Background: Different Ca2+ entry pathways co-exist in endothelial cells.
Results: On artificial basement membrane, endothelial cells displayed Ca2+ oscillations and formed tubes, both processes prevented after silencing TRPC channels, but not Orai1.
Conclusion: TRPC channels are essential for in vitro tubulogenesis.
Significance: TRPC channels represent interesting candidates to modulate angiogenesis.
Keywords: Angiogenesis, Calcium Channels, Calcium Signaling, Endothelial Cell, TRP Channels, STIM1/Orai1
Abstract
In endothelial cells Ca2+ entry is an essential component of the Ca2+ signal that takes place during processes such as cell proliferation or angiogenesis. Ca2+ influx occurs via the store-operated Ca2+ entry pathway, involving stromal interaction molecule-1 (STIM1) and Orai1, but also through channels gated by second messengers like the transient receptor potential canonical (TRPC) channels. The human umbilical vein-derived endothelial cell line EA.hy926 expressed STIM1 and Orai1 as well as several TRPC channels. By invalidating each of these molecules, we showed that TRPC3, TRPC4, and TRPC5 are essential for the formation of tubular structures observed after EA.hy926 cells were plated on Matrigel. On the contrary, the silencing of STIM1 or Orai1 did not prevent tubulogenesis. Soon after being plated on Matrigel, the cells displayed spontaneous Ca2+ oscillations that were strongly reduced by treatment with siRNA against TRPC3, TRPC4, or TRPC5, but not siRNA against STIM1 or Orai1. Furthermore, we showed that cell proliferation was reduced upon siRNA treatment against TRPC3, TRPC5, and Orai1 channels, whereas the knockdown of STIM1 had no effect. On primary human umbilical vein endothelial cells, TRPC1, TRPC4, and STIM1 are involved in tube formation, whereas Orai1 has no effect. These data showed that TRPC channels are essential for in vitro tubulogenesis, both on endothelial cell line and on primary endothelial cells.
Introduction
In response to circulating agents and/or physical forces, endothelial cells synthesize and release several compounds that act on the underlying smooth muscle cells to modulate their contractile status. In addition, endothelial cells control the regulation of vascular permeability, blood coagulation, or the formation of new blood vessels. Elevation of the cytosolic Ca2+ concentration due to Ca2+ release from the endoplasmic reticulum (ER),2 and Ca2+ influx is pivotal for all these functions (1). In nonexcitable cells, Ca2+ can enter via the well described store-operated Ca2+ entry (SOCE) pathway and/or through other routes that do not depend on the filling state of the ER. These routes are linked to the generation of second messengers produced upon agonist stimulation, that we called collectively receptor-activated Ca2+ entry, RACE (2, 3). SOCE was initially described in 1986 by Putney (4) and linked the level of ER Ca2+ store depletion with the opening of plasma membrane Ca2+ channels. Recently, two major SOCE components have been identified: the stromal interacting molecule-1 (STIM1) (5–7), located predominantly in the ER membrane and which senses the luminal Ca2+ concentration, and the Ca2+ channel Orai1 (8–10), which allows Ca2+ entry. STIM1 oligomerizes and forms punctae upon store depletion and translocates close to the plasma membrane where it binds to and activates Orai1 (11). In addition to STIM1 and Orai1, channels from the TRP (transient receptor potential) family were shown to constitute an alternative and/or additional Ca2+ influx pathway in several cellular systems. The TRP channels (12) are composed of 28 members that are divided into six subfamilies. Among them the TRPC (C for canonical) subfamily comprises both store-operated and non-store-operated cation channels (13). Endothelial cells express a great variety of TRP channels, and more and more data link TRP channel isoforms to specific endothelial cell functions, such as control of the vascular tone, vascular permeability, or angiogenesis (14). Angiogenesis, the formation of new blood vessels from preexisting ones, is a complex and multistep process that comprises migration, proliferation, and reorganization of endothelial cells (15). Ca2+ signals are pivotal for many steps that are taking place during angiogenesis (16), and recent data reported that TRPC6 is an important player in angiogenesis (17, 18) whereas others revealed that angiogenesis is severely impaired after Orai1 knockdown (19). In a recent paper, we showed that TRPC1, TRPC3, TRPC4, TRPC5, and TRPC6 isoforms are expressed, together with STIM1 and Orai1, on EA.hy926 cells (20), an endothelial cell line derived from human umbilical vein endothelial cells (HUVECs) fused with human lung adenocarcinoma cell line A549 (21). In this cell line, massive store depletion achieved after thapsigargin stimulation induced Ca2+ entry that is mediated both by store-dependent STIM1/Orai1 and store-independent pathways involving TRPC3 (20). In the present study, we focused on the role played by the different Ca2+ entry channels in angiogenesis, an important physiological but also pathological process involving endothelial cells, using an in vitro approach. We show that TRPC3, TRPC4, and TRPC5 isoforms play a critical role in in vitro tube formation, as cells depleted of these proteins were not able to form tubular structures when plated on the artificial basement membrane Matrigel. The absence of tube formation was correlated with a marked decreased of spontaneous Ca2+ oscillations displayed by control cells seeded on the extracellular matrix. Once the cells have formed tubes, the Ca2+ response elicited by histamine displayed oscillations that depend on the same three TRPC channels. Finally, we showed that on primary HUVEC, TRPC1, TRPC4, and STIM1, but not Orai1, are required for tubulogenesis.
EXPERIMENTAL PROCEDURES
Materials
DMEM, penicillin, and streptomycin were obtained from Invitrogen. Fetal calf serum (FCS) was from PPA Laboratories (Linz, Austria). Histamine, SK&F96365, Pyr3, U73122, and thapsigargin were obtained from Sigma. The acetoxylmethyl ester form of Fura-2 (Fura-2/AM) and BAPTA (BAPTA/AM) were from Molecular Probes Europe (Leiden, The Netherlands). BrdU was from Calbiochem, and the growth factor-reduced Matrigel was from BD Biosciences. The ER-targeted cameleon probe D1ER was kindly provided by Drs. Amy Palmer and Roger Tsien.
Cell Culture and Transfection
Experiments were performed on the HUVEC-derived cell line EA.hy926 (kindly provided by Dr. C. J. S. Edgell) at passages >45. Cells were grown in DMEM containing 15% FCS, 1% HAT (5 mm hypoxanthin, 20 μm aminopterin, 0.8 mm thymidine), 50 units/ml penicillin, 50 μg/ml streptomycin and were maintained at 37 °C in 5% CO2 atmosphere. Primary HUVECs were grown in EGM-2 supplemented with 2% fetal bovine serum (FBS) and Bulletkit (Lonza, Switzerland). For the D1ER (generously provided by Drs. A. Palmer and R. Tsien) experiments, cells were grown until 70–80% confluence and were transiently transfected with 2 μg of cDNA encoding the D1ER construct.
siRNA Knockdown
EA.hy926 and HUVECs were transfected in suspension by incubating 3 × 105 cells in a solution containing 6 μl of Lipofectamine RNAiMax (Invitrogen) and the specific siRNA (100 nm; Ambion, Invitrogen, or Qiagen) according to manufacturer's protocols (Invitrogen). The optimal effect is obtained 48 h after transfection. The siRNA scramble from Ambion was used as a negative control. All siRNA used are the same as published previously (20). The other specific siRNA against TRPC3, TRPC4, and TRPC5 used in supplemental Fig. S3 are described in supplemental Table 1.
