Background: Heparin-induced leukocytosis (HIL) is commonly seen in patients.
Results: In mice, heparin requires 6-O-sulfation to induce leukocytosis. Heparin alters selectin- and/or CXCL12-mediated lymphocyte homing and release, neutrophil release, and distribution in the vasculature.
Conclusion: HIL requires 6-O-sulfation and is caused by blocking selectin- and CXCL12-mediated leukocyte trafficking.
Significance: This study elucidated the structural determinants and the underlying mechanisms of heparin in HIL.
Keywords: Cell Adhesion, Chemokines, Heparin, Leukocyte, Trafficking
Abstract
Leukocytosis refers to an increase in leukocyte count above the normal range in the blood and is a common laboratory finding in patients. In many cases, the mechanisms underlying leukocytosis are not known. In this study, we examined the effects, the structural determinants, and the underlying mechanisms of heparin-induced leukocytosis, a side effect occurring in 0.44% of patients receiving heparin. We observed that heparin induced both lymphocytosis and neutrophilia, and the effects required heparin to be 6-O-sulfated but did not require its anticoagulant activity. Cell mobilization studies revealed that the lymphocytosis was attributable to a combination of blockage of lymphocyte homing and the release of thymocytes from the thymus, whereas the neutrophilia was caused primarily by neutrophil release from the bone marrow and demargination in the vasculature. Mechanistic studies revealed that heparin inhibits L- and P-selectin, as well as the chemokine CXCL12, leading to leukocytosis. Heparin is known to require 6-O-sulfate to inhibit L- and P-selectin function, and in this study we observed that 6-O-sulfate is required for its interaction with CXCL12. We conclude that heparin-induced leukocytosis requires glucosamine 6-O-sulfation and is caused by blockade of L-selectin-, P-selectin-, and CXCL12-mediated leukocyte trafficking.
Introduction
Leukocytosis refers to an increase in leukocyte count above the normal range in the blood and is a common laboratory finding in patients. It occurs in response to a wide variety of pathological conditions, including viral, bacterial, and fungal infection, parasitic invasion, cancer, hemorrhage, trauma, myocardial infarction, malignancies, poisoning, and metabolic disturbances (1). Based on the type of leukocyte that accumulates, leukocytosis is further subcategorized as neutrophilia, lymphocytosis, monocytosis, eosinophilia, and basophilia (1). Clinically, leukocytosis is most commonly caused by neutrophilia, less often caused by lymphocytosis, and rarely is a consequence of monocytosis, eosinophilia, or basophilia (1, 2).
Mechanistic studies have shown that leukocytosis can be attributed to an increase in cell production, an increase in cell release from storage pools, demargination in the vasculature, and/or decreased egress into target tissues. Many molecules that play key roles in inflammation have been implicated in leukocytosis, notably selectins, chemokines, integrins, and proteases (1).
Interestingly, some medications also induce leukocytosis, including corticosteroids, lithium, β-agonists, and heparin (2–5). Heparin, a linear polysaccharide that belongs to the family of glycosaminoglycans, has been used clinically as an anticoagulant/antithrombotic for more than 70 years. It consists of repeating disaccharide units containing a uronic acid and a glucosamine residue that is either N-sulfated, N-acetylated, or occasionally unsubstituted (6). The disaccharides may be further O-sulfated at C6 or C3 of the glucosamine residues and C2 of the uronic acid residues. The potent antithrombotic activity of heparin depends on the specific arrangement of sulfated sugar units and uronic acid epimers, which form a binding site for antithrombin III (6). Heparin also possesses a variety of non-anticoagulant functions such as inhibition of inflammation and tumor metastasis (7–9). Heparin was also reported to induce lymphocytosis in mice, rats, cows, and humans (3–5). Importantly, from a clinical perspective, 0.44% of patients develop leukocytosis when taking therapeutic doses of heparin. In the United States more than 12 million patients receive heparin every year (10), resulting in over 50,000 cases per year and indicating that heparin-induced leukocytosis (HIL)2 is a frequently seen side effect of the drug in hospitals. However, the structural determinants of heparin that cause HIL and the mechanisms behind the effect remain unknown.
In this study, we observed that heparin induced both lymphocytosis and neutrophilia, and the effect required 6-O-sulfation of the glucosamine residues but did not require its anticoagulant activity. Cell mobilization studies revealed that the lymphocytosis was attributable to the blockage of lymphocyte homing and release of thymocytes, whereas the neutrophilia was mainly caused by neutrophil release from bone marrow (BM) and demargination in the vasculature. Molecular mechanism studies revealed that heparin inhibits L- and P-selectin- and chemokine CXCL12-mediated leukocyte trafficking, resulting in HIL.
EXPERIMENTAL PROCEDURES
Heparin and Its Derivatives
Porcine intestinal heparin (average mass = 12,000–15,000 Da) was obtained from Scientific Protein Laboratories (Milwaukee, WI). Heparinoids were prepared as reported previously (9), including N-desulfated/N-acetylated heparin (N-des), 2-O/3-O-desulfated heparin (2/3-des-hep), N-/2-O/3-O-desulfated heparin (N/2/3-des-hep), 6-O-desulfated heparin (6-des-hep), and carboxyl-reduced heparin (CR-hep) (Fig. 2A). The heparin had an anti-factor Xa activity of 149 units/mg, whereas all heparinoids had no detectable anti-factor Xa activity. The heparin and heparinoids were negative for endotoxin.
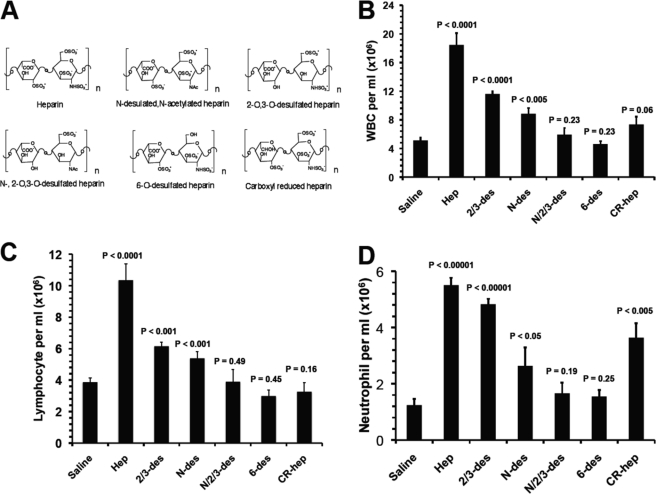
FIGURE 2.
Leukocytosis induced by differently structured heparins (heparinoids). A, structure of heparinoids involved in this study. Representative disaccharide units of unfractionated heparin (Hep), N-desulfated and N-acetylated heparin (N-des), 2-O/3-O-desulfated heparin (2/3-des), N-/2-O/3-O-desulfated heparin (N/2/3-des), 6-O-desulfated heparin (6-des), and carboxyl-reduced heparin (CR-hep). B and D, heparinoid-induced elevation of leukocytes in blood was assessed for leukocytosis (B), lymphocytosis (C), and neutrophilia (D). The data were summarized from three sets of experiments with 8–12 mice per group. Each bar represents the average value ± S.E. The statistical analysis was carried out by paired Student's t test in comparison with saline treatment. WBC, white blood cells.
Mice
Wild-type, P-selectin knock-out (P−/−), and L-selectin knock-out (L−/−) mice, and P- and L-selectin double knock-out (PL−/−) mice on a C57BL/6J background were purchased from The Jackson Laboratory (Bar Harbor, ME). The α(1,3)-fucosyltransferase-IV and -VII double knock-out (FucT-IV/VII−/−) mice were kindly provided by Dr. John B. Lowe (11) and were bred onto C57BL/6J background. Mice were housed at a specific pathogen-free facility, and the experimental protocol was approved by the Institutional Animal Care and Use Committee of the University of Georgia.
