The human follicle-stimulating hormone (hFSH) belongs to a family of hormones responsible for the maintenance of essential reproductive processes (gonadotropins).[1] FSH is produced in the anterior pituitary, and the binding of FSH to its receptor stimulates the maturation of follicles and the production of estrogen in females, and maintains spermatogenesis in males.[2] Consequently, FSH is clinically used in the treatment of anovulatory disorders associated with infertility.[3] Administration is usually in the form of subcutaneous injections, often once a day, over prolonged periods of time. Side effects of this treatment range from allergic reactions and nausea, to mood swings and fatigue.[4] Presently, FSH is mainly derived from recombinant technologies, specifically, from Chinese Hamster Ovary (CHO) cells.[5] The material so obtained is a complex mixture of hormone glycoforms, i.e. highly heterogeneous vis-à-vis the carbohydrates on the peptide backbone.[6] In normal adult humans, the FSH receptor (FSH-R) is expressed only on the ovarian granulosa cells of females and the testicular Sertoli cells of males. However, in a recent discovery it was found that the FSH-R is ubiquitously expressed on the endothelial cells of the peripheries of the tumors of the breast, prostrate, colon, pancreas, kidney, stomach, testis and ovary.[7] Earlier studies in mice have indicated that the effect of FSH on the growth of tumors is at least as potent as that of epidermal growth factor (EGF).[8] Although there was a concentration dependency of this effect, what might be an interesting study of the relative roles of the various glycoforms is presently stymied by the unavailability of homogeneous forms of FSH.
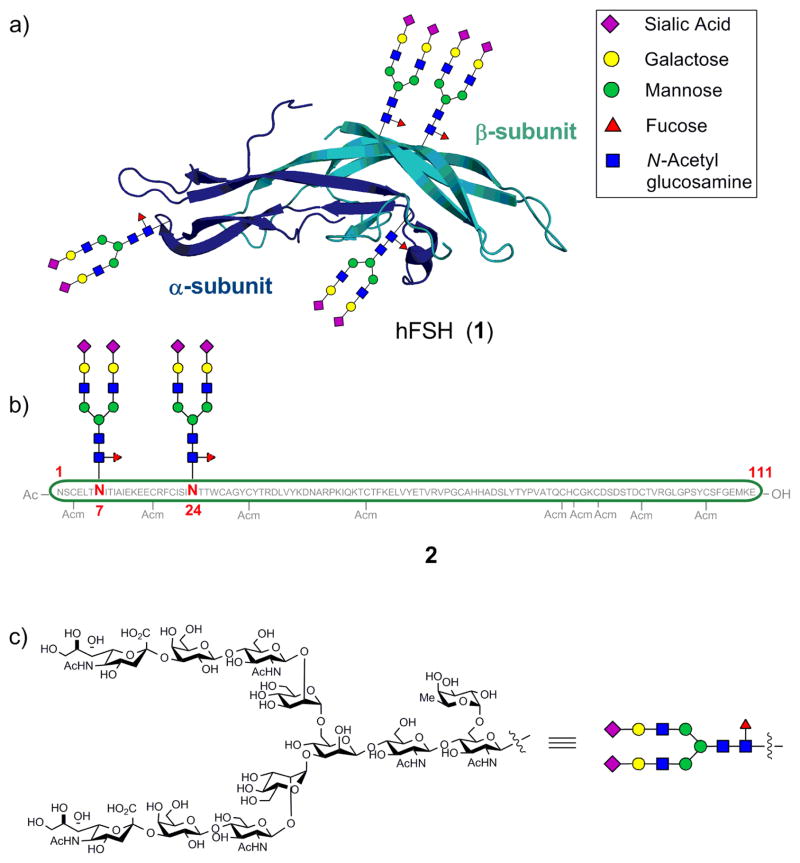
Structurally, FSH is a heterodimeric glycoprotein composed of two non-covalently associated subunits (α and β) (1, Figure 1).[9] Each of the subunits contains two N-linked oligosaccharides—the α-subunit at Asn52 and Asn78, and the β-subunit at Asn7 and Asn24, which are incorporated in the rough endoplasmic reticulum (RER) through co-translational modifications of the peptide backbone. The structures of the oligosaccharides play a crucial role in the proper folding, subunit assembly, secretion, and activation of the target receptor and, ultimately, the metabolic fate of the molecule.[10] Clearly, a method for gaining access to homogeneous glycoforms of FSH would be highly desirable for establishing a structure-activity relationship (SAR). Due to a lack of viable techniques for separating such complex mixtures of glycoforms,[11] chemical and chemoenzymatic methods[12] have emerged as a viable option for the preparation of homogeneous glycoproteins.
