Background: We determined how nerve injury affects calcium channel β (Cavβ) subunits in neuropathic pain.
Results: Nerve injury increases Cavβ3 expression level, and Cavβ3 knockdown reduces increased calcium channel activity in primary sensory neurons and pain hypersensitivity.
Conclusion: Cavβ3 subunit up-regulation augments calcium channel activity in neuropathic pain.
Significance: Understanding molecular changes in sensory neurons is important for developing new treatments for neuropathic pain.
Keywords: Nerve, Neurochemistry, Neurological Diseases, Neuroscience, Neurotransmitter Release, Synaptic Plasticity
Abstract
High voltage-activated calcium channels (HVACCs) are essential for synaptic and nociceptive transmission. Although blocking HVACCs can effectively reduce pain, this treatment strategy is associated with intolerable adverse effects. Neuronal HVACCs are typically composed of α1, β (Cavβ), and α2δ subunits. The Cavβ subunit plays a crucial role in the membrane expression and gating properties of the pore-forming α1 subunit. However, little is known about how nerve injury affects the expression and function of Cavβ subunits in primary sensory neurons. In this study, we found that Cavβ3 and Cavβ4 are the most prominent subtypes expressed in the rat dorsal root ganglion (DRG) and dorsal spinal cord. Spinal nerve ligation (SNL) in rats significantly increased mRNA and protein levels of the Cavβ3, but not Cavβ4, subunit in the DRG. SNL also significantly increased HVACC currents in small DRG neurons and monosynaptic excitatory postsynaptic currents of spinal dorsal horn neurons evoked from the dorsal root. Intrathecal injection of Cavβ3-specific siRNA significantly reduced HVACC currents in small DRG neurons and the amplitude of monosynaptic excitatory postsynaptic currents of dorsal horn neurons in SNL rats. Furthermore, intrathecal treatment with Cavβ3-specific siRNA normalized mechanical hyperalgesia and tactile allodynia caused by SNL but had no significant effect on the normal nociceptive threshold. Our findings provide novel evidence that increased expression of the Cavβ3 subunit augments HVACC activity in primary sensory neurons and nociceptive input to dorsal horn neurons in neuropathic pain. Targeting the Cavβ3 subunit at the spinal level represents an effective strategy for treating neuropathic pain.
Introduction
Chronic pain, such as neuropathic pain induced by peripheral nerve injury, leads to prolonged excruciating suffering and a reduced quality of life. However, effective treatment of chronic pain remains a major challenge, with only half of patients receiving adequate pain relief (1). Pain can occur spontaneously or as a result of exposure to mildly painful stimuli (hyperalgesia) or stimuli not normally perceived as painful (allodynia). Our understanding of the mechanisms that sustain chronic neuropathic pain conditions is incomplete. It is likely that neuropathic pain becomes chronic as a result of the plasticity of both peripheral nerves and spinal dorsal horn neurons. Clinical studies of patients with neuropathic pain have indicated that the altered central processing associated with pain is maintained dynamically by ongoing peripheral input (2, 3). However, the molecular mechanisms underlying persistent nociceptive input from primary afferent nerves in neuropathic pain are not fully known.
High voltage-activated calcium channels (HVACCs)2 are essential for many physiological functions, such as neurotransmitter release, membrane excitability, gene transcription, and synaptic plasticity (4, 5). HVACCs in neurons are typically composed of a pore-forming α1 subunit (the principal component of HVACCs) and accessory β (Cavβ), α2δ, and possibly γ subunits (5, 6). HVACCs are clarified as the L (Cav1.1–1.4)-type, N (Cav2.2)-type, P/Q (Cav2.1)-type, and R (Cav2.3)-type according to the different biophysical and pharmacological properties of their α1 subunits (5, 7). The α2δ subunit can augment functional expression of the α1 subunit (8) and is up-regulated in the dorsal root ganglion (DRG) after nerve injury (9). Another important auxiliary subunit, the Cavβ subunit, is involved in promoting the surface expression of the α1 subunit and the gating properties of HVACCs (10–12). There are four Cavβ subunits, each with splice variants, encoded by four distinct genes. The Cavβ1 subunit appears to be the only Cavβ subunit expressed in the skeletal muscle (13), whereas the Cavβ2 subunit is present primarily in the heart (14). The Cavβ3 subunit is strongly expressed in smooth muscle and brain, and the Cavβ4 is the predominant subunit in cerebellum (14, 15). Mice deficient in Cavβ1 or Cavβ2 are not viable (16, 17), and knock-out of Cavβ3 or Cavβ4 results in a wide range of abnormalities (18, 19). Despite the importance of Cavβ subunits in regulating HVACC activity, little is known about the contribution of Cavβ subunits to nerve injury-induced changes in HVACC activity in primary sensory neurons and neuropathic pain.
Therefore, we used a rat model of neuropathic pain to determine how nerve injury affects the expression level of Cavβ subunits in the DRG and dorsal spinal cord. We also determined the contribution of the Cavβ3 subunit to increased HVACC activity in DRG neurons and nociceptive input to the spinal cord induced by nerve injury. Our findings indicate that among the four Cavβ subunits in the DRG and spinal cord, Cavβ3 and Cavβ4 showed the greatest expression. Nerve injury up-regulated the Cavβ3, but not Cavβ4, subunit in the DRG. Furthermore, siRNA knockdown of Cavβ3 greatly reduced increased HVACC activity in the DRG, glutamatergic input to the spinal cord, and pain hypersensitivity caused by nerve injury without impairing normal nociception. Our study highlights the important role of the Cavβ3 subunit in increased HVACC activity in primary sensory neurons in neuropathic pain. Therefore, targeting the Cavβ3 subunit at the spinal level represents a new strategy for treating neuropathic pain with improved therapeutic profiles.
EXPERIMENTAL PROCEDURES
Neuropathic Pain Model and Intrathecal Cannulation
Neuropathic pain was induced by SNL in rats, as described previously (20, 21). In brief, male Sprague-Dawley rats initially weighing ∼250 g (Harlan, Indianapolis, IN) were anesthetized with use of 2% isoflurane. The left L5 and L6 spinal nerves were isolated under a surgical microscope and ligated with 4.0 silk suture. Sham surgery was performed in the contralateral side of the same rats or separate rats by using the same procedure except that the spinal nerves were not ligated. In some rats, intrathecal cannulation was performed 7 days after SNL. After rats were anesthetized with isoflurane, catheters (PE-10 polyethylene tubing, 8 cm) were inserted through an incision in the cisternal membrane and advanced caudally so that the tip of each catheter was positioned at the lumbar spinal level (22, 23). The catheters were externalized to the back of the neck and sutured to the musculature and skin at the incision site. All experiments were approved by the Animal Care and Use Committee of the University of Texas MD Anderson Cancer Center and conformed to National Institutes of Health guidelines on the ethical use of animals.
Nociceptive Behavioral Tests
To assess mechanical nociception, paw withdrawal thresholds were measured by applying a noxious pressure stimulus using an Ugo Basil Analgesimeter (Varese, Italy). The nociceptive threshold was recorded when rats displayed pain by paw withdrawal or vocalization. The cutoff of 400 g was used to avoid potential tissue injury (22).
The tactile withdrawal threshold was measured by application of von Frey filaments to both hind paws of SNL rats. Rats were placed individually in plastic cages on a mesh floor and then allowed to acclimate for at least 30 min. A series of calibrated von Frey filaments (Stoelting Co., Wood Dale, IL) were applied perpendicularly to the plantar surface of the hind paw. Brisk withdrawal or paw flinching was considered a positive response. The tactile stimulus that could produce a 50% likelihood of withdrawal was determined by using the “up-down” calculating method (21, 24).
Isolation of DRG Neurons and Electrophysiological Recording of HVACCs
Dissociation of DRG neurons was performed essentially as we described previously (25, 26). Rats were anesthetized with isoflurane and then rapidly decapitated. The lumbar segment of the vertebral column was removed quickly. The L5 and L6 DRGs were obtained and transferred into cold DMEM (Invitrogen). After DRGs were minced, they were placed into 5 ml of DMEM containing trypsin (type I, 0.2 mg/ml; Sigma) and collagenase (type I, 1 mg/ml; Sigma). After incubation at 34 °C for 40 min, 10% FBS was used to stop trypsin digestion. Neurons were plated onto a 35-mm culture dish containing poly-l-lysine (50 μg/ml) precoated coverslips. The cells were incubated in fresh medium for at least another hour before they were used for electrophysiological recording of HVACC activity.
