Background: Because genetic linkage studies identified mutations in TRPV4 in patients with peripheral neuropathies, the function of TRPV4 in peripheral neurons is questioned.
Results: TRPV4 was found to promote neurotrophic factor-driven neuritogenesis.
Conclusion: TRPV4 mediates neurotrophic factor-driven neuritogenesis in peripheral neurons.
Significance: This explains molecular mechanisms underlying neuritogenesis and maintenance of peripheral nerves.
Keywords: Actin, Neurite Outgrowth, Neurodegenerative Diseases, Neurotrophic Factor, TRP Channels, Peripheral Neuropathy, TRPV4
Abstract
Spinal muscular atrophy and hereditary motor and sensory neuropathies are characterized by muscle weakness and atrophy caused by the degenerations of peripheral motor and sensory nerves. Recent advances in genetics have resulted in the identification of missense mutations in TRPV4 in patients with these hereditary neuropathies. Neurodegeneration caused by Ca2+ overload due to the gain-of-function mutation of TRPV4 was suggested as the molecular mechanism for the neuropathies. Despite the importance of TRPV4 mutations in causing neuropathies, the precise role of TRPV4 in the sensory/motor neurons is unknown. Here, we report that TRPV4 mediates neurotrophic factor-derived neuritogenesis in developing peripheral neurons. TRPV4 was found to be highly expressed in sensory and spinal motor neurons in early development as well as in the adult, and the overexpression or chemical activation of TRPV4 was found to promote neuritogenesis in sensory neurons as well as PC12 cells, whereas its knockdown and pharmacologic inhibition had the opposite effect. More importantly, nerve growth factor or cAMP treatment up-regulated the expression of phospholipase A2 and TRPV4. Neurotrophic factor-derived neuritogenesis appears to be regulated by the phospholipase A2-mediated TRPV4 pathway. These findings show that TRPV4 mediates neurotrophic factor-induced neuritogenesis in developing peripheral nerves. Because neurotrophic factors are essential for the maintenance of peripheral nerves, these findings suggest that aberrant TRPV4 activity may lead to some types of pathology of sensory and motor nerves.
Introduction
Spinal muscular atrophy, Charcot-Marie-Tooth disease, and hereditary motor and sensory neuropathy type II are heterogeneous genetic disorders that affect peripheral nerves (1, 2). Patients with these congenital neuropathies experience muscle weakness in scapular and peroneal muscles or the diaphragm, or develop laryngeal palsy (3). These neuropathies are classified as axonal, nondemyelinating degenerations of sensory and motor nerves because nerve conduction velocities are unchanged (1, 2, 4). Recent genetic linkage studies in patients indicate that mutations in the ankyrin repeat domains of TRPV4 are implicated in the neuropathies (5–8). The gain-of-function mutations in the ankyrin repeat domain of TRPV4 are known to cause degenerative diseases involving peripheral nerves (6, 7, 9). Gain-of-function mutants such as R269H and R316C elicit increased current responses to TRPV4 agonists as well as Ca2+ loading to cells. These Ca2+ overloads caused by constitutive activities of TRPV4 mutants are believed to be a leading cause of neurodegeneration of peripheral nerves (6, 9). Although the association between TRPV4 mutation and motor/sensory neuropathies has been clearly established, the physiological function of TRPV4 in sensory and motor neurons in the normal condition remains unknown.
TRPV4 is a nonselective cationic channel with six transmembrane domains and six ankyrin repeats in its N terminus, and was originally found to be an osmosensor (10, 11). However, TRPV4 is now known to be a versatile channel that is activated by a variety of physical stimuli, such as, innocuous heat and mechanical stress, and by various endogenous or synthetic chemicals (12). In particular, arachidonic acid and its metabolite, 5,6-epoxyeicosatrienoic acid (5,6-EET)2 and anandamide are endogenous activators of TRPV4 (13), as are phorbol esters like 4α-phorbol 12,13-didecanoate (4α-PDD) (14). Besides peripheral neuropathy, mutations in TRPV4 are known to cause skeletal dysplasias and arthropathy of hands and feet (15, 16). TRPV4 is widely expressed in many cell types, such as, urothelial cells, airway epithelial cells, epidermal keratinocytes, smooth muscle cells, and chondrocytes (12), but its expression in brain is limited only to the hippocampus and circumventricular organs (2, 17, 18). In contrast to its weak expression in the brain, TRPV4 is well expressed in sensory neurons such as dorsal root and trigeminal ganglion neurons and autonomic neurons in the sympathetic ganglia (12).
Neurite sprouting, elongation, and neuritogenesis are important events in early neuronal differentiation, and form the basis of proper neuronal connectivity and brain function. Furthermore, neurotrophins, such as, nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and glial cell-derived neurotrophic factor (GDNF), are important regulators of neuritogenesis and closely connected with the pathophysiological mechanisms of human neuropathies (19). NGF is a multimeric protein that promotes nerve growth and differentiation in the nervous system (20), and is widely expressed in the developing brain (21). Moreover, mutations of TrKA (a NGF receptor) have been found in hereditary and developmental neuropathies (22). On the other hand, BDNF is known to promote neurite growth and the differentiation of cochleovestibular ganglion neurons (23), and to decrease preganglionic synaptic innervation to sympathetic neurons in BDNF-deficient mice (24). GDNF has been reported to induce neurotrophic activity in developing and mature motor neurons of the brain and spinal cord (25), and to enhance neurite branching and elongation from developing dopamine neurons (26). Thus, it appears that neurotrophins are indispensable for the genesis and maintenance of peripheral nerves.
Control of cytoskeleton assembly is essential for axonal growth (27), and NGF induces rapid ruffling and cytoskeletal rearrangements (28), whereas BDNF increases the filopodial dynamics of growth cone regulating actin depolymerization factor (29). Furthermore, BDNF- and GDNF-induced neuronal differentiations require cAMP up-regulation (30), whereas NGF promotes neurite growth in a cAMP-independent manner (31). It has also been established that these neurotrophins use the mitogen-activated protein kinase (MAPK) pathway to promote neurite growth (32), and this pathway is known to regulate scaffold and cytoskeletal dynamics in response to neurotrophic factors (33). However, the molecular mechanisms responsible for actin assembly downstream of MAPK have not been established in the context of neurotrophin-induced neuritogenesis.
Because TRPV4 dysfunction is linked to peripheral congenital neuronal degeneration, we considered that TRPV4 might control neuritogenesis in the developing peripheral neurons. Thus, the main goal of the present study was to determine whether TRPV4 relates axonal growth in peripheral nerves.
