Background: Sox2 and TLX are essential for the self-renewal of adult neural stem cells (NSCs).
Results: Sox2 positively regulates TLX expression and antagonizes the negative feedback system of TLX by a physical interaction between Sox2 and TLX.
Conclusion: Sox2 and TLX form a molecular network regulating adult NSCs.
Significance: This molecular network is a target to discover new ways regulate endogenous neurogenesis.
Keywords: Molecular biology, Neural stem cell, Neuroprogenitor cell, Nuclear receptors, Transcription factors, Neural stem cell, Self-renew, Sox2, TLX, Transcription factors
Abstract
Adult neurogenesis is maintained by self-renewable neural stem cells (NSCs). Their activity is regulated by multiple signaling pathways and key transcription factors. However, it has been unclear whether these factors interplay with each other at the molecular level. Here we show that SRY-box-containing gene 2 (Sox2) and nuclear receptor tailless (TLX) form a molecular network in adult NSCs. We observed that both Sox2 and TLX proteins bind to the upstream region of Tlx gene. Sox2 positively regulates Tlx expression, whereas the binding of TLX to its own promoter suppresses its transcriptional activity in luciferase reporter assays. Such TLX-mediated suppression can be antagonized by overexpressing wild-type Sox2 but not a mutant lacking the transcriptional activation domain. Furthermore, through regions involved in DNA-binding activity, Sox2 and TLX physically interact to form a complex on DNAs that contain a consensus binding site for TLX. Finally, depletion of Sox2 revealed the potential negative feedback loop of TLX expression that is antagonized by Sox2 in adult NSCs. These data suggest that Sox2 plays an important role in Tlx transcription in cultured adult NSCs.
Introduction
Adult neurogenesis includes a series of sequential developmental events that are all necessary for the generation of new neurons from adult neural stem cells (NSCs)3 in two distinct brain areas, the subventricular zone of the lateral ventricles and the subgranular zone of the hippocampal dentate gyrus. New neurons undergo distinct developmental steps before they become functionally integrated into the existing circuitry. Parental NSCs produce intermediate progenitor cells and are identified by proliferative activity and the absence of mature neuronal markers. In the lateral ventricles, these intermediate progenitor cells migrate through the rostral migratory stream to the olfactory bulbs, where they differentiate into interneurons. In the hippocampus, the progenitor cells give rise to new granule cell neurons or glial cells in the subgranular zone of the dentate gyrus (1, 2). Elucidation of the properties of NSCs requires the identification of molecules that determine the self-renewal and multipotent characteristics of these cells. Self-renewing adult neural stem cells express a distinct set of stem-cell-associated proteins. Among these intrinsic factors, Sox2 and TLX are essential for the self-renewal of adult NSCs (3, 4).
Sox2 is a member of the sex-determining Y-box-related high-mobility group (HMG) box gene family, which encodes transcription factors with an HMG DNA-binding domain (5, 6). Sox2 is essential for the pluripotency of epiblast stem cells, embryonic stem cells, and induced pluripotent stem cells (7, 8). Sox2 is also required for adult NSC maintenance in the central nervous system (9–11). It has been hypothesized that Sox2 regulates epidermal growth factor receptor (Egfr) and sonic hedgehog (Shh) expression for self-renewal of NSCs by a positive feedback loop (12–14). The nuclear receptor tailless (TLX), also known as NR2E1, is an orphan nuclear receptor that is expressed in vertebrate forebrains (15, 16). TLX is an important regulator of neural stem cell maintenance and self-renewal in both embryonic and adult brains (4, 16). Global deletion of TLX during development leads to retinal dystrophy, blindness, and aggression (16). Conditional gene disruption of Tlx results in a significant reduction of NSC proliferation and a marked decrease in spatial learning (17). TLX-positive cells in the subventricular zone have been identified as slowly dividing type B NSCs. The inducible deletion of Tlx leads to complete loss of subventricular zone neurogenesis, and it has been suggested that TLX is required for the establishment of astrocyte-like NSCs in the adult brain (18).
The way in which Tlx expression is maintained in adult NSCs remains unclear. In the present study, we investigated the molecular relationship between Sox2 and TLX, key factors for maintaining the stemness of NSCs.
EXPERIMENTAL PROCEDURES
Adult Neural Stem/Progenitor Cell Cultures and BrdU Treatment
The dentate gyrus and subventricular zone of adult female rats or mice were dissected, and progenitors were isolated and propagated as described previously (19). Rat adult hippocampus neural stem/progenitor cells (AHPs) were cultured in DMEM/F-12 medium containing N2 supplement plus FGF-2 (20 ng/ml) on poly-l-ornithine/laminin-coated dishes (20, 21). Adult NSCs prepared from Tlxf/Z mouse whole brains by FACS with the endogenous Tlx promoter activity were cultured in DMEM/F-12 medium with N2 supplement plus FGF-2 (20 ng/ml), EGF (20 ng/ml), and heparin (5 μg/ml) (17). For gene depletion analysis, adult NSCs were transfected by the Nucleofection system (Amaxa Inc., Gaithersburg, MD) with vectors that express scramble sequence of short hairpin RNA (shRNA) or shRNA for Sox2 or Tlx, with pCAG-mock or pCAG-TLX. Cells were cultured for 3 days, and transfected EGFP-positive cells were collected by flow cytometry. For gene complementation assay of Tlx, retrovirus-expressing mock or TLX were infected into mouse NSCs with Puromycin (Sigma), and the selected cells were transfected with scrambled control vector or shRNA for Sox2 for 48 h, and cultured with 10 μm BrdU for 1 h. The BrdU-treated cells were fixed and acid-treated, followed by immunofluorescence analysis with BrdU-specific antibody (Accurate), or non-acid-treated cells were stained with an anti-phospho-histone H3 (Ser10) antibody (Cell Signaling Technology), and nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI).
ChIP-qPCR
The ChIP assay was performed with an EZ-ChIP kit (Upstate/Millipore) and 5 μg of anti-Sox2 antibody (Chemicon), anti-TLX antibody (Perseus Proteomics), and an anti-acetyl-lysine histone H3 antibody (Millipore), an anti-trimethyl histone H3 (Lys-4) antibody (MAB Institute, Inc.), and an anti-trimethyl histone H3 (Lys-27) antibody (MAB Institute, Inc.) per reaction. DNA-relative enrichment was determined by normalizing to an input genomic DNA. All ChIP experiments were obtained from independent chromatin preparations, and all quantitative real-time PCR reactions were performed in quadruplicate for each sample on each amplicons. Primers for the ChIP-qPCR are listed in the supplemental Table S1.