HVACC currents were measured using barium as the charge carrier (IBa) (25, 26). Electrodes with a resistance of 2–3 megohms were pulled from glass capillaries and fire-polished. After establishing the whole-cell configuration, the cell membrane capacitance and series resistance were electronically compensated. Signals were processed with use of an EPC-10 amplifier (HEKA Instruments, Lambrecht, Germany), filtered at 1 kHz, digitized at 10 kHz, and acquired by using the Pulse program (HEKA Instruments). The extracellular solution consisted of 140 mm tetraethylammonium, 2 mm MgCl2, 3 mm BaCl2, 10 mm glucose, and 10 mm HEPES (pH 7.4, osmolarity of 320 mosm). The pipette internal solution consisted of 120 mm CsCl, 1 mm MgCl2, 10 mm HEPES, 10 mm EGTA, 4 mm MgATP, and 0.3 mm NaGTP (pH 7.2, osmolarity of 300 mosm). The voltage-dependent activation of HVACCs was tested at a holding potential of −90 mV and elicited by a series of command potentials from −70 to 50 mV for 150 ms in 10-mV steps (5-s intervals). The voltage-dependent inactivation of HVACCs was assessed by depolarizing cells to a series of prepulse potentials from −90 to 10 mV for 500 ms, followed by a command potential to 0 for 150 ms (25, 26). All experiments were performed at room temperature (∼25 °C).
Spinal Cord Slice Preparation and Electrophysiological Recording of Synaptic Activity
After rats were anesthetized with 2–3% isoflurane, the lumbar segment of the spinal cord at the L5/L6 level was removed through laminectomy. The spinal cord tissue was immediately placed in ice-cold sucrose artificial cerebrospinal fluid presaturated with 95% O2 and 5% CO2. The sucrose artificial cerebrospinal fluid contained 234 mm sucrose, 3.6 mm KCl, 1.2 mm MgCl2, 2.5 mm CaCl2, 1.2 mm NaH2PO4, 12 mm glucose, and 25 mm NaHCO3. The tissue was then placed in a shallow groove formed in a gelatin block and glued onto the stage of a vibratome. Transverse spinal cord slices (400 μm) were cut in the ice-cold sucrose artificial cerebrospinal fluid and preincubated in Krebs' solution oxygenated with 95% O2 and 5% CO2 at 34 °C for at least 1 h before they were transferred to the recording chamber. The Krebs' solution contained 117 mm NaCl, 3.6 mm KCl, 1.2 mm MgCl2, 2.5 mm CaCl2, 1.2 mm NaH2PO4, 11 mm glucose, and 25 mm NaHCO3 gassed with 95% O2 and 5% CO2.
Each spinal cord slice was placed in a glass-bottomed chamber and continuously perfused with Krebs' solution at 5.0 ml/min at 34 °C maintained by an in-line solution heater and a temperature controller. The neurons in lamina II (substantia gelatinosa) at the L5 and L6 levels were identified under a fixed-stage microscope (BX50WI; Olympus, Tokyo, Japan) with differential interference contrast/infrared illumination. Lamina II neurons were selected for recording because they primarily receive nociceptive input from peripheral nerve fibers (27, 28). Recordings of excitatory postsynaptic currents (EPSCs) were performed by using the whole-cell voltage clamp method, as we described previously (23, 29, 30). The impedance of the electrode was 5–10 megohms when filled with internal solution containing 135 mm potassium gluconate, 5 mm KCl, 2 mm MgCl2, 0.5 mm CaCl2, 5 mm HEPES, 5 mm EGTA, 5 mm ATP-Mg, 0.5 mm sodium GTP, and 10 mm lidocaine N-ethyl bromide (QX314) adjusted to pH 7.2–7.4 with 1 m KOH (290–300 mosm). EPSCs of lamina II neurons were recorded at a holding potential of −60 mV and evoked by electrical stimulation (0.8 mA, 0.2 ms, 0.2 Hz) of the dorsal root (31). At the stimulation intensity used, both A- and C-fiber afferents that were in close contact with the electrode tip were stimulated (30, 32). Signals were processed by an amplifier (MultiClamp 700B; Molecular Devices, Sunnyvale, CA), filtered at 1–2 kHz, digitized at 10 kHz, and stored into a computer with pCLAMP 9.2 (Molecular Devices).
PCR Analysis
We used agarose gel electrophoresis to determine which Cavβ subunits are expressed in the DRG and dorsal spinal cord. Because Cavβ subunits have a high “G-C” content, a “slow-down” PCR method was used for PCR amplification as reported previously (33). PCR products were generated with LA TaqTM DNA polymerase (Takara), amplified with use of Cavβ subunit-specific primers, and analyzed with use of agarose gels. The sequence primers of the four Cavβ subunits that were used are shown in Table 1.
TABLE 1.
List of primers used in PCR-agarose gel analysis
| Gene (accession no.) | Primers |
|---|---|
| CaVβ1 (NM_017346.1) | Fwd: 5′-CACACCCGCCTAGCAGGACG-3′ |
| Rev: 5′-CGGCCCCGGTTCCTGTTGTC-3′ | |
| CaVβ2 (NM_053851.1) | Fwd: 5′-AGTGGTGTTGGTGGGCCCCT-3′ |
| Rev: 5′-ACTCCTGCGGGGGACACTGG-3′ | |
| CaVβ3 (NM_012828.2) | Fwd: 5′-AACCACCCAGCACAGCTAGCC-3′ |
| Rev: 5′-CACTGGGCGGACCCAGCATC-3′ | |
| CaVβ4 (NM_012828.2) | Fwd: 5′-CACCAGATCCAGCCTAGCGGAA-3′ |
| Rev: 5′-TTGTGTGGGTGGCACGCCAAT-3′ | |
| GAPDH (NM_012828.2) | Fwd: 5′-TGCCACTCAGAAGACTGTGG-3′ |
| Rev: 5′-TTCAGCTCTGGGATGACCTT-3′ |
We also used real-time PCR to determine the relative mRNA level of the Cavβ subunits. In brief, total RNA was extracted by using an RNeasy mini kit (Qiagen). Reverse transcription was performed with use of SuperScript II (Invitrogen). After treatment with RNase H, the resultant cDNA was quantified by using an IQ5 system (Bio-Rad). The sequence primers of the Cavβ subunits were listed in Table 2. The mRNA level of the Cavβ subunits was calculated by using the 2−ΔΔCt method and normalized by GAPDH (used as an internal control). The mean values of DRGs and spinal cord tissues contralateral to SNL were considered as 1.
TABLE 2.
List of primers used in quantitative PCR
Fwd means forward; Rev means reverse.
| Gene (accession no.) | Primers |
|---|---|
| CaVβ1 (NM_017346.1) | Fwd: 5′-GCGAGCACCTGGCGGAGTAC-3′ |
| Rev: 5′-GCGGTAGCCATGGTGCGGTT-3′ | |
| CaVβ2 (NM_053851.1) | Fwd: 5′-AGTGGGACAGGTCGAGGCCT-3′ |
| Rev: 5′-CACGGTGTGGAACATAGCGGTC-3′ | |
| CaVβ3 (NM_012828.2) | Fwd: 5′-TTCACCCCTGGAGCGGGACA-3′ |
| Rev: 5′-ACGGTGAGGCTGGTACAGGTC-3′ | |
| CaVβ4 (NM_012828.2) | Fwd: 5′-ACCTGGAGGCATATTGGCGTGC-3′ |
| Rev: 5′-TGGTTGCTATGCCTCATCCGCT-3′ | |
| GAPDH (NM_012828.2) | Fwd: 5′-TGCCACTCAGAAGACTGTGG-3′ |
| Rev: 5′-TTCAGCTCTGGGATGACCTT-3′ |
Western Blot Analysis
Tissues were sonicated in RIPA buffer and a mixture of protease inhibitors (Sigma). Total protein was extracted by centrifuge at 16,000 × g for 10 min at 4 °C. Equal amounts of proteins (20 μg) were subjected to SDS-PAGE and transferred onto polyvinylidene difluoride membrane (Immobilon P, Millipore). The blot was probed with anti-Cavβ2 (NeuroMab, Davis, CA; 1:1000 dilution), anti-Cavβ3 antibody (Santa Cruz Biotechnology; 1:1000 dilution), anti-Cavβ4 (NeuroMab; 1:1000 dilution), and anti-GAPDH (Millipore; 1:1000 dilution). ImageJ was used to quantify the band intensities. The amounts of Cavβ subunit proteins were normalized by GAPDH, and the mean values of the DRG or spinal cord tissues in the contralateral side of nerve injury were considered as 1.