Heparin/Heparinoid/Anti-CXCL12-neutralizing Antibody Injection and Analysis of Leukocytosis
Previous studies showed that a single bolus injection of sulfated polysaccharide fucoidan (i.v., 1.25–2.5 mg/25 g BW) induced robust leukocytosis in mice and that the leukocytosis peaked at 1.5 h after the fucoidan injection and coincided with reduced CXCL12 concentration in BM (12). Glycosaminoglycan mimetics injected intraperitoneally at 1.25 mg/25 g BW similarly induced leukocytosis (13). Our pilot study showed that the CXCL12 concentration in BM bottomed out at 90 min after heparin injection (Fig. 6, B and C). Considering that heparin at 0.5–1.25 mg/25 g BW potently blocks selectin-mediated leukocyte trafficking in mice (9) and that disruption of CXCL12 signaling in BM and selectin functions may represent the major molecular mechanisms underlying HIL, we rationalized injecting i.v. heparin at 1 mg/25 g BM, a dose close to glycosaminoglycan mimetics and fucoidan administration (12, 13), and we used 90 min post-heparin injection as the time point for HIL analysis in our studies. In brief, mice at 8–10 weeks old were injected i.v. via tail vein with the heparinoids at 1 mg/25 g BW. Blood samples were taken from the retroorbital plexus during isoflurane anesthesia 90 min after heparinoid injection. Total leukocytes were counted separately by two investigators using hemocytometers. The leukocyte subpopulations were analyzed by flow cytometry (9, 14). Neutrophils and monocytes were identified by high expression of Gr-1 and Mac-1, respectively. The percentage of lymphocyte in leukocyte population was quantified based on forward and right angle light scattering in the cytogram. B cells and NK cells were counted following staining with FITC-conjugated rat anti-mouse CD45R/B220 and pan-NK antibodies, respectively. The subpopulations of T cells were determined by quantifying CD4+/CD8−, CD4−/CD8+, and CD4+/CD8+ cells. For some studies, the mice were preinjected with anti-CXC12-neutralizing antibody (12) (R&D Systems, 2 mg/kg BW) i.v. 90 min prior to heparin administration.
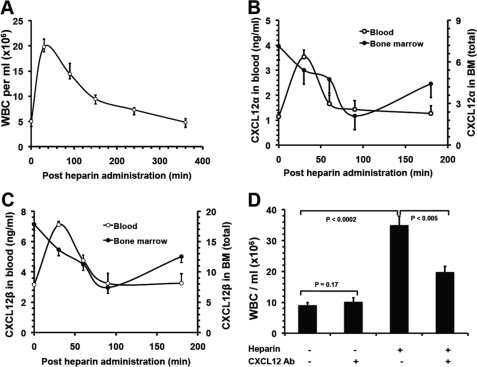
FIGURE 6.
Heparin-induced leukocytosis depends on blockade of CXCL12 function. The kinetics of leukocytosis (A) and the levels of CXCL12α (B) and CXCL12β (C) in peripheral blood and in BM after heparin administration. The data were summarized from three experiments with 10 mice per group. D, blocking CXCL12 function with neutralizing antibody attenuated HIL response. The mice were preinjected i.v. with neutralizing anti-CXCL12 antibody prior to heparin or saline injection and then analyzed for HIL. The data were summarized from three experiments with 7–9 mice per group. Each bar represents the average value ± S.E. The statistical analysis was carried out by paired Student's t test. WBC, white blood cells. Ab, antibody.
Lymphocyte Homing
Lymphocyte suspensions from peripheral lymph nodes were prepared in DMEM, and erythrocytes were lysed with a 0.83% NH4Cl solution. After washing with PBS, the cells (5–10 × 106 cells/ml) were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE, 5 μm) (9). Heparin at 1 mg/25 g BW in 100 μl of saline or only saline was injected into the tail vein of the recipient mice. Five min later, 7 × 106 CFSE-labeled cells in 100 μl of saline were similarly injected. Ninety minutes after heparin administration, the mice were sacrificed. Peripheral blood and single cell suspensions prepared from peripheral (cervical and axillary) and mesenteric lymph nodes were counted, and the percentage of CFSE+ cells was determined by flow cytometry.
Thymocyte Release from Thymus
The chests of anesthetized mice were opened; 10 μl of FITC (Sigma) at 600 μg/ml in PBS was injected directly into each thymic lobe with a 28-gauge needle, and then the chests were closed with surgical clips in the overlying skin (15). This method labeled 30% of the thymocytes in the thymus. Thirty minutes after intrathymic injection, mice received heparin at 1 mg/25 g BW or only PBS via tail vein injection. Ninety minutes later, the FITC+ cells in the circulation were quantified by flow cytometry. The total of thymocytes released from the thymus was calculated as the (number of FITC+ cells)/(30%).
Neutrophil Release from Bone Marrow
Mice were injected with 0.6 mg of BrdU i.p. twice, separated by 12 h (16–18). At 24 h after the first injection, 35% Gr-1+-BM cells were labeled with BrdU, and the mice were injected with heparin at 1 mg/25 g BW in 100 μl of saline or saline only via tail vein. Ninety min later, blood was collected, and total leukocytes were counted. The Gr-1+/BrdU+ cells were quantified by flow cytometry, and the neutrophils released from the BM were calculated as (Gr-1+/BrdU+ cells)/35%.
Neutrophil Demargination
Mice were injected intraperitoneally with 0.6 mg of BrdU daily for 7 days at which point all neutrophils in circulation were BrdU+ (19). Fresh blood was collected into citrate-containing tubes, washed with PBS, and then transfused to the recipient mice at 150 μl/mouse via tail vein. Thirty min after the transfusion, heparin at 1 mg/25 g BW in 100 μl of saline or saline only was injected similarly. Ninety min after the heparin/saline administration, the Gr-1+ and BrdU+/Gr-1+ cells in the peripheral blood were quantified by leukocyte count and flow cytometry.
Measurement of CXCL12 Concentration in Blood and BM
CXCL12α and CXCL12β levels in mouse plasma and BM were determined by ELISA using monoclonal antibodies specific for CXCL12β (BAF351) or CXCL12α (BAF310) with capture antibody MAB350 and recommended protocols from R&D Systems (12). BM samples were prepared by flushing the content of two femurs directly into 0.4 ml of sample buffer (0.1% BSA, 0.05% Tween 20 in 20 mm Trizma (Tris base), 150 mm NaCl, pH 7.3) and centrifuged, and the supernatants were collected and used for CXCL12 concentration determination.
Transendothelial Cell Migration Assay
Transendothelial cell migration was performed using trans-wells containing 3-μm pore-polycarbonate filters to separate the upper and lower chambers in 24-well plates (Costar, Cambridge, MA) (20, 21). Immortalized mouse lung endothelial cells (22, 23) were seeded at 1.5 × 104 per well in the upper chamber and became confluent after 3–4 days in culture. The integrity of the endothelium-covered trans-wells was controlled after each migration by May-Grunwald-Giemsa staining and followed by visualization by microscopy. BM cells were freshly prepared in single cell suspensions from mouse femurs and tibias by flushing in PBS. Red blood cells were lysed with 0.83% NH4Cl solution before rinsing. The BM cells were added at 1 × 105 cells per upper compartment. The migration was initiated by supplying CXCL12α (10 ng/ml) in the lower chamber to determine CXCL12-induced chemotaxis. Only the active migrating cells were able to get through the pores and into the lower chamber. After 4 h, cells that migrated into the lower chamber were counted. To determine whether heparin inhibits the CXCL12-induced transendothelial chemotaxis of BM cells, the experiment was similarly carried out with heparin added in the upper and lower chambers prior to initiation of cell migration. In additional experiments to determine the effect of immobilized CXCL12 on random migration of BM cells (chemokinesis), CXCL12α was preincubated at both apical and basal sides of endothelial cell-covered trans-wells for 30 min at RT. Nonbound CXCL12α was washed away by rinsing with PBS, and the transendothelial cell migration experiments were carried out as described above.
CXCL12α Binds to Endothelial Heparan Sulfate
Primary human umbilical vein endothelial cells plated at 104 cells/well in 96-well tissue culture plates were grown in EMB-2 media supplemented with growth factors and 2% FBS according to the supplier's recommendations (Lonza Inc., Walkersville, MD) until confluency. The cells were then fixed with 4% paraformaldehyde for 10 min at RT and washed in PBS. After fixation, some wells were incubated with a mixture of heparinases I–III (20–50 milliunits/ml of each enzyme) for 1 h at RT. Nonspecific binding sites were blocked with 5% BSA in PBS, and the cells were then incubated with CXCL12α at 10 μg/ml in PBS with or without heparin, heparan sulfate, or heparinoid at 20 μg/ml each for 1.5 h at RT. Bound CXCL12α was detected by incubation with a mouse anti-CXCL12 IgG antibody (R&D Systems, Minneapolis, MN) and then with an HRP-conjugated goat anti-mouse IgG antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and finally with the 3,3′,5,5′-tetramethylbenzidine chromogen (Thermo Fisher Scientific, Waltham, MA). The absorbance at 450 nm was read using a microplate reader (Molecular Devices, Inc., Menlo Park, CA) equipped with Softmax software.