Cytosolic Calcium Measurements
For Ca2+ imaging, EA.hy926 and HUVECs were plated on 30-mm glass coverslips. The changes in cytosolic Ca2+ concentration were measured with Fura-2. Cells were loaded with 2 μm Fura-2/AM plus 1 μm pluronic acid for 30 min in the dark at room temperature in a medium containing 135 mm NaCl, 5 mm KCl, 2 mm CaCl2, 1 mm MgCl2, 10 mm Hepes, 2.6 mm NaHCO3, 0.44 mm KH2PO4, 10 mm glucose with 0.1% vitamins and 0.2% amino acids, pH adjusted to 7.45 with NaOH. Cells were washed twice and equilibrated for 10–15 min in the same buffer to allow deesterification. Ratiometric images of Ca2+ signals were obtained using a microscope (Axio Observer, Zeiss) equipped with a Lambda DG4 illumination system (Sutter Instrument Company, Novato, CA), which rapidly changed the excitation wavelengths between 340 nm (340AF15; Omega Optical) and 380 nm (380AF15; Omega Optical). Emission was collected through a 415DCLP dichroic mirror and a 510WB40 filter (Omega Optical), by a cooled, 12-bit CCD camera (CoolSnap HQ; Ropper Scientific, Trenton, NJ). Image acquisition and analysis were performed with the Metafluor 6.3 software (Universal Imaging, West Chester, PA, USA). Experiments were performed at room temperature in Hepes-buffered solution containing 135 mm NaCl, 5 mm KCl, 1 mm MgCl2, 2 mm CaCl2, 10 mm Hepes, 10 mm glucose, pH adjusted at 7.45 with NaOH. The Ca2+-free solution contained 1 mm EGTA instead of 2 mm CaCl2.
Spontaneous Ca2+ signals of cells plated on Matrigel were recorded during 10 min, in culture medium without phenol red at 37 °C. The signal was analyzed as the percentage of responding cells, and the number of Ca2+ transients recorded during 10 min, considered only for cells that responded at least with one oscillation.
ER Ca2+ Measurements
EA.hy926 cells were transiently transfected with 2 μg of cDNA encoding the D1ER construct 48 h before the experiments. Cells were illuminated at 440 nm (440AF21; Omega Optical), and emission was collected through a 455DRLP dichroic mirror, alternatively at 480 nm (480AF30; Omega Optical) and 535 nm (535AF26; Omega Optical). When required, photobleaching was corrected and data were expressed as R/Ro, Ro being the mean ratio values obtained during the first minute of the recording.
Western Blotting
Western blotting was performed as described previously (20). Briefly, the endothelial cells were lysed using modified Nonidet P-40 cell lysis buffer (Invitrogen), 50 mm Tris, pH 7.4, 250 mm NaCl, 5 mm EDTA, 50 mm NaF, 1 mm Na3VO4, and 1% Nonidet P-40. Total proteins were separated on SDS-PAGE and transferred to nitrocellulose membranes. Membranes were incubated in T-TBS (0.1% Tween 20, 20 mm Tris-HCl, pH 7.5, 137 mm NaCl) and 5% nonfat milk. Blots were incubated with the primary antibodies diluted in T-TBS and nonfat milk as follows: rabbit anti-TRPC1 polyclonal antibody (1:200; Alomone), rabbit anti-TRPC3 polyclonal antibody (1:200; Sigma), rabbit anti-TRPC4 polyclonal antibody (1:200; Alomone), rabbit anti-TRPC5 polyclonal antibody (1:200; Alomone), rabbit anti-TRPC6 polyclonal antibody (1:200; Alomone), and mouse monoclonal antibody against α-tubulin (clone DM1A, Sigma) 1:10,000. Blots were incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse diluted 1:10,000 (Bio-Rad) or with HRP-conjugated goat anti-rabbit diluted 1:10,000 (Bio-Rad), respectively. Antibodies were revealed using ECL reagents and hyperfilm ECL (Amersham Biosciences). ImageJ software was used to quantify the level of protein expression.
Quantitative Real-time PCR
Real-time PCR experiments were performed at the genomics platform of the NCCR Frontiers in Genetics (Geneva). For each PCR, 1/10 of the cDNA template was PCR-amplified in a 7900HT SDS System using Power SYBR Green PCR master mix (both from Applied Biosystems, Foster City, CA). We obtained raw threshold cycle (Ct) values using SDS 2.0 software (Applied Biosytems). A mean quantity was calculated from triplicate PCRs for each sample, and this quantity was normalized to the average of three endogenous control genes (glucuronidase B, GAPDH, and TBP) as described in Ref. 22. Primers used for the real-time PCR are described in Ref. 20.
Cell Proliferation Assay
EA.hy926 cells were cultured in 96-well plates at a density of 10000 cells/well. The Calbiochem BrdU cell proliferation assay is a nonisotopic immunoassay for the quantification of BrdU incorporation into newly synthesized DNA of actively proliferating cells. BrdU reagent was added 48 h after cell transfection, for 24 h. Absorbance was measured at 450 nm in each well using a spectrophotometric plate reader, and background was corrected by removing absorbance measured at 590 nm.
Morphogenesis Assay on Matrigel
24-well plates were precoated with a growth factor-reduced Matrigel, and 48 h after transfection EA.hy926 cells (4.5 × 105 cells/well) or HUVECs (1 × 105 cells/well) were seeded onto the Matrigel surface. Cells were incubated for 12–14 h at 37 °C, in the normal culture medium with serum but without addition of extra growth factors, and tubular network visualization was performed using ImageXpress Micro (MDS Analytical Technologies) automated fluorescence imaging system. Tube formation was quantified as the percentage of the cell surface covered by tubular structures.
Statistics
Results are expressed as mean ± S.E. of n observations. Sets of data were compared with a Student's t test. Differences were considered statistically significant when p < 0.05. ns, nonsignificant difference; *, p < 0.05; **, p < 0.01; ***, p < 0.001. All statistical tests were performed using GraphPad Prism version 5.0 for Windows (GraphPad Software).