Double Immunofluorescence Labeling of Cavβ3 Subunit and NF200 or Peripherin in the DRG
To determine the cellular distribution of the Cavβ3 subunit in the DRG, we performed double immunofluorescence labeling of this Cavβ3 subunit with a marker for small neurons (peripherin) (34) or a marker for medium and large neurons (NF200) (35). The DRGs from sham and SNL rats were cut to 30 μm and collected free floating in 0.1 m PBS. Sections were rinsed in Tris-HCl buffer and incubated with 1% H2O2 in TBS for 30 min to quench the endogenous peroxidase. Sections were blocked with 5% blocking reagent (PerkinElmer Life Sciences) in 0.1 m Tris-HCl for 1 h at 25 °C. Then the sections were incubated with the primary antibody mixture as follows: rabbit anti-Cavβ3 (Alomone Labs, Jerusalem, Israel; dilution 1:100) and mouse anti-NF200 (Sigma; dilution 1:200) or mouse anti-peripherin (Abcam, Cambridge, MA; dilution 1:100) at 25 °C for 2 h and at 4 °C overnight. Subsequently, sections were rinsed and incubated with the secondary antibody mixture as follows: peroxidase-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch; dilution 1:100) and Alexa Fluor-594-conjugated donkey anti-mouse IgG (Molecular Probes, Eugene, OR; dilution 1:400) for 2 h at room temperature. Then the sections were rinsed and incubated with fluorescein tyramide (PerkinElmer Life Sciences; dilution 1:100) for 10 min. Finally, the sections were rinsed, mounted on slides, dried, and coverslipped. The negative control was established by omitting the primary antibody.
The sections were examined on a laser-scanning confocal microscope (Carl Zeiss, Jena, Germany), and the areas of interest were photo-documented. To quantify changes in the distribution of Cavβ3 in peripherin- and NF200-immunoreactive DRG neurons by nerve injury, four confocal images were randomly selected from each DRG (two DRGs/rat) in three control and three nerve-injured rats, and the total number of peripherin- and NF200-immunoreactive cell bodies with and without Cavβ3 labeling was counted from each section.
Chitosan-siRNA Preparation and Intrathecal Injection
All of the siRNA was purchased from Integrated DNA Technologies (San Diego). Two Cavβ3-specific siRNAs (IDT catalog numbers 57372397 and 57372400) and a control siRNA (IDT catalog number 58008661; target sense, CGUUAAUCGCGUAUAAUACGCGUAT) were diluted by using RNA-free duplex buffer (1 μg/μl), incubated at 94 °C for 2 min for annealing, and then cooled slowly to room temperature (∼25 °C). We used chitosan to conjugate the siRNA for intrathecal injections in rats as we described previously (36). Chitosan is a biodegradable cationic polysaccharide that binds tightly with the negatively charged siRNA to form the chitosan-siRNA nanoparticles. After recovery from catheter cannulation for 2 days, siRNA (5 μg/20 μl) was intrathecally injected daily for 3 consecutive days. One day after the last chitosan-siRNA injection, we removed the dorsal spinal cords and DRGs at the L5-L6 level.
Data Analysis
The HVACC current data were analyzed by using the PulseFit software program (HEKA Instruments). The amplitude of monosynaptic EPSCs was analyzed by using Clampfit 9.2 (Molecular Devices). The whole-cell current voltage (I-V) curves for individual neurons were constructed by calculating the peak inward current at each testing potential and normalizing to the cell capacitance. Conductance voltage (G-V) curves were fit with the Boltzmann equation as follows: G/Gmax = 1/(1 + exp(V0.5 − Vm/k)), where V0.5 is the voltage for 50% activation or inactivation of HVACCs, and k is a voltage-dependent slope factor. Results were expressed as mean ± S.E. Student's t test was used to compare two groups, and one-way analysis of variance (with Tukey's or Dunnett's post hoc test) was used to compare multiple groups. p < 0.05 was considered statistically significant.
RESULTS
High Expression Levels of Cavβ3 and Cavβ4 Subunits in the DRG and Spinal Cord
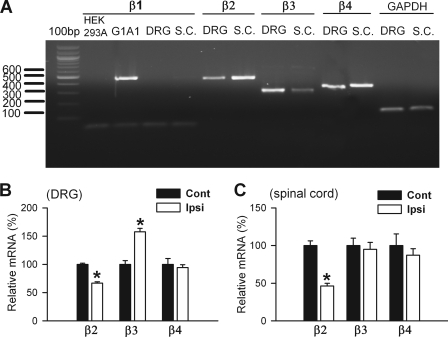
To determine which Cavβ subunits are present in the DRG and dorsal spinal cord, we first used reverse transcriptase-PCR and agarose gel electrophoresis to detect the presence of Cavβ1-Cavβ4 mRNA in these tissues. We detected the specific PCR products for Cavβ2 (461 bp), Cavβ3 (301 bp), and Cavβ4 (362 bp) subunits in DRG and spinal cord tissues (Fig. 1A), but Cavβ1 mRNA was not detected in the DRG or spinal cord on the agarose gel. However, Cavβ1 (463 bp) was clearly present in G1A1 cells, a HEK293-derived cell line that stably expresses Cav2.2, Cavβ1, and α2δ subunits (37).
FIGURE 1.
Effect of nerve injury on the mRNA level of Cavβ subunits in the DRG and dorsal spinal cord tissues. A, agarose gel electrophoresis showing the presence of the DNA products of Cavβ2, Cavβ3, and Cavβ4 subunits in the DRG and dorsal spinal cord (S.C.) tissues from control rats. G1A1 cells stably expressing Cavβ1 were used as a positive control for the Cavβ1 subunit, and the HEK293A cell line was used as a negative control. B, group data show the effect of SNL on the mRNA level of Cavβ2, Cavβ3, and Cavβ4 subunits in the DRG (n = 4 in each group). C, summary data show the effect of SNL on the mRNA level of Cavβ2, Cavβ3, and Cavβ4 subunits in the dorsal spinal cord (SC) (n = 4 in each group). Ipsi, ipsilateral (nerve injury) side; Cont, contralateral (control) side. *, p < 0.05, compared with the corresponding value on the contralateral (control) side.
We then quantified the mRNA level of individual Cavβ subunits in the DRG and dorsal spinal cord by using real time PCR. In the L5/L6 DRGs obtained from control rats, the Ct values of Cavβ1, Cavβ2, Cavβ3, and Cavβ4 subunits were 28.32, 24.92, 21.74, and 21.59, respectively. Thus, Cavβ3 and Cavβ4 were the most abundant Cavβ subtypes present in the DRG. We calculated the relative mRNA levels of Cavβ subunits by using the 2−ΔΔCt method (the Ct value of GAPDH was 22.59 in the DRG). When we considered the mRNA level of Cavβ4 to be 100%, the relative mRNA levels of Cavβ1, Cavβ2, and Cavβ3 subtypes were 0.94, 9.94, and 90.13%, respectively, of Cavβ4 in the DRG tissue.
In the dorsal spinal cord obtained from control rats, the Ct values of the Cavβ1, Cavβ2, Cavβ3, and Cavβ4 were 29.88, 23.53, 23.18, and 21.19, respectively. The Ct value of GAPDH in the spinal cord was 22.61. When we considered the mRNA level of Cavβ4 to be 100%, the relative mRNA levels of Cavβ1, Cavβ2, and Cavβ3 subtypes were 0.48, 19.75, and 25.17%, respectively, of Cavβ4 in the spinal cord tissue. Because the expression level of Cavβ1 in the DRG and spinal cord is very low, we did not study the effect of nerve injury on Cavβ1 subunit expression in the following experiments.
Nerve Injury Increases the Expression Level of the Cavβ3, but Not Cavβ4, Subunit in the DRG and Spinal Cord
We next used quantitative PCR to determine how nerve injury changes the expression levels of Cavβ2, Cavβ3, and Cavβ4 subunits in the DRG and dorsal spinal cords contralateral and ipsilateral to SNL. All of the rats developed tactile allodynia and hyperalgesia within 7 days after SNL surgery. We analyzed changes in the mRNA and protein levels of Cavβ subunits in the DRG and dorsal spinal cord 9 days after SNL. The Cavβ2 mRNA level in the DRG ipsilateral to SNL was significantly reduced (23.13% of control level). In contrast, the mRNA level of the Cavβ3 subunit was about 1.57 times greater in the DRG ipsilateral to SNL than in the contralateral DRG. The Cavβ4 mRNA level in the DRG did not differ significantly between the ipsilateral and contralateral sides (Fig. 1B). In the dorsal spinal cord, the Cavβ2 mRNA level was 53.64% lower in the ipsilateral side than the contralateral side of SNL rats. The mRNA level of Cavβ3 and Cavβ4 in the dorsal spinal cord did not differ significantly between the ipsilateral and the contralateral sides (Fig. 1C).