Binding of CXCL12α to Heparin and Heparinoids Studied by Surface Plasmon Resonance (SPR)
SPR analyses were conducted using a BIACORE T100 apparatus (Biacore AB, Piscataway, NJ) with biotinylated heparin immobilized on streptavidin-coated chips. Biotinylated heparin was prepared using a two-stage procedure with biotin coupled at the reducing end of heparin (24, 25). The biotinylated heparin carried 1.3 biotin/heparin and was immobilized on streptavidin-coated CM5 chip surface. Briefly, the surface of a CM5 chip was activated by injecting a freshly prepared mixture (1:1) of N-hydroxysuccinimide (50 mm in water) and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (200 mm in water) at a flow rate of 5 μl/min, followed by a 21-min injection of streptavidin (400 mg/ml). Any remaining activated carboxylic acid groups were blocked by incubation with 1 m ethanolamine hydrochloride, pH 8.6, for 21 min. Approximately 1000 response units of streptavidin was coupled to the cell surface using this method. Heparin immobilization was achieved by injecting biotinylated heparin at 1 ml/min for 21 min over the streptavidin-coated surface and was optimized to give ∼500 response units. As a reference surface for refractive index and correction of nonspecific binding, a second flow cell was treated in the same manner as above, but without the injection of biotinylated heparin. To determine the interaction of CXCL12α with heparinoids, CXCL12α (125 nm) was premixed with heparin (1 μg/ml) or 6-des-hep (1 μg/ml) in HBS-EP buffer (10 mm Hepes, 150 mm NaCl, 3 mm EDTA, 0.005% v/v surfactant P20) at RT. The mixture was immediately injected at a flow rate of 50 μl/min. CXCL12α (125 nm) was used as a positive control, whereas HBS-EP buffer alone was background control. Data were collected with a contact time of 120 s and a dissociation time of 300 s. The response unit for binding was presented with the background response subtracted. Data were evaluated using BIAevaluation Software 3.1, Biacore.
RESULTS
Heparin Induces Both Lymphocytosis and Neutrophilia
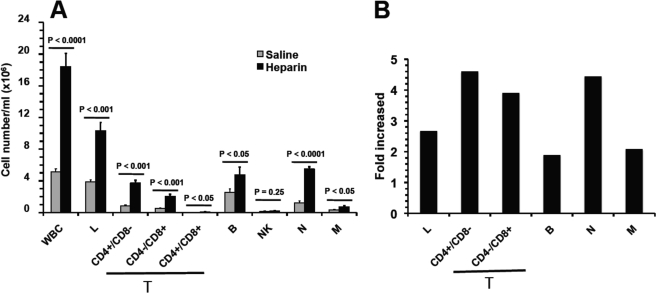
Heparin has been reported to cause rapid and transient lymphocytosis in humans, cows, rats, and mice after i.p or i.v. injection (3–5, 26); however, the effect on subpopulations of leukocytes has not been defined. We examined the subpopulation distribution of circulating leukocytes 90 min after heparin treatment in mice. Compared with saline, heparin increased all major subpopulations of leukocytes in the circulation, including neutrophils (4.27 × 106/ml, 4.4-fold), lymphocytes (6.47 × 106/ml, 2.7-fold), and monocytes (0.37 × 106/ml, 2.1-fold) (Fig. 1). An analysis of the lymphocyte subpopulations showed the same tendency with the following results: B lymphocytes (2.24 × 106/ml, 1.9-fold), CD4+/CD8− (2.91 × 106/ml, 4.6-fold), CD4−/CD8+ (1.53 × 106/ml, 3.9-fold), and CD4+/CD8+ (0.06 × 106/ml, 12.5-fold) (Fig. 1). Lymphocytes and neutrophils were the two major subpopulations of circulating leukocytes and accounted for ∼62 and 33% of HIL cell population, respectively.
FIGURE 1.
Heparin-induced leukocytosis is mainly attributable to lymphocytosis and neutrophilia. A and B, subpopulation distribution of leukocytes in cell numbers (A) and alteration in ratios over saline control (B). The leukocytosis is mainly attributable to a massive increase of circulating lymphocytes (lymphocytosis) and neutrophils (neutrophilia), which account for roughly 62 and 33% of the total leukocyte elevation in peripheral blood, respectively. The data were summarized from three sets of experiments with 8–12 mice per group. Each bar represents the average value ± S.E. The statistical analysis was carried out by paired Student's t test in comparison with saline treatment. WBC, white blood cells; L, lymphocytes; M, monocytes; N, neutrophils; T, T lymphocytes; B, B lymphocytes.
Induction of Leukocytosis Depends on the Degree of Sulfation, 6-O-Sulfation, and Carboxyl Moiety but Not Anticoagulant Activity of Heparin
To determine the structure required for heparin to induce leukocytosis, we treated mice with structurally different heparins (Fig. 2A). Compared with heparin, N-des-hep and 2/3-des-hep induced attenuated responses, whereas N/2/3-des-hep was unable to induce leukocytosis, indicating that HIL depended on the degree of sulfation (Fig. 2, B–D). Intriguingly, 6-des-hep was also unable to induce leukocytosis (Fig. 2, B–D). Heparin carries more N-sulfate than 6-O-sulfate, implying that the inefficacy of 6-des-hep to induce leukocytosis was not due to a reduction in the overall degree of sulfation, but specifically to the lack of 6-O-sulfate, revealing that heparin uniquely requires 6-O-sulfation to induce leukocytosis. CR-hep induced attenuated HIL response too, showing that the presence of the carboxyl moiety is also required for heparin to induce leukocytosis (Fig. 2, B–D). Intriguingly, subpopulation analysis observed that CR-hep had a slightly reduced efficacy for inducing neutrophilia, but its ability to cause lymphocytosis was abrogated (Fig. 2, C and D), highlighting that heparin requires carboxyl moiety to induce lymphocytosis but not neutrophilia. Furthermore, N-des-hep, 2/3-des-hep. and CR-hep all were capable of inducing leukocytosis (Fig. 2, B–D), but they were devoid of anticoagulant activities, indicating that the anticoagulant activity of heparin is not required for HIL. Collectively, these observations indicate that HIL depends on the degree of sulfation and carboxylation of heparin, and there is a specific requirement for 6-O-sulfation, but anticoagulant activity is not required.
Heparin Blocks Lymphocyte Homing to Lymph Nodes and Thymocyte Release from the Thymus
The leukocyte numbers in the peripheral blood are controlled by hematopoiesis in the BM and thymus, emigration from these tissues, distribution in the vasculature, and migration to target organs. HIL occurred minutes post-heparin injection and peaked at 30 min (Fig. 6A). The time frame makes hematopoiesis unlikely to contribute significantly to HIL; we therefore focused on examining the effects of heparin on leukocyte mobilization. We began by examining the role of heparin in lymphocyte homing to peripheral lymph nodes. Lymphocytes from peripheral and mesenteric lymph nodes were labeled with CFSE and then injected via the tail vein 5 min after heparin administration. Ninety minutes post-heparin injection, the mice were sacrificed, and the lymphocytes of peripheral and mesenteric lymph nodes as well as the leukocytes in the peripheral blood were counted and analyzed for CFSE+ cells by flow cytometry. As shown in Fig. 3A, the numbers of CFSE+ lymphocytes homing to the peripheral and mesenteric lymph nodes were reduced by ∼90% in heparin-treated mice compared with saline-treated controls. Furthermore, a corresponding increase in the CFSE+ lymphocytes was observed in the peripheral blood compared with the saline-treated controls (Fig. 3B), indicating that heparin blocked lymphocyte homing to peripheral lymph nodes. In rats, recirculating lymphocytes enter the vascular compartment at a rate sufficient to replace the blood population of lymphocytes about 15 times each day (27). Assuming that lymphocyte recirculation in mice occurred similarly, a complete block should cause an ∼0.94-fold increase in the number of lymphocytes in circulation within 90 min. Based on a 90% inhibition efficiency (Fig. 3A), heparin blockage should increase 0.84-fold of the lymphocytes in circulation. Heparin elevated a 1.7-fold of lymphocytes in HIL (Fig. 1B). Thus, blockage of homing accounted for ∼50% of the lymphocyte elevation in HIL and represented a major mechanism underlying heparin-induced lymphocytosis.
FIGURE 3.