RESULTS
Role of Ca2+ Entry Channels for in Vitro Tube Formation on EA.hy926 Cells
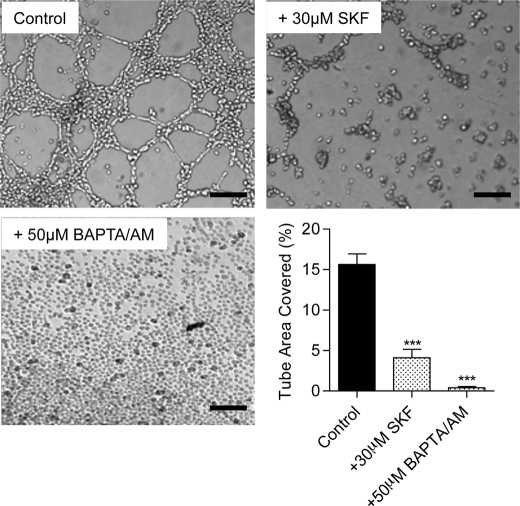
To study the role of Ca2+ entry channels in tubulogenesis, EA.hy926 cells were subjected to an in vitro tube formation assay. When endothelial cells were grown on a reconstituted basement membrane matrix Matrigel (23), they spontaneously formed tubular structures in the control condition (Fig. 1). The quantification of this process was performed by measuring the percentage of the surface covered by tubular structures in different conditions. We first evaluated the importance of Ca2+ signaling and Ca2+ entry in the process of tube formation. As expected, 50 μm BAPTA-AM (an intracellular Ca2+ chelator) completely abolished tube formation. Moreover, the nonspecific Ca2+ entry blocker SK&F96365 (30 μm), decreased tube formation by 70% (Fig. 1). These results confirmed the essential role of Ca2+ entry/Ca2+ signaling in the in vitro tube formation process.
FIGURE 1.
Ca2+ dependence of the in vitro tube formation process on EA.hy926 cells. The EA.hy926 were seeded on growth factor-reduced Matrigel and visualized 10–14 h later, in control conditions, or in the presence of 30 μm SK&F96365 (a Ca2+ entry blocker), or 50 μm BAPTA/AM (an intracellular Ca2+ chelator). Bar graph shows the percent of the surface covered by tubes in control condition and upon SK&F96365 or BAPTA/AM treatment. Data are mean ± S.E. (error bars) of three independent experiments. Scale bars, 200 μm. ***, p < 0.001.
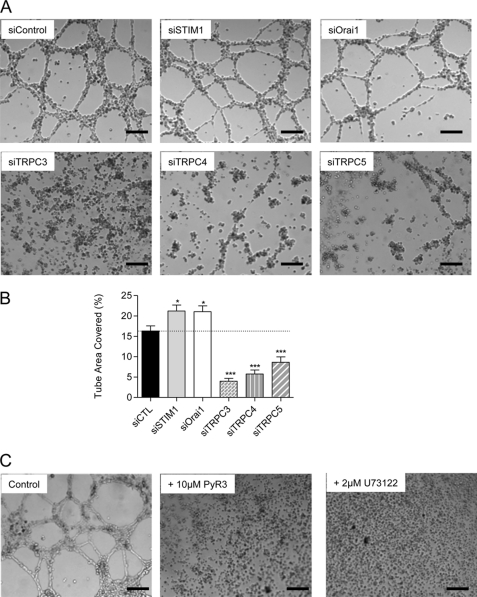
EA.hy926 expressed TRPC1, TRPC3, TRPC4, TRPC5, and TRPC6 together with STIM1 and Orai1 (20). We thus knocked down each of these proteins involved in Ca2+ entry and quantified the impact on tube formation. Knockdown of STIM1 or Orai1 proteins evoked a small but significant increase in the surface covered by tubes by approximately 30% (Fig. 2, A and B). On the contrary, the knockdown of TRPC3 reduced the tube formation by 80% (Fig. 2, A and B). The pharmacological TRPC3 inhibitor PyR3 (24) completely abolished tube formation, confirming the importance of TRPC3 for tube formation (Fig. 2C). TRPC4 and TRPC5 were also important for the in vitro tube formation process, as the silencing of these proteins reduced tube formation by 65 and 50%, respectively. On the other hand, TRPC1 silencing had no significant impact on this process, and TRPC6 knockdown marginally reduced tube formation (supplemental Fig. 1A). We previously reported that STIM1, Orai1, and TRPC3 silencing decreased protein levels by 67–80% (20), and siRNA against TRPC4 and TRPC5 also reduced protein levels by 65 and 70%, respectively (supplemental Fig. 2, A and B,). We verified, at the mRNA level, the absence of cross-effect among TRPC3, TRPC4, and TRPC5 following their respective knockdown (supplemental Fig. 3). We obtained similar effects on tube formation using different siRNA against TRPC3, TRPC4, and TRPC5 (supplemental Fig. 4B).
FIGURE 2.
Effects of protein silencing on tube formation on EA.hy926 cells. A, 48 h after transfection with siRNA (against STIM1, Orai1, TRPC3, TRPC4, and TRPC5), the EA.hy926 cells were seeded on growth factor-reduced Matrigel and visualized 10–14 h later. B, tube formation was quantified in control condition and upon siRNA treatment. Data are mean ± S.E. (error bars) of three to five independent experiments. *, p < 0.05; ***, p < 0.001. C, cells were seeded on growth factor-reduced Matrigel in control conditions and in the presence of 10 μm TRPC3 inhibitor Pyr3 or 2 μm phospholipase C inhibitor U73122. Scale bars, 200 μm.
TRPC channels are known to be activated downstream from the phospholipase C pathway (12). Consequently, we applied the general phospholipase C inhibitor U73122 during the process of tube formation. As shown on Fig. 2C, this treatment completely abolished Matrigel-induced tube formation.