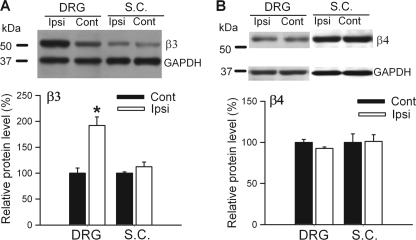
Similarly, the Cavβ3 protein level in the DRG ipsilateral to SNL was about 1.9 times of that in the contralateral side (Fig. 2A). However, the Cavβ4 protein level in the DRG did not differ significantly between the ipsilateral and contralateral sides (Fig. 2B). In the dorsal spinal cord, the protein level of Cavβ3 and Cavβ4 did not differ significantly between the ipsilateral and contralateral sides of SNL rats (Fig. 2, A and B). Because the Cavβ2 protein band of Cavβ1 and Cavβ2 in the DRG and dorsal spinal cord was below the detection level in the immunoblots, we did not quantify nerve injury-induced changes in the protein level of these two subunits. Therefore, our findings indicate that peripheral nerve injury primarily increases the expression level of the Cavβ3 subunit in the DRG.
FIGURE 2.
Effect of nerve injury on the protein level of Cavβ3 and Cavβ4 subunits in the DRG and dorsal spinal cord tissues. A, Western blots (top) and quantification (bottom) of the protein amount of Cavβ3 and Cavβ4 subunits in the DRG ipsilateral and contralateral to SNL (n = 4 in each group). B, Western blots (top) and quantification (bottom) of the protein amount of Cavβ3 and Cavβ4 subunits in the dorsal spinal cord (SC) ipsilateral and contralateral to SNL (n = 4 in each group). Ipsi, ipsilateral (nerve injury) side; Cont, contralateral (control) side. *, p < 0.05, compared with the corresponding value on the contralateral (control) side.
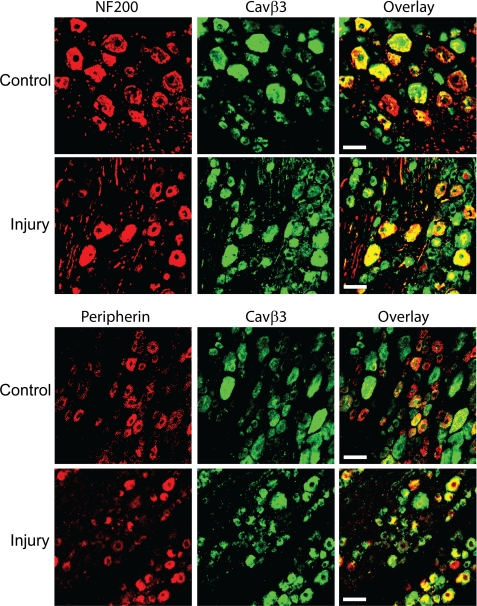
Changes in Distribution of the Cavβ3 Subunit in DRG Neurons after Nerve Injury
We then used double immunofluorescence labeling and confocal microscopy to examine changes in the distribution of the Cavβ3 subunit in NF200- and peripherin-immunoreactive DRG neurons in SNL rats. The Cavβ3 immunoreactivity was present in most DRG neurons immunoreactive to NF200 in control rats (511/547, 93.42%). However, only 44.82% (389/868) peripherin-immunoreactive DRG neurons are co-localized with the Cavβ3 subunit in sham control rats (Fig. 3). In DRG sections from nerve-injured rats, Cavβ3 immunoreactivity was present in 95.31% (447/469) NF200-immunoreactive neurons. Notably, the number of peripherin-immunoreactive DRG neurons co-localized with Cavβ3 (783/822, 95.26%) was significantly increased in nerve-injured rats compared with that in control rats (Fisher's exact test, Fig. 3).
FIGURE 3.
Nerve injury induces increased Cavβ3 in peripherin-immunoreactive DRG neurons. Representative confocal images show the distribution of Cavβ3 immunoreactivity (green) in small and large DRG neurons obtained from a sham control and an SNL rat. Neurons that are immunoreactive to NF200 or peripherin are indicated in red. Scale bar, 50 μm. All images are single confocal optical sections.
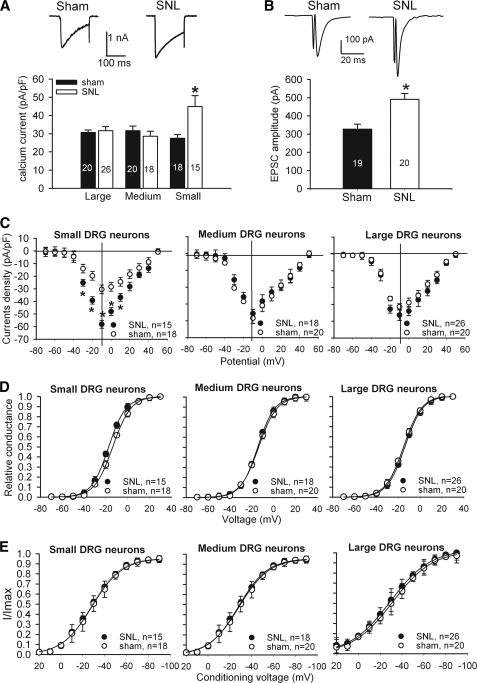
Nerve Injury Increases HVACC Activity in Small DRG Neurons and Glutamatergic Input to Spinal Dorsal Horn Neurons
Although complete nerve transection (axotomy) can reduce HVACC activity in DRG neurons (38), the effect of spinal nerve ligation injury on HVACC activity has not been specifically examined. To determine the changes in the HVACC activity in DRG neurons by SNL, we recorded and compared HVACC currents (IBa) in rat DRG neurons 9–10 days after SNL and sham surgery. Neurons were voltage-clamped at −90 mV and depolarized to 0 mV for 200 ms at 1-s intervals. In small (< 30 μm) DRG neurons, the peak amplitude of IBa was 1.6 times greater in SNL rats than in sham control rats (Fig. 4A). However, in medium (30–40 μm) and large (> 40 μm) DRG neurons, there were no significant differences in the current density of IBa between SNL and sham rats (Fig. 4A).
FIGURE 4.
Effects of nerve injury on HVACC activity of DRG neurons and monosynaptic EPSCs of spinal dorsal horn neurons. A, original current traces (upper panel) show HVACC currents in small DRG neurons from a control and a nerve-injured rat. Group data (lower panel) show that SNL increased HVACC currents in small, but not large and medium, DRG neurons. Neurons were voltage-clamped at −90 mV and depolarized to 0 mV for 200 ms. The number of cells in each group is indicated in the column. B, representative traces and group data show the monosynaptic EPSCs of lamina II neurons in spinal cords obtained from sham control (n = 19) and SNL (n = 20) rats. C, effect of SNL on the current-voltage relationship of HVACCs in small, medium, and large DRG neurons. D, effect of SNL on voltage-dependent activation of HVACCs in small, medium, and large DRG neurons. Note that SNL shifted voltage-dependent activation of HVACCs to the left only in small DRG neurons. The V0.5 in control and SNL rats was −13.7 ± 0.1 and −17.5 ± 0.1 mV (p < 0.05), respectively. The slope factor in control and SNL rats was 8.33 ± 0.2 and 7.80 ± 0.1 mV (p > 0.05), respectively. E, effect of SNL on voltage-dependent inactivation of HVACCs in small, medium, and large DRG neurons. *, p < 0.05, compared with the corresponding value in the sham group. pF, picofarad.
We also determined whether SNL increases glutamatergic input from primary afferent terminals to spinal dorsal horn neurons. EPSCs of lamina II neurons were evoked by electrical stimulation of the dorsal root. The EPSCs were considered monosynaptic if the latency was constant after electrical stimulation (0.2 Hz) and if no conduction failure or increased latency occurred when stimulation frequency was increased to 20 Hz (30, 32). The amplitude of monosynaptic EPSCs of lamina II neurons was significantly increased in the ipsilateral side compared with the contralateral side in SNL rats (Fig. 4B). Blocking glutamate AMPA receptors with 20 μm 6-cyano-7-nitroquinoxaline-2,3-dione abolished EPSCs in all lamina II neurons tested (39).
Next, we determined whether SNL affects activation and inactivation kinetics of IBa in DRG neurons. The whole-cell HVACC currents in DRG neurons were evoked by a series of depolarizing pulses (from −70 to 50 mV for 150 ms in 10-mV increments) from the holding potential of −90 mV. The current density of IBa in small DRG neurons was largely increased in SNL rats compared with that in sham control rats (Fig. 4C). Although SNL did not alter the current-voltage relationship of IBa, it caused a significant hyperpolarizing shift in V0.5 in small DRG neurons (SNL, −17.5 ± 0.1 mV; control, −13.7 ± 0.1 mV) (Fig. 4D). However, SNL did not significantly alter the voltage-dependent inactivation of IBa in DRG neurons (n = 15–26 in each group, see Fig. 4E).