Heparin blocks lymphocyte homing to peripheral lymph nodes and releases thymocytes from thymus. A and B, lymphocytes homing to peripheral lymph nodes. CSFE+-labeled lymphocytes were injected into mice via tail vein, and their homing to lymph nodes was assessed 90 min post-heparin injection by quantifying CSFE+-lymphocytes in lymph nodes (A) and remaining in circulation (B). C, heparin released thymocytes from thymus. Thymocytes were intrathymically labeled with FITC 30 min prior to heparin injection, and FITC+ cells in blood were quantified 90 min post-heparin injection. The data were summarized from two sets of experiments with 4–5 mice per group. Each bar represents the average value ± S.E. The statistical analysis was carried out by paired Student's t test in comparison with saline treatment. PLN, peripheral lymph node; LN, lymph node.
At steady state, T cells emigrate from the thymus to supply and maintain normal levels of these cells in the circulation. The heparin-induced elevation of T lymphocytes may also be due to increased release from thymus. This hypothesis was evaluated by intrathymic injection of FITC to label thymocytes in situ, followed by determining the level of FITC+ thymocytes in the circulation 90 min post-heparin injection. The FITC injection labeled 30% of thymocytes in the thymus, and the thymocytes released from the thymus were calculated as (FITC+ cells in circulation)/(30%). Heparin induced a 1.7-fold increase of thymocytes released into the circulation including CD4+/CD8−, CD4−/CD8+, and CD4+/CD8+ cells (Fig. 3C), indicating that heparin induced thymic release of both mature and immature thymocytes. Of the total T lymphocytes (4.44 × 106/ml) increased by heparin, only 1.9 × 105/ml cells (including those that were CD4+/CD8−, 8 × 104/ml; CD4−/CD8+, 2.3 × 104/ml; CD4+/CD8+, 6.0 × 104/ml; CD4−/CD8−, 3.1 × 104/ml) were from the thymus and accounted for 4.3%, indicating that thymic release is a minor mechanism underlying heparin-induced lymphocytosis.
Heparin Releases Neutrophils from Bone Marrow and Demarginates Neutrophils in Vasculature
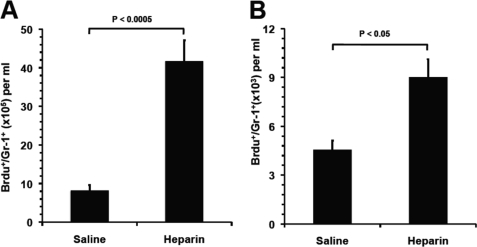
The BM is the site for neutrophil production and contains a large storage pool of mature neutrophils known as the BM reserve (28). These neutrophils can be rapidly mobilized during an inflammatory episode or in response to infection, resulting in neutrophilia within hours (29). To determine whether heparin mobilized neutrophils from the BM leading to neutrophilia, BM neutrophils were labeled by BrdU injection (two times, 12-h intervals). Twenty four hours after the first injection, 35% Gr-1+-BM cells were BrdU+. At the 24-h time point, the mice were given heparin i.v., and BrdU+/Gr-1+ cells in the peripheral blood were counted 90 min post-heparin injection. The neutrophils released from the BM were calculated as the (number of BrdU+/Gr-1+ cells)/35%. As shown in Fig. 4A, compared with the saline control that showed no effect on leukocyte numbers in the circulation, heparin mobilized 3.35 × 106/ml BM neutrophils into the circulation. The total increase of neutrophils in HIL was 4.27 × 106/ml (Fig. 1A), and therefore the BM neutrophils accounted for 75% of the elevation, revealing BM release as the major mechanism underlying heparin-induced neutrophilia.
FIGURE 4.
Heparin released neutrophils from the bone marrow and demarginated neutrophils in the vasculature. A, neutrophil release from BM into blood circulation. BrdU-labeled BM neutrophils in blood as Gr-1+/BrdU+ cell were quantified 90 min post-heparin injection. B, heparin mobilized neutrophils from marginating pool to circulating pool in the vasculature. Transfused BrdU-labeled neutrophils (BrdU+/Gr-1+) were allowed to reach the dynamic equilibrium of distribution between circulating and marginating pools, and then the BrdU+/Gr-1+ cells in circulating pool were quantified 90 min post-heparin injection. The data were summarized from two sets of experiments with 5–6 mice per group. Each bar represents the average value ± S.E. The statistical analysis was carried by paired Student's t test in comparison with saline treatment.
In the vasculature, neutrophils are divided approximately evenly between the circulating pool and the marginating pool (19, 28), which are in dynamic equilibrium. The neutrophils in the marginating pool can be swept rapidly into the circulation by treatments such as epinephrine (30). To test whether heparin mobilized the marginating pool of neutrophils into the circulating pool contributing to neutrophilia, BrdU-labeled leukocytes from the blood of donor mice were transfused into recipient mice via i.v. injection. Thirty min after transfusion, heparin was administered, and 90 min later the BrdU+/Gr-1+ cells in the circulation were quantified. As shown in Fig. 4B, heparin induced a 0.98-fold increase of BrdU+/Gr-1+ cells in the circulation over the saline group. Calculated from the base value of the saline group (1.24 × 106/ml), demargination increased 1.24 × 106/ml neutrophils in the circulation and accounted for 25% of neutrophil elevation, revealing it as the second major mechanism underlying heparin-induced neutrophilia.
Heparin Blocks P- and L-selectin-mediated Leukocyte Trafficking
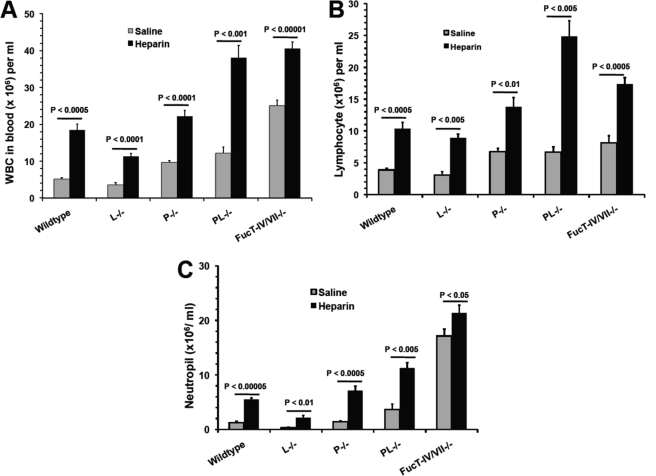
Lymphocyte homing, neutrophil retention in the BM, and margination in the vasculature are mediated by selectins (31). We and others reported previously that heparin bound to L- and P-selectins but not E-selectin and blocked L- and P-selectin-mediated leukocyte trafficking in vivo (7, 9). We also observed that 6-O-sulfation of glucosamine residues was required for heparin to interact with L- and P-selectin and for blockage of their functions in vitro and in vivo (9), similar to heparin's structural requirement for HIL (Fig. 2). Therefore, we hypothesized that heparin functioned similarly to inhibit L- and P-selectin-mediated leukocyte trafficking leading to HIL. To test this hypothesis, we examined L−/− and P−/− mice. L−/− mice showed normal leukocyte counts, whereas P−/− mice displayed spontaneous leukocytosis (Fig. 5), indicating that inhibition of P-selectin represented the major selectin-dependent molecular mechanism underlying HIL. To determine whether inhibition of L-selectin also contributed to HIL, we examined PL−/− mice. PL−/− mice displayed a spontaneous leukocytosis that was not different from P−/− mice (Fig. 5A). However, heparin treatment induced a more profound leukocytosis response, including both lymphocytosis and neutrophilia, in PL−/− mice than in P−/− mice (Fig. 5), demonstrating that the absence of L-selectin sensitized the HIL response. Together, these observations suggest that heparin inhibits both P- and L-selectin-mediated leukocyte trafficking, which leads to leukocytosis. However, the profound HIL response in PL−/− mice also suggests that there was a selectin-independent mechanism involved (Fig. 5). This was confirmed by observing the HIL response occurring in FucT-IV/VII−/− mice that lacked all three selectin ligands (Fig. 5). It should be noted that the heparin-induced neutrophilia was not as dramatic as that of PL−/− mice, which may be due to the high spontaneous neutrophilia present in FucT-IV/VII−/− mice (11, 32).
FIGURE 5.