Figure 1.
(a) Structure of hFSH heterodimer (1) with glycans (see inset legend). (b) β-subunit (2) displaying the N-linked consensus sequence oligosaccharide at the wild-type sites. (c) Structure of the consensus sequence oligosaccharide.
The seminal work by Kent and coworkers in developing the native chemical ligation (NCL) [13] reaction has led to the synthesis of very challenging protein targets such as a ubiquitin diastereomer,[14] RNase,[15] integral membrane kinase,[16] and tetraubiquitin protein,[17] among others, that are inaccessible by conventional peptide synthesis. The scope of the NCL reaction has been vastly expanded now, to enable ligations at Met, Ala, Phe, Ser, Val, Thr, Lys, Leu and Pro.[18] Yet, the ability to gain access to complex glycoproteins through de novo chemical synthesis remains a rather daunting task.[12] The difficulties can be attributed in part to the challenges of obtaining complex carbohydrates, particularly those containing sialic acid, fucose and high mannose residues, by chemical synthesis, in sufficient quantities. With respect to a possible synthesis of FSH, we had recently described an approach to the synthesis of the β-subunit using the disaccharide chitobiose as a model building block for our glycoprotein assembly.[19] Herein, we present the results of a rather ambitious undertaking, i.e. the synthesis of the β-subunit of FSH (2) containing a consensus sequence oligosaccharide at each of the two N-linkage sites, endowed with high mannose, fucose and sialic acid presentation, using natural motifs in all of the glycosidic linkages. The system has been synthesized with protected cysteines anticipating folding and association with the α-subunit.[20] The particular biantennary consensus dodecasaccharide chosen for this purpose was found to be abundant in batches of recombinant FSH displaying high bioactivity. The Manα1—6[Manα1—3]Manβ1—4GlcNAcβ1—4GlcNAc core of this oligosaccharide is also found to exist on other glycoprotein hormones such as, Chorionic gonadotropin (hCG), Luteinizing hormone (hLH), and Thyroid-stimulating hormone (hTSH) as well a s α-fetoprotein (associated with human hepatocellular carcinoma).[21] Consequently, the protocol presented here should also be extendable to the synthesis of these and other glycoproteins.
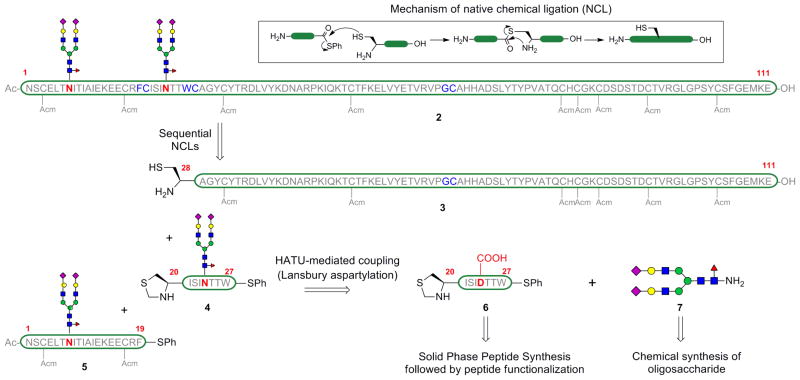
The β-subunit of FSH consists of 111 amino acids and the N-linked sugars are present at Asn7 and Asn24.[9a] The abundance of cysteine residues in the peptide backbone, at fairly regular intervals, speaks to the possibility of assembling the glycoprotein using NCL. The key disconnections are depicted in Scheme 1. The two key ligation sites chosen were Phe19-Cys20 and Trp27-Cys28. To enable the incorporation of the more precious glycopeptides in the final stages of assembly, the construction was performed from the C- to the N-terminus of the subunit. In order to engage in NCL, the C-terminus of each individual peptide fragment was functionalized as a thioester by single amino acid coupling. The peptide fragments were obtained by Fmoc-based solid phase peptide synthesis (SPPS) and the protecting groups on the amino acid side-chains during SPPS were chosen in a way that the aspartic acid residue that will bear the dodecasaccharide was protected orthogonally to those residues that were likely to interfere during the glycan attachment. Of the twelve cysteines on the protein, the nine that were not required for NCL were protected with acetamidomethyl (Acm) protecting groups. Additionally, these cysteine side chain protections prevent undesirable cross-linkages, via oxidation, during the course of the synthesis. Cleavage from the resin and selective deprotection of the aspartic acid side chain provided a free carboxylic acid, which was then coupled with the glycosylamine 7 by HATU-mediated Lansbury aspartylation.[22] The dodecasaccharide 7 was obtained by chemical synthesis, following the program adumbrated in Scheme 2.[19,23]
Scheme 1.
