Background: IL-32 is involved in several cell processes, most likely through integrin signaling.
Results: Modeling of IL-32 revealed a structure similar to FAT and binds to integrins, paxillin, and FAK-1, all members of the integrin-signaling pathway.
Conclusion: IL-32 interacts with members of the focal adhesion protein complex.
Significance: IL-32 might be a key protein in integrin signaling and downstream processes.
Keywords: Bioinformatics, Cell Death, Cytokine, Integrins, Signal Transduction
Abstract
IL-32 can be expressed in several isoforms. The amino acid sequences of the major IL-32 isoforms were used to predict the secondary and tertiary protein structure by I-TASSER software. The secondary protein structure revealed coils and α-helixes, but no β sheets. Furthermore, IL-32 contains an RGD motif, which potentially activates procaspase-3 intracellular and or binds to integrins. Mutation of the RGD motif did not result in inhibition of the IL-32β- or IL-32γ-induced cytotoxicity mediated through caspase-3. Although IL-32α interacted with the extracellular part of αVβ3 and αVβ6 integrins, only the αVβ3 binding was inhibited by small RGD peptides. Additionally, IL-32β was able to bind to αVβ3 integrins, whereas this binding was not inhibited by small RGD peptides. In addition to the IL-32/integrin interactions, we observed that IL-32 is also able to interact with intracellular proteins that are involved in integrin and focal adhesion signaling. Modeling of IL-32 revealed a distinct α-helix protein resembling the focal adhesion targeting region of focal adhesion kinase (FAK). Inhibition of FAK resulted in modulation of the IL-32β- or IL-32γ-induced cytotoxicity. Interestingly, IL-32α binds to paxillin without the RGD motif being involved. Finally, FAK inhibited IL-32α/paxillin binding, whereas FAK also could interact with IL-32α, demonstrating that IL-32 is a member of the focal adhesion protein complex. This study demonstrates for the first time that IL-32 binds to the extracellular domain of integrins and to intracellular proteins like paxillin and FAK, suggesting a dual role for IL-32 in integrin signaling.
Introduction
IL-32, a predominantly intracellular proinflammatory mediator (1–7), is associated with viral (8–13) and bacterial (3, 14–16) infections but also with cancers (17–21). Furthermore, IL-32 is associated with autoimmune diseases like rheumatoid arthritis (3–6, 22, 23) and Crohn disease (24). IL-32 is a multifunctional cytokine, capable of regulating important cell functions; inducer of proinflammatory cytokines (3–7), apoptosis (3, 14, 25), and differentiation (26–28). Recently, it was reported that induction of endogenous IL-32 by TNFα/IFNγ costimulation resulted in colocalization of IL-32 with membrane lipid structures (29), whereas overexpression of splice-resistant IL-32γ resulted in efficient secretion of IL-32γ in rheumatoid arthritis FLS without cell death being involved (5). Furthermore, stimulation with IL-1β or TNFα resulted in enhanced IL-32γ secretion (5). Although several reports show that silencing of intracellular IL-32 results in abrogation of cytokine production (4, 30), demonstrating a major intracellular role for IL-32, extracellular IL-32 can be found (5, 29, 31). Several mechanisms have been suggested. Extracellular IL-32 by secretion (5), potentially cleaved from the cell membrane by proteinase 3 (PR3) (32, 33) or in membrane-like structures (29) or caused by cell death (25), could signal via an unknown IL-32 receptor. To date, only PR3 is recognized as an IL-32 binding partner (33) and seems to process IL-32 into a more active cytokine (32). Finding IL-32 binding partners is not as strait forward as for other cytokines, because IL-32 does not contain any conserved domains that could help to predict possible binding candidates. When IL-32 was described as NK4 (34), it was reported that the IL-32 amino acid sequence contains an RGD motif, likely being involved in cell adhesion processes (34). Previously, it was reported that RGD motifs bind to integrins, which is essential for many cell processes such as adhesion, migration, proliferation, cytokine production, survival, and differentiation (35–37). Therefore, blocking the integrin-signaling pathway is an attractive therapeutic target (38–44). Furthermore, it was reported that small peptides expressing RGD motifs are capable to induce caspase-3-dependent apoptosis (45–47). Interestingly, procaspase-3 contains, besides an RGD motif, a DDM motif (46), and it is suggested that the two motifs can bind to each other, resulting in intramolecular interactions that prevent autoactivation of procaspase-3 (48). Small peptides with RGD motifs could potentially interfere with this engagement and lead to autoactivation of procaspase-3 (46, 48).
In this study, we used I-TASSER software to predict the secondary and tertiary structure of the three major isoforms of IL-32. Next, we investigated the role of the RGD motif in the IL-32β- and IL-32γ-induced cell death by developing several mutants. Furthermore, IL-32 binding assays were conducted to investigate whether IL-32 would bind to integrins and intracellular proteins that are involved in the integrin-signaling pathway. Moreover, a small molecule inhibitor for the integrin-signaling pathway was used to investigate the IL-32-induced cytotoxicity. Finally, we propose a model for the possible IL-32 integrin-signaling cascade.
EXPERIMENTAL PROCEDURES
Modeling of IL-32
I-TASSER online software (49, 50) was used for predicting the secondary and tertiary structure of IL-32α, IL-32β, and IL-32γ. With the freely available Swiss-PdbViewer (51), a model for IL-32α and IL-32β was made. HMMTOP software (52) was used to predict whether IL-32γ contains a transmembrane helix.
RGD Mutants
pCDNA3 expression plasmids containing human IL-32α, IL-32β, or IL-32γ sequences were used to mutate the RGD motif into RGE. Primers (forward, 5′-CAAGCTTGCCACCATGTGCTTCCCGAAGGTCCTCTCTGATGACATGAAGAAGCTGAAGG-3′, and reverse, 5′-GTCTAGATCATTTTGAGGATTGGGGTTCAGAGCACTTCTGGGGTGTCAGCTCCTCCTTCTC-3′) were manufactured by Biolegio (Nijmegen, The Netherlands), which contained the RGD to RGE single nucleotide mutation. Subsequently, the primers were used to construct the mutants by conventional PCR. The RGD deletion mutants were produced by digesting the pCDNA3-IL-32β/IL-32γ expression plasmids with HindIII (New England Biolabs, Ipswich, MA) and XbaI (New England Biolabs) to separate the backbone and insert. The inserts were digested with BtgI (New England Biolabs) to remove the GD amino acid sequence until the stop codon. Subsequently, the modified IL-32β/IL-32γ inserts were ligated at the HindIII overhang into the previously isolated backbone, which also contained a HindIII overhang, by using T4 DNA ligase (New England Biolabs). Next, the BtgI and XbaI sites were both treated with T4 DNA polymerase (New England Biolabs) together with 100 μm dNTPs to create blunt ends to facilitate ligation of both ends with T4 DNA ligase (New England Biolabs). Exactly after the arginine amino acid, a stop codon was present because of the restriction and ligation reaction. Finally, all of the mutants were verified by sequencing.
Overexpression of IL-32α, IL-32β, and IL-32γ in HEK293T Cells to Investigate Cytotoxicity
HEK293T cells were cultured at 37 °C with 5% carbon dioxide in DMEM-GlutaMAX medium (Invitrogen, Carlsbad, CA) containing 10% FCS, pyruvate, and penicillin/streptomycin. The cells were transfected with plasmids (pCDNA3) expressing eGFP, IL-32α, IL-32β, or IL-32γ by using Lipofectamine 2000 (Invitrogen). Briefly, 2 × 105 cells were seeded in 24-well Costar plates (Corning, New York, NY) in 0.5 ml/well with DMEM-GlutaMAX containing 10% FCS and pyruvate (no antibiotics). The next day, the cells were washed with saline and 0.5 ml of RPMI 1640 (Invitrogen) without phenol red but containing 5% FCS was added per well. Next, the cells were transfected by adding 100 μl of DNA-Lipofectamine complexes containing 1 μg of plasmid DNA and 2 μl of Lipofectamine 2000/well in serum free RPMI 1640 without phenol red. Cytotoxicity was determined by using the Cytotox96 assay (Promega, Madison, WI), 72 h after transfection. A specific caspase-3 inhibitor (Z-DEVD-FMK) (Biovision, Mountain View, CA) was used as recommended by the manufacturer (1:1000) to investigate the IL-32-induced cytotoxicity.