Heparin-induced leukocytosis depends on blockade of P- and L-selectin function. Mice deficient in selectin (L−/−, P−/−, and PL−/−) or selectin ligand (FucT-IV/VII−/−) were injected i.v. with heparin or saline and then analyzed for leukocytosis (A), lymphocytosis (B), and neutrophilia (C). The data were summarized from three sets of experiments with 6–10 mice per group. Each bar represents the average value ± S.E. The statistical analysis was carried out by paired Student's t test in comparison with saline treatment. WBC, white blood cells.
Heparin Modulates CXCL12 Function
Chemokines are small secreted proteins whose primary function is regulating leukocyte trafficking. The chemokines, including KC, MIP-2, and CXCL12, potentially mobilize neutrophils and lymphocytes from the BM (12, 33–37). As these chemokines bind heparin (38), we hypothesized that heparin modulates these chemokine functions, leading to HIL. The levels of KC and MIP-2 in the peripheral blood peaked at 150 min post-heparin injection (data not shown) and did not correlate with the HIL kinetics that peaked at 30 min post-heparin injection (Fig. 6A), suggesting that HIL is not associated with these chemokines. However, the levels of CXCL12 in blood strongly correlated with HIL (Fig. 6, A–C), implying that heparin might disrupt CXCL12 function leading to HIL. Mice lacking either CXCL12 or CXCR4 are embryonic lethal and thus are not an option for examining the effect of heparin-induced CXCL12 release on leukocyte mobilization. We chose to treat mice with anti-CXCL12-neutralizing antibody. Co-injection of fucoidan with anti-CXCL12 neutralizing antibody was reported to inhibit fucoidan-induced mobilization of hematopoietic progenitor cells (HPCs) but not mature leukocytes (12). This was probably due to the higher responsiveness of HPCs to CXCL12 than mature leukocytes (39). Also, because mature leukocytes express significantly higher levels of CXCR4 than HPCs (39), the latter may result in higher affinities of mature leukocytes than HPCs for CXCL12 when competing with the anti-SDF-1-neutralizing antibody (12). To avoid this competition problem, we treated mice with an anti-CXCL12-neutralizing antibody 90 min prior to heparin administration, allowing sufficient time for the antibody to bind and to neutralize CXCL12 prior to heparin-induced leukocyte release. Under these experimental conditions, anti-CXCL12 antibody did not affect leukocyte numbers at steady state as reported (12, 40) but attenuated HIL by 44% (Fig. 6D), revealing that disruption of CXCL12 function by heparin is another major contributor to HIL.
Heparin Disrupts CXCL12 Binding to Heparan Sulfate and the Heparin-CXCL12 Interaction Requires 6-O-Sulfation
In contrast to CXCL12 in the blood, reverse kinetics were observed in the BM with a gradual decrease in CXCL12, which plateaued at the lowest levels 90 min after heparin administration (Fig. 6, B and C). CXCL12 is mainly expressed in BM (12, 41). Thus the coincidence of its increase in the blood and decrease in the BM suggests that heparin released CXCL12 from the BM into the circulation. In BM, CXCL12 binds to heparan sulfate on cell surfaces and forms a gradient in the extracellular matrix (12, 21, 25, 42). We therefore hypothesized that heparin displaced the binding of CXCL12 to BM heparan sulfate, thereby releasing it into the blood. In addition, previous studies reported that O-sulfation but not N-sulfation was required for heparin to interact with CXCL12 (43), but the particular type of the O-sulfation required for the interaction was not known. We observed that HIL essentially depended on 6-O-sulfation (Fig. 2, B–D), suggesting that 6-O-sulfation may be required for heparin to interact with CXCL12. To answer these questions, we examined the binding of CXCL12 to endothelial cells in the presence of heparin or heparinoids or to endothelial cells pretreated with heparinases. In agreement with previous reports (12, 21, 37), CXCL12 bound potently to the endothelial cells, but the binding was decreased by heparinase treatment, confirming that the binding was heparan sulfate-dependent (Fig. 7A). Soluble heparin and heparan sulfate both potently inhibited CXCL12 binding to the endothelial cell surface, suggesting that heparin displaced CXCL12 binding from BM heparan sulfate to lead to release into the peripheral blood. 2/3-des-hep retained the same inhibitory potency as that of heparin, but 6-des-hep did not show inhibition, indicating that 6-O- but not 2/3-O-sulfation is required for heparin to interact with CXCL12. To confirm this finding, we also carried out competitive SPR analysis, and we observed that heparin at 1 μg/ml inhibited CXCL12 binding to immobilized heparin by 70%, whereas 6-des-hep at the same concentration did not show any inhibition (Fig. 7B). Together, these observations suggest that heparin releases CXCL12 into the blood by disrupting the binding of CXCL12 to BM heparan sulfate and that the release requires heparin to be 6-O-sulfated.
FIGURE 7.
Heparin disrupted CXCL12 binding to heparan sulfate in a competitive and 6-O-sulfation-dependent manner and abolished the CXCL12 gradient-directed bone marrow cell migration. A, effects of heparinoids on the binding of CXCL12 to endothelial cell surface. CXCL12α was preincubated with heparan sulfate (HS), Hep, N-des-hep, 2/3-des-hep, 6-des-hep, or CR-hep. CXCL12α alone or the preincubated CXCL12a solution was incubated with untreated endothelial cells (control) or cells pretreated with heparinases. CXCL12α bound to the cell surface was detected by an ELISA. The data were summarized from three independent experiments. Bars represent mean ± S.E. The p values were calculated using the paired Student's t test in comparison with saline treatment. B, heparin but not 6-desulfated-heparin competitively inhibited CXCL12 binding to immobilized heparin in the SPR assay. CXCL12α was premixed with Hep or 6-des-hep and then the mixture was injected over heparin-immobilized CM5 chip surface. The SPR sensorgram shown is representative of three experiments. C, CXCL12 induced transendothelial cell migration of BM cells via both chemotaxis and chemokinesis, and heparin inhibited the CXCL12-induced chemotaxis of BM cells. The transendothelial cell migration assay was carried out with BM cells placed in the upper chamber and CXCL12 supplemented in the lower chamber (to determine chemotaxis) or immobilized to both abluminal and luminal surfaces (to determine chemokinesis). The BM cells that migrated into the lower chamber were counted. To determine the effect of heparin on CXCL12-induced chemotaxis of BM cells, heparin was added in both upper and lower chamber prior to initiation of cell migration. The data were summarized from three experiments. Each bar represents the average value ± S.E. The statistical analysis was carried by paired Student's t test.
Heparin Disrupts CXCL12-mediated Neutrophil Retention
In the BM, CXCL12 forms a stable haptotactic gradient to retain newly produced neutrophils (28, 36). It is possible that the release of CXCL12 from BM by heparin might interfere with the CXCL12 gradient, leading to neutrophil egress into the blood. This was determined by examining CXCL12-induced transendothelial migration of BM cells with CXCL12 placed in the lower chamber and heparin added to both the upper and lower chambers prior to initiation of cell migration. Heparin at 100 μg/ml completely inhibited CXCL12 gradient-directed chemotaxis of BM cells (Fig. 7C), supporting the idea that heparin disrupts the CXCL12 gradient in BM, and in this way contributes to neutrophil egress from the BM into the blood.
Heparin-released CXCL12 Promotes BM Cell Migration
CXCL12 is a strong chemoattractant for immature and mature neutrophils and lymphocytes. Elevation of CXCL12 levels over 2 ng/ml in circulation via either injection with an adenovirus expressing CXCL12 (44) or treating mice with AMD3100, a specific CXCR4 inhibitor (40), potentially mobilized both BM mature and immature leukocytes. Heparin elevated CXCL12 in circulation to >10 ng/ml (CXCL12α at 3.6 ng/ml and CXCL12β at 6.9 ng/ml, Fig. 6, B and C). Furthermore, heparin binding protects CXCL12 from carboxyl-terminal cleavage to preserve its biological function in serum (45). Therefore, it is conceivable that the heparin-released CXCL12 in the circulation might create a chemoattractant signal to promote BM cells to egress into the circulation, thereby contributing to leukocytosis. This idea was tested using the transendothelial cell migration assay with CXCL12 supplemented in the lower chamber at 10 ng/ml, a concentration equivalent to peak CXCL12 levels in circulation in HIL. Compared with BSA (10 ng/ml) that showed no effect on BM cell migration (<0.1% of input cells), CXCL12 induced 5% of the input BM cells to migrate into the lower chamber (Fig. 7C). Interestingly, when CXCL12 was added to both the upper and lower chamber at the same concentration such that no CXCL12 gradient existed, it still induced 1.75% of the input BM cells to migrate into the lower chamber (Fig. 7C), indicating that CXCL12 promoted random migration of BM cells (chemokinesis). These data suggest that CXCL12 in the blood induces BM cell egress into the circulation through enhancement of both chemotaxis and chemokinesis of BM cells.
