were preincubated (1 h, 37 °C) with C. difficile toxin B (2 ng/ml) or blank diluent alone. Then the medium was replaced by fresh medium containing 1 μm or no thapsigargin, and the cells were again incubated (30 min, 37 °C). At the end of that incubation interval, cell lysates were prepared and analyzed by SDS-PAGE and immunoblotting with antibody directed at phosphorylated p38 MAPK or total p38 MAPK, as in Fig. 1. The densitometric ratios of the bands for phospho-p38 and total p38 were then determined. Mean values (n = 3) are displayed, and S.E. values are indicated. *, p < 0.05 for the value at the indicated condition versus the condition with 1 μm thapsigargin and 2 ng/ml toxin B.
DISCUSSION
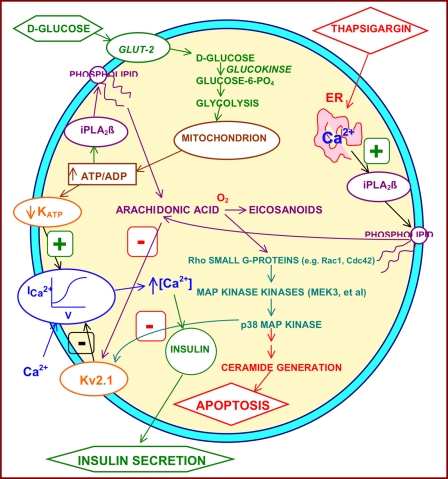
This is the first report of which we are aware that demonstrates participation of p38 MAPK in downstream signaling events that follow iPLA2β activation in β-cells. Fig. 9 provides a schematic summary of the findings reported here and places them in the context of previous observations on the participation of iPLA2β in β-cell signaling and events involved in glucose-induced insulin secretion and in ER stress-induced β-cell apoptosis. Both processes are critically involved in the development of T2DM (8–14).
FIGURE 9.
Schematic diagram of signaling pathways in β-cells in which iPLA2β participates. Signals that induce iPLA2β activation in insulin-secreting β-cells include 1) incubation with concentrations of d-glucose sufficient to stimulate insulin secretion and 2) incubation with SERCA inhibitors (e.g. thapsigargin) that deplete ER Ca2+ content, induce ER stress, and trigger β-cell apoptosis. Activation of iPLA2β occurs in signaling pathways involved both in d-glucose-stimulated insulin secretion and in SERCA inhibitor-induced apoptosis because both sets of responses are attenuated by pharmacologic or genetic reductions in iPLA2β activity, and, conversely, both are amplified by iPLA2β overexpression. The involvement of iPLA2β in insulin secretion may reflect the effect of one of its products, arachidonic acid, to inhibit membrane Kv2.1 channel activity and thereby to prolong the d-glucose-induced action potential in β-cells (62). Amplification of ceramide generation by iPLA2β may represent a component of its participation in apoptosis (65, 71). Data presented in the current paper indicate that p38 MAPK is activated during both glucose-stimulated insulin secretion and SERCA inhibitor-induced apoptosis in β-cells and that this event is downstream of and requires prior iPLA2β activation. Data here and in other studies (87–89) suggest that intermediate events between iPLA2β activation and p38 MAPK activation include arachidonic acid release, its enzymatic oxygenation to bioactive eicosanoids (e.g. 12/15-lipoxygenase products), activation of Rho family small G-proteins (e.g. Rac1 and Cdc42), and activation of MAPK kinases (e.g. MEK3).
As summarized in Fig. 9, d-glucose induces insulin secretion by a process that involves its transport into the β-cell and glycolytic and mitochondrial metabolism to yield signals that include a rise in the ratio of [ATP] to free [ADP] that causes closure of KATP channels in the β-cell plasma membrane (15–21). This causes a rise in membrane potential, activation of voltage-operated Ca2+ channels, influx of Ca2+ from the extracellular space, and a rise in [Ca2+] in cytoplasm and other intracellular compartments, which activates Ca2+-sensitive effectors that trigger insulin exocytosis (21–24). d-Glucose also stimulates hydrolysis of arachidonic acid from β-cell membrane phospholipids under these conditions (44–47), and this is mediated at least in part by iPLA2β (49–52), which may be activated by a rise in [ATP/[ADP] (50, 51). That this process contributes to the glucose-induced rise in β-cell cytosolic [Ca2+] and amplifies the insulin secretory response is reflected by the facts that genetic or pharmacologic suppression of iPLA2β activity impairs glucose-induced insulin secretion and the rise in β-cell cytosolic [Ca2+] (49–52, 58–62) and that iPLA2β overexpression in β-cells augments both the rise in [Ca2+] and secretion (58, 62).
The mechanism by which iPLA2β activation amplifies insulin secretion is incompletely understood, but recent observations indicate that modulation of Kv2.1 voltage-operated K+ channel activity in the β-cell plasma membrane may be involved (62, 97–98). After d-glucose-induced depolarization of the plasma membrane by the sequence of events described above, Kv2.1 channels are activated and serve to repolarize the membrane and limit the duration of the glucose-induced action potential and of the rise in cytosolic [Ca2+] (98).