EXPERIMENTAL PROCEDURES
Cell Culture
Pheochromocytoma cell 12 (PC12) cells were grown on collagen-coated plates in DMEM supplemented with 10% horse serum and 5% fetal bovine serum. Differentiation was conducted in DMEM supplemented with 2% horse serum, 1% fetal bovine serum, and nerve growth factor (100 ng/ml, Invitrogen), which was changed every 2 or 3 days, until differentiation was complete (5–7 days). Dorsal root ganglion (DRG) neurons were dissected in neonatal rats, plated on poly-d-lysine and laminin-treated coverslips, and incubated at 37 °C in 95% air, 5% CO2.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Total RNA was extracted using easy-BLUETM solution (iNtRON Biotech), and reverse transcribed using a Transcriptor First Strand cDNA Synthesis Kit (Roche Applied Science). RT-PCR was performed as per the manufacturer's instructions using sets of specific primers, namely TRPV4 (5′-ACC ACG GTG GAC TAC CTG AG-3′ and 5′-GAT TCA GCA GGG TCA CCA GA-3′) and cPLA2 (5′-ATT CTC CGG TGT GAT GAA GG-3′ and 5′-GTC CGG TTT GAC ATG GAG AC-3′).
Small Interfering RNA (siRNA) Treatment
Nucleotide oligomer siRNA (40 μm) targeting rat TRPV4 (5′-AGA UCG AGA ACC GCC AUG A-3′) or scrambled siRNA (5′-CCU ACG CCA CCA AUU UCG U-3′) labeled with fluorescein were transfected into PC12 cells using Lipofectamine 2000TM (Invitrogen) 1 day after plating.
Measurement of Neurite Lengths
The lengths of neurites of PC12 cells and DRG neurons were measured using ImageJ software (National Institute of Health) after acquiring images using a fluorescent microscope. Percentages of cells bearing neurites were determined by counting more than 100 cells in randomly chosen fields. Cells with processes longer than a cell diameter were counted as positive. Cells with multiple processes were counted only once, and cell aggregates were disregarded. The neurite lengths of cortical neurons were determined using NeuriteTracer software.
Calcium Imaging
Cells were loaded with Fluo3-AM (5 μm, Invitrogen) containing 0.1% Pluronic F-127 (Invitrogen). To follow the temporal course of [Ca2+]i, intensities of fluorescent images were measured at 488 nm every 3 s. Twenty-four hours after transfection with human TRPV4 wild type or mutant TRPV4P19S using FuGENE® HD (Roche Applied Science), basal [Ca2+]i levels were determined by measuring fluorescent intensities after incubating cells with Fluo3-AM for 40 min.
Immunocytochemistry
PC12 cells and DRG neurons cultured on round coverslips were fixed with 4% paraformaldehyde. Mouse monoclonal antibody raised against rat Tuj-1 (neuron-specific class III β-tubulin; 1:1000 dilution; Abcam) was incubated overnight at 4 °C. The primary antibodies were bathed for 1 h at 37 °C with Alexa Fluor 488-conjugated goat anti-mouse IgG (diluted 1:1000; Molecular Probes).
Immunohistochemistry
After being anesthetized, mice were perfused transcardially with saline followed by a phosphate-buffered saline containing 4% paraformaldehyde. Spinal cords and DRGs were excised and placed in 4% paraformaldehyde for 24 h. Fixed tissues were then embedded in wax, sectioned at 5 μm, and placed on slides. After de-waxing, immunohistochemical staining was performed using antisera to TRPV4 (Abcam), NF-M (Santa Cruz Biotechnology), TRPV1 (Millipore), NeuN (Millipore), or GFAP (Millipore). Sections were then preincubated for 1 h in a solution containing 4% bovine serum albumin and 0.05% Tween 20, and incubated overnight at 4 °C in a solution containing primary antibodies. Finally, they were incubated for 1 h at 37 °C with Alexa Fluor 488-conjugated goat anti-rabbit IgG or Alexa Fluor 546-conjugated goat anti-mouse IgG (diluted 1:1000; Molecular Probes).
Western Blot
PC12 cells were lysed with PRO-PREPTM protein extraction solution (iNtRON Biotech). The lysates were subjected to a 8% SDS-PAGE gel and transferred to PVDF membrane using the semi-dry transfer cell (Bio-Rad). The membranes were treated for 1 h with TBS-T solution (20 mm Tris/HCl, 500 mm NaCl, 0.1% Tween 20) containing 2–5% skimmed milk powder and then incubated with polyclonal antibodies against cPLA2 (Santa Cruz Biotechnology), α-tubulin (Millipore), and TRPV4 (Abcam) overnight at 4 °C on a rotary shaker. The membranes were washed three times (10 min each) in the TBS-T solution, incubated with secondary antibody for 1 h, and washed three times with the TBS-T solution. The West-oneTM ECL solution (iNtRON Biotech) was poured onto the membranes and exposed to x-ray film.
Plate Shaking Assay
PC12 cells were cultured on 6-well plates to a maximum confluence of 80%, and then treated with NGF and/or TRPV4 activators for 1 day. Plates were rotated on a shaker at 250 rpm for 1 h (NGF un-treated cells were rotated for 30 min), and cells were washed three times with phosphate-buffered saline. The remaining adherent cells were fixed with 4% paraformaldehyde, stained with Hoechst 33342, and counted.
Statistical Analysis
All results are expressed as mean ± S.E. Significances of differences were determined by one-way analysis of variance followed by the Turkey's post-hoc test for multiple comparisons. Statistical significance was accepted at p values of: *, < 0.05; **, < 0.01; or ***, < 0.001, as indicated.
RESULTS
TRPV4 Was Highly Expressed in Developing Peripheral Neurons
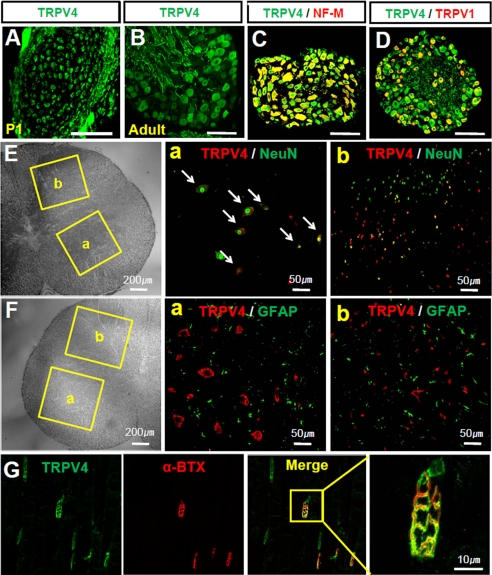
Initially, we examined the expression of TRPV4 in sensory neurons in DRGs and motor neurons in the spinal cord of neonatal or adult mice. Dense TRPV4 immunoreactivity was observed in DRG neurons at postnatal day 1 (P1) (Fig. 1A), and a similar pattern was also found in the DRGs of adult mice, in which TRPV4 immunoreactivity was found in the somata and nerve trunks of DRG neurons (Fig. 1B). TRPV4-positive cells were widely distributed in both small and large DRG neurons in adult mice, in which it co-localized with neurofilament M (a marker of myelinated DRG neurons; Fig. 1C) and with TRPV1 (a marker of nociceptors; Fig. 1D). In neonatal spinal cords, TRPV4 was expressed in neurons of the ventral and dorsal horn. In the ventral horn, TRPV4 immunoreactivity was observed in large motor neurons, which also stained for NeuN, a neuron marker (Fig. 1E). However, TRPV4 immunoreactivity was not colocalized with GFAP (a glial cell marker; Fig. 1F). Thus, TRPV4 was found to be expressed primarily in large motor neurons but not glial cells in the ventral horn of the spinal cord in neonates. In the dorsal horn, small TRPV4 positive interneurons were visible in deep laminae. However, a small number of neurons in superficial layers stained for TRPV4. As was observed in the ventral horn, TRPV4 immunoreactivity in the dorsal horn rarely co-localized with GFAP (Fig. 1F). Furthermore, TRPV4 immunoreactivity is also present in the neuromuscular junction as colocalized with fluorophore-conjugated α-bungarotoxin, a marker for the neuromuscular junction (Fig. 1G). These results suggest that TRPV4 is highly expressed in sensory and motor neurons in neonatal as well as adult mice.