Role of Ca2+ Entry Channels in EA.hy926 Cell Proliferation
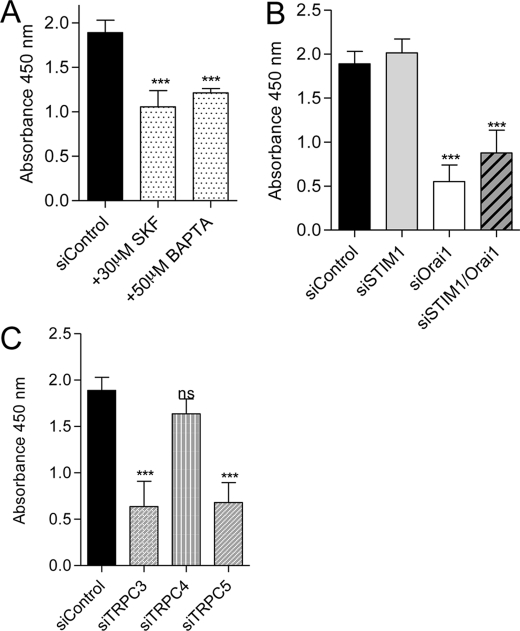
In vivo, angiogenesis requires cell proliferation, in addition to cell migration and organization of tubular structures. Endothelial cell proliferation was evaluated by measuring BrdU incorporation for 24 h. First, we incubated the cells with 50 μm BAPTA-AM to prevent Ca2+ elevations, or with 30 μm SK&F96365 to prevent Ca2+ entry. In both conditions, we observed a decreased in cell proliferation by approximately 50% (Fig. 3A). Then, we estimated the involvement of STIM1/Orai and the different TRPC channels in endothelial cell proliferation. The cell proliferation was not affected by STIM1 knockdown, whereas the silencing of Orai1 decreased cell proliferation by 70% (Fig. 3B). The double knockdown (Orai1/STIM1) reduced the proliferation to the same level as siRNA against Orai1 alone (Fig. 3B). TRPC3 and TRPC5 knockdown decreased cell proliferation by 65%, whereas the silencing of TRPC4 had no significant impact (Fig. 3C). TRPC1 or TRPC6 silencing did not alter endothelial cell proliferation (supplemental Fig. 1B).
FIGURE 3.
EA.hy926 cell proliferation assay. A, quantification of BrdU incorporation in control conditions and in the presence of 30 μm SK&F96365 and 50 μm BAPTA/AM. B, quantification of BrdU incorporation in EA.hy926 cells transfected with control siRNA and siRNA against STIM1, Orai1, and STIM1/Orai1. C, quantification of BrdU incorporation in EA.hy926 cells transfected with control siRNA and siRNA against TRPC3, TRPC4, and TRPC5. Data are mean ± S.E. (error bars) of minimum three independent experiments in duplicate. ns, not significant; ***, p < 0.001.
Spontaneous Ca2+ Transients during the Early Phase of in Vitro Tubulogenesis on EA.hy926 Cells
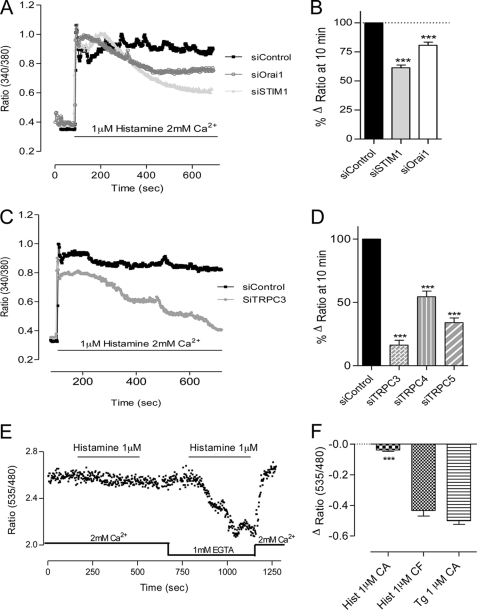
During the first hours after plating on Matrigel, we observed spontaneous Ca2+ oscillations in about 55% of the cells (Fig. 4, A, E, and F). To quantify the impact of gene silencing on these Ca2+ oscillations, we imaged the cells for 10 min and analyzed the oscillation frequency and the percentage of cells presenting Ca2+ transients. In STIM1- or Orai1-depleted cells, the frequency and the percentage of responding cells were not significantly modified. In TRPC3, TRPC4, or TRPC5 knockdown cells, the spontaneous Ca2+ pattern was strongly affected (Fig. 4, C–F), as shown by the decrease in the number of oscillations (Fig. 4E). The percentage of cell oscillating was decreased by 50% in TRPC3- and TRPC5-depleted cells, whereas siRNA against TRPC4 did not significantly affect this parameter (Fig. 4F). In line with the effect observed on tube formation, the silencing of TRPC1 or TRPC6 did not alter the spontaneous Ca2+ signal (supplemental Fig. 5, A and B). Similar effects were obtained using the other siRNA against TRPC3, TRPC4, and TRPC5 (supplemental Fig. 4, C and D). Hence, the alteration of spontaneous Ca2+ signals after gene silencing correlated well with the effect observed on tube formation.
FIGURE 4.
Spontaneous Ca2+ oscillations on EA.hy926 cells during tube formation. A–D, cytosolic Ca2+ measured at 37 °C on EA.hy926 cells loaded with 2 μm Fura-2 (supplemental Movies 1 and 2). Intracellular Ca2+ variations were measured after the first 1–2 h of tube formation on Matrigel, after siControl treatment (A), siSTIM1 (B), siTRPC3 (C) or siTRPC4 (D). Acquisition was done for 10 min, and each panel displays the responses of a representative coverslip. E, number of Ca2+ oscillations during 10 min in cells treated with siControl, siSTIM1, siOrai1, siTRPC3, siTRPC4, and siTRPC5. F, percentage of cells showing at least one Ca2+ transient during the 10-min recording time (n ranges from 64 to 168 cells). Error bars, S.E. ns, not significant; **, p < 0.01; ***, p < 0.001.
Histamine Induced Ca2+ Oscillations in EA.hy926 Cells Forming Tubular Structures
10–14 h after adhesion on Matrigel, once the tubular structures are formed, the cells did not display spontaneous Ca2+ transients any more (data not shown). Hence, we verified whether the cells were still able to respond to an agonist like histamine. Indeed, the addition of 1 μm histamine induced regular Ca2+ oscillations in control cells (Fig. 5A). We thus wanted to explore the molecules important for this Ca2+ response, even though tube formation was not affected by the presence of histamine (data not shown). The Ca2+ oscillatory phenotype was not significantly affected after STIM1 knockdown (Fig. 5, B, E, and F). On the contrary, after Orai1, TRPC3, TRPC4, or TRPC5 silencing, the frequency of Ca2+ oscillations was decreased by 32, 80, 40, and 91%, respectively (Fig. 5E). The amplitude of Ca2+ oscillations was not affected by Orai1 knockdown, whereas the silencing of TRPC3, TRPC4, or TRPC5 channels decreased the average amplitude of Ca2+ oscillations by 50, 20, and 20%, respectively (Fig. 5F). In TRPC1 and TRPC6 knockdown cells the response to histamine was similar to the one obtained in control cells (supplemental Fig. 5, C and D). The percentage of cells responding to histamine was significantly reduced after TRPC3 and TRPC5 knockdown (data not shown).
FIGURE 5.
Histamine-induced Ca2+ response on EA.hy926 cells after tube formation. Cytosolic Ca2+ responses were measured at 37 °C once the tubular structures were formed (14 h after cell adhesion on Matrigel). Before stimulation, no Ca2+ oscillations were detected. A–D, Ca2+ responses elicited by histamine after siControl treatment (A), siSTIM1 (B), siOrai1 (C), or siTRPC3 (D). E, number of Ca2+ oscillations during 10 min in cells treated with siControl, siSTIM1, siOrai1, siTRPC3, siTRPC4, and siTRPC5. F, amplitude of Ca2+ oscillations for each conditions shown in E (n ranges between 25 and 91 cells). Error bars, S.E. ns, not significant; **, p < 0.01; ***, p < 0.001.