Accordingly, genetic deletion of Kv2.1 channels in mice results in prolongation of the action potential and [Ca2+] transient and in amplification of glucose-induced insulin secretion from isolated islets and in a corresponding improvement of glucose tolerance in Kv2.1-null mice (98). Arachidonic acid attenuates β-cell Kv2.1 activity, and genetic deletion or pharmacologic inhibition of iPLA2β eliminates the glucose-induced diminution of β-cell Kv2.1 activity that occurs otherwise (97). Moreover, overexpression of iPLA2β in insulinoma cells or islets amplifies the effect of glucose to reduce Kv2.1 activity, and islets from transgenic mice that overexpress iPLA2β in β-cells exhibit a phenotype that resembles that of Kv2.1-null mice and is characterized by prolonged glucose-induced action potentials and amplified insulin secretory responses that are associated with improved glucose tolerance (62, 97–98).
In this context, it is of interest that several findings reported here suggest the possibility that some downstream effects of iPLA2β in β-cells (e.g. Kv2.1 channel modulation) might be mediated by arachidonic acid-induced p38 MAPK activation. These findings include the facts that: 1) d-glucose activates p38 MAPK (Fig. 2); 2) this effect is mimicked by overexpression of iPLA2β (Fig. 1) or the addition of arachidonic acid (supplemental Fig. S1) and suppressed by the iPLA2β inhibitor BEL (Fig. 2); and 3) d-glucose-induced insulin secretion is suppressed by a p38 MAPK inhibitor (Fig. 3) or BEL (49–52, 55, 58–61). Consistent with our observations that arachidonic acid activates p38 MAPK in β-cells, arachidonic acid has also been reported to activate p38 MAPK in a variety of other cells and to affect insulin sensitivity by that mechanism (99, 100). Kv2.1 channel activity in neurons is regulated by graded phosphorylation-dephosphorylation events at residues that include MAPK consensus sites (101–102), and p38 MAPK-catalyzed phosphorylation at residue Ser800 of the Kv2.1 channel is required for oxidant-induced apoptosis of neurons (103).
Similarly, iPLA2β-mediated p38 MAPK activation appears to be involved in ER stress-induced β-cell apoptosis (Figs. 4–8) because inhibition of either iPLA2β (65, 71, 72) or p38 MAPK attenuates the apoptotic response to SERCA inhibition with thapsigargin (Fig. 5), and inhibition of iPLA2β also prevents ER stress-induced p38 MAPK activation (Figs. 7 and 8). Induction of ER stress in β-cells results in iPLA2β phosphorylation at residue Tyr616, its association with the ER-resident chaperone protein calnexin (68), and catalytic activation of iPLA2β (63–67).
Events downstream of iPLA2β in the signaling pathway that links induction of ER stress in β-cells and apoptosis include ceramide accumulation, and this appears to be required for induction of β-cell apoptosis (65, 71, 72, 104). This requirement is reflected by the fact that suppressing ceramide generation by pharmacologic inhibition or molecular biologic knockdown of neutral sphingomyelinase greatly diminishes the apoptotic response of β-cells to SERCA inhibition (65, 71, 72, 104). Findings reported here indicate that p38 MAPK activation is also required for ER stress-induced ceramide generation in β-cells because ceramide accumulation is prevented by inhibition of p38 MAPK (Fig. 4), just as it is inhibited by BEL (65, 71, 72).
This could reflect effects of p38 MAPK on transcriptional cascades because ER stress-induced ceramide accumulation in β-cells involves transcriptional up-regulation of the enzyme neutral sphingomyelinase (71, 72), and p38 MAPK is known to affect other transcriptional events (100, 105). In other settings, iPLA2β activation is also known to participate in stimulus-induced transcriptional up-regulation, including angiotensin II-induced RGS2 up-regulation in vascular smooth muscle cells (87) and encephalomyocarditis virus-induced inducible nitric-oxide synthase induction in macrophages (106). CREB phosphorylation occurs downstream of iPLA2β activation in both of those cases and is required for transcriptional activation to occur (87, 106). CREB is also a recognized downstream target of p38 MAPK (105).
In summary, as illustrated in Fig. 9, findings reported here indicate that activation of iPLA2β in β-cells that occurs during the processes of glucose-induced insulin secretion and ER stress-induced apoptosis is associated with activation of p38 MAPK and that this is prevented by the iPLA2β inhibitor BEL (Figs. 2, 7, and 8) and mimicked by arachidonic acid (supplemental Fig. S1), which is released from β-cell membrane phospholipids by the action of iPLA2β (44–52). Moreover, adding exogenous arachidonic acid reverses the inhibition of thapsigargin-induced p38 MAPK phosphorylation by BEL (Fig. 7).
In addition, p38 MAPK appears to be required for the functional responses of insulin secretion and apoptosis in these settings because these events are prevented or attenuated by pharmacologic inhibition of p38 MAPK (Figs. 3–5). These observations suggest that p38 MAPK activation is among the signaling events that lie downstream of iPLA2β activation in β-cells. Recent reports indicate that small GTP-binding proteins of the Rho family, including Rac and Cdc42, are activated downstream of iPLA2β by eicosanoids in signaling pathways (88, 89). Rac1 and Cdc42 are upstream activators of p38 MAPK in a number of settings (105), and small G proteins of the Rho class may also lie downstream of iPLA2β in β-cell signaling and participate in p38 MAPK activation via upstream kinases (Fig. 8), thus representing a link between iPLA2β action and p38 MAPK activation (Fig. 9).
Others have recently reported that in cultured prostate cancer cells, inhibition of iPLA2β with BEL or (S)-BEL results in activation of p38 MAPK (107). This stands in contrast to our findings in β-cells that inhibition of iPLA2β with BEL or (S)-BEL prevents activation of p38 MAPK otherwise induced by stimulation with d-glucose or by induction of ER stress with thapsigargin. Our findings point to an activating effect of iPLA2β action on p38 MAPK in β-cells, whereas reported data point to an opposite effect in prostate cancer cells (107).