FIGURE 1.
TRPV4 was expressed in early developing peripheral neurons. A, immunostaining of neonatal mouse (P1) DRG neurons with anti-TRPV4, showing TRPV4 enrichment in sensory neurons. Scale bar, 100 μm. B, immunostaining of adult mouse (P1) DRG neurons with anti-TRPV4, showing TRPV4 enrichment in soma and nerve fibers. Scale bar, 25 μm. C and D, adult mouse DRG neurons were double stained with anti-TRPV4, neurofilament M (NF-M, a marker of myelinated neurons), and TRPV1 (a marker of nociceptors). TRPV4 was found to be expressed in both small and large neurons. Scale bar, 25 μm. E, immunostaining of adult mouse spinal cord with anti-TRPV4 and NeuN. Immunofluorescence for TRPV4 was enhanced in large motor neurons in the ventral horn (a) and in small interneurons in the dorsal horn of the spinal cord (b). F, immunostaining of an adult mouse spinal cord with anti-TRPV4 and GFAP (a glial cell marker). TRPV4 immunoreactivity was not found to colocalize with that of GFAP in the ventral (a) and dorsal horn (b) of the spinal cord. G, immunostaining of an adult mouse gastrocnemius muscles with anti-TRPV4 and α-bungarotoxin (α-BTX), a neuromuscular junction marker. α-Bungarotoxin was conjugated with Alexa Fluor® 555.
TRPV4 Transcripts Levels Were Increased during Neurite Outgrowth
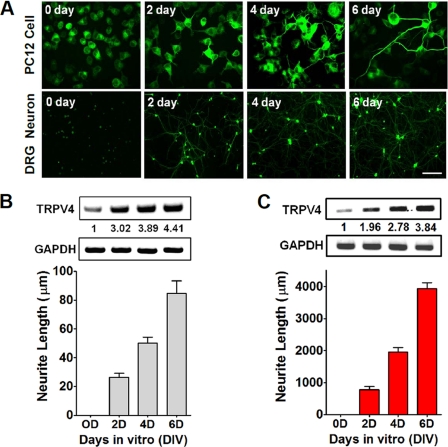
Because TRPV4 mutations are associated with peripheral neuropathy, we considered that the TRPV4 channel would be associated with neuritogenesis. To address this, we investigated the temporal expression patterns of the TRPV4 gene during neuronal outgrowth in PC12 cells and DRG neurons after neurites had been stimulated with NGF. When NGF was applied to cells for 6 days, outgrowing neurites stained for β-tubulin were visible (Fig. 2A). Furthermore, TRPV4 transcript levels increased in proportion to the lengths of dendritic or axonic neurites of PC12 cells or neonatal DRG neurons (Fig. 2, B and C).
FIGURE 2.
TRPV4 transcripts increased neurite outgrowth. A, NGF induced-neuritogenesis in PC12 cells and primary cultures of DRG neurons for 6 days. To better visualize neurites (green), cells were stained with anti-TuJ1 conjugated with Alexa Fluor® 488 goat anti-mouse IgG. Scale bar, 10 μm. B, increase of TRPV4 transcript levels in NGF-treated PC12 cells. mRNA levels were quantified by RT-PCR. Data were normalized versus GAPDH. C, increases in TRPV4 transcript levels in primary cultured DRG neurons. mRNA levels were quantified by RT-PCR. Data were normalized versus GAPDH.
Effects of Knockdown and Overexpression of TRPV4 on Neuritogenesis
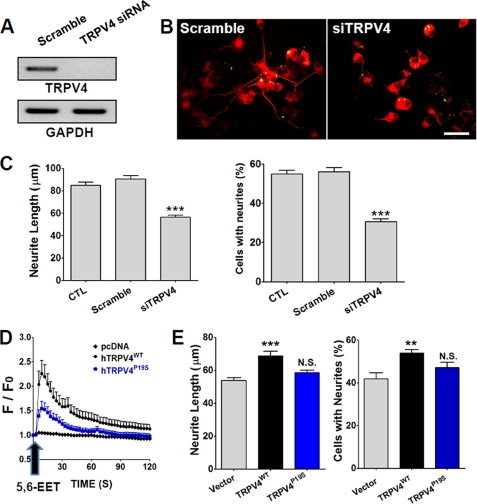
The positive correlation found between the TRPV4 transcript level and neurite length suggested that TRPV4 promotes neuritogenesis. To determine the functional role of TRPV4 in this process further, we knocked down TRPV4 using siRNA and examined its effects on neurite growth in PC12 cells. TRPV4 siRNA-treated cells showed an obvious reduction in transcript level as compared with scrambled siRNA-treated cells (Fig. 3A). Furthermore, TRPV4 siRNA significantly reduced (p < 0.001, n = 222–318) neurite length and the percentage of PC12 cells with neurites (Fig. 3, B and C). On the other hand, the application of scrambled siRNA failed to affect neurite outgrowth or the percentage of cells with neurites, indicating that the TRPV4 channel is required for normal neuritogenesis.
FIGURE 3.
Effects of TRPV4 siRNA and TRPV4 overexpression on neurite outgrowth. A, TRPV4 mRNA levels were measured by RT-PCR in control and RNAi-treated groups in PC12 cells. GAPDH was used as a positive control. B and C, TRPV4 siRNA-transfected cells showed less neurite outgrowth and fewer cells with neurites than scrambled siRNA-treated PC12 cells. Scale bar, 10 μm. D, when treated with 5,6-EET, cells expressing TRPV4 (TRPV4WT) showed an immediate increase in [Ca2+]i. In contrast, a loss of function mutant of TRPV4 (TRPV4P19S) evoked a markedly lower Ca2+ response to 5,6-EET. E, the overexpression of TRPV4WT promoted neurite growth and increased the number of cells with neurites.
TRPV4 was then overexpressed in PC12 cells and its effect on neuritogenesis examined. TRPV4 channel activities were confirmed by Ca2+ imaging using Fluo-3AM (a Ca2+-sensitive fluorescent dye). TRPV4 expressing cells displayed a robust Ca2+ increase after treatment with 5,6-EET, an endogenous activator of TRPV4 (13) (Fig. 3D), whereas a Ca2+ increase was barely detected in cells transfected with vector alone. Furthermore, TRPV4P19S (a loss of function mutant of human TRPV4) elicited a significant reduction in Ca2+ influx (34). After culture for 6 days with NGF supplement, transfection of TRPV4WT into PC12 cells significantly increased neurite length and the percentage of cells bearing neurites as compared with control PC12 cells transfected with empty vector (Fig. 3E). Furthermore, transfection of TRPV4P19S failed to increase neurite length or the percentage of cells bearing neurites. These results show that TRPV4 promotes neuritogenesis.