Down-regulation of Cavβ3 Subunit Reduces HVACC Activity in DRG Neurons and Glutamatergic Input to Spinal Dorsal Horn Neurons
Small interfering RNA (siRNA) (20–30 nucleotides) can silence target genes by degrading mRNA with the matching sequence in vivo and in vitro. It has been shown that 27 nucleotides are more effective in suppressing a target gene than are 21 nucleotides (40). We thus designed two sequences of siRNA with 27 nucleotides targeting the Cavβ3 subunit. To ensure the efficient delivery of siRNA into the spinal cord and DRG neurons, we incorporated the siRNA into chitosan (36). In our preliminary experiments, intrathecal treatment with the first Cavβ3-siRNA (5′-AUGUCUCUUCCUCAUGCUACAUUGCCU-3′) reduced the Cavβ3 protein level in the DRG by ∼50%. However, the second Cavβ3-siRNA (5′-AUCCACCAGUCAUUGCUGUACUUCUCU-3′) decreased the Cavβ3 protein level by only 20%. Because the first Cavβ3 siRNA had a much better effect than did the second Cavβ3 siRNA, we used the first Cavβ3-specific siRNA in the following experiments. We injected siRNA (5 μg) via intrathecal catheters starting on day 10 after SNL for 3 consecutive days.
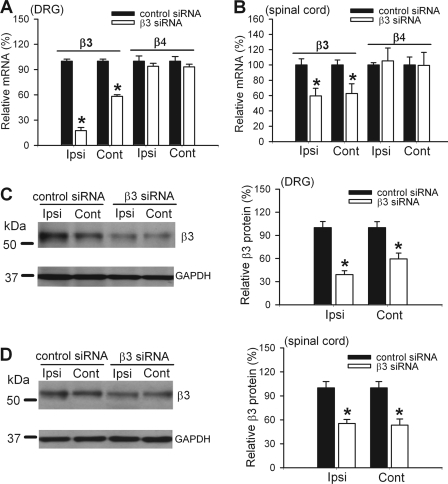
In the DRG tissues, intrathecal treatment with the Cavβ3-specific siRNA caused an 82.44% reduction in the Cavβ3 mRNA level in the ipsilateral side and a 41.75% reduction in the contralateral side of SNL rats, compared with that in control siRNA-treated rats (Fig. 5A). In the dorsal spinal cord, intrathecal treatment with Cavβ3-specific siRNA decreased the Cavβ3 mRNA level by 40% in both sides, compared with that in control siRNA-treated rats (Fig. 5B). To ensure the specificity of the Cavβ3 siRNA, we also determined the effect of Cavβ3-specific siRNA on the Cavβ4 mRNA level in the DRG and dorsal spinal cord. The Cavβ3 siRNA had no significant effects on the Cavβ4 mRNA level in the DRG and spinal cord of SNL rats (Fig. 5, A and B)
FIGURE 5.
Effect of intrathecal treatment with Cavβ3-specific siRNA (5 μg per day for 3 days) on the expression level of Cavβ3 in the DRG and spinal cord of SNL rats. A, effect of Cavβ3-specific siRNA on the mRNA level of Cavβ3 and Cavβ4 in the DRG of SNL rats (n = 4 in each group). B, effect of Cavβ3-specific siRNA on the mRNA level of Cavβ3 and Cavβ4 in the dorsal spinal cord of SNL rats (n = 4 in each group). C, Western blots (left) and quantification (right, n = 4 in each group) of the protein amount of the Cavβ3 subunit in the DRG ipsilateral and contralateral to SNL in rats treated with Cavβ3-specific siRNA or control siRNA. D, Western blots (left) and quantification (right, n = 4 in each group) of the protein amount of the Cavβ3 subunit in the dorsal spinal cord ipsilateral and contralateral to SNL in rats treated with Cavβ3-specific siRNA or control siRNA. Ipsi, ipsilateral (nerve injury) side; Cont, contralateral (control) side. *, p < 0.05, compared with the corresponding value in control siRNA-treated group.
Treatment with the Cavβ3-specific siRNA caused a larger reduction in the Cavβ3 protein level in the DRG on the ipsilateral side (60.90% decrease) than on the contralateral side (40.92% decrease) (Fig. 5C). Also, the amount of the Cavβ3 protein on both sides of the dorsal spinal cord was significantly reduced (by ∼40%) in rats treated with the Cavβ3-specific siRNA, compared with that in control siRNA-treated rats (Fig. 5D).
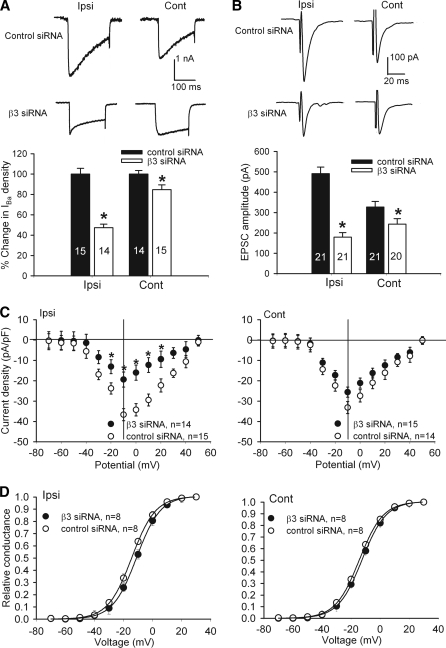
To determine the contribution of the Cavβ3 subunit to increased HVACC activity in DRG neurons of SNL rats, we examined the effect of Cavβ3-specific siRNA on the current density of IBa in DRG neurons. Intrathecal treatment with Cavβ3-siRNA caused a large decrease in the amplitude of IBa in small DRG neurons ipsilateral to SNL, compared with the control siRNA-treated rats (Fig. 6, A and C). By comparison, treatment with Cavβ3-siRNA caused a smaller reduction in the current density of IBa in small DRG neurons on the contralateral side, compared with the control siRNA-treated rats. Also, treatment with Cavβ3-siRNA caused a depolarizing shift of V0.5 in small DRG neurons ipsilateral to SNL (control siRNA, −14.37 ± 0.1; Cavβ3-specific siRNA, −11.02 ± 0.1 mV; see Fig. 6D).
FIGURE 6.
Effects of intrathecal treatment with Cavβ3-specific siRNA on HVACC activity in DRG neurons and monosynaptic EPSCs of spinal dorsal horn neurons of SNL rats. A, original traces and group data show the effect of Cavβ3-specific siRNA on HVACC current in small DRG neurons ipsilateral and contralateral to SNL. The number of cells in each group is indicated in the column. B, representative traces and summary data show the effect of Cavβ3-specific siRNA on the amplitude of monosynaptic EPSCs of lamina II neurons in the spinal cord of SNL rats. C, effect of Cavβ3-specific siRNA on the current-voltage relationship of HVACCs in small DRG neurons ipsilateral and contralateral to SNL. D, effect of Cavβ3-specific siRNA on voltage-dependent activation of HVACCs in small DRG neurons ipsilateral and contralateral to SNL. Note that treatment with Cavβ3-specific siRNA shifted the voltage-dependent activation of HVACCs to the right in small DRG neurons ipsilateral to SNL. The V0.5 in control-siRNA and Cavβ3-specific siRNA groups was −14.37 ± 0.1 and −11.02 ± 0.1 mV (p < 0.05), respectively. The slope factor in control-siRNA and Cavβ3-specific siRNA groups was 8.20 ± 0.1 and 8.20 ± 0.2 mV (p > 0.05), respectively. Ipsi, ipsilateral (nerve injury) side; Cont, contralateral (control) side. *, p < 0.05, compared with the corresponding value in control siRNA-treated group.
Intrathecal treatment with Cavβ3-specific siRNA also significantly decreased the amplitude of monosynaptic EPSCs of spinal lamina II neurons evoked from the dorsal root in SNL rats (Fig. 6B). This Cavβ3 siRNA effect was much more prominent in the ipsilateral than in the contralateral side of the spinal cord in SNL rats. Collectively, these data indicate that up-regulation of the Cavβ3 subunit in primary afferent neurons contributes to increased activity of HVACCs and glutamatergic input from primary afferents to spinal dorsal horn neurons caused by nerve injury.