When cells were seeded on glass instead than on Matrigel, they formed a monolayer and displayed a different pattern of Ca2+ response to histamine. Indeed, 1 μm histamine induced a rapid increase of the cytosolic Ca2+ concentration, which was followed by a sustained plateau during the whole stimulation. The silencing of STIM1 and Orai1 decreased the plateau phase (estimated after 10 min of stimulation) by 40 and 25%, respectively (Fig. 6, A and B). Knockdown of TRPC3, TRPC4, and TRPC5 had a stronger effect and reduced histamine-induced Ca2+ response by 80, 50, and 75%, respectively (Fig. 6, C and D). The knockdown of TRPC1 decreased Ca2+ entry by about 20% whereas TRPC6 did not appear to play a role in histamine-induced Ca2+ entry (supplemental Fig. 1C).
FIGURE 6.
Histamine-induced Ca2+ entry on EA.hy926 cells grown on glass. Cytosolic Ca2+ concentration was assessed on EA.hy926 cells loaded with 2 μm Fura-2 and stimulated with 1 μm histamine in 2 mm Ca2+-containing medium. A, EA.hy926 cells were transiently transfected with control siRNA (scramble, siControl), or siRNA against STIM1 or Orai1. Each trace is the mean of a representative coverslip. B, quantification of the effects of siSTIM1 and siOrai1 on the difference between the ratio at 10 min and the basal ratio (n ranges from 81 to 201 cells). C, representative traces of 1 μm histamine stimulation of cells transiently transfected with siControl or siTRPC3. Each trace represents the mean of a representative coverslip. D, quantification of the effects of siTRPC3, siTRPC4, and siTRPC5 on the difference between the ratio after 10 min of stimulation and the basal ratio (n ranges from 67 to 85 cells). E, EA.hy926 cells were transiently transfected with the ER-targeted cameleon probe D1ER. Representative recording of a cell stimulated sequentially with 1 μm histamine in Ca2+-containing medium and in Ca2+-free medium is shown. F, quantification of ER Ca2+ release in EA.hy926 cells stimulated by 1 μm histamine in Ca2+-containing medium (CA), Ca2+-free medium (CF), and by 1 μm thapsigargin (Tg) (in CA). Error bars, S.E. ***, p < 0.001.
Because STIM1 and Orai1 are implicated in histamine-induced Ca2+ entry, we investigated whether this low dose of agonist leads to store depletion. To this end, we transfected the EA.hy926 cells with a genetically encoded Ca2+ probe targeted to the ER (D1ER). We hardly could measure a decrease of the ER Ca2+ concentration upon 1 μm histamine stimulation in Ca2+ containing medium (Fig. 6, E and F), which is in line with the small ER depletion obtained with 100 μm histamine (3). In Ca2+-free medium, 1 μm histamine strongly depleted the store to a level similar to thapsigargin (Fig. 6, E and F), pointing to an efficient cycling of Ca2+ from and to the ER when extracellular Ca2+ is available (25, 26). Hence, a low dose of histamine activated STIM1/Orai1 even if the ER Ca2+ depletion was almost undetectable with the D1ER (see “Discussion”).
Role of Ca2+ Entry Channels in Primary Cultured Endothelial Cells
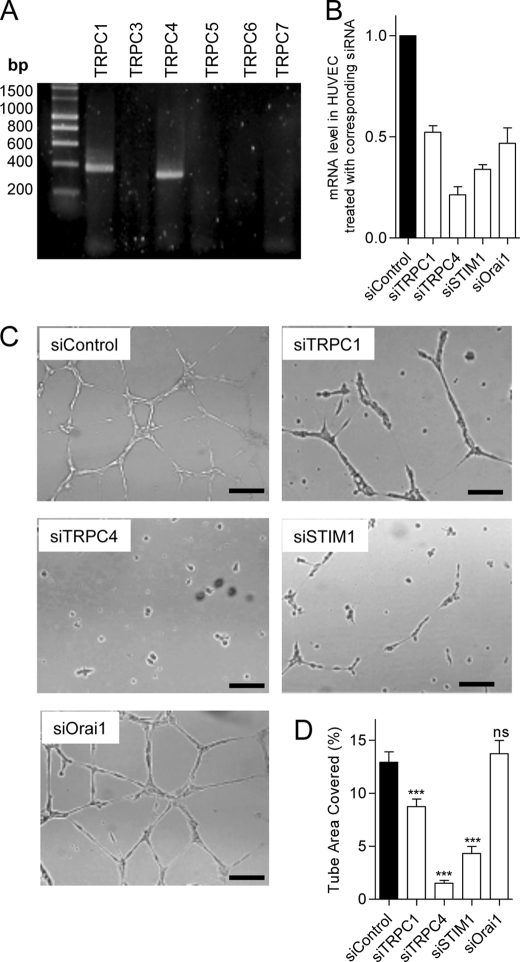
We used the primary cultured HUVECs to assess the role of TRPC channels and STIM1/Orai1 during in vitro tube formation and spontaneous Ca2+ signals. We first determined that TRPC1 and TRPC4 are expressed on HUVECs (Fig. 7A) as well as STIM1 and Orai1. The efficiency of gene silencing was assessed at the mRNA level and ranged between 50 and 85% of mRNA reduction (Fig. 7B). The knockdown of STIM1, TRPC1, and TRPC4 reduced tube formation, whereas siRNA against Orai1 had no effect (Fig. 7, C and D). To ensure that the absence of effect of siOrai1 during tube formation was not due to a moderate efficiency of our siOrai1, we performed Western blotting and found that the protein level of Orai1 was decreased by approximately 80% after siRNA treatment (data not shown). Similar to what we observed on EA.hy926 cells, the HUVECs displayed spontaneous Ca2+ oscillations when plated on Matrigel, at the beginning of tubulogenesis (Fig. 8A). siRNA against TRPC4 strongly reduced both the frequency and the percentage of responding cells, and the knockdown of TRPC1 and STIM1 reduced Ca2+ oscillations to a lesser extend (Fig. 8, B–D). siOrai1 minimally reduced the frequency of Ca2+ oscillations (Fig. 8C). Hence, on HUVEC (as on EA.hy926 cells), the impact of gene silencing on the spontaneous Ca2+ response correlated well with the effect on tube formation.
FIGURE 7.