Retrosynthetic strategy for the construction of the fully elaborated β-subunit of hFSH (2).
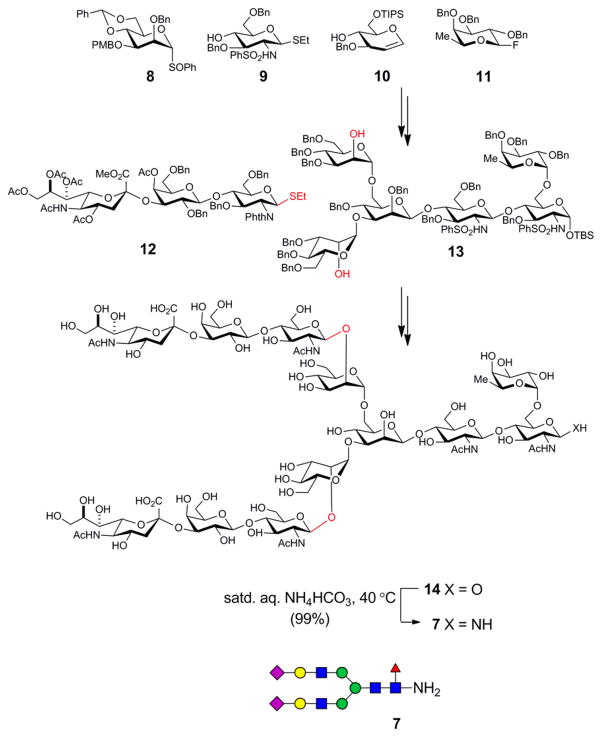
Scheme 2.
Schematic representation of the synthesis of the dodecasaccharide 7.
The strategy exploited the symmetry in the dodecasaccharide, in that a bis-glycosylation reaction on the hexasaccharide acceptor 13 with the trisaccharide donor 12 was executed in the late stages of the synthesis. The hexasaccharide core 13 was obtained through a highly convergent series of glycosylations using the known building blocks 8–11. In each case, the protecting groups were carefully selected to maximize the diastereoselectivity during the glycosylations and to minimize the number of deprotection steps that would need to be performed towards the end of the synthesis. Global deprotection of all the protecting groups present on the sugar moieties provided the dodecasaccharide 14. The anomeric hydroxyl group was converted into a primary amine under Kochetkov amination conditions[19,24] by treatment with saturated ammonium bicarbonate solution. Excess ammonium bicarbonate was removed by repeated lyophilization of the material with minimal exposure to moisture.[25]
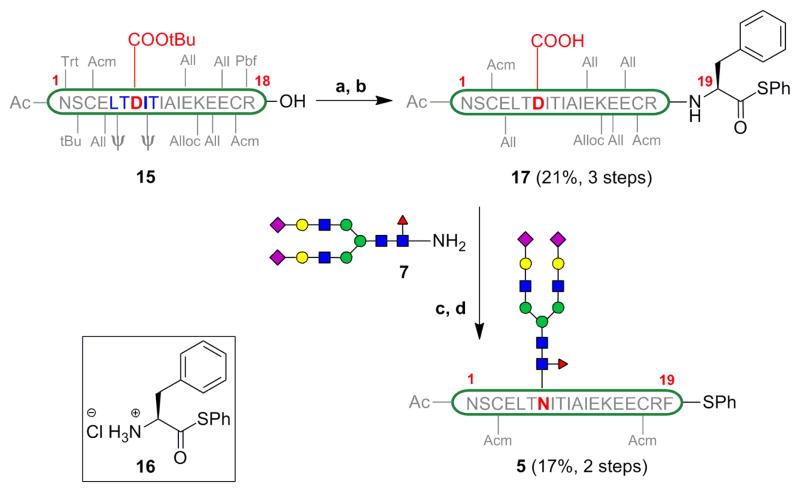
The protected [βFSH1–18] 15 was obtained via Fmoc-based SPPS starting from the commerically available Fmoc-Arg(Pbf)-TGT resin, Pseudoproline dipeptides (denoted by ψ) were incorporated into the peptide synthesis sequence to improve the yield of this aggregation-prone fragment.