What explains these different patterns of responses in the two cell types is not clear at present, but differential effects of the products of iPLA2β action, which include free fatty acids and 2-lysophospholipids, via interaction with different sets of effectors may occur in the two cell types. Alternatively or in addition, iPLA2β may act on a different set of substrates in distinct membranous compartments in the two cell types to produce opposing responses.
Supplementary Material
Acknowledgments
We thank Robert Sanders for assistance in preparing the manuscript and its figures and Alan Bohrer for technical assistance.
This work was supported, in whole or in part, by National Institutes of Health United States Public Health Service Grants R37-DK34388, P41-RR00954, P60-DK20579, and P30-DK56341.

This article contains supplemental Figs. S1–S4.
- T2DM
- type 2 diabetes mellitus
- PLA2
- phospholipase A2
- ER
- endoplasmic reticulum
- iPLA2β
- Group VIA PLA2
- MS/MS
- tandem mass spectrometry
- OE
- overexpressing
- SERCA
- sarco(endo)plasmic reticulum ATPase
- BEL
- bromoenol lactone
- KRB
- Krebs-Ringer bicarbonate buffer
- ESI
- electrospray ionization
- CREB
- cAMP-response element-binding protein.
REFERENCES
- 1. Mahler R. J., Adler M. L. (1999) Type 2 diabetes mellitus. Update on diagnosis, pathophysiology, and treatment. J. Clin. Endocr. Metab. 84, 1165–1171 [DOI] [PubMed] [Google Scholar]
- 2. Hossain P., Kawar B., El Nahas M. (2007) Obesity and diabetes in the developing world. A growing challenge. N. Engl. J. Med. 356, 213–215 [DOI] [PubMed] [Google Scholar]
- 3. Hotamisligil G. S., Shargill N. S., Spiegelman B. M. (1993) Adipose expression of tumor necrosis factor-α. Direct role in obesity-linked insulin resistance. Science 259, 87–91 [DOI] [PubMed] [Google Scholar]
- 4. Ogden C. L., Carroll M. D., Curtin L. R., Lamb M. M., Flegal K. M. (2010) Prevalence of high body mass index in US children and adolescents. JAMA 303, 242–249 [DOI] [PubMed] [Google Scholar]
- 5. Laing S. P., Swerdlow A. J., Slater S. D., Burden A. C., Morris A., Waugh N. R., Gatling W., Bingley P. J., Patterson C. C. (2003) Mortality from heart disease in a cohort of 23,000 patients with insulin-treated diabetes. Diabetologia 46, 760–765 [DOI] [PubMed] [Google Scholar]
- 6. Shulman GI. (2000) Cellular mechanisms of insulin resistance. J. Clin. Invest. 106, 171–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kahn B. (1998) Type 2 diabetes: When insulin secretion fails to compensate for insulin resistance. Cell 92, 593–596 [DOI] [PubMed] [Google Scholar]
- 8. Gerich J. E. (1998) The genetic basis of type 2 diabetes mellitus. Impaired insulin secretion versus impaired insulin sensitivity. Endocr. Rev. 19, 491–503 [DOI] [PubMed] [Google Scholar]
- 9. Weyer C., Bogardus C., Mott D. M., Pratley R. E. (1999) The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J. Clin. Invest. 104, 787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Klöppel G., Löhr M., Habich K., Oberholzer M., Heitz P. U. (1985) Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Surv. Synth. Pathol. Res. 4, 110–125 [DOI] [PubMed] [Google Scholar]
- 11. Stefan Y., Orci L., Malaisse-Lagae F., Perrelet A., Patel Y., Unger R. H. (1982) Quantitation of endocrine cell content in the pancreas of nondiabetic and diabetic humans. Diabetes 31, 694–700 [DOI] [PubMed] [Google Scholar]
- 12. Butler A. E., Janson J., Bonner-Weir S., Ritzel R., Rizza R. A., Butler P. C. (2003) Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 52, 102–110 [DOI] [PubMed] [Google Scholar]
- 13. Oyadomari S., Araki E., Mori M. (2002) Endoplasmic reticulum stress-mediated apoptosis in pancreatic beta-cells. Apoptosis 7, 335–345 [DOI] [PubMed] [Google Scholar]
- 14. Araki E., Oyadomari S., Mori M. (2003) Impact of endoplasmic reticulum stress pathway on pancreatic beta cells and diabetes mellitus. Exp. Biol. Med. 228, 1213–1217 [DOI] [PubMed] [Google Scholar]
- 15. Ashcroft S. J. (1980) Glucoreceptor mechanisms and the control of insulin release and biosynthesis. Diabetologia 18, 5–15 [DOI] [PubMed] [Google Scholar]
- 16. Meglasson M. D., Matschinsky F. M. (1986) Pancreatic islet glucose metabolism and the regulation of insulin secretion. Diabetes Metab. Rev. 2, 163–214 [DOI] [PubMed] [Google Scholar]
- 17. Garvey W. T. (1992) Glucose transport and NIDDM. Diabetes Care 15, 396–417 [DOI] [PubMed] [Google Scholar]
- 18. Matschinsky F. M. (1990) Glucokinase as glucose sensor and metabolic signal generator in pancreatic beta cells and hepatocytes. Diabetes 39, 647–652 [DOI] [PubMed] [Google Scholar]
- 19. Cook D. L., Hales C. N. (1984) Intracellular ATP directly blocks K+ channels in pancreatic β-cells. Nature 311, 271–273 [DOI] [PubMed] [Google Scholar]