Quantitative Real-time RT-PCR
Total RNAs were extracted using the RNeasy kit (Qiagen) and reverse-transcribed using ReverTra Ace-α- kit (TOYOBO). Quantitative real-time RT-PCR and ChIP-qPCR were performed with a SYBR Green Q-PCR analysis kit (TaKaRa) according to the manufacturer's instructions. All samples were run in quadruplicate for each experiment (Applied Biosystems). The primers used for qRT-PCR are listed in supplemental Table S1, and values were normalized to that for glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Luciferase Reporter Assay
The reporter for the mouse Tlx/Nr2e1 gene proximal promoter (spanning from −1319 to −477) was constructed by PCR with subsequent ligation into pGL3 basic vector (pTLX-P1-luciferase). The luciferase reporters for transcription factor-specific analysis, with repeats of the sequence, 5′-ACCAACAATGAAC-3′, containing the putative Sox2 binding consensus S1, and repeats of the sequence, 5′-GGCACAAAGTCACAG-3′ or 5′-CCAGAAAGTCATCA-3′ containing the putative TLX binding consensus T1 or T2 in the TLX promoter region, were introduced into a firefly luciferase construct driven by tk minimal promoter. These constructs were designated 3×ST-tk-luciferase (three repeats of S1 with T1 or T2) and 6×T-tk-luciferase (six repeats of T1 or T2), respectively. Rat AHPs were seeded in N2 supplement plus FGF-2 (20 ng/ml) in 12-well plates coated with poly-l-ornithine/laminin and transiently transfected with Tlx promoter reporters along with the expression vector for transgenes and pEF-Rluc or pCMV-Rluc using LT-1 (Mirus) according to the manufacturer's instruction. After 48 h, cells were lysed in passive lysis buffer (Promega, Madison, WI). Luciferase activity was measured using the dual luciferase reporter assay system (Promega). All reporter assays were performed in triplicate, and the bars in the figures denote the standard deviation.
Immunoblotting and Gel Shift Analysis
293T cells transfected with FLAG-, HA-, or Myc-tagged transgenes were lysed by sonication in Nonidet P-40 lysis buffer (0.5% Nonidet P-40, 10 mm Tris-HCl, pH 7.4, 150 mm NaCl, protease inhibitor mixture; Pierce). Lysates were immunoprecipitated and subjected to SDS-PAGE, followed by transfer and Western blotting as described previously (22). Mouse monoclonal anti-FLAG antibody (Sigma), anti-Myc tag 9E10 antibody (Upstate), and anti-HA tag 16B12 antibody (BABCO) were used. Detection was performed using the ECL detection system (Amersham Biosciences). For GST pulldown assay, recombinant protein fused with GST and TLX-(1–186) was expressed from the pGEX-TLX-(1–186) construct in BL21(DE3) Escherichia coli cells. The bacterial lysate containing recombinant GST fusion protein that was prepared by sonication in B-PER II Bacterial Protein Extraction Reagent (Pierce), was purified with glutathione-Sepharose 4B (GE Healthcare). Recombinant Sox2 protein, prepared by TnT Quick Coupled Transcription/Translation Systems (Promega), was mixed with the GST-fusion protein/beads and rotated for 2 h at 4 °C. After washing the beads with Nonidet P-40 lysis buffer, the protein complex was eluted by Laemmli SDS sample buffer and subjected to Western immunoblotting with anti-Sox2 antibody as described above. To examine the effects of endogenous Sox2 interaction with TLX, mouse NSCs transfected with pEF-FLAG-TLX (FLAG-TLX) or pEF-FLAG (Mock) were harvested and lysed by using a Nuclear Complex Co-IP Kit (Active Motif). The nuclear extracts were immunoprecipitated with anti-FLAG antibody (Sigma) and subjected to Western blotting with anti-Sox2 (Cell Signaling Technology) according to the instructions. The gel-shift assay was performed with proteins prepared by the transfection of the same amount of mock vector, FLAG-tagged TLX, or Sox2 expression constructs into 293T cells, respectively. For titrations of TLX and Sox2, protein lysates were mixed at a protein ratio of 1:1 to 1:8, and incubated for 10 min at room temperature before separation in the native gel. The DNA probes for the gel-shift assay were annealed oligonucleotides with the following sequences: 5′-bio-CGG CCC GCA GCG ACA GGC ACA AAG TCA CAG GGT AAT GAA CTT CG-3′ and 5′-CGA AGT TCA TTA CCC TGT GAC TTT GTG CCT GTC GCT GCG GGC CG-3′. Detection was performed using a Lightshift chemiluminescence EMSA kit (Pierce).
RESULTS
Sox2 Binds to the Tlx 5′ UTR Chromatin and Activates the Tlx Promoter in Adult NSCs
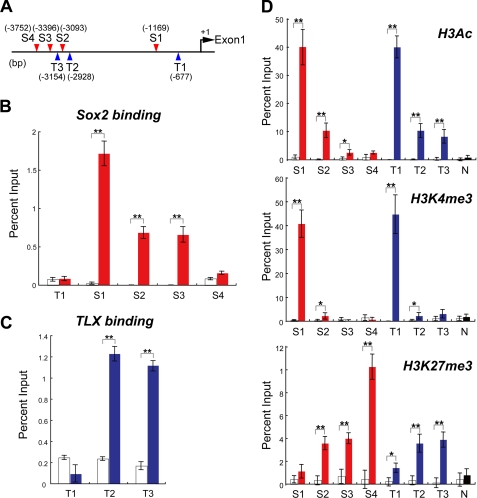
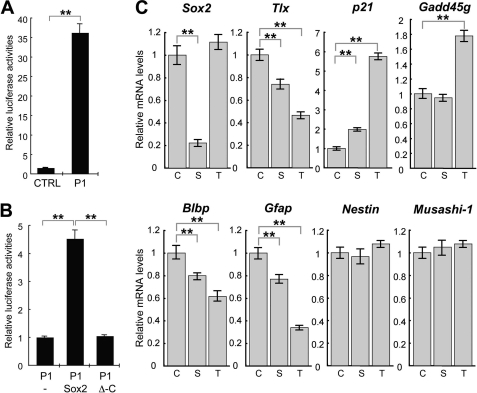
TLX is essential for maintenance of the undifferentiated and self-renewable state of NSCs in the adult brain (4, 17). To identify potential regulators of TLX, we analyzed the upstream proximal region of Tlx gene for transcription factor binding sites. We found several putative Sox2 binding consensus sequences (5′-(A/T)(A/T)CAA(A/T)G-3′) on both strands, named S1–S4, in the 4-kb upstream region of the Tlx gene. We also found TLX binding consensus sequences (5′-AAGTCA-3′) in the same 4-kb region, designated T1–T3 (Fig. 1A). To investigate whether Sox2 could directly regulate Tlx expression, chromatin immunoprecipitation (ChIP) assays were performed. As shown in Fig. 1B, the ChIP-qPCR analysis revealed that Sox2 significantly binds to the putative Sox2 binding site, S1. Sox2 also binds to the S2 and S3 sites, but not the S4 site or the T1 site that contains an imperfect Sox2 binding sequence, 5′-ACAAAG-3′. Next, we investigated TLX binding to T1–T3 sites by ChIP-qPCR. As shown in Fig. 1C, TLX binds T2 and T3, but not T1-putative TLX binding sites. Because TLX has been reported to act as a transcriptional repressor, we analyzed the chromatin status of the proximal promoter region of Tlx in adult NSCs (Fig. 1D). The chromatin status of T1, T2, and T3 regions