Pharmacological Perturbation of TRPV4 Alters Neuritogenesis
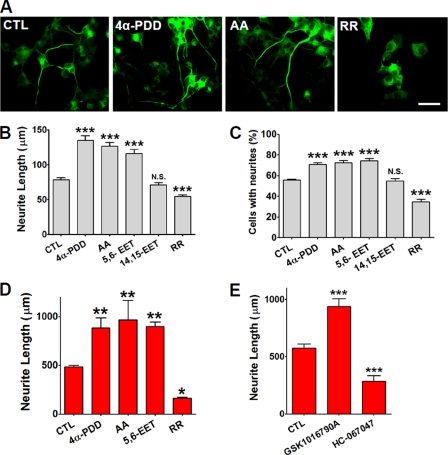
To determine whether TRPV4 actively controls neuritogenesis in primary cultures of DRG neurons or PC12 cells, pharmacological agonists or antagonists of TRPV4 were applied. 4α-PDD, 5,6-EET, and arachidonic acid (AA) are synthetic or endogenous activators of TRPV4 (13, 14). Treatment with 10 μm 4α-PDD or AA significantly increased neurite length and the percentages of cells bearing neurites in NGF-treated PC12 cells and DRG neurons (p < 0.001, n = 196–226) (Fig. 4, A–D). Furthermore, treatment with 5,6-EET also significantly increased neurite lengths and the percentages of neurite bearing cells in both cell types (Fig. 4, B–D). However, 14,15-EET, an inactive metabolite of AA in terms of activating TRPV4, failed to increase neurite length (Fig. 4, B and C) (13). On the other hand, pharmacological blocking with ruthenium red (a pan-TRP channel blocker) completely prevented neurite extension in DRG and PC12 cells (Fig. 4, A–D). Moreover, application of GSK1016790A, a newly synthesized agonist increases the neurite length in DRG neurons (Fig. 4E) and PC12 cells (supplemental Fig. S1A), whereas HC-067047, a newly synthesized antagonist of TRPV4 reversely decreases the neuritogenesis in DRG neurons (Fig. 4E) and PC12 cells (supplemental Fig. S1B) (35, 36). These results further suggest that TRPV4 participates in the control of neurite length.
FIGURE 4.
Modulation of neuritogenesis with agonists or antagonists of TRPV4. A, neurite outgrowth was observed when PC12 cells were treated with 4α-PDD or AA, but was rarely observed in cells treated with ruthenium red (RR) (a pan-TRP channel blocker). PC12 cells were immunostained with anti-Tuj1 conjugated with Alexa Fluor 488 goat anti-mouse IgG (green), and cultured for 6 days with NGF. B-D, treatment with 4α-PDD, AA, or 5,6-EET (all TRPV4 activators) significantly increased neurite lengths (B) and the percentages of PC12 cells bearing neurites (C). In contrast, 14,15-EET (an inactive metabolite of cytochrome P450 in terms of activating TRPV4) failed to increase the neurite lengths of PC12 cells or DRG neurons in primary culture (D). Scale bar, 10 μm. E, treatment of DRG neurons with GSK1016790A, a selective activator of TRPV4 significantly augmented, whereas treatment with HC-067047, a selective antagonist reduced neuritogenesis.
NGF-induced Neuritogenesis Required TRPV4 Activity via PLA2 Pathway
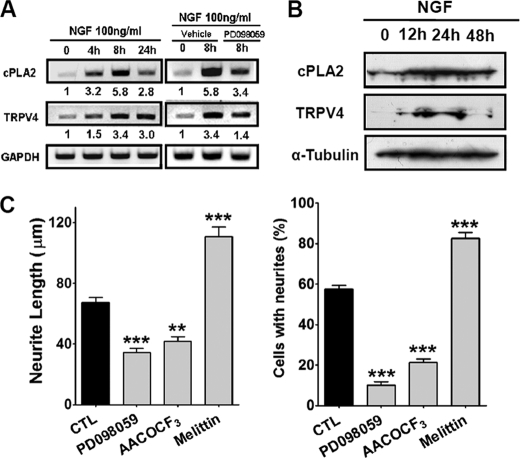
We then sought to determine the signals upstream of TRPV4. Because NGF is required for neurite growth of sensory neurons (22) and NGF-treated neuronal cells showed elevated TRPV4 expression during neurite growth (Fig. 2), we hypothesized that NGF utilizes TRPV4 to control neurite growth. Furthermore, because NGF would stimulate cytosolic phospholipase A2 (PLA2, an enzyme that releases AA from phosphoglycerides) (37), we considered that AA and its metabolite, 5,6-EET, were found to stimulate neuritogenesis. Therefore, we examined whether NGF regulates the expression levels of PLA2 and TRPV4. Remarkably, when PC12 cells were treated with NGF, the PLA2 and TRPV4 transcript levels were markedly increased (Fig. 5A). Furthermore, this increase in PLA2 transcript levels was reduced by disrupting the NGF signaling pathway with PD98059, a MEK inhibitor (Fig. 5A). The increase in protein expression of PLA2 and TRPV4 in Western blots was also observed after NGF treatment (Fig. 5B). We then examined whether perturbation of the activity of PLA2 affects neuritogenesis. Application of melittin (a PLA2 activator) markedly stimulated NGF-induced neurite growth, whereas AACOCF3 (a PLA2 inhibitor) significantly retarded this growth (p < 0.01, n = 76) (Fig. 5C). These results suggest that NGF promotes neuritogenesis via the PLA2/TRPV4 pathway.
FIGURE 5.
The NGF-induced MAPK pathway regulated TRPV4. A, NGF (100 ng/ml) increased the levels of PLA2 and TRPV4 transcripts in PC12 cells, and these increases were inhibited by PD098059 (a MEK inhibitor). GAPDH mRNA was use to normalize PLA2 and TRPV4 mRNA levels. B, NGF (100 ng/ml) increased the protein expression of PLA2 and TRPV4 in PC12 cells probed by Western blot. α-Tubulin was blotted as a control. C, inhibitors of PLA2 (AACOCF3) and MEK (PD098059) suppressed NGF-induced neurite growth in PC12 cells, whereas an activator of PLA2 (melittin) promoted NGF-induced neurite growth.