- 20. Kakei M., Kelly R. P., Ashcroft S. J., Ashcroft F. M. (1986) The ATP sensitivity of K+ channels in rat pancreatic β-cells is modulated by ADP. FEBS Lett. 208, 63–66 [DOI] [PubMed] [Google Scholar]
- 21. Ghosh A., Ronner P., Cheong E., Khalid P., Matschinsky F. M. (1991) The role of ATP and free ADP in metabolic coupling during fuel-stimulated insulin release from islet β-cells in the isolated perfused rat pancreas. J. Biol. Chem. 266, 22887–22892 [PubMed] [Google Scholar]
- 22. Arkhammar P., Nilsson T., Rorsman P., Berggren P. O. (1987) Inhibition of ATP-regulated K+ channels precedes depolarization-induced increase in cytoplasmic free Ca2+ concentration in pancreatic beta cells. J. Biol. Chem. 262, 5448–5454 [PubMed] [Google Scholar]
- 23. Misler S., Barnett D. W., Pressel D. M., Gillis K. D., Scharp D. W., Falke L. C. (1992) Stimulus-secretion coupling in β-cells of human islets of Langerhans. Evidence for a critical role for Ca2+ entry. Diabetes 41, 662–670 [DOI] [PubMed] [Google Scholar]
- 24. Easom RA. (1999) CaM kinase II. A protein kinase with extraordinary talents germane to insulin exocytosis. Diabetes 48, 675–684 [DOI] [PubMed] [Google Scholar]
- 25. Dukes I. D., Roe M. W., Worley J. F., Philipson L. H. (1997) Glucose-induced alterations in beta cell cytoplasmic Ca2+ involving the coupling of intracellular Ca2+ stores and plasma membrane ion channels. Curr. Opin. Endocrinol. Diabetes Obes. 4, 262–271 [Google Scholar]
- 26. Gilon P., Arredouani A., Gailly P., Gromada J., Henquin J. C. (1999) Uptake and release of Ca2+ by the endoplasmic reticulum contribute to the oscillations of the cytosolic Ca2+ concentration triggered by Ca2+ influx in the electrically excitable pancreatic beta cell. J. Biol. Chem. 274, 20197–20205 [DOI] [PubMed] [Google Scholar]
- 27. Owada S., Larsson O., Arkhammar P., Katz A. I., Chibalin A. V., Berggren P. O., Bertorello A. M. (1999) Glucose decreases Na+,K+-ATPase activity in pancreatic beta cells. An effect mediated by Ca2+-independent phospholipase A2 and protein kinase C-dependent phosphorylation of the α-subunit. J. Biol. Chem. 274, 2000–2008 [DOI] [PubMed] [Google Scholar]
- 28. Brown L. J., MacDonald M. J., Lehn D. A., Moran S. M. (1994) Sequence of rat mitochondrial glycerol-3-phosphate dehydrogenase cDNA. Evidence for EF-hand calcium-binding domains. J. Biol. Chem. 269, 14363–14366 [PubMed] [Google Scholar]
- 29. MacDonald MJ. (1995) Feasibility of a mitochondrial pyruvate-malate shuttle in pancreatic islets. Further implication of cytosolic NADPH in insulin secretion. J. Biol. Chem. 270, 20051–20058 [PubMed] [Google Scholar]
- 30. Eto K., Tsubamoto Y., Terauchi Y., Sugiyama T., Kishimoto T., Takahashi N., Yamauchi N., Kubota N., Murayama S., Aizawa T., Akanuma Y., Aizawa S., Kasai H., Yazaki Y., Kadowaki T. (1999) Role of NADH shuttle system in glucose-induced activation of mitochondrial metabolism and insulin secretion. Science 283, 981–985 [DOI] [PubMed] [Google Scholar]
- 31. Ravier M. A., Eto K., Jonkers F. C., Nenquin M., Kadowaki T., Henquin J. C. (2000) The oscillatory behavior of pancreatic islets from mice with mitochondrial glycerol-3-phosphate dehydrogenase knockout. J. Biol. Chem. 275, 1587–1593 [DOI] [PubMed] [Google Scholar]
- 32. Wollheim C. B. (2000) Beta cell mitochondria in the regulation of insulin secretion. A new culprit in type II diabetes. Diabetologia 43, 265–277 [DOI] [PubMed] [Google Scholar]
- 33. Sato Y., Anello M., Henquin J. C. (1999) Glucose regulation of insulin secretion independent of the opening or closure of adenosine triphosphate-sensitive K+ channels in beta cells. Endocrinology 140, 2252–2257 [DOI] [PubMed] [Google Scholar]
- 34. Yajima H., Komatsu M., Schermerhorn T., Aizawa T., Kaneko T., Nagai M., Sharp G. W., Hashizume K. (1999) cAMP enhances insulin secretion by an action on the ATP-sensitive K+ channel-independent pathway of glucose signaling in rat pancreatic islets. Diabetes 48, 1006–1012 [DOI] [PubMed] [Google Scholar]
- 35. Seino S., Shibasaki T. (2005) PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol. Rev. 85, 1303–1342 [DOI] [PubMed] [Google Scholar]
- 36. Prentki M., Matschinsky F. M. (1987) Ca2+, cAMP, and phospholipid-derived messengers in coupling mechanisms of insulin secretion. Physiol. Rev. 67, 1185–1248 [DOI] [PubMed] [Google Scholar]
- 37. Laychock S. G. (1982) Phospholipase A2 activity in pancreatic islets is calcium-dependent and stimulated by glucose. Cell Calcium 3, 43–54 [DOI] [PubMed] [Google Scholar]
- 38. Dunlop M. E., Larkins R. G. (1984) Activity of endogenous phospholipase C and phospholipase A2 in glucose stimulated pancreatic islets. Biochem. Biophys. Res. Commun. 120, 820–827 [DOI] [PubMed] [Google Scholar]
- 39. Metz S. A. (1991) The pancreatic islet as Rubik's cube. Is phospholipid hydrolysis a piece of the puzzle? Diabetes 40, 1565–1573 [DOI] [PubMed] [Google Scholar]