or the S1–S4 region were analyzed by the ChIP-qPCR using an anti-acetyl-lysine histone H3 (AcH3) antibody, anti-trimethyl-lysine 4 histone H3 (H3K4me3), and anti-trimethyl-lysine 27 histone H3 (H3K27me3). The chromatin status of T1 and S1 regions was active based on highly enriched AcH3 or H3K4me3 signals. Interestingly, the T2 and T3 regions showed not only H3K27 trimethylation marks that suggest repressive functions, but also AcH3-positive chromatin status. On the other hand, the chromatin status of the S4 region is inactive based on H3K27me3-positive but not AcH3 and H3K4me3 ChIP-qPCR results. We next confirmed that the 5′-proximal region of the Tlx gene has promoter activity in rat AHPs, using a luciferase reporter assay (19) (Fig. 2A). Furthermore, Sox2 increased the Tlx promoter activity up to 5-fold in a C-terminal-dependent fashion (Fig. 2B). Collectively, we document that Sox2 bound to the Tlx promoter and contributed to the activation of regulatory elements of the Tlx locus. To investigate how Sox2 and TLX may control each other in adult NSCs, we constructed short hairpin RNA (shRNA) expression vectors that contained EGFP and the shRNA sequence of interest: Scrambled (CTRL), Tlx, and Sox2. Adult NSCs were transfected with these shRNA vectors, and flow cytometry was used to purify EGFP-positive cells. The EGFP-positive cells were analyzed for expression of Sox2, Tlx, and TLX-downstream genes, Sox2, Sox2, and a neural stem cell marker gene, Blbp, Gfap, Nestin, and Musashi-1 expression by qRT-PCR (Fig. 2C). Expression of Sox2 shRNA resulted in a 24.6 (±4)% decrease in TLX expression compared with CTRL shRNA in adult NSCs. Reduced expression of endogenous Sox2, Blbp, and Gfap and increased expression of p21 were observed in Sox2 shRNA-transfected adult NSCs after 72 h, but Gadd45g, Nestin, and Musashi-1 expression were not changed. Tlx shRNA led to the reduction of endogenous Tlx, Blbp, and Gfap and increased expression of TLX targets p21 and Gadd45g; however, it had no effect on Sox2, Nestin, and Musashi-1 mRNA levels. As shown in Fig. 2C, the decrease in Tlx expression by Sox2 knockdown is ∼25%, and these data suggest that expression of Tlx may require another transcriptional activator in addition to Sox2. Sox2 belongs to the SoxB1 subfamily, and they show overlapping biological functions in neural stem/progenitor cells (23, 24). We have confirmed the expression of Sox3 in the cultured adult NSCs by qRT-PCR (data not shown). Therefore, Tlx gene expression could potentially be controlled by the Sox gene subfamily, such as Sox3 in addition to Sox2.
FIGURE 1.
TLX is a direct target of Sox2 in adult NSCs. A, Sox2 (S1–S4; red triangles) and TLX/NR2E1 (T1–T3; blue triangles) consensus sites in the 5′ region of mouse Tlx gene. B, Sox2 binds to the 5′ region of mouse Tlx gene in adult NSCs. Sox2 binding was determined by ChIP-qPCR, performed with normal rabbit IgG (white bars) or Sox2 antibody (red bars). C, ChIP-qPCR analysis using anti-TLX antibody (blue bars) or normal mouse IgG (white bars) showed that TLX binds to T2 and T3 sites in the 5′ region of mouse Tlx gene in adult NSCs. D, the analysis of histone modifications at the 5′ region of mouse Tlx gene. ChIP-qPCR analysis of S1–S4 and T1–T3 sites with normal rabbit and mouse IgG (white bars), or anti-AcH3 (top graph), anti-H3K4me3 (middle), and anti-H3K27me3 (bottom) antibodies (red, blue, and black bars) in proliferating adult NSCs. For negative control, the p21 upstream region (top and middle graphs) and Actin (bottom) are shown as N in each graph. Data are means ±S.E. of value from four samples. *, p < 0.05; **, p < 0.01; t test and error bars show the standard deviation (±S.D.).
FIGURE 2.
Sox2 activates Tlx gene expression in adult NSCs. A, luciferase activity in AHP cells transfected with control luciferase reporter vector (CTRL), or luciferase reporter gene (P1) that was placed downstream of the Tlx promoter region (−1319 to −477), was measured and normalized with pCMV-RLuc. Assays were performed in triplicate, and the error bars indicate the standard deviation. B, the P1-luciferase construct (P1) was transfected with the pCAG mock vector for control (−), pCAG-Sox2 (Sox2), or pCAG-Sox2ΔC (Δ-C) into AHPs. Luciferase activity was measured as described above. C, adult NSCs were transfected with vectors for scrambled Control shRNA, Sox2 shRNA, or Tlx shRNA and cultured for 3 days. Transfected EGFP-positive cells were collected by flow cytometry and were analyzed by quantitative RT-PCR. Data are means ±S.E. of value from four samples. **, p < 0.01; t test and error bars represent ±S.D. shRNA treatments are denoted: C, control; S, Sox2; T, TLX.
TLX Represses Its Own Promoter and Is Antagonized by Sox2
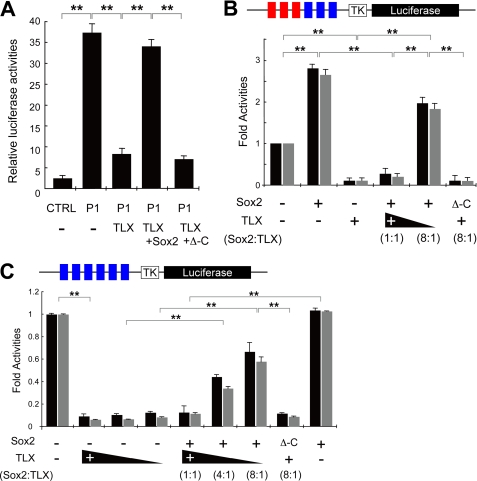
As shown in Fig. 1A, there are many putative TLX binding sites in the 5′-proximal region of the Tlx gene. TLX protein is detected on T2 and T3 sites in the Tlx promoter region in the proliferating adult NSCs (Fig. 1C). To test the influence of TLX on its own promoter in adult NSCs, the pCAG control vector or pCAG-TLX (TLX) expression vector was transfected into AHPs with a P1-luciferase vector, which contains the Tlx proximal promoter upstream of a firefly luciferase gene, along with a vector encoding a Renilla luciferase for normalization. As shown in Fig. 3A, TLX overexpression repressed the P1 promoter activity. When Sox2 was co-transfected with TLX, Sox2 antagonized the repressive activity of TLX. However, Sox2, which lacked a C-terminal domain, Sox2Δ-C (Δ-C), did not inhibit TLX activity (Fig. 3A). These data indicate that TLX could repress its own promoter activity and that Sox2 antagonizes the repression activity of TLX in a C-terminal domain-dependent manner.