DISCUSSION
Early studies established that heparin induces lymphocytosis (3–5, 26). In this study, we observed that heparin induced both lymphocytosis and neutrophilia. We also observed monocytosis induced by heparin. These observations demonstrate that heparin actually functions as a pan-leukocytosis inducer in mice. Clinical observation reveals that ∼1 out of every 230 patients develops HIL when taking heparin. In the United States, more than 12 million patients receive heparin every year (10); thus HIL is a frequent side effect of the drug. Further study is needed to determine whether pan-leukocytosis occurs in HIL patients. This study revealed that heparin disrupts key leukocyte trafficking molecules to lead to HIL. HIL patients demonstrate sensitivity to heparin treatment by manifesting leukocytosis. This suggests that some molecules that modulate leukocyte trafficking may function abnormally in the patients. Further studies with HIL patients may uncover novel mechanisms of leukocyte trafficking.
Sulfated glycans, including dextran sulfate and fucoidan, have been reported to induce lymphocytosis or leukocytosis, and their effects are dependent on sulfation (3, 5, 12, 26, 37). Similarly, we observed that sulfation was essential for heparin to induce leukocytosis. Furthermore, our study illustrated that 6-O-sulfation is uniquely required for induction of leukocytosis by heparin. We previously showed that heparin requires 6-O-sulfation to inhibit L- and P-selectin function (9), and in this study we observed that heparin requires 6-O-sulfation to bind to CXCL12. Therefore, the requirement of 6-O-sulfation for induction of leukocytosis is consistent with our finding that heparin blocks L- and P-selectin and CXCL12-mediated leukocyte trafficking, leading to HIL. Interestingly, we also observed that reduction of the carboxyl groups of heparin diminished its effect on lymphocytosis but not on neutrophils, suggesting that some lymphocyte mobilization molecules are critically modulated by carboxylated heparin, and further study of this issue may reveal novel mechanisms of lymphocyte trafficking. Meanwhile, it has been recognized that clinical heparins from different manufacturers differ in their levels of sulfation and/or carboxylation modifications (46). Therefore, it is conceivable that different heparins may affect HIL differently.
Leukocytosis is a disruption of normal homeostasis of leukocytes that leads to enhanced production, enhanced release, and/or reduced emigration into tissues (1). We uncovered that neutrophil release from BM and demargination in the vasculature are the major mechanisms underlying heparin-induced neutrophilia. We also determined that heparin inhibited lymphocyte homing accounted for ∼50% of heparin-induced lymphocytosis, whereas thymic release only accounted for 4.3%, indicating that heparin must also disrupt other pathways of lymphocyte trafficking. Under physiological conditions, 20% of the recirculating lymphocytes are in the spleen (47), and a large number of differentiating B cells (B220+) cells are present in the BM. Therefore, it is possible that heparin may inhibit homing of recirculating lymphocytes to spleen and release B220+ cells from BM contributing to lymphocytosis.
Leukocyte trafficking has been well dissected at the molecular level. Selectins are specialized in capturing leukocytes from the bloodstream onto the blood vessel wall, thereby critically modulating leukocyte margination, lymphocyte recirculation, and leukocyte extravasation (31). In this study, we showed that inhibition of L- and P-selectin represents one major mechanism underlying HIL. This observation is consistent with previous studies showing that heparin binds to L- and P-selectin and inhibits L- and P-selectin-mediated leukocyte trafficking in inflammation (7, 9). This observation also agrees with the observation that fucoidan blocks L- and P-selectin to cause leukocytosis (37). Selectins contain an amino-terminal calcium-dependent carbohydrate recognition domain that binds to a sialylated, fucosylated carbohydrate antigen related to sialyl Lewisx. The selectin-ligand interactions involve sulfate residues either on the carbohydrate or in the context of sulfated tyrosines in the proteins that are in proximity to the carbohydrate chain (48). It is currently thought that the sulfate of heparin competes with the endogenous sulfate residues of selectin and/or their ligands to disrupt selectin-ligand interactions thereby inhibiting L- and P-selectin functions.
In this study, heparin was injected at 1 mg/25 g BW. We estimate that the heparin dose in our study was higher than the clinical dose of heparin in humans. Recent studies by Stevenson et al. (49, 50) observed that clinically relevant levels of heparins block tumor metastasis via selectin inhibition. In line with these observations, we believe that blockage of L- and P-selectin function contributes to HIL at clinically relevant levels.
Our studies also revealed that release of CXCL12 from BM to circulation contributes to the mechanisms underlying HIL. This finding is in agreement with previous studies examining other sulfated glycans, including fucoidan, dextran sulfate, and glycosaminoglycan mimetics (12, 13, 20, 21, 42). CXCL12 is a CXC chemokine that binds to the G protein-coupled receptor, CXCR4. CXCL12 is constitutively expressed at high levels by stromal cells, osteoblasts, and endothelial cells in BM, and the CXCL12/CXCR4 axis provides a major retention signal for neutrophils in the BM where more than 90% of the mature neutrophils in the body reside (2, 18, 29, 33, 36). Similarly, CXCL12 also acts as an inhibitory factor that deters emigration of premature B cells out of the BM (51). CXCL12 is sequestered by heparan sulfate in the BM (20, 21, 42). In this study, we observed that heparin released CXCL12 from the BM, competitively inhibiting CXCL12 binding to heparan sulfate, and that the released CXCL12 reached a concentration that could potently induce egress of BM cells, together resulting in HIL. CXCL12 is also expressed in spleen and lymph where it functions as a chemoattractant for both progenitor and mature T and B lymphocytes (51). It is likely that heparin disrupts the CXCL12 gradient in the spleen and lymph nodes thereby contributing to lymphocytosis by interfering with lymphocyte homing to these secondary lymphoid organs. CXCL12 is also expressed in the thymus (51). In this study, we observed that heparin released thymocytes that may be due to heparin disruption of CXCL12 function in the thymus. Overall, our results show that heparin alters CXCL12-mediated leukocyte trafficking in multiple organs, resulting in neutrophilia and lymphocytosis.
Heparin is known to interact with multiple leukocyte trafficking-related molecules, including selectins, chemokines, integrins, cytokines such as granulocyte colony-stimulating factor (G-CSF), and granulocyte-macrophage colony-stimulating factor (GM-CSF) (6, 9, 14, 38, 52). Our study revealed that inhibition of L- and P-selectin and CXCL12 represent the main molecular mechanisms underlying HIL. It is possible that leukocytosis could be caused by heparin interactions with other molecules, including other chemokines and integrins. However, we postulate that these contributions to leukocytosis are less significant due to the critical and unique roles of L- and P-selectin as well as CXCL12 in leukocyte trafficking. During immune surveillance, P- and L-selectins mediate the first essential steps of leukocyte margination and homing prior to transendothelial migration mediated by chemokines and integrins (31). It is conceivable that heparin inhibition of P- and L-selectin diminishes the apparent effect of chemokines and integrins in HIL, consistent with our previous report that heparin's anti-inflammatory function is achieved primarily by blockage of L- and P-selectin functions (9). The CXCL12 pathway acts as the major retention signal to deter BM cell egress, and interruption of the CXCL12/CXCR4 signaling rapidly induces leukocytosis (12, 13, 33, 40). G-CSF and GM-CSF function to stimulate proliferation and differentiation of HPCs into granulocytes and macrophages, and both can mobilize mature and HPCs from BM to peripheral blood also (53). GM-CSF and G-CSF require days to elevate leukocytes in blood (41, 54, 55), whereas mobilization of leukocytes by heparin occurs within minutes and the effect is short lived. Thus, it is unlikely that heparin induces leukocytosis via modulating GM-CSF and/or G-CSF functions.
Blockage of L- and P-selectin or CXCL12 has been shown to potently mobilize HPCs from the BM to the peripheral blood (12, 37, 53, 54). Thus, it would be interesting to know whether and how heparin mobilizes HPCs and the structural features of heparin required for the effect. Furthermore, the unique expression pattern of chemokines, such as CXCL12, MIP-3β, SLC, MIP-1β, TECK, BLC, BLC, and/or DC-CK1, in specialized microenvironments determines the location of lymphoid progenitors in primary lymphoid tissues, circulation of naive T cells and B cells to secondary lymphoid tissues, and tissue-specific infiltration of effector lymphocytes (38, 51, 56). It is therefore also interesting to know whether and how heparin affects chemokine-directed trafficking of T and B cells for development and effector functions.