- 40. Thams P., Capito K. (1997) Inhibition of glucose-induced insulin secretion by the diacylglycerol lipase inhibitor RHC80267 and the phospholipase A2 inhibitor ACA through stimulation of K+ permeability without diminution by exogenous arachidonic acid. Biochem. Pharm. 53, 1077–1086 [DOI] [PubMed] [Google Scholar]
- 41. Simonsson E., Ahren B. (2000) Phospholipase A2 and its potential regulation of islet function. Int. J. Pancreatol. 27, 1–11 [DOI] [PubMed] [Google Scholar]
- 42. Song K., Zhang X., Zhao C., Ang N. T., Ma Z. A. (2005) Inhibition of Ca2+-independent phospholipase A2 results in insufficient insulin secretion and impaired glucose tolerance. Mol Endocrinol. 19, 504–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McDonald P., Veluthakal R., Kaur H., Kowluru A. (2007) Biologically active lipids promote trafficking and membrane association of Rac1 in insulin-secreting INS 832/13 cells. Am. J. Physiol. Cell. Physiol. 292, C1216–C1220 [DOI] [PubMed] [Google Scholar]
- 44. Poitout V. (2008) Phospholipid hydrolysis and insulin secretion. A step toward solving the Rubik's cube. Am. J. Physiol. Endocrinol. Metab. 294, E214–E216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wolf B. A., Turk J., Sherman W. R., McDaniel M. L. (1986) Intracellular Ca2+ mobilization by arachidonic acid. Comparison with myo-inositol 1,4,5-triphosphate in isolated pancreatic islets. J. Biol. Chem. 261, 3501–3511 [PubMed] [Google Scholar]
- 46. Wolf B. A., Pasquale S. M., Turk J. (1991) Free fatty acid accumulation in secretagogue-stimulated pancreatic islets and effects of arachidonate on depolarization-induced insulin secretion. Biochemistry 30, 6372–6379 [DOI] [PubMed] [Google Scholar]
- 47. Turk J., Mueller M., Bohrer A., Ramanadham S. (1992) Arachidonic acid metabolism in isolated pancreatic islets VI. Carbohydrate insulin secretagogues must be metabolized to induce eicosanoid release. Biochim. Biophys. Acta 1125, 280–291 [DOI] [PubMed] [Google Scholar]
- 48. Ramanadham S., Gross R., Turk J. (1992) Arachidonic acid induces an increase in cytosolic calcium concentration in single pancreatic islet beta cells. Biochem. Biophys. Res. Commun. 184, 647–653 [DOI] [PubMed] [Google Scholar]
- 49. Ramanadham S., Gross R. W., Han X., Turk J. (1993) Inhibition of arachidonate release by secretagogue-stimulated pancreatic islets suppresses both insulin secretion and the rise in beta cell cytosolic calcium concentration. Biochemistry 32, 337–346 [DOI] [PubMed] [Google Scholar]
- 50. Gross R. W., Ramanadham S., Kruszka K. K., Han X., Turk J. (1993) Rat and human pancreatic islet cells contain a calcium-independent phospholipase A2 activity selective for hydrolysis of arachidonate which is stimulated by ATP and specifically localized to islet beta cells. Biochemistry 32, 327–336 [DOI] [PubMed] [Google Scholar]
- 51. Ramanadham S., Wolf M. J., Jett P. A., Gross R. W., Turk J. (1994) Characterization of an ATP-stimulatable, Ca2+-independent phospholipase A2 from clonal insulin-secreting HIT cells and rat pancreatic islets. A possible molecular component of the beta cell fuel sensor. Biochemistry 33, 7442–7452 [DOI] [PubMed] [Google Scholar]
- 52. Ramanadham S., Wolf M. J., Li B., Bohrer A., Turk J. (1997) Glucose responsivity and expression of an ATP-stimulatable, Ca2+-independent phospholipase A2 enzyme in clonal insulinoma cell lines. Biochim. Biophys. Acta 1344, 153–164 [DOI] [PubMed] [Google Scholar]
- 53. Ma Z., Ramanadham S., Kempe K., Chi X. S., Ladenson J., Turk J. (1997) Pancreatic islets express a Ca2+-independent phospholipase A2 enzyme that contains a repeated structural motif homologous to the integral membrane protein binding domain of ankyrin. J. Biol. Chem. 272, 11118–11127 [PubMed] [Google Scholar]
- 54. Ma Z., Wang X., Nowatzke W., Ramanadham S., Turk J. (1999) Human pancreatic islets express mRNA species encoding two distinct catalytically active isoforms of group VI phospholipase A2 (iPLA2) that arise from an exon-skipping mechanism of alternative splicing of the transcript from the iPLA2 gene on chromosome 22q13.1. J. Biol. Chem. 274, 9607–9616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ramanadham S., Hsu F. F., Bohrer A., Ma Z., Turk J. (1999) Studies of the role of group VI phospholipase A2 (iPLA2) in fatty acid incorporation, phospholipid remodeling, lysophosphatidylcholine generation, and secretagogue-induced arachidonic acid release in pancreatic islets and insulinoma cells. J. Biol. Chem. 274, 13915–13927 [DOI] [PubMed] [Google Scholar]
- 56. Turk J., Ramanadham S. (2004) Expression and function of Group VIA Phospholipase A2 (iPLA2β) in β-cells. Can J. Physiol. Pharmacol. 82, 824–832 [DOI] [PubMed] [Google Scholar]