FIGURE 3.
Sox2 is a molecular competitor of TLX with cis and trans effects. A, Sox2 functions as a positive regulator of transcription and antagonizes TLX suppressor activity in cis with C-terminal domain dependence in a reporter assay. The luciferase reporter P1 that contains both putative Sox2 and TLX binding consensus sequences (S1 and T1, respectively) were transfected into AHPs along with pCAG-TLX (TLX) or both TLX and Sox2 or both TLX and Δ-C. The firefly luciferase activity was measured and normalized to pTK-RLuc. Assays were performed in triplicate, and the error bars indicate the standard deviation. B, the luciferase reporter with three repeats of the putative Sox2 binding sequence (S1; red squares), and the putative TLX binding sequence (T1 or T2; blue squares) with the tk minimal promoter, was transfected into AHPs along with either Sox2 or TLX, or both Sox2 and TLX such that the molar ratio of Sox2 to TLX was 1:1 or 8:1. Note that the same amount of Sox2 expression construct was used in contrast to the variable amount of TLX expression constructs. Luciferase activity was measured as described above. The values represent the mean in black bars (T1) or gray bars (T2), and error bars indicate the standard deviation, with assays performed in triplicate. C, Sox2 antagonizes TLX suppressor activity in trans with C-terminal domain dependence in the artificial reporter assay. The luciferase reporter containing six repeats of the putative TLX binding sequence (T1 or T2; blue squares) with the tk minimal promoter, was transfected into AHPs along with a combination of indicated vectors (note the constant amount of Sox2 transgene and variable amount of Tlx transgene). Reporter luciferase activity was measured as described above. The values represent the mean in black bars (T1) or gray bars (T2), and error bars indicate the standard deviation, with assays performed in triplicate. A luciferase analysis with this reporter showed that the repression activity of Tlx transgene was lost in the presence of Sox2 transgene using both T1 (black bars) and T2 (gray bars) sequence reporters. **, p < 0.01, t test and error bars represent ±S.D.
Sox2 Functions as a Molecular Competitor of TLX Repression in cis and trans
To examine the molecular relationship between Sox2 and TLX in more detail, we prepared luciferase reporter constructs that contain a minimal tk promoter with multimerized Sox2 and TLX (3×ST-tk-luciferase; Fig. 3B) or TLX only (6×T-tk-luciferase; Fig. 3C) binding sites. As shown in Fig. 3B, Sox2 functioned as a cis activator, whereas TLX repressed the transcriptional activity of 3×ST-tk-luciferase in AHPs. When the same molar ratio of Sox2 and TLX expression constructs were transfected into AHPs, Sox2-mediated activation was canceled. A molar ratio of Sox2:TLX plasmids, at 8:1 or greater, was required to overcome TLX repressor activity with dependence on the C-terminal domain of Sox2 transgene (Fig. 3B: black bar, T1; gray bar, T2). To further characterize this Sox2-dependent activity on TLX, we constructed 6x T1 or T2-tk-luciferase. As shown in Fig. 3C, TLX repressed the reporter activity of 6×T-tk-luciferase even in the presence of low levels of the Tlx transgene. Interestingly, the repression activity of TLX was lost in a dose-dependent manner, in the presence of a constant amount of the Sox2 even though the reporter has no Sox2 binding site (Fig. 3C: black bar, T1; gray bar, T2). This activity also required the C-terminal domain of Sox2. These results indicate that Sox2 functions as a counterforce to TLX with respect to transcriptional activity in AHPs, and Sox2 can antagonize TLX without Sox2 binding sequences in the DNA.
Sox2 Interacts with TLX and Forms Complexes on the DNA without Sox2 Binding Sequence
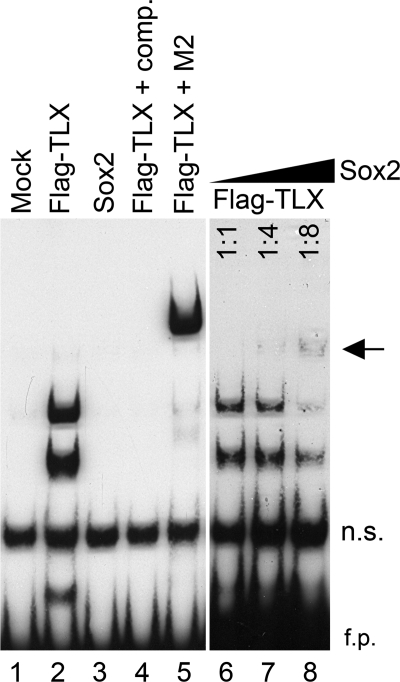
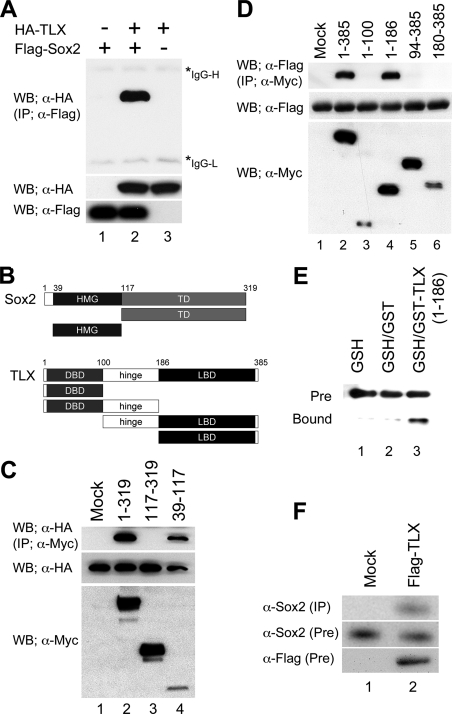
How is such derepression by Sox2 on the suppressor activity of TLX achieved without a Sox2 binding sequence? To clarify the molecular mechanism of this trans-inhibition of TLX by Sox2, we performed a gel-shift analysis with an oligonucleotide probe that contained a TLX binding consensus sequence. As shown in Fig. 4, FLAG-TLX, which could form homo-oligomers, but not Sox2, specifically bound to this probe (lanes 2 and 3). It is possible that there is also an interaction between Sox2 and TLX off the DNA probe during the gel-shift analysis, because a constant amount of FLAG-TLX protein and an increasing amount of Sox2 protein in the reaction mixture resulted in an increase of the formation of a Sox2·TLX·DNA complex as indicated with an arrow as well as a decrease in the TLX·DNA complexes (lanes 6–8, Fig. 4). Furthermore, to investigate these molecular mechanisms involved, we tested whether the interaction between Sox2 and TLX was direct. We first performed immunoprecipitation analysis by expressing HA-tagged TLX and/or FLAG-tagged Sox2 in 293T cells. When the lysates expressing both HA-TLX and FLAG-Sox2 were immunoprecipitated with an anti-FLAG antibody, a significant amount of HA-TLX protein was co-precipitated (lane 2, Fig. 5A). These data suggest a direct molecular interaction between Sox2 and TLX. Sox2 contains two major functional domains, an HMG and a C-terminal serine-rich domain. A hallmark function of the HMG domain is to bind the minor groove of the target DNA. The C-terminal serine-rich domain is able to activate target gene expression (25). TLX belongs to the so-called orphan nuclear receptor family, for which