Acknowledgments
We thank Dr. John B. Lowe (University of Michigan) for providing us the FucT-IV/VII−/− mice. We also thank Dr. Jeffrey Esko (University of California San Diego) for discussion of the project and Karen Howard for the English revision in the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants R01HL093339 from NHLBI (to L. W.), RR005351 from NIAID (to L. W./James Prestegard), and R01AI37113 (to T. M. H.). This work was also supported by American Heart Association postdoctoral fellowship (to S. Y. Z.) and a scientist development grant (to L. W.).
- HIL
- heparin-induced leukocytosis
- BM
- bone marrow
- BW
- body weight
- N-des
- N-desulfated
- des-hep
- desulfated heparin
- CR-hep
- carboxyl-reduced heparin
- CFSE
- carboxyfluorescein diacetate succinimidyl ester
- SPR
- surface plasmon resonance
- HPC
- hematopoietic progenitor cell.
REFERENCES
- 1. Opdenakker G., Fibbe W. E., Van Damme J. (1998) The molecular basis of leukocytosis. Immunol. Today 19, 182–189 [DOI] [PubMed] [Google Scholar]
- 2. Abramson N., Melton B. (2000) Leukocytosis. Basics of clinical assessment. Am. Fam. Physician 62, 2053–2060 [PubMed] [Google Scholar]
- 3. Bradfield J. W., Born G. V. (1974) Lymphocytosis produced by heparin and other sulfated polysaccharides in mice and rats. Cell. Immunol. 14, 22–32 [DOI] [PubMed] [Google Scholar]
- 4. Godlowski Z. Z. (1951) Prevention of hormona eosinopenia and lymphopenia by inhibition of clotting in blood; preliminary report. Br. Med. J. 1, 854–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sasaki S. (1967) Production of lymphocytosis by polysaccharide polysulfates (heparinoids). Nature 214, 1041–1042 [DOI] [PubMed] [Google Scholar]
- 6. Esko J. D., Selleck S. B. (2002) Order out of chaos. Assembly of ligand-binding sites in heparan sulfate. Annu. Rev. Biochem. 71, 435–471 [DOI] [PubMed] [Google Scholar]
- 7. Nelson R. M., Cecconi O., Roberts W. G., Aruffo A., Linhardt R. J., Bevilacqua M. P. (1993) Heparin oligosaccharides bind L- and P-selectin and inhibit acute inflammation. Blood 82, 3253–3258 [PubMed] [Google Scholar]
- 8. Borsig L., Wong R., Feramisco J., Nadeau D. R., Varki N. M., Varki A. (2001) Heparin and cancer revisited. Mechanistic connections involving platelets, P-selectin, carcinoma mucins, and tumor metastasis. Proc. Natl. Acad. Sci. U.S.A. 98, 3352–3357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang L., Brown J. R., Varki A., Esko J. D. (2002) Heparin's anti-inflammatory effects require glucosamine 6-O-sulfation and are mediated by blockade of L- and P-selectins. J. Clin. Invest. 110, 127–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fahey V. A. (1995) Heparin-induced thrombocytopenia. J. Vasc. Nurs. 13, 112–116 [DOI] [PubMed] [Google Scholar]
- 11. Weninger W., Ulfman L. H., Cheng G., Souchkova N., Quackenbush E. J., Lowe J. B., von Andrian U. H. (2000) Specialized contributions by α(1,3)-fucosyltransferase-IV and FucT-VII during leukocyte rolling in dermal microvessels. Immunity 12, 665–676 [DOI] [PubMed] [Google Scholar]
- 12. Sweeney E. A., Lortat-Jacob H., Priestley G. V., Nakamoto B., Papayannopoulou T. (2002) Sulfated polysaccharides increase plasma levels of SDF-1 in monkeys and mice. Involvement in mobilization of stem/progenitor cells. Blood 99, 44–51 [DOI] [PubMed] [Google Scholar]
- 13. Albanese P., Caruelle D., Frescaline G., Delbé J., Petit-Cocault L., Huet E., Charnaux N., Uzan G., Papy-Garcia D., Courty J. (2009) Glycosaminoglycan mimetics-induced mobilization of hematopoietic progenitors and stem cells into mouse peripheral blood. Structure/function insights. Exp. Hematol. 37, 1072–1083 [DOI] [PubMed] [Google Scholar]
- 14. Wang L., Fuster M., Sriramarao P., Esko J. D. (2005) Endothelial heparan sulfate deficiency impairs L-selectin- and chemokine-mediated neutrophil trafficking during inflammatory responses. Nat. Immunol. 6, 902–910 [DOI] [PubMed] [Google Scholar]
- 15. Scollay R. G., Butcher E. C., Weissman I. L. (1980) Thymus cell migration. Quantitative aspects of cellular traffic from the thymus to the periphery in mice. Eur. J. Immunol. 10, 210–218 [DOI] [PubMed] [Google Scholar]
- 16. Horwitz B. H., Mizgerd J. P., Scott M. L., Doerschuk C. M. (2001) Mechanisms of granulocytosis in the absence of CD18. Blood 97, 1578–1583 [DOI] [PubMed] [Google Scholar]
- 17. Basu S., Hodgson G., Katz M., Dunn A. R. (2002) Evaluation of role of G-CSF in the production, survival, and release of neutrophils from bone marrow into circulation. Blood 100, 854–861 [DOI] [PubMed] [Google Scholar]
- 18. Suratt B. T., Petty J. M., Young S. K., Malcolm K. C., Lieber J. G., Nick J. A., Gonzalo J. A., Henson P. M., Worthen G. S. (2004) Role of the CXCR4/SDF-1 chemokine axis in circulating neutrophil homeostasis. Blood 104, 565–571 [DOI] [PubMed] [Google Scholar]
- 19. Nakagawa M., Terashima T., D'yachkova Y., Bondy G. P., Hogg J. C., van Eeden S. F. (1998) Glucocorticoid-induced granulocytosis. Contribution of marrow release and demargination of intravascular granulocytes. Circulation 98, 2307–2313 [DOI] [PubMed] [Google Scholar]
- 20. Netelenbos T., Zuijderduijn S., Van Den Born J., Kessler F. L., Zweegman S., Huijgens P. C., Dräger A. M. (2002) Proteoglycans guide SDF-1-induced migration of hematopoietic progenitor cells. J. Leukocyte Biol. 72, 353–362 [PubMed] [Google Scholar]
- 21. Netelenbos T., van den Born J., Kessler F. L., Zweegman S., Merle P. A., van Oostveen J. W., Zwaginga J. J., Huijgens P. C., Dräger A. M. (2003) Proteoglycans on bone marrow endothelial cells bind and present SDF-1 towards hematopoietic progenitor cells. Leukemia 17, 175–184 [DOI] [PubMed] [Google Scholar]
- 22. Wijelath E., Namekata M., Murray J., Furuyashiki M., Zhang S., Coan D., Wakao M., Harris R. B., Suda Y., Wang L., Sobel M. (2010) Multiple mechanisms for exogenous heparin modulation of vascular endothelial growth factor activity. J. Cell. Biochem. 111, 461–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhou B., Honor L. B., He H., Ma Q., Oh J. H., Butterfield C., Lin R. Z., Melero-Martin J. M., Dolmatova E., Duffy H. S., Gise A., Zhou P., Hu Y. W., Wang G., Zhang B., Wang L., Hall J. L., Moses M. A., McGowan F. X., Pu W. T. (2011) Adult mouse epicardium modulates myocardial injury by secreting paracrine factors. J. Clin. Invest. 121, 1894–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Osmond R. I., Kett W. C., Skett S. E., Coombe D. R. (2002) Protein-heparin interactions measured by BIAcore 2000 are affected by the method of heparin immobilization. Anal. Biochem. 310, 199–207 [DOI] [PubMed] [Google Scholar]
- 25. Amara A., Lorthioir O., Valenzuela A., Magerus A., Thelen M., Montes M., Virelizier J. L., Delepierre M., Baleux F., Lortat-Jacob H., Arenzana-Seisdedos F. (1999) Stromal cell-derived factor-1α associates with heparan sulfates through the first β-strand of the chemokine. J. Biol. Chem. 274, 23916–23925 [DOI] [PubMed] [Google Scholar]
- 26. Bradfield J. W., Born G. V. (1969) Inhibition of lymphocyte recirculation by heparin. Nature 222, 1183–1184 [DOI] [PubMed] [Google Scholar]