[26] The C-terminal carboxylic acid of the peptide was coupled to phenylalanine phenylthioester 16 under Sakakibara conditions,[27] which are known to be epimerization-free. Cocktail B[28] treatment removed all the acid labile protecting groups on the amino acid side chains. This three step protocol afforded [βFSH1–19] 17 in 21% yield after HPLC purification. The material so obtained was treated with the glycosyl amine 7 under HATU-mediated coupling conditions. The aspartylation proceeded in 73% conversion as indicated by LC-MS, along with a trace amount of undesired aspartimide formation. Treatment with Pd(PPh3)4 and phenylsilane removed all the allyl and alloc protecting groups on the amino acid side chains, in a one-flask procedure. The mixture was purified by HPLC to provide the [βFSH1–19] glycopeptide 5 in 17% yield (averaged over 9 trials).
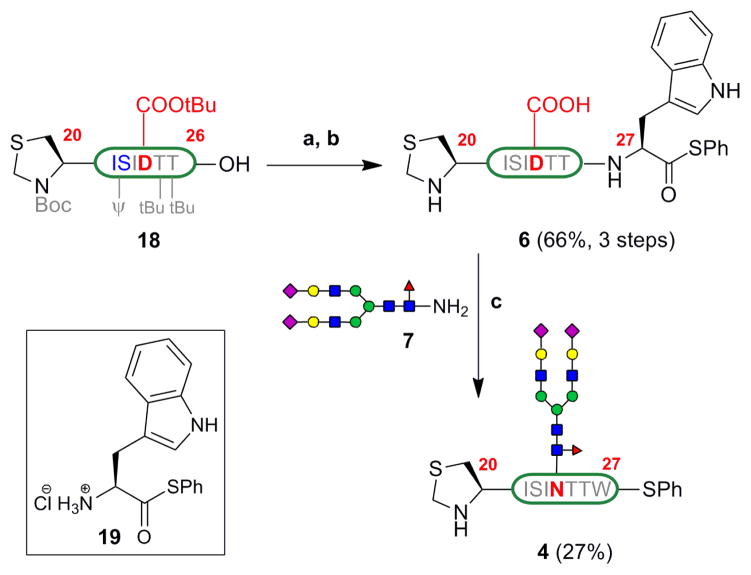
Similarly, [βFSH20–26] 18 was obtained by Fmoc-based SPPS on Fmoc-Thr(tBu)-TGT resin and cleavage from the resin. Treatment of peptide 18 with tryptophan phenylthioester 19, and subsequent deprotection with Cocktail B, provided [βFSH20–27] 6 in 66% yield (3 steps) (Scheme 4). Lansbury aspartylation of this peptide with the glycosyl amine 7 proceeded in 50% conversion with some aspartimide formation (ca. 8%). The [βFSH20–27] glycopeptide 4 was isolated by HPLC purification in 27% yield (averaged over 5 trials).
Scheme 4.
Synthesis of [βFSH20–27] 4 (a) 19, HOOBt, EDC, TFE/CHCl3 1:3 (b) Cocktail B (c) 7, HATU, DIEA, DMSO.