ligands have not yet been identified. TLX consists of a DNA-binding domain (DBD), a ligand-binding domain (LBD), and a hinge region between DBD and LBD (15). To map the interaction domain between Sox2 and TLX, a series of Sox2 and TLX deletion mutants was tagged with the Myc epitope (Fig. 5B). When the full length of HA-TLX was co-expressed with a series of Myc-tagged Sox2 deletion constructs, the TLX protein co-precipitated with the full-length (1–319; lane 2) and HMG domain of Sox2 (39–117; lane 4), but not the C terminus of Sox2 (117–319; lane 3) (Fig. 5C). The interaction between Sox2 and TLX was also confirmed using a series of Myc-tagged TLX deletion constructs and a FLAG-Sox2 construct. As shown in Fig. 5D, Sox2 binds to the full-length TLX (1–385; lane 2) and amino acids 1–186 (lane 4) that contain the DBD and hinge region of the TLX protein, but not the DBD protein alone or the hinge/LBD region of the protein (Fig. 5D). To further confirm this interaction, we conducted a GST pulldown assay using purified, bacterially expressed GST-TLX (amino acids 1–186) and in vitro translated Sox2 proteins. In these assays, Sox2 specifically associated with the N terminus region of TLX containing both the DBD and hinge regions (lane 3, Fig. 5E). We also show that overexpressed TLX protein formed a complex with endogenous Sox2 protein in the adult NSCs (lane 2, Fig. 5F). Taken together, these data suggest that Sox2 interferes with the TLX repressor function through direct protein-protein interaction.
FIGURE 4.
Sox2 and TLX form a complex on DNA. Gel-shift analyses were performed with the biotin-labeled T1 region using whole cell extracts prepared from 293T cells in which the FLAG-tagged TLX, Sox2 expression vectors were individually introduced (lanes 2 and 3), in the presence of a 50-fold molar excess of the competitor (lane 4) or in the presence of anti-FLAG M2 monoclonal antibody (lane 5). FLAG-TLX proteins prepared from transfected 293T cell lysates were incubated with variable amounts of Sox2 proteins at a ratio of 1:1 to 1:8 (lanes 6–8). The amount of Sox2 protein of lanes 3 and 8 in the reaction was the same. n.s., nonspecific binding proteins; f.p., free probes.
FIGURE 5.
Sox2 and TLX physically interact through the HMG domain of Sox2 and the DBD-hinge region of TLX, with Sox2 binding to the DNA through TLX interacting complexes. A, FLAG-tagged Sox2 and HA-tagged TLX proteins were transiently expressed in 293T cells. Equal amounts of total extracts were immunoprecipitated with anti-FLAG antibody. Co-immunoprecipitated TLX was detected by an anti-HA immunoblot (top panel). The expression of HA-TLX and FLAG-Sox2 proteins in total cell lysates was determined by anti-HA (middle) or anti-FLAG (bottom) immunoblots, respectively. B, schematic diagrams of Sox2 and TLX. Sox2 contains the high-mobility group DNA-binding domain (HMG), and C-terminal transactivation domain (TD). TLX contains the N-terminal DNA binding domain (DBD), hinge region, and C-terminal ligand binding domain (LBD). C, N- or C-terminal truncated Myc-Sox2 and HA-TLX proteins were transiently expressed in 293T cells. Equal amounts of total extracts were immunoprecipitated with anti-Myc antibody. Co-immunoprecipitated TLX was detected by an anti-HA immunoblot (top panel). The expression of Myc-Sox2 and HA-TLX proteins in total cell lysates was determined by anti-HA (middle) or anti-Myc (bottom) immunoblots, respectively. D, N- or C-terminal truncated Myc-TLX and FLAG-Sox2 were transiently expressed in 293T cells. Equal amounts of total extracts were immunoprecipitated with anti-Myc antibody. Co-immunoprecipitated Sox2 was detected by an anti-FLAG immunoblot (top panel). The expression of Myc-TLX and FLAG-Sox2 proteins in total cell lysates was determined by anti-FLAG (middle) or anti-Myc (bottom) immunoblots. E, physical association between Sox2 and TLX protein in vitro. Bacterially expressed GST or GST-TLX-(1–186) and in vitro translated Sox2 protein were used in pulldown assays. Co-precipitated Sox2 proteins were detected by an anti-Sox2 immunoblot (bottom). F, interaction of endogenous Sox2 protein with TLX. FLAG-tagged TLX protein was transiently expressed in adult NSCs. Equal amounts of nuclear extracts were immunoprecipitated with anti-FLAG antibody. Co-immunoprecipitated Sox2 was detected with anti-Sox2 antibody (top panel). The expression of Sox2 and FLAG-TLX proteins in nuclear extracts was determined by anti-Sox2 (middle) or anti-FLAG (bottom) immunoblots.
Sox2 Interferes with TLX-mediated Self-repression on Its Own Promoter in Adult NSCs
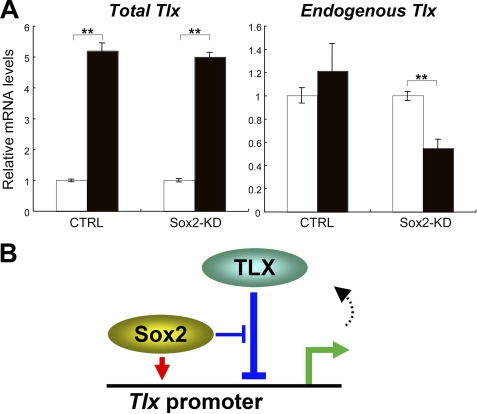
Several observations in our study suggested that TLX-mediated transcriptional self-repression is controlled by Sox2. To assess the molecular system in endogenous adult NSCs, we performed transient overexpression of TLX in adult NSCs. As shown in Fig. 6A, transient TLX expression increases total TLX mRNA levels up to 5-fold, but endogenous expression of TLX was not affected. These data suggest that TLX is already at saturating levels in adult NSCs. However, depletion of Sox2 by gene-specific knockdown reduced the expression level of endogenous Tlx when exogenous TLX was overexpressed transiently in adult NSCs (Fig. 6A). Collectively, our data indicate that TLX is part of a negative feedback loop for its own expression, and this loop is antagonized through interactions with Sox2 in addition to activation of Tlx gene expression by Sox2 in adult NSCs (Fig. 6B).