Overexpression of IL-32 Wild Type and RGD Mutants in HEK293T Cells
Cytotoxicity was investigated in HEK293T cells that were transfected with plasmids (pCDNA3) expressing eGFP,4 wild type, or RGD mutants of IL-32α, IL-32β, and IL-32γ by using Lipofectamine 2000 (Invitrogen) and cytotoxicity was determined as described earlier.
IL-32/Integrin Binding Assays
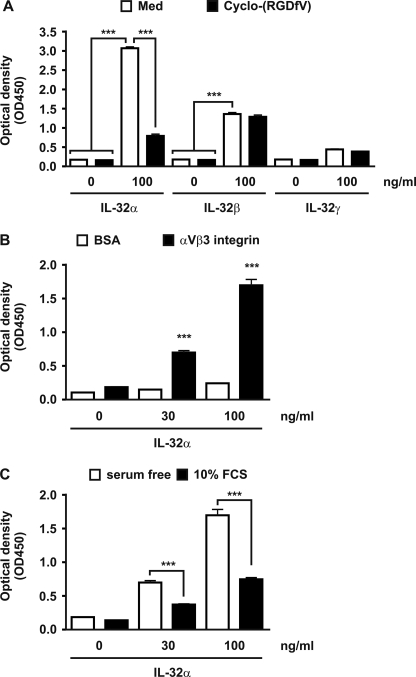
MaxiSorp flat-bottomed 96-well plates (Nunc, Roskilde, Denmark) were coated with 1 μg/ml recombinant αVβ3 integrin (R & D Systems, Minneapolis, MN), 1 μg/ml recombinant αVβ6 integrin (R & D Systems), 1 μg/ml αVβ8 integrin (R & D Systems), or 1 μg/ml BSA all diluted in PBS and incubated overnight at 4 °C. Next, the wells were blocked with 1% BSA (Invitrogen) in PBS at 37 °C for 1 h followed by three wash steps with PBS containing 0.05% Tween 20. Subsequently, some wells were preincubated with 10 μm cyclo-(RGDfV) (Peptide Institute Inc, Osaka, Japan) or 10% FCS, whereas other were incubated with wash buffer (PBS with 0.05% Tween 20) at room temperature for 1 h. Next, the wells were incubated with different concentrations recombinant IL-32 proteins (R & D Systems) diluted in PBS, with or without 10 μm cyclo-(RGDfV) or 10% FCS at room temperature for 1 h. The wells were washed three times with wash buffer and incubated with biotinylated anti-IL-32α antibody (R & D Systems) diluted in PBS with a concentration of 0.2 μg/ml at room temperature for 1 h. After the incubation, the wells were washed three times with wash buffer and incubated with streptavidin-poly-HRP (Sanquin, Amsterdam, The Netherlands) at room temperature for 30 min. Next, wells were washed six times with wash buffer and incubated with TMB substrate (Biomérieux, Boxtel, The Netherlands) at room temperature for ∼10 min, and the color reaction was stopped with 2.5 m H2SO4. The absorbance/optical density was measured with an ELISA reader (Tecan Group Ltd., Männedorf, Switzerland) at 450 nm.
Inhibition of FAK Phosphorylation
HEK293T were transfected with plasmids expressing eGFP, IL-32α, IL-32β, or IL-32γ as described earlier. Phosphorylation of FAK was inhibited by using two different concentrations, 1 and 10 μm of FAK inhibitor 14 (Tocris Bioscience, Bristol, UK), which prevents autophosphorylation of FAK tyrosine 397. Cytotoxicity was determined by using the Cytotox96 assay (Promega, Madison, WI), 72 h after transfection.
IL-32/Paxillin/FAK Binding Assays
Maxisorp plates (Nunc) were coated with 1 μg/ml recombinant paxillin (BioLegend, San Diego, CA), or 1 μg/ml recombinant FAK (Biaffin GmbH & Co KG, Kassel, Germany). As a control, recombinant αVβ3 and cyclo-(RGDfV) was included as described before. The IL-32/paxillin binding was inhibited by using 0.5 μg/ml recombinant FAK (Biaffin GmbH & Co KG) in the same way as cyclo-(RGDfV), preincubation, and competition, respectively.
RESULTS
In Silico Modeling of IL-32
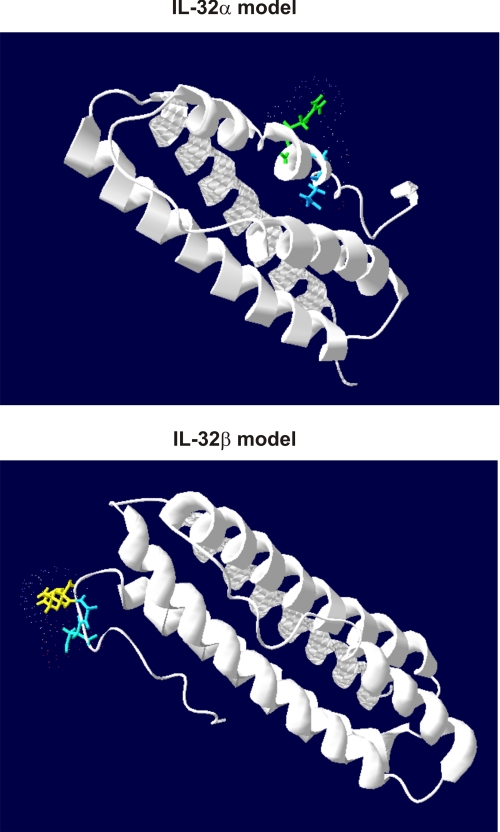
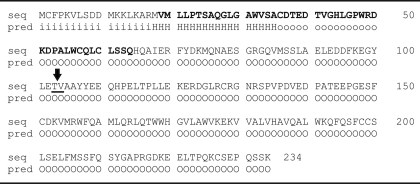
I-TASSER software was used to predict the secondary and tertiary structure of IL-32. IL-32 consists of several isoforms, and we used the amino acid sequences of IL-32α, IL-32β, and IL-32γ for the modeling. Table 1 shows the secondary structure of IL-32α, IL-32β, and IL-32γ. Coils (C) and α-helixes (H) are aligned with the corresponding amino acid together with the confidence score. Remarkably, no β sheets were predicted but only coils, and α-helixes are found in the secondary structure. Furthermore, in the amino acid sequences of IL-32α, IL-32β, and IL-32γ, two known motifs are present (bold and underlined), namely a DDM and an RGD sequence. Interestingly, IL-32α misses the large α-helix located in front of the RGD motif. Fig. 1 shows the tertiary structure of IL-32α and IL-32β with a confidence score (C-score) of −1.89 and −2.19, respectively. Furthermore, the RGD motif is differently located in the IL-32α and IL-32β models, which could lead to a less accessible RGD motif. Predicting the tertiary structure of IL-32γ is rather complicated because of the possibility of a transmembrane domain. The C-score of IL-32γ is −2.61, which is less reliable than the predicted IL-32α/IL-32β models, whereas models with C-scores higher than −1.5 are considered to have correct folds. Table 2 shows the intracellular (i, inside), transmembrane (H, transmembrane helix), and extracellular domains (o, outside) of IL-32γ as predicted by HMMTOP software. In addition, the PR3 cleavage site between threonine and valine is underlined together with an arrow in Table 2, which could be involved in releasing IL-32γ from the membrane. Furthermore, the IL-32γ-specific amino acid sequence is displayed bold.