Expression of TRPC isoforms on HUVEC and effects of protein silencing on tube formation. A, mRNA amplification of TRPC1, TRPC3, TRPC4, TRPC5, TRPC6, and TRPC7 isoforms by real-time PCR in HUVECs. B, mRNA levels assessed by quantitative RT-PCR 48 h after transfection (mean ± S.E. (error bars), 2–4 independent experiments). C, 48 h after transfection with siRNA (against TRPC1, TRPC4, STIM1, and Orai1), the HUVECs were seeded on growth factor-reduced Matrigel and visualized 10–14 h later. D, quantification of tube formation in control condition and upon siRNA treatment. Data are mean ± S.E. of three to five independent experiments. Scale bar, 200 μm. ns, not significant; ***, p < 0.001.
FIGURE 8.
Spontaneous Ca2+ oscillations during the tube formation on HUVECs. A and B, cytosolic Ca2+ measured at 37 °C on HUVECs loaded with 2 μm Fura-2. Intracellular Ca2+ variations were measured after the first 1–2 h of tube formation on Matrigel, after siControl treatment (A) and siTRPC4 (B). Acquisition was done for 10 min, and each panel displays the responses of a representative coverslip. C, percentage of cells showing at least one Ca2+ transient during the 10-min recording time (n ranges from 39 to 135 cells). D, number of Ca2+ oscillations during 10 min in cells treated with siControl, siTRPC1, siTRPC4, siSTIM1, and siOrai1. ns, not significant; *, p < 0.05; **, p < 0.01; ***, p < 0.001.
DISCUSSION
In the present work, we assessed the role of STIM1, Orai1, and TRPC channels on cell proliferation and in vitro tube formation in the HUVEC line, EA.hy926, and on the parental primary cells, the HUVECs. By using a siRNA approach, we showed that on EA.hy926 cells, the knockdown of TRPC3, TRPC4, or TRPC5 strongly decreased Matrigel-induced tube formation, whereas the silencing of STIM1 or Orai1 had no negative impact. Reduction of tube formation was correlated with the decrease of spontaneous Ca2+ oscillations during the early stages of the process. We also demonstrated that the silencing of Orai1, TRPC3, or TRPC5 strongly reduced endothelial cell proliferation. On primary cultured endothelial cells (HUVECs), the knockdown of TRPC1, TRPC4, and STIM1 reduced tube formation and spontaneous Ca2+ oscillations, whereas the siRNA against Orai1 had no effect on these processes. From these results, it appears that TRPC channels play a major role in tubulogenesis, both in EA.hy926 cells and in HUVECs.
In vitro angiogenesis, using Matrigel as the artificial basement membrane, is widely used to study endothelial cell tube formation (27, 28). In addition, the endothelial cell line EA.hy926 was demonstrated to be a suitable model for in vitro studies of tube formation (29–31). Using this assay, we revealed an important role of TRPC3, TRPC4, and TRPC5 channels, which, unlike STIM1 and Orai1, are required for tube formation. On the contrary, the ability to form tubular structures was enhanced upon STIM1/Orai1 gene silencing. The role played by these different Ca2+ entry channels correlated well with their role in spontaneous Ca2+ oscillations recorded during the early phase of tube formation, which strengthen the link between tube formation and Ca2+ entry. An early study reported an increase in cytosolic Ca2+ concentration during the initial phase of endothelial cell sprouting on collagen IV surface (32). The Ca2+ rise and the cell sprouting were prevented by the nonspecific Ca2+ entry blockers carboxyamidotriazole and SK&F96365 (32). Our study confirmed the essential role of Ca2+ entry for endothelial tube formation and shows that TRPC channels are important for the early Ca2+ signals associated with tubulogenesis.
TRPC channels have been shown to participate in in vitro angiogenesis, in particular TRPC6 (17, 18). On EA.hy926 cells, TRPC6 knockdown slightly reduced tube formation, a marginal effect compared with the important effect of TRPC3, TRPC4, and TRPC5 knockdown. TRPC1 was shown to have a prominent role in angiogenesis in zebrafish (33), but in mammals, no further data reported a role of TRPC channels in tube formation (14).
Angiogenesis is a complex process that comprises not only cell migration and organization as tubular structures, but also requires cell proliferation. On Matrigel, the cells do not proliferate (27), but migrate and reorganize to form capillary-like structures. Hence, only part of the angiogenic process is recapitulated using this assay. Consequently, a channel that is important for cell proliferation is likely also important for angiogenesis if we consider the full process. The involvement of Orai1 but not STIM1 in cell proliferation is at odds considering the way Orai1 is getting activated, i.e. upon STIM1 oligomerization, recruitment close to the plasma membrane and binding to Orai1 (11, 34). This, however, was already observed in endothelial cells (35) and in HEK293 cells (36). In both studies, STIM2 was involved in cell proliferation and thus was likely responsible for Orai1 activation. In our model, STIM2 silencing did not impact on cell proliferation (data not shown). Alternatively, Orai1 might be activated independently of STIM1, as was recently reported (37). Furthermore, it was also proposed that Orai1 might interact with cyclin-dependent kinase, pointing to a specific role of this channel in cell proliferation (38).
From our data on EA.hy926 cells, it emerged that STIM1 and Orai1, the major contributors of SOCE, are not required for tubulogenesis, whereas several of the TRPC channels appeared to be. The Ca2+ oscillatory behavior observed after stimulation with 1 μm histamine in cells forming tubes is reminiscent of the spontaneous Ca2+ transients observed at the beginning of tube formation. Furthermore, both responses relied mainly on TRPC3, TRPC4, and TRPC5 channels, and minimally on STIM1 and Orai1. That likely implies that the ER Ca2+ content stayed elevated during Ca2+ oscillations and is in line with previous papers showing that Ca2+ oscillations do not affect the Ca2+ level within the ER (39) and thus do not activate SOCE (40). Previously, we and other have shown that 100 μm histamine led to approximately 15% of store depletion (3), and in these conditions, STIM1 and Orai1 accounted for approximately 35–50% of the sustained Ca2+ entry (data not shown). In the present study, we showed that 1 μm histamine induced also a sustained Ca2+ response of cells forming monolayers on glass coverslip, and STIM1/Orai1 knockdown reduced Ca2+ entry by approximately 20–40%. The level of store depletion after 1 μm histamine was, however, minimal (Fig. 6E). Hence, it appears that the extent of STIM1/Orai1 involvement in cytosolic Ca2+ response is not linearly correlated with the level of store depletion we measured with the D1ER. That might be explained by restricted ER compartments emptied by histamine but not measured by the D1ER. Recently, it was shown by electron microscopy that proteins targeted to the ER through a KDEL sequence were excluded from thin cortical ER structures (41), suggesting that D1ER might also be excluded from these subcompartments. Whether such regions are depleted by low dose of agonists and whether that leads to the activation of STIM1/Orai1 remained an open question. As already mentioned, during Ca2+ oscillations, we can reasonably postulate that the actual level of ER Ca2+ depletion is extremely limited, and that would explain that STIM1/Orai1 do not participate in Ca2+ entry during Ca2+ oscillatory response, at least on EA.hy926 cells. The signaling pathway leading to specific TRPC activation remained, however, to be determined.