FIGURE 6.
Sox2 is involved in the negative feedback system of TLX in adult NSCs. A, adult NSCs were transfected with vectors that express the scramble sequence of shRNA (CTRL), or Sox2 shRNA (Sox2-KD), and with mock (white bars) or TLX expression vectors (black bars). Cells were cultured for 3 days, and transfected EGFP-positive cells were collected by flow cytometry and were analyzed by quantitative RT-PCR. Data are means ± S.E. of value from four samples. **, p < 0.01, t test and error bars represent ±S.D. B, a molecular model of the relationship between Sox2 and TLX. Sox2 activates Tlx transcription. TLX can suppress its own promoter activity, but Sox2 antagonizes such repression in a dose-dependent manner.
DISCUSSION
Sox2 is known to be a self-renewal gene, similar to Oct3/4 and Nanog, and plays a pivotal role in maintaining the stemness of embryonic stem cells (6). Sox2 also plays an essential role in the maintenance of NSCs (23, 26). Sox2 deficiency causes impaired neurogenesis in the adult mouse brain, particularly in the hippocampus (27). TLX has also been reported to function in the maintenance of NSCs in the adult brain. The orphan nuclear receptor tailless, TLX, also called NR2E1, is expressed in astrocyte-like B cells in the subventricular zone and in the subgranular zone of the hippocampus in the adult mouse brain (4, 18). The inactivation of the Tlx gene in adult brains leads to loss of the self-renewal ability of adult NSCs (4, 17, 18), suggesting that TLX is a key regulator of NSC maintenance. Here, we show that Sox2 is an upstream transcription factor of TLX in adult NSCs. Sox2 predominantly functions as a transcriptional activator, and the C-terminal domain is thought to interact with a co-activator, such as CBP/p300 (28–30). We show that the Sox2 protein binds upstream from the Tlx/Nr2e1 gene locus and activates TLX proximal promoter activity. Although knockdown of Sox2 led to a moderate reduction of Tlx expression in adult NSCs as shown in Fig. 2C, Sox2 and TLX are required autonomously in NSCs (11, 31). To examine whether Sox2 controls the proliferation of adult NSCs through the TLX pathway, we transfected shRNA vectors for Sox2 knockdown into mouse adult NSCs that continuously express the exogenous Tlx gene, and measured the cell proliferation by using 5-bromodeoxyuridine (BrdU) labeling and immunostaining with an antibody for phospho-histone H3 (Ser-10), which is a marker for the cell proliferation of dividing cells. Expression of Sox2 shRNA significantly decreased BrdU incorporation and phosho-histone H3 detection compared with the control vector, and exogenous TLX co-expression did not rescue this defect (supplemental Fig. S1). These data indicate that Sox2 plays an important role in Tlx transcription within cultured adult NSCs, but Sox2 may also be part of a TLX-independent pathway involved in regulation of self-renewal in adult NSCs. Interestingly, even though Sox2 regulates Nestin gene expression (32, 33) and controls Nestin-positive NSCs (27), Nestin and Musashi-1 mRNAs were not reduced by Sox2 knockdown within 72 h after shRNA vector transfection (Fig. 2C). In addition we have observed only ∼25% decrease in Tlx mRNA levels upon Sox2 knockdown. These observations could be due to a number of reasons. The delay with which one would expect not only mRNA but also the Sox2 protein levels to decrease can lead to the somewhat subtle effects of Sox2 knockdown in our system. Furthermore, other Sox family members such as Sox1 and Sox3 could contribute to functional redundancy among SoxB1 members (23, 24), and Sox partner proteins such as Brn1, Brn2, or Pax6 could determine the set of regulatory target genes for activation in neural cell fate specification (25). It has been reported that Sox3 expression is elevated in Sox2 mutant cells in the developing brains and embryonic neurosphere cells (34). In addition, besides Sox2, Sox3 could also contribute to Nestin gene expression (33). A recent study has shown that ∼96% overlap exists between Sox2 and Sox3 target loci (35). We have confirmed Sox3 expression in the cultured adult NSCs (data not shown). Taken together with these observations and scientific reports, it is highly probable that the timing of knockdown/analysis and involvement of other SoxB1 proteins contribute to our findings. Again, Sox2 has relatively broader expression than TLX, including differentiated astrocytes where TLX is not expressed. In addition to the need for another activator for TLX expression, it is likely that epigenetic regulation of the Tlx/Nr2e1 gene locus could be involved in regulating TLX transcription. Further research on Tlx regulation is required to address these questions.
TLX functions as a transcriptional repressor through interaction with other co-repressors. TLX interacts with atrophin1, HDAC3/4/5, and LSD1 (36–38). These proteins and TLX form complexes that negatively regulate p21 and phosphatase and tensin homologue deleted on chromosome 10 (PTEN) expression to control the cell-cycle engine and inhibit differentiation of NSCs to maintain stemness. Recently, other functions of TLX have also been reported. TLX directly activates the Ascl1/Mash1 promoter through interaction with Sp1, recruiting co-activators, but dismissing the co-repressor HDAC4 (39). It is suggested that TLX not only acts as a repressor of cell cycle and glial differentiation, but also activates neuronal lineage commitment in adult NSCs. We show that Sox2 antagonizes TLX suppressor activity by cis- and trans- mechanisms, and inhibits TLX function in a C-terminal domain-dependent manner in reporter assays. In addition, depletion of Sox2 revealed TLX-mediated self-repression of its own endogenous mRNA production in adult NSCs as shown in Fig. 6A. Our model proposes a negative feedback loop of TLX transcription by TLX and the regulation of TLX by Sox2 through direct protein-protein interaction between them (Fig. 6B). However, as shown in Fig. 1 (C and D), TLX protein binds to its own promoter, and these regions have a poised chromatin status in adult NSCs. So, it is possible that Sox2 is involved in keeping the Tlx locus poised and not necessarily strongly activated for expression. Furthermore, the TLX regulation by Sox2 may also require other factors, because Tlx promoter construct (P1) is more sensitive to Sox2 levels than the multimerized reporter as shown in Fig. 3 (A and B). Sox2 may regulate such molecular switching, but the biological role of Sox2 on TLX binding sites for derepression of TLX activity remains to be elucidated.