TABLE 1.
IL-32α, IL-32β, IL-32γ; primary and secondary amino acid sequences
FIGURE 1.
IL-32α and IL-32β protein modeling showing different accessibility and orientation of the RGD-motif and a typical α-helix bundle shape protein. Tertiary structure of IL-32α contains a confidence score of −1.89, whereas IL-32β contains a confidence score of −2.19. Accessibility of the RGD motif is shown by coloring the Arg-Gly-Asp amino acids from blue to red (blue = low, red = highly accessible) with the Swiss-PdbViewer.
TABLE 2.
IL-32γ transmembrane prediction and PR3 cleavage site
IL-32β and IL-32γ Induced Cytotoxicity through Caspase-3 Activation
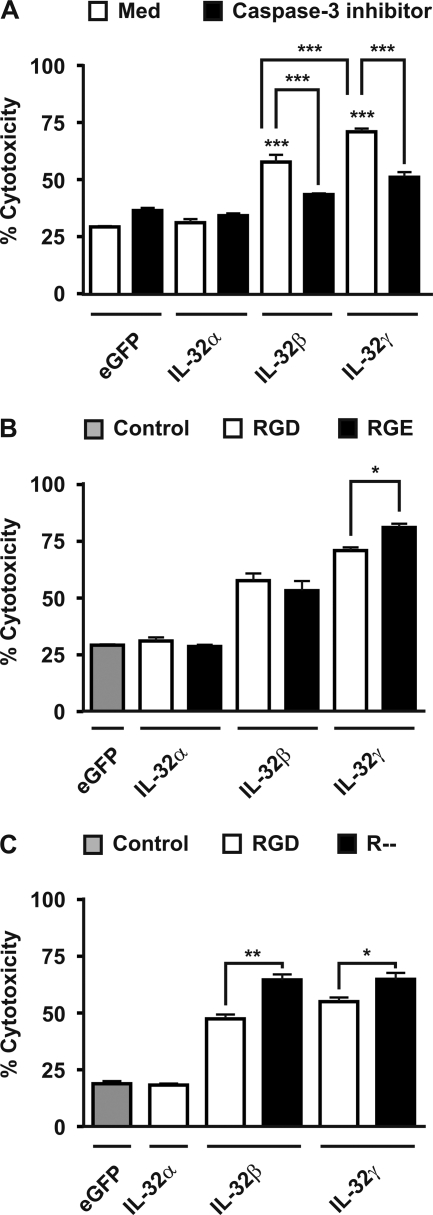
Overexpression of IL-32α in human HEK293T showed comparable amount of cell death compared with control (eGFP) transfected cells (Fig. 2A). Overexpression of IL-32β or IL-32γ resulted in notably enhanced cytotoxicity compared with control or IL-32α transfected HEK293T cells (Fig. 2A, open bars). The IL-32β/IL-32γ-induced cytotoxicity was significantly reduced by caspase-3 inhibitors (Fig. 2A, solid bars). Interestingly, IL-32γ showed more cytotoxic capacity compared with IL-32β-transfected cells (Fig. 2A).
FIGURE 2.
IL-32β- and IL-32γ-driven apoptosis is caspase-3-dependent, although mutation of the RGD motif in IL-32β or IL-32γ does not prevent cell death. A, overexpression of IL-32β or IL-32γ, but not IL-32α, induced cell death (open bars) in HEK293T cells that could be prevented by caspase-3 inhibitors (solid bars). B, mutation of the RGD motif into RGE did not decrease cytotoxicity. B and C, furthermore, GD till stop codon deletion could not prevent the IL-32β/IL-32γ-induced cytotoxicity (C), whereas IL-32α or control (eGFP) showed comparable cytotoxicity (B and C). The values are the means with S.E. One-way ANOVA with Bonferroni's multiple comparison test was used (n = 4; *, p < 0.05; **, p < 0.01; ***, p < 0.001).
Mutation of RGD Motif Present in IL-32β or IL-32γ Does Not Prevent Cell Death
Small soluble peptides containing an RGD motif can induce apoptosis through direct activation of procaspase-3, leading to caspase-3-induced apoptosis (46). Interestingly, an RGD motif is present in IL-32 (Table 1), and therefore, we hypothesized that the RGD motif present in IL-32 could activate procaspase-3 and finally result in apoptosis. Surprisingly, the RGD motif present in IL-32β or IL-32γ is not involved in the IL-32β/γ-induced caspase-3-dependent apoptosis, because mutation of the RGD motif into RGE did not reduce cell death (Fig. 2B). To study whether the lack of the RGD motif instead of RGD to RGE mutation was protective, we deleted the GD until the stop codon of IL-32β and IL-32γ. Overexpression of these deletion mutants did not show inhibition of the IL-32β or IL-32γ-induced cytotoxicity compared with the wild type isoforms of IL-32β and IL-32γ (Fig. 2C). Remarkably, mutating the RGD motif resulted in enhanced IL-32γ-cytotoxicity (Fig. 2B), whereas deletion of the RGD motif increased the IL-32β- and IL-32γ-induced cytotoxicity (Fig. 2C).
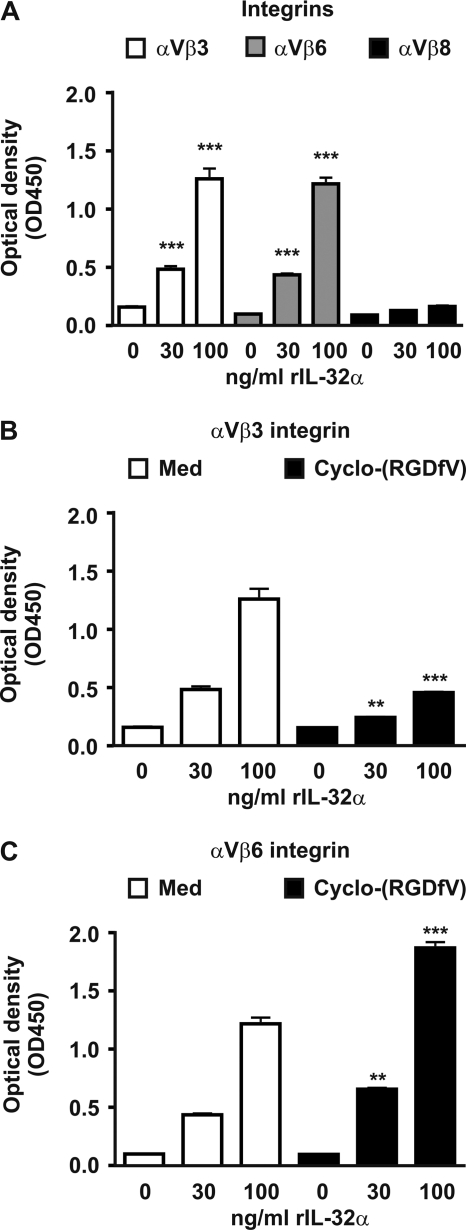
IL-32 Binds to αVβ3 and αVβ6 Integrins
RGD amino acid sequences that are localized in extracellular matrix proteins interact with integrins on cell surfaces and are important for several processes such as adhesion, migration, proliferation, survival, and apoptosis (35–37, 46). These RGD motifs are also present in the IL-32 isoforms (Table 1) and could potentially bind to integrins. Therefore, three integrins were tested: αVβ3, αVβ6, and αVβ8. Fig. 3A shows that IL-32α can bind to αVβ3 and αVβ6, but not to αVβ8 integrins. The interaction between IL-32α and αVβ3 can be inhibited by cyclo-(RGDfV), which is a small peptide containing the RGD motif (Fig. 3B). Interestingly, cyclo-(RGDfV) could not inhibit the IL-32α and αVβ6 binding but increased the interaction between IL-32α and αVβ6 (Fig. 3C).
FIGURE 3.