Recently, it was reported that Orai1 channel is an important player for both in vitro and in vivo angiogenesis (19). The in vitro experiments were performed on HUVECs, but neither the impact of other channels like the TRPC nor the role of STIM1 was evaluated. Hence, we wanted to investigate whether TRPC channels are important for angiogenesis in HUVECs. We found out that the knockdown of TRPC1, TRPC4, and STIM1 reduced tube formation, and, like on EA.hy926 cells, also reduced spontaneous Ca2+ oscillations observed at the beginning of tubulogenesis. However, and on the contrary to the findings of Li et al. (19), we did not observe a defect of in vitro angiogenesis after knocking down Orai1. The main difference between both studies is the use of vascular endothelial growth factor (VEGF) to enhance tube formation. We did not add VEGF in the medium whereas Li et al. added 2 ng/ml VEGF. The application of VEGF activated STIM1 and Orai1 (19), whereas without this growth factor, the level of Ca2+ within the ER likely remained elevated (as we proposed for the EA.hy926 cells) and thus would explain the absence of Orai1 activation. The mechanism leading to the activation of STIM1 that we observed, remains, however, to be elucidated. We should mention that on HUVECs, it was shown that Orai1 is important for cell proliferation (35). Hence, we cannot exclude an implication of Orai1 during in vivo angiogenesis, as in vivo, cell proliferation is part of the process.
In this study, we showed that in vitro tubulogenesis relies on a Ca2+ signaling pathway that engages mainly TRPC channels. The knockdown of several TRPC channels, both on an endothelial cell line (EA.hy926) as well as on primary endothelial cells (HUVECs), severely prevented spontaneous Ca2+ oscillations of cells plated on Matrigel, as well as the subsequent tube formation. Interestingly, Orai1, a major player in Ca2+ signaling, does not seem to be essential for tube formation. Instead, TRPC channels are pivotal and represent very interesting candidates to modulate angiogenesis.
Supplementary Material
Acknowledgments
We thank Cyril Castelbou for excellent technical assistance; Drs. R. Malli, W. F. Graier, N. Demaurex, and C. Vandebrouck for critical reading of the manuscript; Drs. A. Palmer and R. Y. Tsien for the D1ER construct; Dr. C. J. S. Edgell for the EA.hy926 cells; and Drs. B. Imhof and S. Garrido-Urbani for the HUVECs.
This work was supported by the Swiss National Science Foundation Grant 31000-120186/1 and by the Carlos and Elsie de Reuter Foundation.

This article contains supplemental Table 1, Figs. S1–S5, and Movies 1 and 2.
- ER
- endoplasmic reticulum
- SOCE
- store-operated Ca2+ entry
- STIM1
- stromal interaction molecule-1
- STIM2
- stromal interaction molecule-2
- TRP
- transient receptor potential
- TRPC
- TRP canonical.
REFERENCES
- 1. Nilius B., Droogmans G. (2001) Ion channels and their functional role in vascular endothelium. Physiol. Rev. 81, 1415–1459 [DOI] [PubMed] [Google Scholar]
- 2. Girardin N. C., Antigny F., Frieden M. (2010) Electrophysiological characterization of store-operated and agonist-induced Ca2+ entry pathways in endothelial cells. Pflugers Arch. 460, 109–120 [DOI] [PubMed] [Google Scholar]
- 3. Jousset H., Malli R., Girardin N., Graier W. F., Demaurex N., Frieden M. (2008) Evidence for a receptor-activated Ca2+ entry pathway independent from Ca2+ store depletion in endothelial cells. Cell Calcium 43, 83–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Putney J. W., Jr. (1986) A model for receptor-regulated calcium entry. Cell Calcium 7, 1–12 [DOI] [PubMed] [Google Scholar]
- 5. Roos J., DiGregorio P. J., Yeromin A. V., Ohlsen K., Lioudyno M., Zhang S., Safrina O., Kozak J. A., Wagner S. L., Cahalan M. D., Veliçelebi G., Stauderman K. A. (2005) STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. 169, 435–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liou J., Kim M. L., Heo W. D., Jones J. T., Myers J. W., Ferrell J. E., Jr., Meyer T. (2005) STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 15, 1235–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang S. L., Yu Y., Roos J., Kozak J. A., Deerinck T. J., Ellisman M. H., Stauderman K. A., Cahalan M. D. (2005) STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 437, 902–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Feske S., Prakriya M., Rao A., Lewis R. S. (2005) A severe defect in CRAC Ca2+ channel activation and altered K+ channel gating in T cells from immunodeficient patients. J. Exp. Med. 202, 651–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vig M., Peinelt C., Beck A., Koomoa D. L., Rabah D., Koblan-Huberson M., Kraft S., Turner H., Fleig A., Penner R., Kinet J. P. (2006) CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science 312, 1220–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yeromin A. V., Zhang S. L., Jiang W., Yu Y., Safrina O., Cahalan M. D. (2006) Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature 443, 226–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang Y., Deng X., Gill D. L. (2010) Calcium signaling by STIM and Orai: intimate coupling details revealed. Sci. Signal. 3, pe42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Montell C. (2005) The TRP superfamily of cation channels. Sci. STKE 2005, re3. [DOI] [PubMed] [Google Scholar]
- 13. Birnbaumer L. (2009) The TRPC class of ion channels: a critical review of their roles in slow, sustained increases in intracellular Ca2+ concentrations. Annu. Rev. Pharmacol. Toxicol. 49, 395–426 [DOI] [PubMed] [Google Scholar]
- 14. Wong C. O., Yao X. (2011) TRP channels in vascular endothelial cells. Adv. Exp. Med. Biol. 704, 759–780 [DOI] [PubMed] [Google Scholar]
- 15. Carmeliet P. (2005) Angiogenesis in life, disease, and medicine. Nature 438, 932–936 [DOI] [PubMed] [Google Scholar]
- 16. Munaron L., Fiorio Pla A. (2009) Endothelial calcium machinery and angiogenesis: understanding physiology to interfere with pathology. Curr. Med. Chem. 16, 4691–4703 [DOI] [PubMed] [Google Scholar]