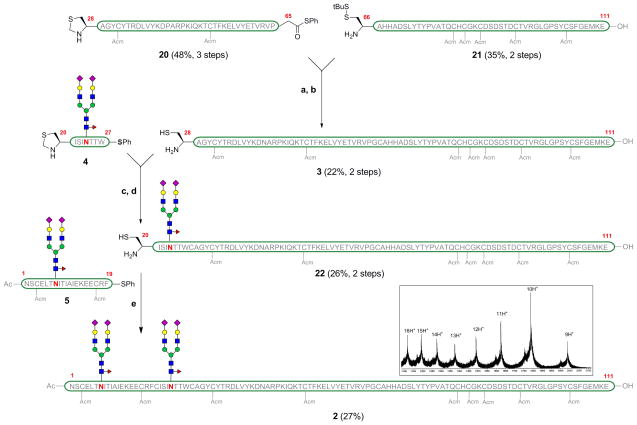
The last segment required for the assembly was [βFSH28–111] (3, Scheme 5). Fragment 3 is devoid of glycosylation sites and could have been obtained entirely by SPPS. However, we were unable to obtain useful yields of this peptide on solid support and we therefore opted to accomplish its synthesis via native chemical ligation of two smaller peptide fragments of roughly equal length. Gly65-Cys66 was chosen as the ligation site, for two reasons. First, ligations at glycines are particularly effective, likely due to the lack of steric bulk at the α-position. Addionally, conversion of terminal glycine carboxylic acids to their thioesters is not subject to epimerization. Thus, two shorter peptide fragments were synthesized. [βFSH28–65] was obtained by SPPS on Fmoc-Gly-TGT resin. The terminal glycine residue is converted to glycine phenylthioester and treated with Cocktail B to remove all the acid labile protecting groups, providing fragment 20 in 48% yield (over 3 steps). The fully deprotected peptide [βFSH66–111] 21 was obtained on solid support using Fmoc-Glu(OtBu)-TGT resin, cleavage and treatment with Cocktail B. The two fragments, 20 and 21, were coupled under native chemical ligation conditions, using thiophenol as an additive. Upon completion of the reaction as monitored by LC-MS, the terminal Thz protecting group was removed using methoxylamine hydrochloride, in a one-flask procedure, to provide [βFSH28–111] 3 in 22% yield after HPLC purification.
Scheme 5.
Final ligation of the glycopeptide fragments. (a) 20, 21, PhSH, Gnd·HCl, Na2HPO4, TCEP·HCl, H2O (pH = 7.4) (b) NH2OMe·HCl, Gnd·HCl, TCEP·HCl (pH = 4.8) (c) 4, PhSH, Gnd·HCl, Na2HPO4, TCEP·HCl, H2O (pH = 7.4) (d) NH2OMe·HCl, Gnd·HCl, TCEP·HCl (pH = 4.8). (e) 5, PhSH, Gnd·HCl, Na2HPO4, TCEP·HCl, H2O (pH = 7.3). Mass spectrum of compound 2 (inset).
The final assembly of the individual peptide fragments commenced with the coupling of fragment [βFSH20–27] 4 with the fragment [βFSH28–111] 3 under NCL conditions in a pH 7.3 buffer, Scheme 5. Subsequently, the N-terminal Thz group was cleaved using methoxylamine hydrochloride at pH 4.8, to free the N-terminal cysteine required for the final ligation, in a one-flask procedure. Purification by HPLC provided the desired glycopeptide [βFSH20–111] 22 bearing the dodecasaccharide, in 26% yield (averaged over 3 trials).
Finally, the glycopeptide [βFSH1–19] 5 was coupled to the glycopeptide [βFSH20–111] 22 under NCL conditions. Gratifyingly, we obtained the full chain β-subunit of FSH (2) containing the two dodecasaccharides in 27% yield (averaged over 3 trials) after HPLC purification. Interestingly, the ligation was facile despite the fact that the two bulky dodecasaccharides are merely 16 residues apart during the ligation event. Also noteworthy, is the fact that the final compound 2 represents the largest realistically glycosylated glycoprotein with all natural-type linkages to have been synthesized in a homogeneous state, using strictly chemical methods.