cAMP-induced Neuritogenesis Required TRPV4 Activity
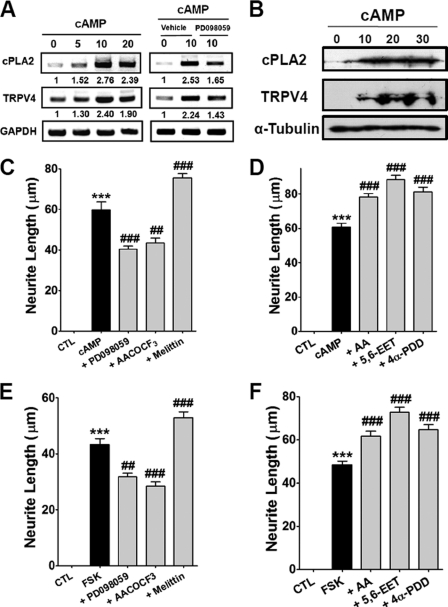
The up-regulation of intracellular cAMP is known to promote neuronal survival, cell growth, and differentiation, and cAMP induces neuronal outgrowth via the B-Raf/MAP kinase pathway (38). In addition, combined stimulation with cAMP and NGF has been reported to have an additive effect on neuronal differentiation via MAPK activation (39), which raises questions regarding whether cAMP-induced neuritogenesis also utilizes TRPV4 for neuronal outgrowth. To test this possibility, we examined the effects of the pharmacological disruption of the PLA2/TRPV4 pathway on cAMP-stimulated neurite outgrowth. As shown in Fig. 6, A and B, the transcript and protein levels of PLA2 and TRPV4 progressively increased after cAMP treatment. Furthermore, these increases were reduced by PD098059 (Fig. 6A).
FIGURE 6.
TRPV4 regulated cAMP-dependent neurite growth. A, treatment of PC12 cells with 1 mm cAMP increased the transcript levels of PLA2 and TRPV4, but co-treatment with PD098059 inhibited these transcript level increases. B, treatment of 1 mm cAMP increased the protein expression of PLA2 and TRPV4. C and D, PC12 cells treated with 1 mm cAMP showed markedly enhanced neurite growth, and co-treatment with 250 nm melittin augmented this growth, whereas co-treatment with 10 μm PD098095 or 20 μm AACOCF3 suppressed cAMP-induced neurite growth (C). The TRPV4 activators, AA, 5,6-EET, and 4α-PDD augmented cAMP-induced neurite growth (D). ***, p < 0.001 versus untreated cells (CTL); ###, p < 0.001 versus cAMP-treated cells (cAMP). E and F, treatment of PC12 cells with 10 μm forskolin (FSK) significantly promoted neurite growth, and co-treatment with melittin augmented this growth, but co-treatment with PD098095 or AACOCF3 suppressed FSK-induced neurite growth (E). The TRPV4 activators, AA, 5,6-EET, and 4α-PDD augmented cAMP-induced neurite growth (F).
As was observed for NGF, cAMP treatment markedly promoted neurite growth (Fig. 6, C and D), and cAMP-induced neurite growth was significantly suppressed by PD098059 and AACOCF3. In contrast, melittin significantly augmented neurite growth to a level greater than that shown by cAMP-treated cells (Fig. 6, C and D). Forskolin (an activator of adenylyl cyclase) also promoted neurite growth, and this was significantly suppressed by preincubation with PD098059 or AACOCF3 (Fig. 6, E and F). In addition, all endogenous or synthetic activators of TRPV4 augmented cAMP- and forskolin-induced neurite growth (Fig. 6F and supplemental Fig. S2).
TRPV4 Increased Actin Polymerization and Cell Adhesion
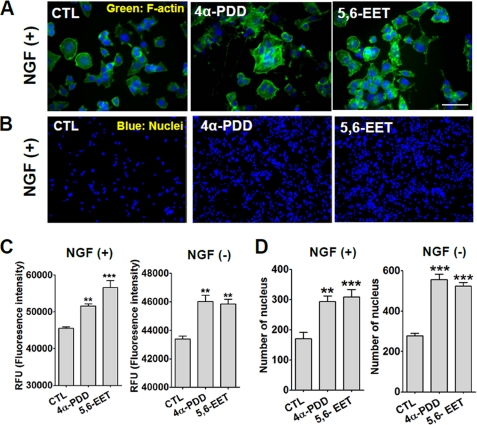
Microtubule assembly and disassembly are required for cytoskeleton rearrangement (27), and NGF-induced neuritogenesis is accompanied by cytoskeleton reorganization (40). If TRPV4 regulates NGF-induced neuritogenesis, it probably affects actin polymerization. We found that when stained with F-actin antibody, treatment of NGF-stimulated PC12 cells with 4α-PDD or 5,6-EET significantly increased actin polymerization (Fig. 7, A–C). Notably even in the absence of NGF, these TRPV4 agonists increased actin polymerization (Fig. 7C), although to a lesser degree than in the presence of NGF, implying that active TRPV4 is a potent cytoskeleton modifier.
FIGURE 7.
TRPV4 activation increased actin polymerization and cell adhesion. A, fluorescent photomicrograph of phalloidin, a marker for F-actin. In the presence of NGF, treatment with 10 μm 4α-PDD or 300 nm 5,6-EET induced morphological change and increased phalloidin fluorescence intensities. Phalloidin was conjugated with Alexa Fluor 488. Nuclei were stained with Hoechst 33342. Scale bar, 10 μm. NGF and TRPV4 activators were applied to cells for 24 h before fixation for staining. B, after shaking, numbers of attached cells were markedly greater after treating cells with 4α-PDD or 5,6-EET. Cell numbers were determined by staining nuclei with Hoechst 33342. C, quantification of phalloidin fluorescence intensities. 4α-PDD and 5,6-EET increased the phalloidin fluorescence intensities of cells treated with or without NGF. D, summary of the effect of treatment with TRPV4 agonists (4α-PDD or 5,6-EET) on numbers of attached cells. Note that TRPV4 activators significantly increased the number of cells attached to plates after shaking.
Cell adhesion is also required for neuritogenesis during brain development (41), which suggests that if TRPV4 regulates neuritogenesis, it might also affect cell adhesion. To examine this possibility, we counted numbers of cells (by nuclear staining) that remained attached to a plate after shaking. When PC12 cells were treated 10 μm 4α-PDD or 300 nm 5,6-EET for 24 h, the number of attached cells after shaking for 1 h was significantly increased (p < 0.01, n = 15) (Fig. 7, B–D). This increase in attached cell numbers by 4α-PDD or 5,6-EET was observed in cells not treated with NGF, which confirmed that TRPV4 is the downstream signal to NGF that promotes actin polymerization and cell adhesion.
DISCUSSION
The present study shows that TRPV4 is required for regulating neuritogenesis in developing neurons. TRPV4 was observed in sensory and motor neurons in neonatal and adult mice, and TRPV4 transcript levels increased as the neurites of DRG neurons grew. Furthermore, knockdown of TRPV4 retarded NGF-induced neurite growth, whereas its overexpression enhanced neurite growth, and the pharmacological inhibition of TRPV4 activity inhibited neuritogenesis. Furthermore, NGF, which is essential for peripheral neuron survival, up-regulated TRPV4 and PLA2, presumably via the MAPK pathway. Thus, PLA2 up-regulation presumably resulted in the release of AA and 5,6-EET, which activated TRPV4 and increased [Ca2+]i for neuritogenesis.