The hippocampal developmental defects in Sox2 loss-of-function mutants resemble those caused by late Shh loss (40). Sox2-deleted NSCs did not express Shh in vitro (13), and it is suggested that Shh is controlled, at least partly, through a Sox2-dependent autocrine mechanism. A recent study has revealed that Gli2, a key mediator of Shh signaling, functions as a regulator of Sox2 expression in telencephalic neuroepithelial cells (14). Thus, the endogenous co-regulatory loop between Shh and Sox2 is an essential regulatory component for stemness in adult NSCs. A chemical inhibitor of Shh signaling, cyclopamine, can block the growth of gliomas (41), suggesting that Shh-Gli-Sox2 protein signaling is involved in the formation of both NSCs and glioma-initiating cells (glioma stem cells) of malignant brain tumors. The knockdown of Sox2 resulted in decreased tumorigenic activity of glioma stem cells (42); however, direct target genes of Sox2 that may be involved in brain tumor formation have not been identified. It has been reported that NSC-specific overexpression of TLX using bacterial artificial chromosome-based technology is sufficient to induce NSC expansion and glioma-like lesions, containing glioma stem cells in the adult mouse brain (43). In this report, we propose a novel transcriptional cascade, Sox2-TLX, which maintains the undifferentiated state of adult NSCs. Thus, the biological relevance of self-repression of TLX expression may be to prevent uncontrolled growth by limiting the protein level of TLX in adult NSCs. The similarity of NSCs and glioma stem cells suggests that they may use similar machinery for their maintenance. Thus it will be of great interest to investigate whether the molecular interplay between Sox2 and TLX plays an important part in inducing gliomas from NSCs. Controlling both Sox2 and TLX expression by specific antisense oligonucleotides or RNA interference may help develop novel approaches toward targeted brain tumor therapy. Furthermore, other HMG domain proteins and nuclear hormone receptors may interact as well. This interplay may be involved in a number of developmental, adult stem cell, and neoplastic processes.
Supplementary Material
Acknowledgments
We thank S. Christenson for secretarial assistance; J. Ray, L. Moore, B. Miller, D. Sepp, and Y. Hamaguchi for technical help; and T. Kondo, I. Nobuhisa, K. Nakashima, and Y. Soda for technical advice.
This work was supported, in whole or in part, by National Institutes of Health Grant NIMH-MH090258. This work was also supported by the McDonnell Foundation, the Mothers Foundation, and the Lookout Foundation, a Grant-in-Aid from the Japan Society for the Promotion of Science, a Grant-in-Aid for Research Activity Start-up from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, and the Uehara Memorial Foundation of Japan.

This article contains supplemental Fig. S1 and Table S1.
- NSC
- neural stem cell
- Sox2
- SRY-box-containing gene 2
- HMG
- high-mobility group
- Shh
- sonic hedgehog
- TLX
- nuclear receptor tailless
- AHP
- adult hippocampus neural stem/progenitor cell
- AcH3
- anti-acetyl-lysine histone H3
- H3K27me3
- anti-trimethyl-lysine 27 histone H3
- H3K4me3
- anti-trimethyl-lysine 4 histone H3
- DBD
- DNA-binding domain
- LBD
- ligand-binding domain
- CTRL
- control vector.
REFERENCES
- 1. Gage F. H. (2000) Mammalian neural stem cells. Science 287, 1433–1438 [DOI] [PubMed] [Google Scholar]
- 2. Alvarez-Buylla A., Lim D. A. (2004) For the long run. Maintaining germinal niches in the adult brain. Neuron 41, 683–686 [DOI] [PubMed] [Google Scholar]
- 3. Cavallaro M., Mariani J., Lancini C., Latorre E., Caccia R., Gullo F., Valotta M., DeBiasi S., Spinardi L., Ronchi A., Wanke E., Brunelli S., Favaro R., Ottolenghi S., Nicolis S. K. (2008) Impaired generation of mature neurons by neural stem cells from hypomorphic Sox2 mutants. Development 135, 541–557 [DOI] [PubMed] [Google Scholar]
- 4. Shi Y., Chichung Lie D., Taupin P., Nakashima K., Ray J., Yu R. T., Gage F. H., Evans R. M. (2004) Expression and function of orphan nuclear receptor TLX in adult neural stem cells. Nature 427, 78–83 [DOI] [PubMed] [Google Scholar]
- 5. Avilion A. A., Nicolis S. K., Pevny L. H., Perez L., Vivian N., Lovell-Badge R. (2003) Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 17, 126–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Masui S., Nakatake Y., Toyooka Y., Shimosato D., Yagi R., Takahashi K., Okochi H., Okuda A., Matoba R., Sharov A. A., Ko M. S., Niwa H. (2007) Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat Cell Biol. 9, 625–635 [DOI] [PubMed] [Google Scholar]
- 7. Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 [DOI] [PubMed] [Google Scholar]
- 8. Takahashi K., Yamanaka S. (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 [DOI] [PubMed] [Google Scholar]
- 9. Catena R., Tiveron C., Ronchi A., Porta S., Ferri A., Tatangelo L., Cavallaro M., Favaro R., Ottolenghi S., Reinbold R., Schöler H., Nicolis S. K. (2004) Conserved POU binding DNA sites in the Sox2 upstream enhancer regulate gene expression in embryonic and neural stem cells. J. Biol. Chem. 279, 41846–41857 [DOI] [PubMed] [Google Scholar]
- 10. Suh H., Consiglio A., Ray J., Sawai T., D'Amour K. A., Gage F. H. (2007) In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell 1, 515–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pevny L. H., Nicolis S. K. (2010) Sox2 roles in neural stem cells. Int. J. Biochem. Cell Biol. 42, 421–424 [DOI] [PubMed] [Google Scholar]
- 12. Hu Q., Zhang L., Wen J., Wang S., Li M., Feng R., Yang X., Li L. (2010) The EGF receptor-sox2-EGF receptor feedback loop positively regulates the self-renewal of neural precursor cells. Stem Cells 28, 279–286 [DOI] [PubMed] [Google Scholar]
- 13. Favaro R., Valotta M., Ferri A. L., Latorre E., Mariani J., Giachino C., Lancini C., Tosetti V., Ottolenghi S., Taylor V., Nicolis S. K. (2009) Hippocampal development and neural stem cell maintenance require Sox2-dependent regulation of Shh. Nat. Neurosci. 12, 1248–1256 [DOI] [PubMed] [Google Scholar]
- 14. Takanaga H., Tsuchida-Straeten N., Nishide K., Watanabe A., Aburatani H., Kondo T. (2009) Gli2 is a novel regulator of sox2 expression in telencephalic neuroepithelial cells. Stem Cells 27, 165–174 [DOI] [PubMed] [Google Scholar]
- 15. Yu R. T., McKeown M., Evans R. M., Umesono K. (1994) Relationship between Drosophila gap gene tailless and a vertebrate nuclear receptor Tlx. Nature 370, 375–379 [DOI] [PubMed] [Google Scholar]