A, IL-32α binds to the extracellular domain of recombinant αVβ3 and αVβ6 integrin, whereas αVβ8 integrin does not bind to IL-32α. B, IL-32α to αVβ3 binding can be inhibited by cyclo-(RGDfV), a small peptide expressing an RGD motif. C, binding of IL-32α to αVβ6 could not be inhibited by small RGD peptides but increased IL-32α to αVβ6 binding. The values are the means with S.E. One-way ANOVA with Bonferroni's multiple comparison test was used (n = 4; **, p < 0.01; ***, p < 0.001).
IL-32α-, IL-32β-, and IL-32γ-αVβ3 Integrin Interactions
The amino acid sequence of IL-32α contains an RGD motif; however, by modeling it appeared that the localization of this motif is different compared with IL-32β and IL-32γ (Fig. 1). For that reason, it might be possible that the binding of the different IL-32 isoforms to RGD-integrins is different. As a control, binding of IL-32α to αVβ3 was verified again, besides the binding of IL-32β and IL-32γ to αVβ3 integrin. It appeared that IL-32β and to a lesser extent IL-32γ binds to αVβ3, whereas the IL-32α/αVβ3 binding was observed again (Fig. 4A). Furthermore, cyclo-(RGDfV) could not inhibit the IL-32β/αVβ3 interaction, whereas the IL-32α/αVβ3 interaction was inhibited by cyclo-(RGDfV) (Fig. 4A). Binding of IL-32α to αVβ3 is specific, because IL-32α does not bind to the same amount of control protein (BSA) (Fig. 4B). The inhibiting effects of serum toward IL-32 responses were previously observed5; therefore, we investigated whether the IL-32α/αVβ3-binding could be inhibited by serum. Fig. 4C shows that 10% FCS inhibited the IL-32α/αVβ3 binding remarkably.
FIGURE 4.
IL-32α-, IL-32β-, and IL-32γ-αVβ3 engagement. A, IL-32α as well as IL-32β bind to αVβ3; however, only the IL-32α/αVβ3 interaction is inhibited by small RGD peptides. B, as a control, interactions between IL-32α and BSA were investigated but showed no significant binding compared with the IL-32α/αVβ3 binding. C, FCS did inhibit the IL-32α/αVβ3 interactions. The values are the means with S.E. One-way ANOVA with Bonferroni's multiple comparison test was used (n = 4 for A and n = 6 for B and C; **, p < 0.01; ***, p < 0.001).
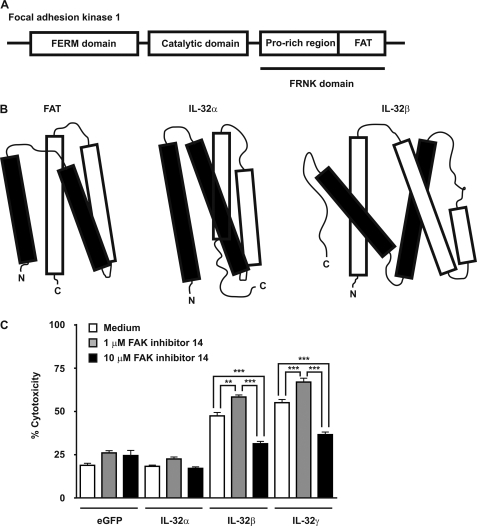
IL-32 Resembles Focal Adhesion Targeting Region of Focal Adhesion Kinase 1
FAK-1 contains three domains: a FERM domain, which binds to β-integrins; a catalytic domain, which phosphorylates several tyrosine substrates; and a FAK-related nonkinase (FRNK) domain, which acts as a natural inhibitor of FAK-1 signaling and contains a proline-rich region and a focal adhesion targeting (FAT) region that targets FAK-1 toward focal adhesions through binding to paxillin or talin (Fig. 5A) (53, 54). FAT resembles IL-32α, both having a typical four α-helix bundle structure (54), whereas IL-32β contains an additional α-helix but still resembles FAT (Fig. 5B). Inhibition of FAK-1 autophosphorylation (Y397) resulted in a dual effect; low concentration of the inhibitor enhanced the IL-32β/IL-32γ-induced cytotoxicity, whereas high concentration of the inhibitor remarkably reduced the IL-32β/IL-32γ-induced cytotoxicity (Fig. 5C).
FIGURE 5.
IL-32α and IL-32β protein models display a typical α-helix bundle structure similar as the focal adhesion targeting region of FAK. A, FAK-1 contains three domains: FERM, catalytic, and FRNK domains. The FRNK domain is divided into two regions: proline-rich and FAT regions. B, IL-32α and IL-32β modeling revealed a typical α-helix bundle structure as observed for FAT. C, overexpression of IL-32β or IL-32γ together with inhibition of FAK tyrosine 397 phosphorylation modulated the IL-32β/IL-32γ-induced cytotoxicity in human HEK293T cells. The values are the means with S.E. One-way ANOVA with Bonferroni's multiple comparison test was use (n = 4; **, p < 0.01; ***, p < 0.001).
IL-32 Binds to FAK and Paxillin, Both Members of Focal Adhesion Protein Complex
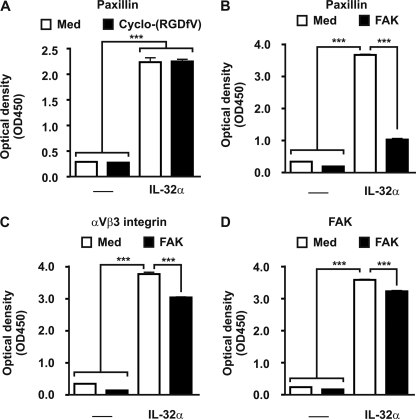
Modeling of IL-32 revealed a typical structure of α-helixes, which resembles FAT. FAT localizes FAK-1 toward focal adhesions that are formed after integrin/extracellular matrix engagement. FAT binds to focal adhesion protein paxillin, which results in intracellular signaling (53, 54). Fig. 6A shows that IL-32α can bind to paxillin, whereas the interaction could not be inhibited by cyclo-(RGDfV), demonstrating that the RGD motif is not involved in the IL-32α/paxillin engagement. Moreover, the IL-32α/paxillin interaction was inhibited by recombinant FAK-1, containing the FAT region that binds to paxillin (Fig. 6B). Remarkably, the IL-32α/αVβ3 interaction was slightly inhibited by FAK, indicating that IL-32α also binds to FAK (Fig. 6C). Fig. 6D demonstrates that IL-32α and FAK interact with each other.
FIGURE 6.
IL-32/paxillin/FAK interactions. A, IL-32α binds to paxillin without the RGD motif binding involved. B, the IL-32α/paxillin engagement can be inhibited by FAK that contains the FAT region that binds to paxillin. C, additionally, the IL-32α/αVβ3 integrin binding was inhibited by FAK, indicating that FAK can bind to IL-32α. D, IL-32α binds to FAK, whereas additional FAK scavenged the IL-32α/FAK complex. The values are the means with S.E. One-way ANOVA with Bonferroni's multiple comparison test was used (n = 4; ***, p < 0.001).
DISCUSSION
IL-32 does not contain any conversed domains in its amino acid sequence, which complicated the modeling. Nowadays, sophisticated modeling software can predict secondary and tertiary structures based on amino acid sequences. By I-TASSER, the secondary structure of IL-32α, IL-32β, and IL-32γ was predicted and revealed α-helixes with short coils but no β sheets. Subsequently, I-TASSER predicted the tertiary structure by comparing the secondary structure of known proteins with the IL-32 predicted secondary structure that resulted in an α-helix bundle shape-like protein. IL-32γ contains a potential transmembrane helix specific for IL-32γ, which complicated the modeling. HMMTOP software predicted a transmembrane helix specific for IL-32γ and not for IL-32α or IL-32β. Supporting evidence for a transmembrane helix in IL-32γ is provided by the observation that IL-32γ secretion is enhanced by TNFα or IL-1β stimulation in rheumatoid arthritis fibroblast-like synoviocytes without cell death being involved (4). More evidence is provided by the observation that intestinal epithelial cells that were stimulated with TNFα and INFγ showed subcellular colocalization of IL-32 with cell membranes of lipid droplet-like structures (29). Interestingly, PR3, a serine proteinase binds to IL-32 (33) and can cleave IL-32γ into a more active form (32). Moreover, expression of membrane PR3 is elevated in rheumatoid arthritis patients (55), which might release transmembrane IL-32γ. PR3-released IL-32γ could be an explanation for the increased IL-32 levels observed in synovial fluid (31), although cell death could be involved.