- 17. Hamdollah Zadeh M. A., Glass C. A., Magnussen A., Hancox J. C., Bates D. O. (2008) VEGF-mediated elevated intracellular calcium and angiogenesis in human microvascular endothelial cells in vitro are inhibited by dominant negative TRPC6. Microcirculation 15, 605–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ge R., Tai Y., Sun Y., Zhou K., Yang S., Cheng T., Zou Q., Shen F., Wang Y. (2009) Critical role of TRPC6 channels in VEGF-mediated angiogenesis. Cancer Lett. 283, 43–51 [DOI] [PubMed] [Google Scholar]
- 19. Li J., Cubbon R. M., Wilson L. A., Amer M. S., McKeown L., Hou B., Majeed Y., Tumova S., Seymour V. A., Taylor H., Stacey M., O'Regan D., Foster R., Porter K. E., Kearney M. T., Beech D. J. (2011) Orai1 and CRAC channel dependence of VEGF-activated Ca2+ entry and endothelial tube formation. Circ. Res. 108, 1190–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Antigny F., Jousset H., König S., Frieden M. (2011) Thapsigargin activates Ca2+ entry both by store-dependent, STIM1/Orai1-mediated, and store-independent, TRPC3/PLC/PKC-mediated pathways in human endothelial cells. Cell Calcium 49, 115–127 [DOI] [PubMed] [Google Scholar]
- 21. Edgell C. J., McDonald C. C., Graham J. B. (1983) Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc. Natl. Acad. Sci. U.S.A. 80, 3734–3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A., Speleman F. (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3, RESEARCH0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kleinman H. K., Martin G. R. (2005) Matrigel: basement membrane matrix with biological activity. Semin. Cancer Biol. 15, 378–386 [DOI] [PubMed] [Google Scholar]
- 24. Kiyonaka S., Kato K., Nishida M., Mio K., Numaga T., Sawaguchi Y., Yoshida T., Wakamori M., Mori E., Numata T., Ishii M., Takemoto H., Ojida A., Watanabe K., Uemura A., Kurose H., Morii T., Kobayashi T., Sato Y., Sato C., Hamachi I., Mori Y. (2009) Selective and direct inhibition of TRPC3 channels underlies biological activities of a pyrazole compound. Proc. Natl. Acad. Sci. U.S.A. 106, 5400–5405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arnaudeau S., Kelley W. L., Walsh J. V., Jr., Demaurex N. (2001) Mitochondria recycle Ca2+ to the endoplasmic reticulum and prevent the depletion of neighboring endoplasmic reticulum regions. J. Biol. Chem. 276, 29430–29439 [DOI] [PubMed] [Google Scholar]
- 26. Malli R., Frieden M., Osibow K., Zoratti C., Mayer M., Demaurex N., Graier W. F. (2003) Sustained Ca2+ transfer across mitochondria is essential for mitochondrial Ca2+ buffering, store-operated Ca2+ entry, and Ca2+ store refilling. J. Biol. Chem. 278, 44769–44779 [DOI] [PubMed] [Google Scholar]
- 27. Arnaoutova I., George J., Kleinman H. K., Benton G. (2009) The endothelial cell tube formation assay on basement membrane turns 20: state of the science and the art. Angiogenesis 12, 267–274 [DOI] [PubMed] [Google Scholar]
- 28. Arnaoutova I., Kleinman H. K. (2010) In vitro angiogenesis: endothelial cell tube formation on gelled basement membrane extract. Nat. Protoc. 5, 628–635 [DOI] [PubMed] [Google Scholar]
- 29. Bauer J., Margolis M., Schreiner C., Edgell C. J., Azizkhan J., Lazarowski E., Juliano R. L. (1992) In vitro model of angiogenesis using a human endothelium-derived permanent cell line: contributions of induced gene expression, G-proteins, and integrins. J. Cell. Physiol. 153, 437–449 [DOI] [PubMed] [Google Scholar]
- 30. Tapia V., Gabler F., Muñoz M., Yazigi R., Paredes A., Selman A., Vega M., Romero C. (2011) Tyrosine kinase A receptor (trkA): a potential marker in epithelial ovarian cancer. Gynecol. Oncol. 121, 13–23 [DOI] [PubMed] [Google Scholar]
- 31. Piqueras L., Reynolds A. R., Hodivala-Dilke K. M., Alfranca A., Redondo J. M., Hatae T., Tanabe T., Warner T. D., Bishop-Bailey D. (2007) Activation of PPARβ/δ induces endothelial cell proliferation and angiogenesis. Arterioscler. Thromb. Vasc. Biol. 27, 63–69 [DOI] [PubMed] [Google Scholar]
- 32. Alessandro R., Masiero L., Lapidos K., Spoonster J., Kohn E. C. (1998) Endothelial cell spreading on type IV collagen and spreading-induced FAK phosphorylation is regulated by Ca2+ influx. Biochem. Biophys. Res. Commun. 248, 635–640 [DOI] [PubMed] [Google Scholar]
- 33. Yu P. C., Gu S. Y., Bu J. W., Du J. L. (2010) TRPC1 is essential for in vivo angiogenesis in zebrafish. Circ. Res. 106, 1221–1232 [DOI] [PubMed] [Google Scholar]
- 34. Park C. Y., Hoover P. J., Mullins F. M., Bachhawat P., Covington E. D., Raunser S., Walz T., Garcia K. C., Dolmetsch R. E., Lewis R. S. (2009) STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell 136, 876–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Abdullaev I. F., Bisaillon J. M., Potier M., Gonzalez J. C., Motiani R. K., Trebak M. (2008) Stim1 and Orai1 mediate CRAC currents and store-operated calcium entry important for endothelial cell proliferation. Circ. Res. 103, 1289–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. El Boustany C., Katsogiannou M., Delcourt P., Dewailly E., Prevarskaya N., Borowiec A. S., Capiod T. (2010) Differential roles of STIM1, STIM2 ,and Orai1 in the control of cell proliferation and SOCE amplitude in HEK293 cells. Cell Calcium 47, 350–359 [DOI] [PubMed] [Google Scholar]
- 37. Feng M., Grice D. M., Faddy H. M., Nguyen N., Leitch S., Wang Y., Muend S., Kenny P. A., Sukumar S., Roberts-Thomson S. J., Monteith G. R., Rao R. (2010) Store-independent activation of Orai1 by SPCA2 in mammary tumors. Cell 143, 84–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Capiod T. (2011) Cell proliferation, calcium influx, and calcium channels. Biochimie 93, 2075–2079 [DOI] [PubMed] [Google Scholar]
- 39. Park M. K., Petersen O. H., Tepikin A. V. (2000) The endoplasmic reticulum as one continuous Ca2+ pool: visualization of rapid Ca2+ movements and equilibration. EMBO J. 19, 5729–5739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shuttleworth T. J. (2004) Receptor-activated calcium entry channels: who does what, and when? Sci. STKE 2004, pe40. [DOI] [PubMed] [Google Scholar]
- 41. Orci L., Ravazzola M., Le Coadic M., Shen W. W., Demaurex N., Cosson P. (2009) From the cover: STIM1-induced precortical and cortical subdomains of the endoplasmic reticulum. Proc. Natl. Acad. Sci. U.S.A. 106, 19358–19362 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.