Down-regulation of Cavβ3 Subunit at the Spinal Level Reduces Neuropathic Pain
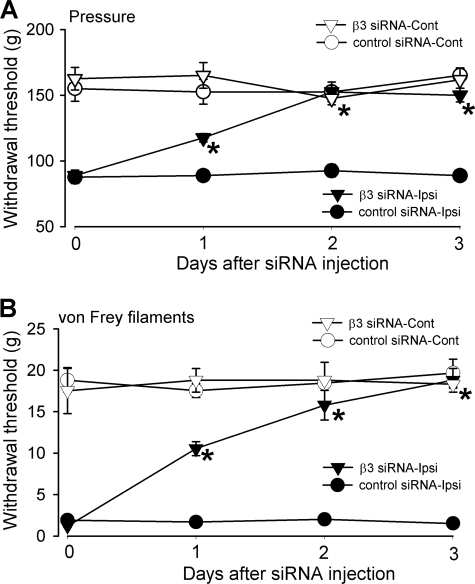
In addition, we performed nociceptive behavioral tests to determine whether up-regulation of the Cavβ3 subunit in DRG neurons contributes to pain hypersensitivity induced by nerve injury. SNL significantly reduced the pressure and tactile withdrawal thresholds of the hind paw on the ipsilateral side compared with the contralateral side (Fig. 7). Intrathecal treatment with Cavβ3-specific siRNA (5 μg/day for 3 days) or control siRNA had no significant effect on the ambulation behavior of rats. Compared with the control siRNA treatment, intrathecal administration of Cavβ3-specific siRNA gradually reversed the pressure and tactile withdrawal thresholds of the hind paw ipsilateral to SNL (Fig. 7). The pressure withdrawal threshold and tactile withdrawal threshold of the hind paw ipsilateral to SNL were completely normalized 3 days after treatment with Cavβ3-specific siRNA. However, treatment with control siRNA did not significantly alter the pressure and tactile withdrawal thresholds of the hind paw ipsilateral to SNL. Also, the Cavβ3-specific siRNA had no significant effect on the pressure and tactile withdrawal thresholds of the hind paw contralateral to SNL (Fig. 7).
FIGURE 7.
Effects of intrathecal treatment with Cavβ3-specific siRNA (5 μg per day for 3 days) on nociceptive and tactile withdrawal thresholds of hind paws in SNL rats. A, time course effects of control siRNA and Cavβ3-specific siRNA on the nociceptive withdrawal threshold, tested with a noxious pressure stimulus, of hind paws ipsilateral and contralateral to SNL (n = 6 rats in each group). B, time course effects of control siRNA and Cavβ3-specific siRNA on the tactile withdrawal threshold, measured with von Frey filaments, of hind paws ipsilateral and contralateral to SNL (n = 6 rats in each group). Note that Cavβ3-specific siRNA, but not control siRNA, increased nociceptive and tactile withdrawal thresholds of hind paws ipsilateral to SNL. Ipsi, ipsilateral (nerve injury) side; Cont, contralateral (control) side. *, p < 0.05, compared with the base-line value in Cavβ3-specific siRNA-treated group.
DISCUSSION
In this study, we determined how nerve injury affects the expression of Cavβ subunits in primary sensory neurons and whether such changes contribute to increased HVACC currents and pain hypersensitivity induced by nerve injury. Persistent increases in nociceptive input from injured peripheral nerves are an important feature of chronic neuropathic pain (3, 22, 23). We found that SNL significantly increased the current density of HVACCs in small DRG neurons. SNL also shifted the voltage-dependent activation of HVACCs in the hyperpolarizing direction, suggesting that HVACCs open more readily in these DRG neurons in the neuropathic pain condition. We found that SNL also significantly increased the amplitude of monosynaptic EPSCs of dorsal horn neurons evoked from the dorsal root. Our data suggest that glutamatergic input from primary afferents to spinal dorsal horn neurons is potentiated in neuropathic pain. Blocking N-type HVACCs at the spinal level is highly effective in the treatment of severe pain in patients (41, 42), and knock-out of N-type HVACCs in mice reduces neuropathic pain symptoms (43). Also, reducing the expression of Cav1.2 (one of the L-type HVACCs) attenuates neuropathic pain in SNL rats (44). Although L- and N-type HVACCs are involved in the generation of abnormal ectopic discharges of DRG neurons caused by nerve injury (45), the molecular mechanisms underlying increased HVACC activity in DRG neurons and nociceptive input in neuropathic pain are not fully known.
The pore-forming α1 subunit is often associated with two core auxiliary subunits consisting of the membrane-anchored, largely extracellular α2δ subunit and the cytoplasmic Cavβ subunit (5). The α1 subunits are the primary determinants of both channel biophysics and pharmacology, whereas Cavβ, α2δ, and γ subunits are regarded as regulators of HVACC functions. There are four subfamilies of Cavβ subunits that are mainly expressed in the excitable tissues such as the brain, heart, and muscles. Cavβ1 is mainly expressed in the hippocampus and skeletal muscle (46), whereas Cavβ2 is mostly expressed in the heart (47, 48). Cavβ3 is expressed not only in several brain regions but also in the heart and lungs (47, 49). Cavβ4 is strongly expressed in the cerebellum (14). It has been reported that the mRNA level of several α1 subunits in DRG tissues is reduced by sciatic nerve injury (50). Although nerve injury can increase α2δ expression in DRG neurons (51, 52), it is uncertain whether this change is casually related to increased HVACC activity in DRG neurons by nerve injury. α1B (N-type) is the predominant α1 subtype that mediates HVACC currents in DRG neurons (25). The β3 subunit regulates both L- and N-type HVACCs (53, 54). By using quantitative PCR and Western blot analyses, we found that Cavβ3 and Cavβ4 are the most predominant Cavβ subtypes distributed in the DRG and dorsal spinal cord tissues. Furthermore, we found that SNL caused a significant increase in the mRNA and protein levels of the Cavβ3, but not Cavβ4, subunit in the DRG, suggesting that the Cavβ3 expression level is increased in primary afferent neurons in neuropathic pain. Our immunolabeling results provide further evidence that increased Cavβ3 expression occurs predominantly in peripherin-positive small-sized DRG neurons after nerve injury. We noted that nerve injury significantly decreased the mRNA level of Cavβ2 in the DRG and spinal cord. Because the basal expression level of Cavβ2 in the DRG and spinal cord was extremely low, the reduced Cavβ2 expression level may have little impact on HVACC activity in primary sensory neurons. Alternatively, the reduced Cavβ2 level may free up HVACC α1 subunits and allow them to form more α1-Cavβ3 complexes in injured DRG neurons.
Although the important role of the Cavβ3 subunit in the regulation of HVACCs has been shown in cell lines (12, 26), their contribution to increased HVACC activity in neuropathic pain has not been demonstrated in native neurons due to the lack of specific pharmacological approaches. In this study, we demonstrated that intrathecal treatment with Cavβ3-specific siRNA conjugated to chitosan selectively and efficiently knocked down the expression level of the Cavβ3 subunit in the DRG and dorsal spinal cord. Importantly, down-regulation of Cavβ3 by siRNA caused a large reduction in the current density of HVACCs and a depolarizing shift in the voltage-dependent activation of HVACCs in small DRG neurons ipsilateral to SNL. By comparison, Cavβ3-specific siRNA had little effect on HVACC currents in small DRG neurons on the control side of SNL rats. Cavβ3-specific siRNA also reduced voltage-dependent activation and inactivation of HVACC currents in small DRG neurons, which is consistent with the role of Cavβ3 in the control of HVACC gating (15). Thus, our results indicate that up-regulation of the β3 subunit plays a key role in increased HVACC activity in injured DRG neurons in neuropathic pain. Because treatment with Cavβ3-specific siRNA attenuated the amplitude of monosynaptic EPSCs of dorsal horn neurons more on the ipsilateral side than on the contralateral side of SNL rats, this finding provides further evidence that up-regulation of the Cavβ3 subunit contributes to increased HVACC activity and neurotransmitter release from the primary afferent terminals in the spinal dorsal horn in neuropathic pain. An increase in the Cavβ3 subunit could increase the α1-Cavβ3 binding and augment HVACC activity by promoting α1 subunit expression on the plasma membrane after nerve injury.
Another salient finding of our study is that down-regulation of the Cavβ3 subunit at the spinal level by chitosan-siRNA profoundly reduced mechanical hyperalgesia and tactile allodynia induced by SNL in rats. We found that intrathecal treatment with Cavβ3-specific siRNA for 3 days completely normalized pain hypersensitivity in SNL rats. Interestingly, although knockdown of Cavβ3 also decreased the expression level of Cavβ3 in DRG neurons and spinal cord contralateral to SNL, intrathecal treatment with Cavβ3-specific siRNA had no significant effect on the nociceptive and tactile thresholds of the hind paw contralateral to SNL. This finding suggests that down-regulation of Cavβ3 at the spinal level alone does not impair normal nociception. Consistent with our finding with the siRNA approach, it has been reported that knock-out of Cavβ3 in mice reduces acute inflammatory pain but has little effect on normal nociceptive thresholds in response to noxious heat and mechanical stimuli (55). The profound effect of Cavβ3-specific siRNA on SNL-induced pain hypersensitivity suggests that the increased Cavβ3 expression level in DRG neurons becomes critically important for augmented HVACC activity and nociceptive input in neuropathic pain. This notion is supported by our finding that the Cavβ3-siRNA effect on the HVACC currents of small DRG neurons was much more pronounced on the ipsilateral (injury) side than the contralateral (control side) of SNL rats. Furthermore, the inhibitory effect of Cavβ3-specific siRNA on the evoked EPSC amplitude of spinal dorsal horn neurons was much greater on the ipsilateral side than on the contralateral side of SNL rats. Another possibility is that nerve injury may increase the uptake of chitosan-siRNA by injured primary afferent neurons. This is because Cavβ3-specific siRNA treatment produced a greater effect on the Cavβ3 expression level in the DRG on the ipsilateral side than on the contralateral side of SNL rats.