IL-32 contains two interesting amino acid motifs, namely DDM and RGD. These two motifs are involved in integrin signaling and activation of procaspase-3 (46, 48). By modeling, it became clear that the IL-32α RGD motif is differently orientated and probably less accessible for binding compared with the IL-32β or IL-32γ RGD motif. In integrin signaling, the RGD motifs expressed by extracellular matrix proteins (37) can bind to DDM motifs present in β-integrins (56). Moreover, RGD and DDM motifs are also present in procaspase-3, and small peptides expressing RGD motifs can directly activate procaspase-3, resulting in active caspase-3 leading to apoptosis (46). In addition, RGD-induced cell death, probably by activation of caspase-3, was reported in chondrocytes and synovial cells that were exposed to small RGD peptides (47). Because RGD motifs can bind to DDM motifs in the integrin signaling, it is plausible that the RGD and DDM motifs located in procaspase-3 bind to each other, leading to a self-inhibitory conformational state, whereas small RGD peptides probably interfere with this binding and cause conformational changes leading to autoactivation of procaspase-3 as suggested by Porter (48). However, the RGD motif present in IL-32β and IL-32γ is not directly involved in the IL-32-induced cell death, because deletion of the RGD motif did not show decreased cytotoxicity.
The RGD motif was first found in fibronectin (57), which is an extracellular matrix protein. The fibronectin receptor was discovered through binding with fibronectin in an RGD-dependent manner (58, 59), which demonstrated the importance of the RGD motif. This fibronectin receptor is now known as α5β1 integrin (60). Soon after that initial report, it became clear that the RGD motif is the cell adhesion site of many other adhesion proteins such as vitronectin, fibrinogen, von Willebrand factor, thrombospondin, and osteopontin (60) that bind to integrins. Integrins are membrane-bound receptors containing an α and β subunit forming a heterodimer (35). Furthermore, integrins belong to the type I transmembrane glycoproteins containing a short cytoplasmic tail (35), and nowadays, 18 different α subunits and 8 different β subunits are known that can form at least 24 heterodimers (61, 62). The crystal structure of αVβ3 together with small RGD peptides demonstrated that the RGD motif binds at the major interface between the αV and β3 subunits and interacts with both (63). Integrins can signal bidirectional, which mean that binding to the extracellular matrix is regulated from the inside of the cell, whereas binding to the extracellular matrix triggers signals that are transferred into the cell (37). Integrin signaling is essential for many important cell processes such as anchorage, proliferation, migration, survival, cytokine production, and differentiation (35, 37). Many of these properties are observed for IL-32, and the question arose regarding whether the RGD motif present in IL-32 could bind to integrins. In the present study, we show that IL-32α binds to αVβ3, αVβ6, but not to αVβ8 integrins. All three integrins can bind RGD motifs; however, only the αVβ3/IL-32α interaction could be inhibited by small RGD peptides. It is possible that the αVβ6/IL-32α interaction is not mediated via the RGD motif or that the small RGD peptides bind to the DDM motif present in IL-32α, thereby altering the tertiary structure of IL-32α and resulting in more free RGD motifs that can bind to αVβ6. Furthermore, IL-32β was able to bind to αVβ3, but the interaction could not be inhibited by small RGD peptides, suggesting that besides the RGD motif, other sites in IL-32β can bind to the αVβ3 integrin. Previous experiments showed reduced production of cytokines by human peripheral blood mononuclear cells that were stimulated with recombinant IL-32γ in combination with serum, in contrast to serum-free conditions. Culturing cells often require attachment to a surface, which is facilitated by serum adhesive proteins. In FCS, serum adhesive proteins such as fibronectin and vitronectin are present (64, 65) that contain RGD motifs. Presumably, these serum adhesive proteins inhibit the IL-32/integrin interactions through their RGD motifs, resulting in reduced cytokine production. As expected, by adding 10% FCS, the interaction between IL-32α and αVβ3 integrins was significantly inhibited. When RGD motif-containing proteins bind to integrins, a signal is transduced through the cell membrane, which activates intracellular kinases such as FAK. FAK is targeted to focal adhesion formations, which are formed by extracellular matrix/integrin clustering, guided via the FAT region. Interestingly, inhibition of FAK tyrosine 397 phosphorylation regulates the IL-32β/IL-32γ-induced cytotoxicity. A low concentration of the FAK inhibitor resulted in increased cytotoxicity, which reflects other reports describing increased cell death when FAK is inhibited (41, 42). On the contrary, a high concentration of the FAK inhibitor significantly reduced the IL-32β/-32γ-induced cytotoxicity. This dual effect is rather difficult to understand; however, others reported successful inhibition of FAK tyrosine 397 phosphorylation by a small inhibitor but failed to induce apoptosis (43). On the other hand, the FERM domain of FAK can bind to the catalytic domain of FAK, resulting in autoinhibition (66). Nevertheless, the IL-32β/IL-32γ-induced cytotoxicity includes FAK activity.
Several reports show that IL-32 can synergize with bacterial fragments for the production of proinflammatory cytokines (3, 15). Could there be a link between bacteria and FAK activation? The answer is yes, it is reported that FLS stimulated with protein I/II, a modulin from Streptococci, which binds to α5β1 integrins, induces IL-6 and CXCL8 production via the ERK1/2 and JNK pathways that requires FAK activation (67). Interestingly, the important tyrosine 397 of FAK is not involved in this increased IL-6 and CXCL8 production, indicating that other tyrosines present in FAK are important too. Integrin α5β1 can also bind to RGD motifs (68), whether the RGD motif present in IL-32 can bind to α5β1 remains unknown, and it would be interesting to demonstrate that extracellular IL-32 can interact with integrins and thereby inducing proinflammatory cytokines. Furthermore, IL-32 can synergize with TLR-2 and NOD2 fragments by enhancing both receptors (3). Interestingly, FAK and TLR pathways are interconnected via MyD88 (69, 70), and IL-32 fulfills a key role in this process as shown by IL-32 silencing or overexpression (4, 30).
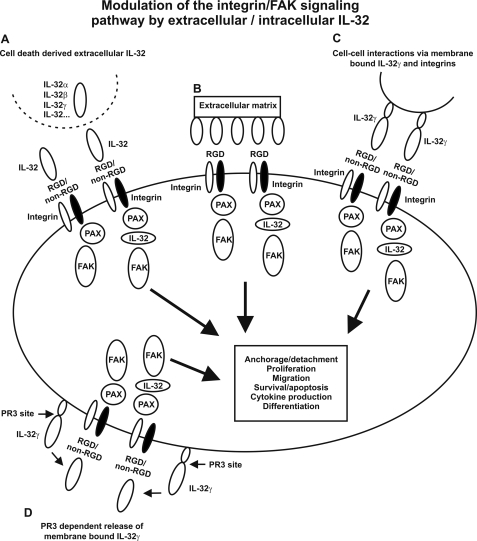
Modeling of IL-32 revealed a typical α-helix bundle protein that resembles FAT (54). The FRNK domain contains the FAT region that guides FAK to focal adhesions and thereby connects the integrin-signaling pathway. FRNK can be transcribed by using an alternative promoter present in the FAK gene (71), which inhibits FAK signaling (72–74). Possibly, IL-32 that resembles FAT acts like an intracellular inhibitor of FAK signaling as observed for FRNK, which explains IL-32-induced cell death. IL-32 can also induce proinflammatory cytokines involved in rheumatoid arthritis (3–6), which indicates that IL-32 can enhance integrin signaling. Perhaps membrane-bound IL-32γ signals via cell-cell contact using its RGD motif to interact with integrins and thereby inducing FAK activation. Another possibility is that membrane-bound IL-32γ is processed by PR3, because PR3 binds (33) and processes/activates (32) IL-32γ, resulting in integrin binding and FAK activation. A different option is that IL-32 modulates FAK activation, as a member of the focal adhesion protein complex, because we demonstrated that IL-32 interacts with both paxillin and FAK. The involvement of IL-32 in the integrin-signaling pathway as proposed in Fig. 7 would explain the many functions reported for IL-32 such as differentiation (26–28), inflammation (3–7, 15, 16), and apoptosis (25) that stimulates our imagination to think of the many possibilities of IL-32.