Interactions between target tissues and innervated nerves are essential for the maintenance and survival of sensory nerves, and if these interactions are disrupted, the neuropathy results. Neutrophins like NGF, BDNF, and GDNF mediate nerve-tissue interactions and are also required for peripheral nerve survival (24, 42, 43). NGF depletion causes severe sensory and sympathetic neuron loss (44). In mice with peripheral neuropathy, NGF promoted the recovery of peripheral nerves (45). Schwann cells are known to release NGF and promote DRG neuron axonal growth by secreting NGF (46), and thus, Schwann cell dysfunction affects the survival, maintenance, and differentiation of peripheral nerves. The adhesion of Schwann cells to extracellular matrix is crucial for peripheral nerve morphogenesis, growth, and myelination, and mutations in laminin or integrin subunit in Schwann cells have been shown to cause inherited peripheral neuropathy (47, 48).
Cytosolic Ca2+ controls axonal and dendritic growth and guidance, and these processes are critically dependent on the reorganization of the actin cytoskeleton by many actin-binding proteins. Thus, abnormal Ca2+ homeostasis has been implicated in the induction of axonal degeneration in peripheral neuropathies (49). Furthermore, increases in intracellular Ca2+ regulate protein kinases, such as, Rho-associated kinase, myosin light chain kinase, and LIM kinases, which modify actin dynamics (50–52). Local changes in Ca2+ transients involve Ca2+-dependent signals for neurite growth or retraction (53). Thus, because TRPV4 is Ca2+ permeable, the activation of TRPV4 evokes an increase in local Ca2+ transients that probably regulate actin polymerization.
Neurons are differentiated via morphogenetic changes in response to mechanical tension, which is a potent stimulator of neurite growth, and it has been shown that osmotic dilution in culture medium promotes neurite growth by making axons more sensitive to tension (54). Furthermore, osmotic pressure change is known to regulate cell surface area and volume adjustments via F-actin dynamics (55). Originally, TRPV4 was found to be activated by hypotonicity-induced cell swelling (10, 11). Subsequently, it was found that TRPV4-deficient mice show an osmotic sensing impairment in the central nervous system (56). Cell swelling indirectly activates TRPV4 via the production of 5,6-EET by the PLA2/AA pathway (57). Similarly, TRPV2 that is activated by membrane stretching also enhances axon outgrowth in developing sensory and motor neurons (58). Thus, these reports further substantiate the regulation of neuritogenesis by TRPV4.
Summarizing, TRPV4 was found to be highly expressed in peripheral sensory/motor nerves, and its activation was found to regulate NGF or cAMP-induced neurite outgrowth. Furthermore, NGF or cAMP up-regulated TRPV4 and its upstream signal PLA2, and thus, TRPV4 appears to be critical for mediating neurotrophic factor-derived neurite growth in peripheral nerves. Despite the association of aberrant TRPV4 activity with congenital sensory/motor neuropathies, its physiological role in sensory/motor neurons has not been introduced. The present study shows that TRPV4 is essential for axonal growth of sensory/motor nerves in early development as well as for their maintenance in the adult. Thus, congenital alterations in TRPV4 activity are likely to result in some types of hereditary sensory/motor neuropathies, even though they may not be the major one.
Supplementary Material
This work was supported by Grant R31-2008-000-10103-0 from the World Class University program and Creative Research Initiatives Program Grant 20110018358 of the Ministry of Education Science and Technology and the National Research Foundation of Korea.

This article contains supplemental Figs. S1 and S2.
- 5,6-EET
- 5,6-epoxyeicosatrienoic acid
- 4α-PDD
- 4α-phorbol 12,13-didecanoate
- DRG
- dorsal root ganglion
- BDNF
- brain-derived neurotrophic factor
- GDNF
- glial cell-derived neurotrophic factor
- AA
- arachidonic acid
- PLA2
- phospholipase A2.
REFERENCES
- 1. Reilly M. M., Murphy S. M., Laura M. (2011) Charcot-Marie-Tooth disease. J. Peripher. Nerv. Syst. 16, 1–14 [DOI] [PubMed] [Google Scholar]
- 2. Drew A. P., Blair I. P., Nicholson G. A. (2011) Molecular genetics and mechanisms of disease in distal hereditary motor neuropathies. Insights directing future genetic studies. Curr. Mol. Med. 11, 650–665 [DOI] [PubMed] [Google Scholar]
- 3. DeLong R., Siddique T. (1992) A large New England kindred with autosomal dominant neurogenic scapuloperoneal amyotrophy with unique features. Arch. Neurol. 49, 905–908 [DOI] [PubMed] [Google Scholar]
- 4. Shy M. E., Patzkó A. (2011) Axonal Charcot-Marie-Tooth disease. Curr. Opin. Neurol. 24, 475–483 [DOI] [PubMed] [Google Scholar]
- 5. Auer-Grumbach M., Olschewski A., Papi L., Kremer H., McEntagart M. E., Uhrig S., Fischer C., Fröhlich E., Bálint Z., Tang B., Strohmaier H., Lochmüller H., Schlotter-Weigel B., Senderek J., Krebs A., Dick K. J., Petty R., Longman C., Anderson N. E., Padberg G. W., Schelhaas H. J., van Ravenswaaij-Arts C. M., Pieber T. R., Crosby A. H., Guelly C. (2010) Alterations in the ankyrin domain of TRPV4 cause congenital distal SMA, scapuloperoneal SMA, and HMSN2C. Nat. Genet. 42, 160–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deng H. X., Klein C. J., Yan J., Shi Y., Wu Y., Fecto F., Yau H. J., Yang Y., Zhai H., Siddique N., Hedley-Whyte E. T., Delong R., Martina M., Dyck P. J., Siddique T. (2010) Scapuloperoneal spinal muscular atrophy and CMT2C are allelic disorders caused by alterations in TRPV4. Nat. Genet. 42, 165–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Landouré G., Zdebik A. A., Martinez T. L., Burnett B. G., Stanescu H. C., Inada H., Shi Y., Taye A. A., Kong L., Munns C. H., Choo S. S., Phelps C. B., Paudel R., Houlden H., Ludlow C. L., Caterina M. J., Gaudet R., Kleta R., Fischbeck K. H., Sumner C. J. (2010) Mutations in TRPV4 cause Charcot-Marie-Tooth disease type 2C. Nat. Genet. 42, 170–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zimoń M., Baets J., Auer-Grumbach M., Berciano J., Garcia A., Lopez-Laso E., Merlini L., Hilton-Jones D., McEntagart M., Crosby A. H., Barisic N., Boltshauser E., Shaw C. E., Landouré G., Ludlow C. L., Gaudet R., Houlden H., Reilly M. M., Fischbeck K. H., Sumner C. J., Timmerman V., Jordanova A., Jonghe P. D. (2010) Dominant mutations in the cation channel gene transient receptor potential vanilloid 4 cause an unusual spectrum of neuropathies. Brain 133, 1798–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fecto F., Shi Y., Huda R., Martina M., Siddique T., Deng H. X. (2011) Mutant TRPV4-mediated toxicity is linked to increased constitutive function in axonal neuropathies. J. Biol. Chem. 286, 17281–17291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liedtke W., Choe Y., Martí-Renom M. A., Bell A. M., Denis C. S., Sali A., Hudspeth A. J., Friedman J. M., Heller S. (2000) Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 103, 525–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Strotmann R., Harteneck C., Nunnenmacher K., Schultz G., Plant T. D. (2000) OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat. Cell. Biol. 2, 695–702 [DOI] [PubMed] [Google Scholar]