- 16. Monaghan A. P., Bock D., Gass P., Schwäger A., Wolfer D. P., Lipp H. P., Schütz G. (1997) Defective limbic system in mice lacking the tailless gene. Nature 390, 515–517 [DOI] [PubMed] [Google Scholar]
- 17. Zhang C. L., Zou Y., He W., Gage F. H., Evans R. M. (2008) A role for adult TLX-positive neural stem cells in learning and behaviour. Nature 451, 1004–1007 [DOI] [PubMed] [Google Scholar]
- 18. Liu H. K., Belz T., Bock D., Takacs A., Wu H., Lichter P., Chai M., Schütz G. (2008) The nuclear receptor tailless is required for neurogenesis in the adult subventricular zone. Genes Dev. 22, 2473–2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ray J., Gage F. H. (2006) Differential properties of adult rat and mouse brain-derived neural stem/progenitor cells. Mol. Cell Neurosci. 31, 560–573 [DOI] [PubMed] [Google Scholar]
- 20. Ray J., Peterson D. A., Schinstine M., Gage F. H. (1993) Proliferation, differentiation, and long-term culture of primary hippocampal neurons. Proc. Natl. Acad. Sci. U.S.A. 90, 3602–3606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Palmer T. D., Ray J., Gage F. H. (1995) FGF-2-responsive neuronal progenitors reside in proliferative and quiescent regions of the adult rodent brain. Mol. Cell Neurosci. 6, 474–486 [DOI] [PubMed] [Google Scholar]
- 22. Shimozaki K., Namihira M., Nakashima K., Taga T. (2005) Stage- and site-specific DNA demethylation during neural cell development from embryonic stem cells. J. Neurochem. 93, 432–439 [DOI] [PubMed] [Google Scholar]
- 23. Graham V., Khudyakov J., Ellis P., Pevny L. (2003) SOX2 functions to maintain neural progenitor identity. Neuron 39, 749–765 [DOI] [PubMed] [Google Scholar]
- 24. Taranova O. V., Magness S. T., Fagan B. M., Wu Y., Surzenko N., Hutton S. R., Pevny L. H. (2006) SOX2 is a dose-dependent regulator of retinal neural progenitor competence. Genes Dev. 20, 1187–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kondoh H., Kamachi Y. (2010) SOX-partner code for cell specification. Regulatory target selection and underlying molecular mechanisms. Int. J. Biochem. Cell Biol. 42, 391–399 [DOI] [PubMed] [Google Scholar]
- 26. Episkopou V. (2005) SOX2 functions in adult neural stem cells. Trends Neurosci. 28, 219–221 [DOI] [PubMed] [Google Scholar]
- 27. Ferri A. L., Cavallaro M., Braida D., Di Cristofano A., Canta A., Vezzani A., Ottolenghi S., Pandolfi P. P., Sala M., DeBiasi S., Nicolis S. K. (2004) Sox2 deficiency causes neurodegeneration and impaired neurogenesis in the adult mouse brain. Development 131, 3805–3819 [DOI] [PubMed] [Google Scholar]
- 28. Nowling T. K., Johnson L. R., Wiebe M. S., Rizzino A. (2000) Identification of the transactivation domain of the transcription factor Sox-2 and an associated co-activator. J. Biol. Chem. 275, 3810–3818 [DOI] [PubMed] [Google Scholar]
- 29. Bylund M., Andersson E., Novitch B. G., Muhr J. (2003) Vertebrate neurogenesis is counteracted by Sox1–3 activity. Nat. Neurosci. 6, 1162–1168 [DOI] [PubMed] [Google Scholar]
- 30. Nowling T., Bernadt C., Johnson L., Desler M., Rizzino A. (2003) The co-activator p300 associates physically with and can mediate the action of the distal enhancer of the FGF-4 gene. J. Biol. Chem. 278, 13696–13705 [DOI] [PubMed] [Google Scholar]
- 31. Shi Y., Sun G., Zhao C., Stewart R. (2008) Neural stem cell self-renewal. Crit. Rev. Oncol. Hematol. 65, 43–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Josephson R., Müller T., Pickel J., Okabe S., Reynolds K., Turner P. A., Zimmer A., McKay R. D. (1998) POU transcription factors control expression of CNS stem cell-specific genes. Development 125, 3087–3100 [DOI] [PubMed] [Google Scholar]
- 33. Tanaka S., Kamachi Y., Tanouchi A., Hamada H., Jing N., Kondoh H. (2004) Interplay of SOX and POU factors in regulation of the Nestin gene in neural primordial cells. Mol. Cell. Biol. 24, 8834–8846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Miyagi S., Masui S., Niwa H., Saito T., Shimazaki T., Okano H., Nishimoto M., Muramatsu M., Iwama A., Okuda A. (2008) Consequence of the loss of Sox2 in the developing brain of the mouse. FEBS Lett. 582, 2811–2815 [DOI] [PubMed] [Google Scholar]
- 35. Bergsland M., Ramsköld D., Zaouter C., Klum S., Sandberg R., Muhr J. (2011) Sequentially acting Sox transcription factors in neural lineage development. Genes Dev. 25, 2453–2464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang C. L., Zou Y., Yu R. T., Gage F. H., Evans R. M. (2006) Nuclear receptor TLX prevents retinal dystrophy and recruits the corepressor atrophin1. Genes Dev. 20, 1308–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sun G., Yu R. T., Evans R. M., Shi Y. (2007) Orphan nuclear receptor TLX recruits histone deacetylases to repress transcription and regulate neural stem cell proliferation. Proc. Natl. Acad. Sci. U.S.A. 104, 15282–15287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yokoyama A., Takezawa S., Schüle R., Kitagawa H., Kato S. (2008) Transrepressive function of TLX requires the histone demethylase LSD1. Mol. Cell. Biol. 28, 3995–4003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Elmi M., Matsumoto Y., Zeng Z. J., Lakshminarasimhan P., Yang W., Uemura A., Nishikawa S., Moshiri A., Tajima N., Agren H., Funa K. (2010) TLX activates MASH1 for induction of neuronal lineage commitment of adult hippocampal neuroprogenitors. Mol. Cell Neurosci. 45, 121–131 [DOI] [PubMed] [Google Scholar]
- 40. Han Y. G., Spassky N., Romaguera-Ros M., Garcia-Verdugo J. M., Aguilar A., Schneider-Maunoury S., Alvarez-Buylla A. (2008) Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat. Neurosci. 11, 277–284 [DOI] [PubMed] [Google Scholar]
- 41. Clement V., Sanchez P., de Tribolet N., Radovanovic I., Ruiz i Altaba A. (2007) HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr. Biol. 17, 165–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gangemi R. M., Griffero F., Marubbi D., Perera M., Capra M. C., Malatesta P., Ravetti G. L., Zona G. L., Daga A., Corte G. (2009) SOX2 silencing in glioblastoma tumor-initiating cells causes stop of proliferation and loss of tumorigenicity. Stem Cells 27, 40–48 [DOI] [PubMed] [Google Scholar]
- 43. Liu H. K., Wang Y., Belz T., Bock D., Takacs A., Radlwimmer B., Barbus S., Reifenberger G., Lichter P., Schütz G. (2010) The nuclear receptor tailless induces long-term neural stem cell expansion and brain tumor initiation. Genes Dev. 24, 683–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.