In summary, we demonstrated in this study that the Cavβ3 subunit is up-regulated and critically contributes to increased HVACC activity in DRG neurons and nociceptive input to spinal dorsal horn neurons in neuropathic pain. Down-regulation of the Cavβ3 subunit at the spinal level normalizes neuropathic pain but has no significant effect on normal nociception. Directly blocking N-type HVACCs at the spinal level often produces intolerable adverse effects (42, 56) and thus has limited clinical use in the treatment of chronic pain. Our findings suggest the Cavβ3 subunit may represent a new target for neuropathic pain treatment with a reduced adverse effect profile. Many aminopyridine analogs can modulate HVACC activity through the Cavβ subunit (26). These compounds could be modified to inhibit Cavβ3 subunit function to reduce increased HVACC activity and nociceptive input in neuropathic pain. Therefore, our study provides important information about the molecular mechanisms underlying increased HVACC activity in primary sensory neurons by nerve injury. This new information improves our understanding of neuroplastic changes associated with neuropathic pain and helps the design of novel analgesics for neuropathic pain treatment.
This work was supported, in whole or in part, by National Institutes of Health Grants GM064830, NS045602, and NS073935 (to H.-L. P.) and CA151668 (to A. K. S. and G. L.-B.). This work was also supported by the N. G. and Helen T. Hawkins endowment (to H.-L. P.).
- HVACC
- high voltage-activated calcium channels
- DRG
- dorsal root ganglion
- EPSC
- excitatory postsynaptic current
- SNL
- spinal nerve ligation.
REFERENCES
- 1. Dworkin R. H., O'Connor A. B., Backonja M., Farrar J. T., Finnerup N. B., Jensen T. S., Kalso E. A., Loeser J. D., Miaskowski C., Nurmikko T. J., Portenoy R. K., Rice A. S., Stacey B. R., Treede R. D., Turk D. C., Wallace M. S. (2007) Pharmacologic management of neuropathic pain. Evidence-based recommendations. Pain 132, 237–251 [DOI] [PubMed] [Google Scholar]
- 2. Campero M., Serra J., Marchettini P., Ochoa J. L. (1998) Ectopic impulse generation and autoexcitation in single myelinated afferent fibers in patients with peripheral neuropathy and positive sensory symptoms. Muscle Nerve 21, 1661–1667 [DOI] [PubMed] [Google Scholar]
- 3. Gracely R. H., Lynch S. A., Bennett G. J. (1992) Painful neuropathy. Altered central processing maintained dynamically by peripheral input. Pain 51, 175–194 [DOI] [PubMed] [Google Scholar]
- 4. Catterall W. A., Few A. P. (2008) Calcium channel regulation and presynaptic plasticity. Neuron 59, 882–901 [DOI] [PubMed] [Google Scholar]
- 5. Catterall W. A. (2000) Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 16, 521–555 [DOI] [PubMed] [Google Scholar]
- 6. Kang M. G., Chen C. C., Felix R., Letts V. A., Frankel W. N., Mori Y., Campbell K. P. (2001) Biochemical and biophysical evidence for γ2 subunit association with neuronal voltage-activated Ca2+ channels. J. Biol. Chem. 276, 32917–32924 [DOI] [PubMed] [Google Scholar]
- 7. Catterall W. A., Perez-Reyes E., Snutch T. P., Striessnig J. (2005) International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol. Rev. 57, 411–425 [DOI] [PubMed] [Google Scholar]
- 8. Andrade A., Sandoval A., González-Ramírez R., Lipscombe D., Campbell K. P., Felix R. (2009) The α2δ subunit augments functional expression and modifies the pharmacology of Ca(V)1.3 L-type channels. Cell Calcium 46, 282–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Luo Z. D., Chaplan S. R., Higuera E. S., Sorkin L. S., Stauderman K. A., Williams M. E., Yaksh T. L. (2001) Up-regulation of dorsal root ganglion α2δ calcium channel subunit and its correlation with allodynia in spinal nerve-injured rats. J. Neurosci. 21, 1868–1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Waard M., Pragnell M., Campbell K. P. (1994) Ca2+ channel regulation by a conserved β subunit domain. Neuron 13, 495–503 [DOI] [PubMed] [Google Scholar]
- 11. Pragnell M., De Waard M., Mori Y., Tanabe T., Snutch T. P., Campbell K. P. (1994) Calcium channel β-subunit binds to a conserved motif in the I-II cytoplasmic linker of the α1-subunit. Nature 368, 67–70 [DOI] [PubMed] [Google Scholar]
- 12. Yamaguchi H., Hara M., Strobeck M., Fukasawa K., Schwartz A., Varadi G. (1998) Multiple modulation pathways of calcium channel activity by a β subunit. Direct evidence of β subunit participation in membrane trafficking of the α1C subunit. J. Biol. Chem. 273, 19348–19356 [DOI] [PubMed] [Google Scholar]
- 13. Hofmann F., Biel M., Flockerzi V. (1994) Molecular basis for Ca2+ channel diversity. Annu. Rev. Neurosci. 17, 399–418 [DOI] [PubMed] [Google Scholar]
- 14. Ludwig A., Flockerzi V., Hofmann F. (1997) Regional expression and cellular localization of the α1 and β subunit of high voltage-activated calcium channels in rat brain. J. Neurosci. 17, 1339–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buraei Z., Yang J. (2010) The β subunit of voltage-gated Ca2+ channels. Physiol. Rev. 90, 1461–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gregg R. G., Messing A., Strube C., Beurg M., Moss R., Behan M., Sukhareva M., Haynes S., Powell J. A., Coronado R., Powers P. A. (1996) Absence of the β subunit (cchb1) of the skeletal muscle dihydropyridine receptor alters expression of the α1 subunit and eliminates excitation-contraction coupling. Proc. Natl. Acad. Sci. U.S.A. 93, 13961–13966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weissgerber P., Held B., Bloch W., Kaestner L., Chien K. R., Fleischmann B. K., Lipp P., Flockerzi V., Freichel M. (2006) Reduced cardiac L-type Ca2+ current in CaVβ2−/− embryos impairs cardiac development and contraction with secondary defects in vascular maturation. Circ. Res. 99, 749–757 [DOI] [PubMed] [Google Scholar]
- 18. Murakami M., Nakagawasai O., Yanai K., Nunoki K., Tan-No K., Tadano T., Iijima T. (2007) Modified behavioral characteristics following ablation of the voltage-dependent calcium channel β3 subunit. Brain Res. 1160, 102–112 [DOI] [PubMed] [Google Scholar]
- 19. Burgess D. L., Jones J. M., Meisler M. H., Noebels J. L. (1997) Mutation of the Ca2+ channel β subunit gene Cchb4 is associated with ataxia and seizures in the lethargic (lh) mouse. Cell 88, 385–392 [DOI] [PubMed] [Google Scholar]
- 20. Kim S. H., Chung J. M. (1992) An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 50, 355–363 [DOI] [PubMed] [Google Scholar]
- 21. Chen S. R., Eisenach J. C., McCaslin P. P., Pan H. L. (2000) Synergistic effect between intrathecal non-NMDA antagonist and gabapentin on allodynia induced by spinal nerve ligation in rats. Anesthesiology 92, 500–506 [DOI] [PubMed] [Google Scholar]
- 22. Chen S. R., Cai Y. Q., Pan H. L. (2009) Plasticity and emerging role of BKCa channels in nociceptive control in neuropathic pain. J. Neurochem. 110, 352–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhou H. Y., Chen S. R., Chen H., Pan H. L. (2011) Functional plasticity of group II metabotropic glutamate receptors in regulating spinal excitatory and inhibitory synaptic input in neuropathic pain. J. Pharmacol. Exp. Ther. 336, 254–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chaplan S. R., Bach F. W., Pogrel J. W., Chung J. M., Yaksh T. L. (1994) Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 53, 55–63 [DOI] [PubMed] [Google Scholar]
- 25. Wu Z. Z., Pan H. L. (2004) High voltage-activated Ca2+ channel currents in isolectin B(4)-positive and -negative small dorsal root ganglion neurons of rats. Neurosci. Lett. 368, 96–101 [DOI] [PubMed] [Google Scholar]