- 57. Kienesberger P. C., Oberer M., Lass A., Zechner R. (2009) Mammalian patatin domain-containing proteins. A family with diverse lipolytic activities involved in multiple biological functions. J. Lipid Res. 50, (suppl.) S63–S68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ma Z., Ramanadham S., Wohltmann M., Bohrer A., Hsu F. F., Turk J. (2001) Studies of insulin secretory responses and of arachidonic acid incorporation into phospholipids of stably transfected insulinoma cells that overexpress Group VIA Phospholipase A2 (iPLA2β) indicate a signaling rather than a housekeeping role for iPLA2β. J. Biol. Chem. 276, 13198–13208 [DOI] [PubMed] [Google Scholar]
- 59. Ramanadham S., Song H., Hsu F. F., Zhang S., Crankshaw M., Grant G. A., Newgard C. B., Bao S., Ma Z., Turk J. (2003) Pancreatic islets and insulinoma cells express a novel isoform of Group VIA phospholipase A2 (iPLA2β) that participates in glucose-stimulated insulin secretion and is not produced by alternate splicing of the iPLA2β transcript. Biochemistry 42, 13929–13940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bao S., Bohrer A., Ramanadham S., Jin W., Zhang S., Turk J. (2006) Effects of stable suppression of Group VIA phospholipase A2 expression on phospholipid content and composition, insulin secretion, and proliferation of INS-1 insulinoma cells. J. Biol. Chem. 281, 187–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bao S., Song H., Wohltmann M., Ramanadham S., Jin W., Bohrer A., Turk J. (2006) Insulin secretory responses and phospholipid composition of pancreatic islets from mice that do not express Group VIA phospholipase A2 and effects of metabolic stress on glucose homeostasis. J. Biol. Chem. 281, 20958–20973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bao S., Jacobson D. A., Wohltmann M., Bohrer A., Jin W., Philipson L. H., Turk J. (2008) Glucose homeostasis, insulin secretion, and islet phospholipids in mice that overexpress iPLA2β in pancreatic β-cells and in iPLA2β-null mice. Am. J. Physiol. Endocrinol. Metab. 294, E217–E229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wolf M. J., Wang J., Turk J., Gross R. W. (1997) Depletion of intracellular calcium stores activates smooth muscle cell calcium-independent phospholipase A2. A novel mechanism underlying arachidonic acid release. J. Biol. Chem. 272, 1522–1526 [DOI] [PubMed] [Google Scholar]
- 64. Nowatzke W., Ramanadham S., Ma Z., Hsu F. F., Bohrer A., Turk J. (1998) Mass spectrometric evidence that agents which cause loss of Ca2+ from intracellular compartments induce hydrolysis of arachidonic acid from pancreatic islet membrane phospholipids by a mechanism that does not require a rise in cytosolic Ca2+ concentration. Endocrinology 139, 4073–4085 [DOI] [PubMed] [Google Scholar]
- 65. Ramanadham S., Hsu F. F., Zhang S., Jin C., Bohrer A., Song H., Bao S., Ma Z., Turk J. (2004) Involvement of the Group VIA phospholipase A2 (iPLA2β) in endoplasmic reticulum stress-induced apoptosis in insulinoma cells. Biochemistry 43, 918–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bao S., Li Y., Lei X., Wohltmann M., Jin W., Bohrer A., Semenkovich C. F., Ramanadham S., Tabas I., Turk J. (2007) Attenuated free cholesterol loading-induced apoptosis and preserved phospholipid composition of peritoneal macrophages from mice that do not express Group VIA phospholipase A2. J. Biol. Chem. 282, 27100–27114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Moon S. H., Jenkins C. M., Mancuso D. J., Turk J., Gross R. W. (2008) Arachidonic acid release induced by thapsigargin or ionophore is ablated in aortic smooth muscle cells from mice null for Ca2+-independent PLA2β. Attenuation of smooth muscle cell migration and proliferation in iPLA2β−/− mice. J. Biol. Chem. 283, 33975–33987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Song H., Rohrs H., Tan M., Wohltmann M., Ladenson J. H., Turk J. (2010) Effects of ER stress on Group VIA PLA2 (iPLA2β) in beta cells include tyrosine phosphorylation and increased association with calnexin. J. Biol. Chem. 285, 33843–33857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhao Z., Zhang X., Zhao C., Choi J., Shi J., Song K., Turk J., Ma Z. A. (2010) Protection of pancreatic β-cells by group VIA phospholipase A2-mediated repair of mitochondrial membrane peroxidation. Endocrinology 151, 3038–3048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Seleznev K., Zhao C., Zhang X. H., Song K., Ma Z. A. (2006) Calcium-independent phospholipase A2 localizes in and protects mitochondria during apoptotic induction by staurosporine. J. Biol. Chem. 281, 22275–22288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lei X., Zhang S., Bohrer A., Bao S., Song H., Ramanadham S. (2007) The group VIA calcium-independent phospholipase A2 participates in ER stress-induced INS-1 insulinoma cell apoptosis by promoting ceramide generation via hydrolysis of sphingomyelins by neutral sphingomyelinase. Biochemistry 46, 10170–10185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lei X., Zhang S., Bohrer A., Ramanadham S. (2008) Calcium-independent phospholipase A2 (iPLA2β)-mediated ceramide generation plays a key role in the cross-talk between the endoplasmic reticulum (ER) and mitochondria during ER stress-induced insulin-secreting cell apoptosis. J. Biol. Chem. 283, 34819–34832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ron D., Walter P. (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 8, 519–529 [DOI] [PubMed] [Google Scholar]