Much encouraged by this demonstration of feasibility, we continue on the journey through which we hope to reach hFSH itself. There remains deprotection of the Acm protecting groups[29] on the cysteine side chains such as to allow for folding and subsequent association with the α-subunit. The total synthesis of the latter is already well advanced. The results of these ongoing studies will be disclosed in a timely way.
Supplementary Material
Scheme 3.
Synthesis of [βFSH1–19] 5 (a) 16, HOOBt, EDC, TFE/CHCl3 1:3; (b) Cocktail B; (c) 7, HATU, DIEA, DMSO; (d) Pd(PPh3)4, PhSiH3, NMP.
Footnotes
This work was supported by NIH grant CA103823 (to SJD). We thank Ms. Rebecca Wilson for assistance with the preparation of the manuscript.
Supporting information for this article is available on the WWW under http://www.angewandte.org
References
- 1.Fevold HL, Hisaw FL, Leonard SL. Am J Physiol. 1931;97:291–301. [Google Scholar]
- 2.Smith PE. J Am Med Assoc. 1927;88:158–161. [Google Scholar]
- 3.Howles CM. Hum Reprod Update. 1996;2:172–191. doi: 10.1093/humupd/2.2.172. [DOI] [PubMed] [Google Scholar]
- 4.Pang SC. Women’s Health. 2005;1:87–95. doi: 10.2217/17455057.1.1.87. [DOI] [PubMed] [Google Scholar]
- 5.Lobo RA. In: Comprehensive Gynecology. 5. Katz VL, et al., editors. MOSBY; Philadelphia: 2007. pp. 1001–1037. [Google Scholar]
- 6.Gervais A, Hammel YA, Pelloux S, Lepage P, Baer G, Carte N, Sorokine O, Strub JM, Koerner R, Leize E, Van Dorsselaer A. Glycobiology. 2003;13:179–189. doi: 10.1093/glycob/cwg020. [DOI] [PubMed] [Google Scholar]
- 7.Radu A, Pichon C, Camparo P, Antoine M, Allory Y, Couvelard A, Fromont G, Hai MTV, Ghinea N. N Engl J Med. 2010;363:1621–1630. doi: 10.1056/NEJMoa1001283. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Ganta S, Cheng C, Craig R, Ganta RR, Freeman LC. Mol Cell Endocrinol. 2007;267:26–37. doi: 10.1016/j.mce.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.a) Pierce JG, Parsons TF. Annu Rev Biochem. 1981;50:465–495. doi: 10.1146/annurev.bi.50.070181.002341. [DOI] [PubMed] [Google Scholar]; b) Baenziger JU, Green ED. Biochim Biophys Acta. 1988;947:287–306. doi: 10.1016/0304-4157(88)90012-3. [DOI] [PubMed] [Google Scholar]; c) Fan QR, Hendrickson WA. Nature. 2005;433:269–277. doi: 10.1038/nature03206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.a) Hartree AS, Renwick AG. Biochem J. 1992;287:665–679. doi: 10.1042/bj2870665. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Ulloa-Aguirre A, Timossi C. Hum Reprod Update. 1998;4:260–283. doi: 10.1093/humupd/4.3.260. [DOI] [PubMed] [Google Scholar]
- 11.Rudd PM, Dwek RA. Crit Rev Biochem Mol Biol. 1997;32:1–100. doi: 10.3109/10409239709085144. [DOI] [PubMed] [Google Scholar]