- 26. Wu Z. Z., Li D. P., Chen S. R., Pan H. L. (2009) Aminopyridines potentiate synaptic and neuromuscular transmission by targeting the voltage-activated calcium channel β subunit. J. Biol. Chem. 284, 36453–36461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pan H. L., Khan G. M., Alloway K. D., Chen S. R. (2003) Resiniferatoxin induces paradoxical changes in thermal and mechanical sensitivities in rats. Mechanism of action. J. Neurosci. 23, 2911–2919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cervero F., Iggo A. (1980) The substantia gelatinosa of the spinal cord. A critical review. Brain 103, 717–772 [DOI] [PubMed] [Google Scholar]
- 29. Chen S. R., Chen H., Yuan W. X., Pan H. L. (2011) Increased presynaptic and postsynaptic α2-adrenoceptor activity in the spinal dorsal horn in painful diabetic neuropathy. J. Pharmacol. Exp. Ther. 337, 285–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhou H. Y., Chen S. R., Chen H., Pan H. L. (2010) Opioid-induced long term potentiation in the spinal cord is a presynaptic event. J. Neurosci. 30, 4460–4466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhou H. Y., Zhang H. M., Chen S. R., Pan H. L. (2008) Increased C-fiber nociceptive input potentiates inhibitory glycinergic transmission in the spinal dorsal horn. J. Pharmacol. Exp. Ther. 324, 1000–1010 [DOI] [PubMed] [Google Scholar]
- 32. Wang X. L., Zhang H. M., Li D. P., Chen S. R., Pan H. L. (2006) Dynamic regulation of glycinergic input to spinal dorsal horn neurons by muscarinic receptor subtypes in rats. J. Physiol. 571, 403–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Frey U. H., Bachmann H. S., Peters J., Siffert W. (2008) PCR amplification of GC-rich regions. “Slowdown PCR.” Nat. Protoc. 3, 1312–1317 [DOI] [PubMed] [Google Scholar]
- 34. Chen C. C., England S., Akopian A. N., Wood J. N. (1998) A sensory neuron-specific, proton-gated ion channel. Proc. Natl. Acad. Sci. U.S.A. 95, 10240–10245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Perry M. J., Lawson S. N., Robertson J. (1991) Neurofilament immunoreactivity in populations of rat primary afferent neurons. A quantitative study of phosphorylated and nonphosphorylated subunits. J. Neurocytol. 20, 746–758 [DOI] [PubMed] [Google Scholar]
- 36. Cai Y. Q., Chen S. R., Han H. D., Sood A. K., Lopez-Berestein G., Pan H. L. (2009) Role of M2, M3, and M4 muscarinic receptor subtypes in the spinal cholinergic control of nociception revealed using siRNA in rats. J. Neurochem. 111, 1000–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McDavid S., Currie K. P. (2006) G-proteins modulate cumulative inactivation of N-type (Cav2.2) calcium channels. J. Neurosci. 26, 13373–13383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Abdulla F. A., Smith P. A. (2001) Axotomy- and autotomy-induced changes in Ca2+ and K+ channel currents of rat dorsal root ganglion neurons. J. Neurophysiol. 85, 644–658 [DOI] [PubMed] [Google Scholar]
- 39. Pan Y. Z., Pan H. L. (2004) Primary afferent stimulation differentially potentiates excitatory and inhibitory inputs to spinal lamina II outer and inner neurons. J. Neurophysiol. 91, 2413–2421 [DOI] [PubMed] [Google Scholar]
- 40. Kim D. H., Behlke M. A., Rose S. D., Chang M. S., Choi S., Rossi J. J. (2005) Synthetic dsRNA Dicer substrates enhance RNAi potency and efficacy. Nat. Biotechnol. 23, 222–226 [DOI] [PubMed] [Google Scholar]
- 41. Staats P. S., Yearwood T., Charapata S. G., Presley R. W., Wallace M. S., Byas-Smith M., Fisher R., Bryce D. A., Mangieri E. A., Luther R. R., Mayo M., McGuire D., Ellis D. (2004) Intrathecal ziconotide in the treatment of refractory pain in patients with cancer or AIDS. A randomized controlled trial. JAMA 291, 63–70 [DOI] [PubMed] [Google Scholar]
- 42. Schmidtko A., Lötsch J., Freynhagen R., Geisslinger G. (2010) Ziconotide for treatment of severe chronic pain. Lancet 375, 1569–1577 [DOI] [PubMed] [Google Scholar]
- 43. Saegusa H., Kurihara T., Zong S., Kazuno A., Matsuda Y., Nonaka T., Han W., Toriyama H., Tanabe T. (2001) Suppression of inflammatory and neuropathic pain symptoms in mice lacking the N-type Ca2+ channel. EMBO J. 20, 2349–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fossat P., Dobremez E., Bouali-Benazzouz R., Favereaux A., Bertrand S. S., Kilk K., Léger C., Cazalets J. R., Langel U., Landry M., Nagy F. (2010) Knockdown of L calcium channel subtypes. Differential effects in neuropathic pain. J. Neurosci. 30, 1073–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu X., Zhou J. L., Chung K., Chung J. M. (2001) Ion channels associated with the ectopic discharges generated after segmental spinal nerve injury in the rat. Brain Res. 900, 119–127 [DOI] [PubMed] [Google Scholar]
- 46. Powers P. A., Liu S., Hogan K., Gregg R. G. (1992) Skeletal muscle and brain isoforms of a β-subunit of human voltage-dependent calcium channels are encoded by a single gene. J. Biol. Chem. 267, 22967–22972 [PubMed] [Google Scholar]
- 47. Hullin R., Singer-Lahat D., Freichel M., Biel M., Dascal N., Hofmann F., Flockerzi V. (1992) Calcium channel β subunit heterogeneity. Functional expression of cloned cDNA from heart, aorta, and brain. EMBO J. 11, 885–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chu P. J., Larsen J. K., Chen C. C., Best P. M. (2004) Distribution and relative expression levels of calcium channel β subunits within the chambers of the rat heart. J. Mol. Cell. Cardiol. 36, 423–434 [DOI] [PubMed] [Google Scholar]
- 49. Castellano A., Wei X., Birnbaumer L., Perez-Reyes E. (1993) Cloning and expression of a third calcium channel β subunit. J. Biol. Chem. 268, 3450–3455 [PubMed] [Google Scholar]
- 50. Kim D. S., Yoon C. H., Lee S. J., Park S. Y., Yoo H. J., Cho H. J. (2001) Changes in voltage-gated calcium channel α(1) gene expression in rat dorsal root ganglia following peripheral nerve injury. Brain Res. Mol. Brain Res. 96, 151–156 [DOI] [PubMed] [Google Scholar]
- 51. Luo Z. D., Calcutt N. A., Higuera E. S., Valder C. R., Song Y. H., Svensson C. I., Myers R. R. (2002) Injury type-specific calcium channel α2δ-1 subunit up-regulation in rat neuropathic pain models correlates with antiallodynic effects of gabapentin. J. Pharmacol. Exp. Ther. 303, 1199–1205 [DOI] [PubMed] [Google Scholar]
- 52. Newton R. A., Bingham S., Case P. C., Sanger G. J., Lawson S. N. (2001) Dorsal root ganglion neurons show increased expression of the calcium channel α2δ-1 subunit following partial sciatic nerve injury. Brain Res. Mol. Brain Res. 95, 1–8 [DOI] [PubMed] [Google Scholar]
- 53. Murakami M., Yamamura H., Suzuki T., Kang M. G., Ohya S., Murakami A., Miyoshi I., Sasano H., Muraki K., Hano T., Kasai N., Nakayama S., Campbell K. P., Flockerzi V., Imaizumi Y., Yanagisawa T., Iijima T. (2003) Modified cardiovascular L-type channels in mice lacking the voltage-dependent Ca2+ channel β3 subunit. J. Biol. Chem. 278, 43261–43267 [DOI] [PubMed] [Google Scholar]
- 54. Namkung Y., Smith S. M., Lee S. B., Skrypnyk N. V., Kim H. L., Chin H., Scheller R. H., Tsien R. W., Shin H. S. (1998) Targeted disruption of the Ca2+ channel β3 subunit reduces N- and L-type Ca2+ channel activity and alters the voltage-dependent activation of P/Q-type Ca2+ channels in neurons. Proc. Natl. Acad. Sci. U.S.A. 95, 12010–12015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Murakami M., Fleischmann B., De Felipe C., Freichel M., Trost C., Ludwig A., Wissenbach U., Schwegler H., Hofmann F., Hescheler J., Flockerzi V., Cavalié A. (2002) Pain perception in mice lacking the β3 subunit of voltage-activated calcium channels. J. Biol. Chem. 277, 40342–40351 [DOI] [PubMed] [Google Scholar]
- 56. Wallace M. S., Rauck R., Fisher R., Charapata S. G., Ellis D., Dissanayake S. (2008) Intrathecal ziconotide for severe chronic pain. Safety and tolerability results of an open-label, long term trial. Anesth. Analg. 106, 628–637 [DOI] [PubMed] [Google Scholar]