- 74. Ma Y., Brewer J. W., Diehl J. A., Hendershot L. M. (2002) Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J. Mol. Biol. 318, 1351–1365 [DOI] [PubMed] [Google Scholar]
- 75. Harding H. P., Zhang Y., Bertolotti A., Zeng H., Ron D. (2000) Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol. Cell 5, 897–904 [DOI] [PubMed] [Google Scholar]
- 76. Rao R. V., Ellerby H. M., Bredesen D. E. (2004) Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ. 11, 372–380 [DOI] [PubMed] [Google Scholar]
- 77. Cargnello M., Roux P. P. (2011) Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 75, 50–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hsu F. F., Turk J. (2002) Characterization of ceramides by low energy collisional-activated dissociation tandem mass spectrometry with negative-ion electrospray ionization. J. Am. Soc. Mass Spectrom. 13, 558–570 [DOI] [PubMed] [Google Scholar]
- 79. Hsu F. F., Turk J., Stewart M. E., Downing D. T. (2002) Structural studies on ceramides as lithiated adducts by low energy collisional-activated dissociation tandem mass spectrometry with electrospray ionization. J. Am. Soc. Mass Spectrom. 13, 680–695 [DOI] [PubMed] [Google Scholar]
- 80. Song H., Wohltmann M., Bao S., Ladenson J. H., Semenkovich C. F., Turk J. (2010) Mice deficient in Group VIB phospholipase A2 (iPLA2γ) exhibit relative resistance to obesity and metabolic abnormalities induced by a Western diet. Am. J. Physiol. Endocrinol. Metab. 298, E1097–E1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bordin S., Whitfield D. (2003) Cutting edge. Proliferating fibroblasts respond to collagenous C1q with phosphorylation of p38 mitogen-activated protein kinase and apoptotic features. J. Immunol. 170, 667–671 [DOI] [PubMed] [Google Scholar]
- 82. Song H., Ramanadham S., Bao S., Hsu F. F., Turk J. (2006) A bromoenol lactone suicide substrate inactivates Group VIA phospholipase A2 by generating a diffusible bromomethyl ketoacid that alkylates cysteine thiols. Biochemistry 45, 1061–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hazen S. L., Zupan L. A., Weiss R. H., Getman D. P., Gross R. W. (1991) Suicide inhibition of canine myocardial cytosolic calcium-independent phospholipase A2. Mechanism-based discrimination between calcium-dependent and -independent phospholipases A2. J. Biol. Chem. 266, 7227–7232 [PubMed] [Google Scholar]
- 84. Zupan L. A., Weiss R. H., Hazen S. L., Parnas B. L., Aston K. W., Lennon P. J., Getman D. P., Gross R. W. (1993) Structural determinants of haloenol lactone-mediated suicide inhibition of canine myocardial calcium-independent phospholipase A2. J. Med. Chem. 36, 95–100 [DOI] [PubMed] [Google Scholar]
- 85. Khaled A. R., Moor A. N., Li A., Kim K., Ferris D. K., Muegge K., Fisher R. J., Fliegel L., Durum S. K. (2001) Trophic factor withdrawal. p38 mitogen-activated protein kinase activates NHE1, which induces intracellular alkalinization. Mol. Cell. Biol. 21, 7545–7557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Jenkins C. M., Han X., Mancuso D. J., Gross R. W. (2002) Identification of calcium-independent phospholipase A2 (iPLA2) beta, and not iPLA2γ, as the mediator of arginine vasopressin-induced arachidonic acid release in A-10 smooth muscle cells. Enantioselective mechanism-based discrimination of mammalian iPLA2s. J. Biol. Chem. 277, 32807–32814 [DOI] [PubMed] [Google Scholar]
- 87. Xie Z., Gong M. C., Su W., Turk J., Guo Z. (2007) Group VIA phospholipase A2 (iPLA2β) participates in angiotensin II-induced transcriptional up-regulation of regulator of G-protein signaling-2 in vascular smooth muscle cells. J. Biol. Chem. 282, 25278–25289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Xie Z., Gong M. C., Su W., Xie D., Turk J., Guo Z. (2010) Role of calcium-independent phospholipase A2β in high glucose-induced activation of RhoA, Rho kinase, and CPI-17 in cultured vascular smooth muscle cells and vascular smooth muscle hypercontractility in diabetic animals. J. Biol. Chem. 285, 8628–8638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Nikolic D. M., Gong M. C., Turk J., Post S. R. (2007) Class A scavenger receptor-mediated macrophage adhesion requires coupling of calcium-independent phospholipase A2 and 12/15-Lipoxygenase to Rac and Cdc42 activation. J. Biol. Chem. 282, 33405–33411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zhang S., Han J., Sells M. A., Chernoff J., Knaus U. G., Ulevitch R. J., Bokoch G. M. (1995) Rho family GTPases regulate p38 mitogen-activated protein kinase through the downstream mediator Pak1. J. Biol. Chem. 270, 23934–23936 [DOI] [PubMed] [Google Scholar]