- 12. Everaerts W., Nilius B., Owsianik G. (2010) The vanilloid transient receptor potential channel TRPV4. From structure to disease. Prog. Biophys. Mol. Biol. 103, 2–17 [DOI] [PubMed] [Google Scholar]
- 13. Watanabe H., Vriens J., Prenen J., Droogmans G., Voets T., Nilius B. (2003) Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 424, 434–438 [DOI] [PubMed] [Google Scholar]
- 14. Watanabe H., Davis J. B., Smart D., Jerman J. C., Smith G. D., Hayes P., Vriens J., Cairns W., Wissenbach U., Prenen J., Flockerzi V., Droogmans G., Benham C. D., Nilius B. (2002) Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J. Biol. Chem. 277, 13569–13577 [DOI] [PubMed] [Google Scholar]
- 15. Rock M. J., Prenen J., Funari V. A., Funari T. L., Merriman B., Nelson S. F., Lachman R. S., Wilcox W. R., Reyno S., Quadrelli R., Vaglio A., Owsianik G., Janssens A., Voets T., Ikegawa S., Nagai T., Rimoin D. L., Nilius B., Cohn D. H. (2008) Gain-of-function mutations in TRPV4 cause autosomal dominant brachyolmia. Nat. Genet. 40, 999–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lamandé S. R., Yuan Y., Gresshoff I. L., Rowley L., Belluoccio D., Kaluarachchi K., Little C. B., Botzenhart E., Zerres K., Amor D. J., Cole W. G., Savarirayan R., McIntyre P., Bateman J. F. (2011) Mutations in TRPV4 cause an inherited arthropathy of hands and feet. Nat. Genet. 43, 1142–1146 [DOI] [PubMed] [Google Scholar]
- 17. Benfenati V., Amiry-Moghaddam M., Caprini M., Mylonakou M. N., Rapisarda C., Ottersen O. P., Ferroni S. (2007) Expression and functional characterization of transient receptor potential vanilloid-related channel 4 (TRPV4) in rat cortical astrocytes. Neuroscience 148, 876–892 [DOI] [PubMed] [Google Scholar]
- 18. Bai J. Z., Lipski J. (2010) Differential expression of TRPM2 and TRPV4 channels and their potential role in oxidative stress-induced cell death in organotypic hippocampal culture. Neurotoxicology 31, 204–214 [DOI] [PubMed] [Google Scholar]
- 19. Anand P. (2004) Neurotrophic factors and their receptors in human sensory neuropathies. Prog. Brain Res. 146, 477–492 [DOI] [PubMed] [Google Scholar]
- 20. Levi-Montalcini R. (1987) The nerve growth factor. Thirty-five years later. EMBO J. 6, 1145–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Large T. H., Bodary S. C., Clegg D. O., Weskamp G., Otten U., Reichardt L. F. (1986) Nerve growth factor gene expression in the developing rat brain. Science 234, 352–355 [DOI] [PubMed] [Google Scholar]
- 22. Indo Y., Tsuruta M., Hayashida Y., Karim M. A., Ohta K., Kawano T., Mitsubuchi H., Tonoki H., Awaya Y., Matsuda I. (1996) Mutations in the TRKA/NGF receptor gene in patients with congenital insensitivity to pain with anhidrosis. Nat. Genet. 13, 485–488 [DOI] [PubMed] [Google Scholar]
- 23. Avila M. A., Varela-Nieto I., Romero G., Mato J. M., Giraldez F., Van De Water T. R., Represa J. (1993) Brain-derived neurotrophic factor and neurotrophin-3 support the survival and neuritogenesis response of developing cochleovestibular ganglion neurons. Dev. Biol. 159, 266–275 [DOI] [PubMed] [Google Scholar]
- 24. Causing C. G., Gloster A., Aloyz R., Bamji S. X., Chang E., Fawcett J., Kuchel G., Miller F. D. (1997) Synaptic innervation density is regulated by neuron-derived BDNF. Neuron 18, 257–267 [DOI] [PubMed] [Google Scholar]
- 25. Granholm A. C., Srivastava N., Mott J. L., Henry S., Henry M., Westphal H., Pichel J. G., Shen L., Hoffer B. J. (1997) Morphological alterations in the peripheral and central nervous systems of mice lacking glial cell line-derived neurotrophic factor (GDNF). Immunohistochemical studies. J. Neurosci. 17, 1168–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Costantini L. C., Isacson O. (2000) Immunophilin ligands and GDNF enhance neurite branching or elongation from developing dopamine neurons in culture. Exp. Neurol. 164, 60–70 [DOI] [PubMed] [Google Scholar]
- 27. Parisiadou L., Xie C., Cho H. J., Lin X., Gu X. L., Long C. X., Lobbestael E., Baekelandt V., Taymans J. M., Sun L., Cai H. (2009) Phosphorylation of ezrin/radixin/moesin proteins by LRRK2 promotes the rearrangement of actin cytoskeleton in neuronal morphogenesis. J. Neurosci. 29, 13971–13980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Connolly J. L., Greene L. A., Viscarello R. R., Riley W. D. (1979) Rapid, sequential changes in surface morphology of PC12 pheochromocytoma cells in response to nerve growth factor. J. Cell Biol. 82, 820–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gehler S., Shaw A. E., Sarmiere P. D., Bamburg J. R., Letourneau P. C. (2004) Brain-derived neurotrophic factor regulation of retinal growth cone filopodial dynamics is mediated through actin depolymerizing factor/cofilin. J. Neurosci. 24, 10741–10749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cai D., Qiu J., Cao Z., McAtee M., Bregman B. S., Filbin M. T. (2001) Neuronal cyclic AMP controls the developmental loss in ability of axons to regenerate. J. Neurosci. 21, 4731–4739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rydel R. E., Greene L. A. (1988) cAMP analogs promote survival and neurite outgrowth in cultures of rat sympathetic and sensory neurons independently of nerve growth factor. Proc. Natl. Acad. Sci. U.S.A. 85, 1257–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huang E. J., Reichardt L. F. (2001) Neurotrophins. Roles in neuronal development and function. Annu. Rev. Neurosci. 24, 677–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pullikuth A. K., Catling A. D. (2007) Scaffold mediated regulation of MAPK signaling and cytoskeletal dynamics. A perspective. Cell. Signal. 