FIGURE 7.
A proposed model for the role of IL-32 in the integrin/FAK-signaling pathway. A, presence of extracellular IL-32 isoforms released by dying cells that interact with integrins through RGD and non-RGD motifs and thereby activating integrin/FAK-signaling pathways. B, RGD motifs expressed by extracellular matrix that bind to integrins and activate integrin/FAK-signaling pathways. C, cell/cell interactions through membrane-bound IL-32γ, which binds to integrins present on other cells. D, release of membrane-bound IL-32γ by PR3 cleavage that interacts with integrins and activates integrin/FAK-signaling pathways. Besides the activation of the integrin/FAK-signaling pathway by extracellular IL-32, intracellular IL-32 modulates the integrin/FAK-signaling pathway by interacting with paxillin (PAX) and FAK (A–D).
Acknowledgment
We thank R & D Systems for helpful advice and several reagents used in these studies.
This work was supported, in whole or in part, by National Institutes of Health Grant AI-15614 (to C. A. D.).
B. Heinhuis, M. I. Koenders, W. B. van den Berg, M. G. Netea, C. A. Dinarello, and L. A. B. Joosten, unpublished data.
- eGFP
- enhanced GFP
- FAK
- focal adhesion kinase
- PR3
- proteinase 3
- FRNK
- FAK-related nonkinase
- FAT
- focal adhesion targeting
- ANOVA
- analysis of variance.
REFERENCES
- 1. Calabrese F., Baraldo S., Bazzan E., Lunardi F., Rea F., Maestrelli P., Turato G., Lokar-Oliani K., Papi A., Zuin R., Sfriso P., Balestro E., Dinarello C. A., Saetta M. (2008) IL-32, a novel proinflammatory cytokine in chronic obstructive pulmonary disease. Am. J. Respir. Crit Care Med. 178, 894–901 [DOI] [PubMed] [Google Scholar]
- 2. Dinarello C. A., Kim S. H. (2006) IL-32, a novel cytokine with a possible role in disease. Ann. Rheum. Dis. 65, iii61–iii64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heinhuis B., Koenders M. I., van de Loo F. A., van Lent P. L., Kim S. H., Dinarello C. A., Joosten L. A., van den Berg W. B. (2010) IL-32γ and Streptococcus pyogenes cell wall fragments synergise for IL-1-dependent destructive arthritis via upregulation of TLR-2 and NOD2. Ann. Rheum. Dis. 69, 1866–1872 [DOI] [PubMed] [Google Scholar]
- 4. Heinhuis B., Koenders M. I., van Riel P. L., van de Loo F. A., Dinarello C. A., Netea M. G., van den Berg W. B., Joosten L. A. (2011) Tumour necrosis factor α-driven IL-32 expression in rheumatoid arthritis synovial tissue amplifies an inflammatory cascade. Ann. Rheum. Dis. 70, 660–667 [DOI] [PubMed] [Google Scholar]
- 5. Heinhuis B., Koenders M. I., van de Loo F. A., Netea M. G., van den Berg W. B., Joosten L. A. (2011) Inflammation-dependent secretion and splicing of IL-32γ in rheumatoid arthritis. Proc. Natl. Acad. Sci. U.S.A. 108, 4962–4967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Joosten L. A., Netea M. G., Kim S. H., Yoon D. Y., Oppers-Walgreen B., Radstake T. R., Barrera P., van de Loo F. A., Dinarello C. A., van den Berg W. B. (2006) IL-32, a proinflammatory cytokine in rheumatoid arthritis. Proc. Natl. Acad. Sci. U.S.A. 103, 3298–3303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim S. H., Han S. Y., Azam T., Yoon D. Y., Dinarello C. A. (2005) Interleukin-32. A cytokine and inducer of TNFα. Immunity. 22, 131–142 [DOI] [PubMed] [Google Scholar]
- 8. Li W., Liu Y., Mukhtar M. M., Gong R., Pan Y., Rasool S. T., Gao Y., Kang L., Hao Q., Peng G., Chen Y., Chen X., Wu J., Zhu Y. (2008) Activation of interleukin-32 pro-inflammatory pathway in response to influenza A virus infection. PLoS ONE 3, e1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li W., Yang F., Liu Y., Gong R., Liu L., Feng Y., Hu P., Sun W., Hao Q., Kang L., Wu J., Zhu Y. (2009) Negative feedback regulation of IL-32 production by iNOS activation in response to dsRNA or influenza virus infection. Eur. J. Immunol. 39, 1019–1024 [DOI] [PubMed] [Google Scholar]
- 10. Li W., Sun W., Liu L., Yang F., Li Y., Chen Y., Fang J., Zhang W., Wu J., Zhu Y. (2010) IL-32. A host proinflammatory factor against influenza viral replication is upregulated by aberrant epigenetic modifications during influenza A virus infection. J. Immunol. 185, 5056–5065 [DOI] [PubMed] [Google Scholar]
- 11. Nold M. F., Nold-Petry C. A., Pott G. B., Zepp J. A., Saavedra M. T., Kim S. H., Dinarello C. A. (2008) Endogenous IL-32 controls cytokine and HIV-1 production. J. Immunol. 181, 557–565 [DOI] [PubMed] [Google Scholar]
- 12. Rasool S. T., Tang H., Wu J., Li W., Mukhtar M. M., Zhang J., Mu Y., Xing H. X., Wu J., Zhu Y. (2008) Increased level of IL-32 during human immunodeficiency virus infection suppresses HIV replication. Immunol. Lett. 117, 161–167 [DOI] [PubMed] [Google Scholar]
- 13. Zepp J. A., Nold-Petry C. A., Dinarello C. A., Nold M. F. (2011) Protection from RNA and DNA viruses by IL-32. J. Immunol. 186, 4110–4118 [DOI] [PubMed] [Google Scholar]
- 14. Bai X., Kim S. H., Azam T., McGibney M. T., Huang H., Dinarello C. A., Chan E. D. (2010) IL-32 is a host protective cytokine against Mycobacterium tuberculosis in differentiated THP-1 human macrophages. J. Immunol. 184, 3830–3840 [DOI] [PubMed] [Google Scholar]
- 15. Netea M. G., Azam T., Ferwerda G., Girardin S. E., Walsh M., Park J. S., Abraham E., Kim J. M., Yoon D. Y., Dinarello C. A., Kim S. H. (2005) IL-32 synergizes with nucleotide oligomerization domain (NOD) 1 and NOD2 ligands for IL-1β and IL-6 production through a caspase 1-dependent mechanism. Proc. Natl. Acad. Sci. U.S.A. 102, 16309–16314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Netea M. G., Azam T., Lewis E. C., Joosten L. A., Wang M., Langenberg D., Meng X., Chan E. D., Yoon D. Y., Ottenhoff T., Kim S. H., Dinarello C. A. (2006) Mycobacterium tuberculosis induces interleukin-32 production through a caspase-1/IL-18/interferon-γ-dependent mechanism. PLoS Med. 3, e277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Majid S., Dar A. A., Saini S., Yamamura S., Hirata H., Tanaka Y., Deng G., Dahiya R. (2010) MicroRNA-205-directed transcriptional activation of tumor suppressor genes in prostate cancer. Cancer 116, 5637–5649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marcondes A. M., Mhyre A. J., Stirewalt D. L., Kim S. H., Dinarello C. A., Deeg H. J. (2008) Dysregulation of IL-32 in myelodysplastic syndrome and chronic myelomonocytic leukemia modulates apoptosis and impairs NK function. Proc. Natl. Acad. Sci. U.S.A. 105, 2865–2870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nishida A., Andoh A., Inatomi O., Fujiyama Y. (2009) Interleukin-32 expression in the pancreas. J. Biol. Chem. 284, 17868–17876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Seo E. H., Kang J., Kim K. H., Cho M. C., Lee S., Kim H. J., Kim J. H., Kim E. J., Park D. K., Kim S. H., Choi Y. K., Kim J. M., Hong J. T., Yoon D. Y. (2008) Detection of expressed IL-32 in human stomach cancer using ELISA and immunostaining. J. Microbiol. Biotechnol. 18, 1606–1612 [PubMed] [Google Scholar]