- 91. Bagrodia S., Dérijard B., Davis R. J., Cerione R. A. (1995) Cdc42 and PAK-mediated signaling leads to Jun kinase and p38 mitogen-activated protein kinase activation. J. Biol. Chem. 270, 27995–27998 [DOI] [PubMed] [Google Scholar]
- 92. Tibbles L. A., Ing Y. L., Kiefer F., Chan J., Iscove N., Woodgett J. R., Lassam N. J. (1996) MLK-3 activates the SAPK/JNK and p38/RK pathways via SEK1 and MKK3/6. EMBO J. 15, 7026–7035 [PMC free article] [PubMed] [Google Scholar]
- 93. Martin G. A., Bollag G., McCormick F., Abo A. (1995) A novel serine kinase activated by rac1/CDC42Hs-dependent autophosphorylation is related to PAK65 and STE20. EMBO J. 14, 1970–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Manser E., Leung T., Salihuddin H., Zhao Z. S., Lim L. (1994) A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature 367, 40–46 [DOI] [PubMed] [Google Scholar]
- 95. Just I., Selzer J., Wilm M., von Eichel-Streiber C., Mann M., Aktories K. (1995) Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature 375, 500–503 [DOI] [PubMed] [Google Scholar]
- 96. Cheng H., Kartenbeck J., Kabsch K., Mao X., Marqués M., Alonso A. (2002) Stress kinase p38 mediates EGFR transactivation by hyperosmolar concentrations of sorbitol. J. Cell. Physiol. 192, 234–243 [DOI] [PubMed] [Google Scholar]
- 97. Jacobson D. A., Weber C. R., Bao S., Turk J., Philipson L. H. (2007) Modulation of the pancreatic islet beta-cell-delayed rectifier potassium channel Kv2.1 by the polyunsaturated fatty acid arachidonate. J. Biol. Chem. 282, 7442–7449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Jacobson D. A., Kuznetsov A., Lopez J. P., Kash S., Ammälä C. E., Philipson L. H. (2007) Kv2.1 ablation alters glucose-induced islet electrical activity, enhancing insulin secretion. Cell Metab. 6, 229–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Hii C. S., Huang Z. H., Bilney A., Costabile M., Murray A. W., Rathjen D. A., Der C. J., Ferrante A. (1998) Stimulation of p38 phosphorylation and activity by arachidonic Acid in HeLa Cells, HL60 promyelocytic leukemic cells, and human neutrophils. Stimulation of p38 phosphorylation and activity by arachidonic acid in HeLa cells, HL60 promyelocytic leukemic cells, and human neutrophils. Evidence for cell type-specific activation of mitogen-activated protein kinases. J. Biol. Chem. 273, 19277–19282 [DOI] [PubMed] [Google Scholar]
- 100. Talukdar I., Szeszel-Fedorowicz W., Salati L. M. (2005) Arachidonic acid inhibits the insulin induction of glucose-6-phosphate dehydrogenase via p38 MAP kinase. J. Biol. Chem. 280, 40660–40667 [DOI] [PubMed] [Google Scholar]
- 101. Park K. S., Mohapatra D. P., Misonou H., Trimmer J. S. (2006) Graded regulation of the Kv2.1 potassium channel by variable phosphorylation. Science 313, 976–979 [DOI] [PubMed] [Google Scholar]
- 102. Tiran Z., Peretz A., Attali B., Elson A. (2003) Phosphorylation-dependent regulation of Kv2.1 channel activity at tyrosine 124 by Src and by protein-tyrosine phosphatase ϵ. J. Biol. Chem. 278, 17509–17514 [DOI] [PubMed] [Google Scholar]
- 103. Redman P. T., He K., Hartnett K. A., Jefferson B. S., Hu L., Rosenberg P. A., Levitan E. S., Aizenman E. (2007) Apoptotic surge of potassium currents is mediated by p38 phosphorylation of Kv2.1. Proc. Natl. Acad. Sci. U.S.A. 104, 3568–3573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Lei X., Zhang S., Barbour S. E., Bohrer A., Ford E. L., Koizumi A., Papa F. R., Ramanadham S. (2010) Spontaneous development of endoplasmic reticulum stress that can lead to diabetes mellitus is associated with higher calcium-independent phospholipase A2 expression. A role for regulation by SREBP-1. J. Biol. Chem. 285, 6693–6705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zarubin T., Han J. (2005) Activation and signaling of the p38 MAP kinase pathway. Cell Res. 15, 11–18 [DOI] [PubMed] [Google Scholar]
- 106. Moran J. M., Buller R. M., McHowat J., Turk J., Wohltmann M., Gross R. W., Corbett J. A. (2005) Genetic and pharmacologic evidence that calcium-independent phospholipase A2β regulates virus-induced inducible nitric-oxide synthase expression by macrophages. J. Biol. Chem. 280, 28162–28168 [DOI] [PubMed] [Google Scholar]
- 107. Sun B., Zhang X., Yonz C., Cummings B. S. (2010) Inhibition of calcium-independent phospholipase A2 activates p38 MAPK signaling pathways during cytostasis in prostate cancer cells. Biochem. Pharmacol. 79, 1727–1735 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.