19, 1621–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tian W., Fu Y., Garcia-Elias A., Fernández-Fernández J. M., Vicente R., Kramer P. L., Klein R. F., Hitzemann R., Orwoll E. S., Wilmot B., McWeeney S., Valverde M. A., Cohen D. M. (2009) A loss-of-function nonsynonymous polymorphism in the osmoregulatory TRPV4 gene is associated with human hyponatremia. Proc. Natl. Acad. Sci. U.S.A. 106, 14034–14039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thorneloe K. S., Sulpizio A. C., Lin Z., Figueroa D. J., Clouse A. K., McCafferty G. P., Chendrimada T. P., Lashinger E. S., Gordon E., Evans L., Misajet B. A., Demarini D. J., Nation J. H., Casillas L. N., Marquis R. W., Votta B. J., Sheardown S. A., Xu X., Brooks D. P., Laping N. J., Westfall T. D. (2008) N-((1S)-1-{[4-((2S)-2-{[(2,4-Dichlorophenyl)sulfonyl]amino}-3-hydroxypropanoyl)-1-piperazinyl]carbonyl}-3-methylbutyl)-1-benzothiophene-2-carboxamide (GSK1016790A), a novel and potent transient receptor potential vanilloid 4 channel agonist induces urinary bladder contraction and hyperactivity. Part I. J. Pharmacol. Exp. Ther. 326, 432–442 [DOI] [PubMed] [Google Scholar]
- 36. Everaerts W., Zhen X., Ghosh D., Vriens J., Gevaert T., Gilbert J. P., Hayward N. J., McNamara C. R., Xue F., Moran M. M., Strassmaier T., Uykal E., Owsianik G., Vennekens R., De Ridder D., Nilius B., Fanger C. M., Voets T. (2010) Inhibition of the cation channel TRPV4 improves bladder function in mice and rats with cyclophosphamide-induced cystitis. Proc. Natl. Acad. Sci. U.S.A. 107, 19084–19089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Akiyama N., Hatori Y., Takashiro Y., Hirabayashi T., Saito T., Murayama T. (2004) Nerve growth factor-induced up-regulation of cytosolic phospholipase A2α level in rat PC12 cells. Neurosci. Lett. 365, 218–222 [DOI] [PubMed] [Google Scholar]
- 38. Vossler M. R., Yao H., York R. D., Pan M. G., Rim C. S., Stork P. J. (1997) cAMP activates MAP kinase and Elk-1 through a B-Raf- and Rap1-dependent pathway. Cell 89, 73–82 [DOI] [PubMed] [Google Scholar]
- 39. Hansen T. O., Rehfeld J. F., Nielsen F. C. (2000) Cyclic AMP-induced neuronal differentiation via activation of p38 mitogen-activated protein kinase. J. Neurochem. 75, 1870–1877 [DOI] [PubMed] [Google Scholar]
- 40. Dehmelt L., Smart F. M., Ozer R. S., Halpain S. (2003) The role of microtubule-associated protein 2c in the reorganization of microtubules and lamellipodia during neurite initiation. J. Neurosci. 23, 9479–9490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gupton S. L., Gertler F. B. (2010) Integrin signaling switches the cytoskeletal and exocytic machinery that drives neuritogenesis. Dev. Cell 18, 725–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Glebova N. O., Ginty D. D. (2004) Heterogeneous requirement of NGF for sympathetic target innervation in vivo. J. Neurosci. 24, 743–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hiltunen P. H., Airaksinen M. S. (2004) Sympathetic cholinergic target innervation requires GDNF family receptor GFR α2. Mol. Cell. Neurosci. 26, 450–457 [DOI] [PubMed] [Google Scholar]
- 44. Crowley C., Spencer S. D., Nishimura M. C., Chen K. S., Pitts-Meek S., Armanini M. P., Ling L. H., McMahon S. B., Shelton D. L., Levinson A. D. (1994) Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell 76, 1001–1011 [DOI] [PubMed] [Google Scholar]
- 45. Aloe L., Manni L., Properzi F., De Santis S., Fiore M. (2000) Evidence that nerve growth factor promotes the recovery of peripheral neuropathy induced in mice by cisplatin. Behavioral, structural, and biochemical analysis. Auton. Neurosci. 86, 84–93 [DOI] [PubMed] [Google Scholar]
- 46. Tosaki T., Kamiya H., Yasuda Y., Naruse K., Kato K., Kozakae M., Nakamura N., Shibata T., Hamada Y., Nakashima E., Oiso Y., Nakamura J. (2008) Reduced NGF secretion by Schwann cells under the high glucose condition decreases neurite outgrowth of DRG neurons. Exp. Neurol. 213, 381–387 [DOI] [PubMed] [Google Scholar]
- 47. Berti C., Nodari A., Wrabetz L., Feltri M. L. (2006) Role of integrins in peripheral nerves and hereditary neuropathies. Neuromolecular. Med. 8, 191–204 [DOI] [PubMed] [Google Scholar]
- 48. Yu W. M., Yu H., Chen Z. L. (2007) Laminins in peripheral nerve development and muscular dystrophy. Mol. Neurobiol. 35, 288–297 [DOI] [PubMed] [Google Scholar]
- 49. Fernyhough P., Calcutt N. A. (2010) Abnormal calcium homeostasis in peripheral neuropathies. Cell Calcium 47, 130–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jin M., Guan C. B., Jiang Y. A., Chen G., Zhao C. T., Cui K., Song Y. Q., Wu C. P., Poo M. M., Yuan X. B. (2005) Ca2+-dependent regulation of rho GTPases triggers turning of nerve growth cones. J. Neurosci. 25, 2338–2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tang Z., Chen H., Yang J., Dai S., Lin Y. (2005) The comparison of Ca2+/CaM-independent and Ca2+/CaM-dependent phosphorylation of myosin light chains by MLCK. Physiol. Res. 54, 671–678 [PubMed] [Google Scholar]
- 52. Tojima T., Takahashi M., Ito E. (2003) Dual regulation of LIM kinase 1 expression by cyclic AMP and calcium determines cofilin phosphorylation states during neuritogenesis in NG108–15 cells. Brain Res. 985, 43–55 [DOI] [PubMed] [Google Scholar]
- 53. Gu X., Olson E. C., Spitzer N. C. (1994) Spontaneous neuronal calcium spikes and waves during early differentiation. J. Neurosci. 14, 6325–6335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lin C., Lamoureux P., Buxbaum R. E., Heidemann S. R. (1995) Osmotic dilution stimulates axonal outgrowth by making axons more sensitive to tension. J. Biomech. 28, 1429–1438 [DOI] [PubMed] [Google Scholar]
- 55. Herring T. L., Cohan C. S., Welnhofer E. A., Mills L. R., Morris C. E. (1999) F-actin at newly invaginated membrane in neurons. Implications for surface area regulation. J. Membr. Biol. 171, 151–169 [DOI] [PubMed] [Google Scholar]
- 56. Liedtke W., Friedman J. M. (2003) Abnormal osmotic regulation in trpv4−/− mice. Proc. Natl. Acad. Sci. U.S.A. 100, 13698–13703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vriens J., Watanabe H., Janssens A., Droogmans G., Voets T., Nilius B. (2004) Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc. Natl. Acad. Sci. U.S.A. 101, 396–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Shibasaki K., Murayama N., Ono K., Ishizaki Y., Tominaga M. (2010) TRPV2 enhances axon outgrowth through its activation by membrane stretch in developing sensory and motor neurons. J. Neurosci. 30, 4601–4612 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.