- 21. Sorrentino C., Di Carlo E. (2009) Expression of IL-32 in human lung cancer is related to the histotype and metastatic phenotype. Am. J. Respir. Crit Care Med. 180, 769–779 [DOI] [PubMed] [Google Scholar]
- 22. Cagnard N., Letourneur F., Essabbani A., Devauchelle V., Mistou S., Rapinat A., Decraene C., Fournier C., Chiocchia G. (2005) Interleukin-32, CCL2, PF4F1, and GFD10 are the only cytokine/chemokine genes differentially expressed by in vitro cultured rheumatoid and osteoarthritis fibroblast-like synoviocytes. Eur. Cytokine Netw. 16, 289–292 [PubMed] [Google Scholar]
- 23. Shoda H., Fujio K., Yamaguchi Y., Okamoto A., Sawada T., Kochi Y., Yamamoto K. (2006) Interactions between IL-32 and tumor necrosis factor α contribute to the exacerbation of immune-inflammatory diseases. Arthritis Res. Ther. 8, R166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shioya M., Nishida A., Yagi Y., Ogawa A., Tsujikawa T., Kim-Mitsuyama S., Takayanagi A., Shimizu N., Fujiyama Y., Andoh A. (2007) Epithelial overexpression of interleukin-32α in inflammatory bowel disease. Clin. Exp. Immunol. 149, 480–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goda C., Kanaji T., Kanaji S., Tanaka G., Arima K., Ohno S., Izuhara K. (2006) Involvement of IL-32 in activation-induced cell death in T cells. Int. Immunol. 18, 233–240 [DOI] [PubMed] [Google Scholar]
- 26. Kim Y. G., Lee C. K., Oh J. S., Kim S. H., Kim K. A., Yoo B. (2010) Effect of interleukin-32γ on differentiation of osteoclasts from CD14+ monocytes. Arthritis Rheum. 62, 515–523 [DOI] [PubMed] [Google Scholar]
- 27. Mabilleau G., Sabokbar A. (2009) Interleukin-32 promotes osteoclast differentiation but not osteoclast activation. PLoS ONE. 4, e4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Netea M. G., Lewis E. C., Azam T., Joosten L. A., Jaekal J., Bae S. Y., Dinarello C. A., Kim S. H. (2008) Interleukin-32 induces the differentiation of monocytes into macrophage-like cells. Proc. Natl. Acad. Sci. U.S.A. 105, 3515–3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hasegawa H., Thomas H. J., Schooley K., Born T. L. (2011) Native IL-32 is released from intestinal epithelial cells via a non-classical secretory pathway as a membrane-associated protein. Cytokine 53, 74–83 [DOI] [PubMed] [Google Scholar]
- 30. Nold-Petry C. A., Nold M. F., Zepp J. A., Kim S. H., Voelkel N. F., Dinarello C. A. (2009) IL-32-dependent effects of IL-1β on endothelial cell functions. Proc. Natl. Acad. Sci. U.S.A. 106, 3883–3888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mun S. H., Kim J. W., Nah S. S., Ko N. Y., Lee J. H., Kim J. D., Kim D. K., Kim H. S., Choi J. D., Kim S. H., Lee C. K., Park S. H., Kim B. K., Kim H. S., Kim Y. M., Choi W. S. (2009) Tumor necrosis factor α-induced interleukin-32 is positively regulated via the Syk/protein kinase Cδ/JNK pathway in rheumatoid synovial fibroblasts. Arthritis Rheum. 60, 678–685 [DOI] [PubMed] [Google Scholar]
- 32. Kim S., Lee S., Her E., Bae S., Choi J., Hong J., Jaekal J., Yoon D., Azam T., Dinarello C. A., Kim S. (2008) Proteinase 3-processed form of the recombinant IL-32 separate domain. BMB Rep. 41, 814–819 [DOI] [PubMed] [Google Scholar]
- 33. Novick D., Rubinstein M., Azam T., Rabinkov A., Dinarello C. A., Kim S. H. (2006) Proteinase 3 is an IL-32 binding protein. Proc. Natl. Acad. Sci. U.S.A. 103, 3316–3321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dahl C. A., Schall R. P., He H. L., Cairns J. S. (1992) Identification of a novel gene expressed in activated natural killer cells and T cells. J. Immunol. 148, 597–603 [PubMed] [Google Scholar]
- 35. Abram C. L., Lowell C. A. (2009) The ins and outs of leukocyte integrin signaling. Annu. Rev. Immunol. 27, 339–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. D'Souza S. E., Ginsberg M. H., Plow E. F. (1991) Arginyl-glycyl-aspartic acid (RGD). A cell adhesion motif. Trends Biochem. Sci. 16, 246–250 [DOI] [PubMed] [Google Scholar]
- 37. Giancotti F. G., Ruoslahti E. (1999) Integrin signaling. Science 285, 1028–1032 [DOI] [PubMed] [Google Scholar]
- 38. Broxterman H. J., Hoekman K. (1999) Direct activation of caspases by RGD-peptides may increase drug sensitivity of tumour cells. Drug Resist. Updat. 2, 139–141 [DOI] [PubMed] [Google Scholar]
- 39. Wilder R. L. (2002) Integrin alpha V beta 3 as a target for treatment of rheumatoid arthritis and related rheumatic diseases. Ann. Rheum. Dis. 61, (Suppl. 2) ii96–ii99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stupp R., Ruegg C. (2007) Integrin inhibitors reaching the clinic. J. Clin. Oncol. 25, 1637–1638 [DOI] [PubMed] [Google Scholar]
- 41. Beierle E. A., Ma X., Stewart J., Nyberg C., Trujillo A., Cance W. G., Golubovskaya V. M. (2010) Inhibition of focal adhesion kinase decreases tumor growth in human neuroblastoma. Cell Cycle 9, 1005–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hochwald S. N., Nyberg C., Zheng M., Zheng D., Wood C., Massoll N. A., Magis A., Ostrov D., Cance W. G., Golubovskaya V. M. (2009) A novel small molecule inhibitor of FAK decreases growth of human pancreatic cancer. Cell Cycle 8, 2435–2443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Slack-Davis J. K., Martin K. H., Tilghman R. W., Iwanicki M., Ung E. J., Autry C., Luzzio M. J., Cooper B., Kath J. C., Roberts W. G., Parsons J. T. (2007) Cellular characterization of a novel focal adhesion kinase inhibitor. J. Biol. Chem. 282, 14845–14852 [DOI] [PubMed] [Google Scholar]
- 44. Millard M., Odde S., Neamati N. (2011) Integrin targeted therapeutics. Theranostics. 1, 154–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Aguzzi M. S., Fortugno P., Giampietri C., Ragone G., Capogrossi M. C., Facchiano A. (2010) Intracellular targets of RGDS peptide in melanoma cells. Mol. Cancer 9, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Buckley C. D., Pilling D., Henriquez N. V., Parsonage G., Threlfall K., Scheel-Toellner D., Simmons D. L., Akbar A. N., Lord J. M., Salmon M. (1999) RGD peptides induce apoptosis by direct caspase-3 activation. Nature 397, 534–539 [DOI] [PubMed] [Google Scholar]