- 27. Ford W. L., Gowans J. L. (1969) The traffic of lymphocytes. Semin. Hematol. 6, 67–83 [PubMed] [Google Scholar]
- 28. Christopher M. J., Link D. C. (2007) Regulation of neutrophil homeostasis. Curr. Opin. Hematol. 14, 3–8 [DOI] [PubMed] [Google Scholar]
- 29. Furze R. C., Rankin S. M. (2008) Neutrophil mobilization and clearance in the bone marrow. Immunology 125, 281–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Johnson R. C., Mayadas T. N., Frenette P. S., Mebius R. E., Subramaniam M., Lacasce A., Hynes R. O., Wagner D. D. (1995) Blood cell dynamics in P-selectin-deficient mice. Blood 86, 1106–1114 [PubMed] [Google Scholar]
- 31. Springer T. A. (1994) Traffic signals for lymphocyte recirculation and leukocyte emigration. The multistep paradigm. Cell 76, 301–314 [DOI] [PubMed] [Google Scholar]
- 32. Malý P., Thall A., Petryniak B., Rogers C. E., Smith P. L., Marks R. M., Kelly R. J., Gersten K. M., Cheng G., Saunders T. L., Camper S. A., Camphausen R. T., Sullivan F. X., Isogai Y., Hindsgaul O., von Andrian U. H., Lowe J. B. (1996) The α(1,3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell 86, 643–653 [DOI] [PubMed] [Google Scholar]
- 33. Martin C., Burdon P. C., Bridger G., Gutierrez-Ramos J. C., Williams T. J., Rankin S. M. (2003) Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity 19, 583–593 [DOI] [PubMed] [Google Scholar]
- 34. Wang J., Mukaida N., Zhang Y., Ito T., Nakao S., Matsushima K. (1997) Enhanced mobilization of hematopoietic progenitor cells by mouse MIP-2 and granulocyte colony-stimulating factor in mice. J. Leukocyte Biol. 62, 503–509 [DOI] [PubMed] [Google Scholar]
- 35. Zhang P., Quinton L. J., Gamble L., Bagby G. J., Summer W. R., Nelson S. (2005) The granulopoietic cytokine response and enhancement of granulopoiesis in mice during endotoxemia. Shock 23, 344–352 [DOI] [PubMed] [Google Scholar]
- 36. Eash K. J., Greenbaum A. M., Gopalan P. K., Link D. C. (2010) CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J. Clin. Invest. 120, 2423–2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Frenette P. S., Weiss L. (2000) Sulfated glycans induce rapid hematopoietic progenitor cell mobilization. Evidence for selectin-dependent and -independent mechanisms. Blood 96, 2460–2468 [PubMed] [Google Scholar]
- 38. Handel T. M., Johnson Z., Crown S. E., Lau E. K., Proudfoot A. E. (2005) Regulation of protein function by glycosaminoglycans, as exemplified by chemokines. Annu. Rev. Biochem. 74, 385–410 [DOI] [PubMed] [Google Scholar]
- 39. Shen H., Cheng T., Olszak I., Garcia-Zepeda E., Lu Z., Herrmann S., Fallon R., Luster A. D., Scadden D. T. (2001) CXCR-4 desensitization is associated with tissue localization of hemopoietic progenitor cells. J. Immunol. 166, 5027–5033 [DOI] [PubMed] [Google Scholar]
- 40. Dar A., Schajnovitz A., Lapid K., Kalinkovich A., Itkin T., Ludin A., Kao W. M., Battista M., Tesio M., Kollet O., Cohen N. N., Margalit R., Buss E. C., Baleux F., Oishi S., Fujii N., Larochelle A., Dunbar C. E., Broxmeyer H. E., Frenette P. S., Lapidot T. (2011) Rapid mobilization of hematopoietic progenitors by AMD3100 and catecholamines is mediated by CXCR4-dependent SDF-1 release from bone marrow stromal cells. Leukemia 25, 1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Petit I., Szyper-Kravitz M., Nagler A., Lahav M., Peled A., Habler L., Ponomaryov T., Taichman R. S., Arenzana-Seisdedos F., Fujii N., Sandbank J., Zipori D., Lapidot T. (2002) G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat. Immunol. 3, 687–694 [DOI] [PubMed] [Google Scholar]
- 42. Netelenbos T., van den Born J., Kessler F. L., Zweegman S., Huijgens P. C., Drager A. M. (2003) In vitro model for hematopoietic progenitor cell homing reveals endothelial heparan sulfate proteoglycans as direct adhesive ligands. J. Leukocyte Biol. 74, 1035–1044 [DOI] [PubMed] [Google Scholar]
- 43. Sadir R., Baleux F., Grosdidier A., Imberty A., Lortat-Jacob H. (2001) Characterization of the stromal cell-derived factor-1α-heparin complex. J. Biol. Chem. 276, 8288–8296 [DOI] [PubMed] [Google Scholar]
- 44. Hattori K., Heissig B., Tashiro K., Honjo T., Tateno M., Shieh J. H., Hackett N. R., Quitoriano M. S., Crystal R. G., Rafii S., Moore M. A. (2001) Plasma elevation of stromal cell-derived factor-1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood 97, 3354–3360 [DOI] [PubMed] [Google Scholar]
- 45. De La Luz Sierra M., Yang F., Narazaki M., Salvucci O., Davis D., Yarchoan R., Zhang H. H., Fales H., Tosato G. (2004) Differential processing of stromally derived factor-1α and stromal-derived factor-1β explains functional diversity. Blood 103, 2452–2459 [DOI] [PubMed] [Google Scholar]
- 46. Zhang F., Yang B., Ly M., Solakyildirim K., Xiao Z., Wang Z., Beaudet J. M., Torelli A. Y., Dordick J. S., Linhardt R. J. (2011) Structural characterization of heparins from different commercial sources. Anal. Bioanal. Chem. 401, 2793–2803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stekel D. J., Parker C. E., Nowak M. A. (1997) A model of lymphocyte recirculation. Immunol. Today 18, 216–221 [DOI] [PubMed] [Google Scholar]
- 48. Leppänen A., White S. P., Helin J., McEver R. P., Cummings R. D. (2000) Binding of glycosulfopeptides to P-selectin requires stereospecific contributions of individual tyrosine sulfate and sugar residues. J. Biol. Chem. 275, 39569–39578 [DOI] [PubMed] [Google Scholar]
- 49. Stevenson J. L., Varki A., Borsig L. (2007) Heparin attenuates metastasis mainly due to inhibition of P- and L-selectin, but non-anticoagulant heparins can have additional effects. Thromb. Res. 120, S107–S111 [DOI] [PubMed] [Google Scholar]
- 50. Stevenson J. L., Choi S. H., Varki A. (2005) Differential metastasis inhibition by clinically relevant levels of heparins. Correlation with selectin inhibition, not antithrombotic activity. Clin. Cancer Res. 11, 7003–7011 [DOI] [PubMed] [Google Scholar]
- 51. Kim C. H., Broxmeyer H. E. (1999) Chemokines. Signal lamps for trafficking of T and B cells for development and effector function. J. Leukocyte Biol. 65, 6–15 [DOI] [PubMed] [Google Scholar]
- 52. Tyrrell D. J., Horne A. P., Holme K. R., Preuss J. M., Page C. P. (1999) Heparin in inflammation. Potential therapeutic applications beyond anticoagulation. Adv. Pharmacol. 46, 151–208 [DOI] [PubMed] [Google Scholar]
- 53. Papayannopoulou T. (2004) Current mechanistic scenarios in hematopoietic stem/progenitor cell mobilization. Blood 103, 1580–1585 [DOI] [PubMed] [Google Scholar]
- 54. Lapidot T., Petit I. (2002) Current understanding of stem cell mobilization. The roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp. Hematol. 30, 973–981 [DOI] [PubMed] [Google Scholar]
- 55. de Haan G., Ausema A., Wilkens M., Molineux G., Dontje B. (2000) Efficient mobilization of hematopoietic progenitors after a single injection of pegylated recombinant human granulocyte colony-stimulating factor in mouse strains with distinct marrow-cell pool sizes. Br. J. Haematol. 110, 638–646 [DOI] [PubMed] [Google Scholar]
- 56. Thelen M., Stein J. V. (2008) How chemokines invite leukocytes to dance. Nat. Immunol. 9, 953–959 [DOI] [PubMed] [Google Scholar]