- 47. Matsuki K., Sasho T., Nakagawa K., Tahara M., Sugioka K., Ochiai N., Ogino S., Wada Y., Moriya H. (2008) RGD peptide-induced cell death of chondrocytes and synovial cells. J. Orthop. Sci. 13, 524–532 [DOI] [PubMed] [Google Scholar]
- 48. Porter A. G. (2006) Flipping the safety catch of procaspase-3. Nat. Chem. Biol. 2, 509–510 [DOI] [PubMed] [Google Scholar]
- 49. Roy A., Kucukural A., Zhang Y. (2010) I-TASSER. A unified platform for automated protein structure and function prediction. Nat. Protoc. 5, 725–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang Y. (2008) I-TASSER server for protein 3D structure prediction. BMC Bioinformatics 9, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Guex N., Peitsch M. C. (1997) SWISS-MODEL and the Swiss-PdbViewer. An environment for comparative protein modeling. Electrophoresis 18, 2714–2723 [DOI] [PubMed] [Google Scholar]
- 52. Tusnády G. E., Simon I. (2001) The HMMTOP transmembrane topology prediction server. Bioinformatics. 17, 849–850 [DOI] [PubMed] [Google Scholar]
- 53. Parsons J. T., Martin K. H., Slack J. K., Taylor J. M., Weed S. A. (2000) Focal adhesion kinase. A regulator of focal adhesion dynamics and cell movement. Oncogene 19, 5606–5613 [DOI] [PubMed] [Google Scholar]
- 54. Hayashi I., Vuori K., Liddington R. C. (2002) The focal adhesion targeting (FAT) region of focal adhesion kinase is a four-helix bundle that binds paxillin. Nat. Struct. Biol. 9, 101–106 [DOI] [PubMed] [Google Scholar]
- 55. Witko-Sarsat V., Lesavre P., Lopez S., Bessou G., Hieblot C., Prum B., Noël L. H., Guillevin L., Ravaud P., Sermet-Gaudelus I., Timsit J., Grünfeld J. P., Halbwachs-Mecarelli L. (1999) A large subset of neutrophils expressing membrane proteinase 3 is a risk factor for vasculitis and rheumatoid arthritis. J. Am. Soc. Nephrol. 10, 1224–1233 [DOI] [PubMed] [Google Scholar]
- 56. Du X. P., Plow E. F., Frelinger A. L., 3rd, O'Toole T. E., Loftus J. C., Ginsberg M. H. (1991) Ligands “activate” integrin αIIb β3 (platelet GPIIb-IIIa). Cell 65, 409–416 [DOI] [PubMed] [Google Scholar]
- 57. Pierschbacher M. D., Ruoslahti E. (1984) Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature 309, 30–33 [DOI] [PubMed] [Google Scholar]
- 58. Pytela R., Pierschbacher M. D., Ruoslahti E. (1985) A 125/115-kDa cell surface receptor specific for vitronectin interacts with the arginine-glycine-aspartic acid adhesion sequence derived from fibronectin. Proc. Natl. Acad. Sci. U.S.A. 82, 5766–5770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pytela R., Pierschbacher M. D., Ruoslahti E. (1985) Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell 40, 191–198 [DOI] [PubMed] [Google Scholar]
- 60. Ruoslahti E. (1996) RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol. 12, 697–715 [DOI] [PubMed] [Google Scholar]
- 61. Luo B. H., Carman C. V., Springer T. A. (2007) Structural basis of integrin regulation and signaling. Annu. Rev. Immunol. 25, 619–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shimaoka M., Springer T. A. (2003) Therapeutic antagonists and conformational regulation of integrin function. Nat. Rev. Drug Discov. 2, 703–716 [DOI] [PubMed] [Google Scholar]
- 63. Xiong J. P., Stehle T., Zhang R., Joachimiak A., Frech M., Goodman S. L., Arnaout M. A. (2002) Crystal structure of the extracellular segment of integrin αVβ3 in complex with an Arg-Gly-Asp ligand. Science 296, 151–155 [DOI] [PubMed] [Google Scholar]
- 64. Hayman E. G., Pierschbacher M. D., Suzuki S., Ruoslahti E. (1985) Vitronectin. A major cell attachment-promoting protein in fetal bovine serum. Exp. Cell Res. 160, 245–258 [DOI] [PubMed] [Google Scholar]
- 65. Richards S., Leavesley D., Topping G., Upton Z. (2008) Development of defined media for the serum-free expansion of primary keratinocytes and human embryonic stem cells. Tissue Eng. Part C Methods 14, 221–232 [DOI] [PubMed] [Google Scholar]
- 66. Lietha D., Cai X., Ceccarelli D. F., Li Y., Schaller M. D., Eck M. J. (2007) Structural basis for the autoinhibition of focal adhesion kinase. Cell 129, 1177–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Neff L., Zeisel M., Druet V., Takeda K., Klein J. P., Sibilia J., Wachsmann D. (2003) ERK 1/2- and JNKs-dependent synthesis of interleukins 6 and 8 by fibroblast-like synoviocytes stimulated with protein I/II, a modulin from oral streptococci, requires focal adhesion kinase. J. Biol. Chem. 278, 27721–27728 [DOI] [PubMed] [Google Scholar]
- 68. Mould A. P., Symonds E. J., Buckley P. A., Grossmann J. G., McEwan P. A., Barton S. J., Askari J. A., Craig S. E., Bella J., Humphries M. J. (2003) Structure of an integrin-ligand complex deduced from solution x-ray scattering and site-directed mutagenesis. J. Biol. Chem. 278, 39993–39999 [DOI] [PubMed] [Google Scholar]
- 69. Semaan N., Alsaleh G., Gottenberg J. E., Wachsmann D., Sibilia J. (2008) Etk/BMX, a Btk family tyrosine kinase, and Mal contribute to the cross-talk between MyD88 and FAK pathways. J. Immunol. 180, 3485–3491 [DOI] [PubMed] [Google Scholar]
- 70. Zeisel M. B., Druet V. A., Sibilia J., Klein J. P., Quesniaux V., Wachsmann D. (2005) Cross-talk between MyD88 and focal adhesion kinase pathways. J. Immunol. 174, 7393–7397 [DOI] [PubMed] [Google Scholar]
- 71. Nolan K., Lacoste J., Parsons J. T. (1999) Regulated expression of focal adhesion kinase-related nonkinase, the autonomously expressed C-terminal domain of focal adhesion kinase. Mol. Cell Biol. 19, 6120–6129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Richardson A., Parsons T. (1996) A mechanism for regulation of the adhesion-associated proteintyrosine kinase pp125FAK. Nature 380, 538–540 [DOI] [PubMed] [Google Scholar]
- 73. Gilmore A. P., Romer L. H. (1996) Inhibition of focal adhesion kinase (FAK) signaling in focal adhesions decreases cell motility and proliferation. Mol. Biol. Cell 7, 1209–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Xu L. H., Yang X., Bradham C. A., Brenner D. A., Baldwin A. S., Jr., Craven R. J., Cance W. G. (2000) The focal adhesion kinase suppresses transformation-associated, anchorage-independent apoptosis in human breast cancer cells. Involvement of death receptor-related signaling pathways. J. Biol. Chem. 275, 30597–30604 [DOI] [PubMed] [Google Scholar]