- 12.(a) Gamblin DP, Scanlan EM, Davis BG. Chem Rev. 2009;109:131–163. doi: 10.1021/cr078291i. [DOI] [PubMed] [Google Scholar]; b) Kunz H, Unverzagt C. Angew Chem. 1988;100:1763–1765. [Google Scholar]; Angew Chem Int Ed Engl. 1988;27:1697–1699. [Google Scholar]; c) Offer J, Quibell M, Johnson T. J Chem Soc, Perkin Trans 1. 1996:175–182. [Google Scholar]; d) Macmillan D, Bertozzi CR. Tetrahedron. 2000;56:9515–9525. [Google Scholar]; e) Yamamoto N, Ohmori Y, Sakakibara T, Sasaki K, Juneja LR, Kajihara Y. Angew Chem. 2003;115:2641–2644. doi: 10.1002/anie.200250572. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2003;42:2537–2540. doi: 10.1002/anie.200250572. [DOI] [PubMed] [Google Scholar]; f) Hirano K, Macmillan D, Tezuka K, Tsuji T, Kajihara Y. Angew Chem. 2009;121:9721–9724. doi: 10.1002/anie.200904376. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2009;48:9557–9560. doi: 10.1002/anie.200904376. [DOI] [PubMed] [Google Scholar]; g) Chen R, Tolbert TJ. J Am Chem Soc. 2010;132:3211–3216. doi: 10.1021/ja9104073. [DOI] [PubMed] [Google Scholar]
- 13.Dawson PE, Muir TW, Clark-Lewis I, Kent SB. Science. 1994;266:776–779. doi: 10.1126/science.7973629. [DOI] [PubMed] [Google Scholar]
- 14.Bang D, Makhatadze GI, Tereshko V, Kossiakoff AA, Kent SB. Angew Chem. 2005;117:3920–3924. doi: 10.1002/anie.200463040. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2005;44:3852–3856. doi: 10.1002/anie.200463040. [DOI] [PubMed] [Google Scholar]
- 15.(a) Piontek C, Varón Silva D, Heinlein C, Pöhner C, Mezzato S, Ring P, Martin A, Schmid FX, Unverzagt C. Angew Chem. 2009;121:1974–1978. doi: 10.1002/anie.200804735. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2009;48:1941–1945. doi: 10.1002/anie.200804735. [DOI] [PubMed] [Google Scholar]; b) Piontek C, Ring P, Harjes O, Heinlein C, Mezzato S, Lombana N, Pöhner C, Püttner M, Varón Silva D, Martin A, Schmid FX, Unverzagt C. Angew Chem. 2009;121:1968–1973. doi: 10.1002/anie.200804734. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2009;48:1936–1940. doi: 10.1002/anie.200804734. [DOI] [PubMed] [Google Scholar]
- 16.Lahiri S, Brehs M, Olschewski D, Becker CFW. Angew Chem. 2011;123:4074–4078. doi: 10.1002/anie.201006686. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2011;50:3988–3992. doi: 10.1002/anie.201006686. [DOI] [PubMed] [Google Scholar]
- 17.Kumar KSA, Bavikar SN, Spasser L, Moyal T, Ohayon S, Brik A. Angew Chem. 2011;123:6261–6265. doi: 10.1002/anie.201101920. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2011;50:6137–6141. doi: 10.1002/anie.201101920. [DOI] [PubMed] [Google Scholar]
- 18.(a) Tam JP, Yu Q. Biopolymers. 1998;46:319–327. doi: 10.1002/(SICI)1097-0282(19981015)46:5<319::AID-BIP3>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]; b) Yan LZ, Dawson PE. J Am Chem Soc. 2001;123:526–533. doi: 10.1021/ja003265m. [DOI] [PubMed] [Google Scholar]; c) Wan Q, Danishefsky SJ. Angew Chem. 2007;119:9408–9412. [Google Scholar]; Angew Chem Int Ed. 2007;46:9248–9252. doi: 10.1002/anie.200704195. [DOI] [PubMed] [Google Scholar]; d) Crich D, Banerjee A. J Am Chem Soc. 2007;129:10064–10065. doi: 10.1021/ja072804l. [DOI] [PubMed] [Google Scholar]; e) Okamoto R, Kajihara Y. Angew Chem. 2008;120:5482–5486. [Google Scholar]; Angew Chem Int Ed. 2008;47:5402–5406. doi: 10.1002/anie.200801097. [DOI] [PubMed] [Google Scholar]; f) Haase C, Rohde H, Seitz O. Angew Chem. 2008;120:6912–6915. doi: 10.1002/anie.200801590. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2008;47:6807–6810. doi: 10.1002/anie.200801590. [DOI] [PubMed] [Google Scholar]; g) Chen J, Wan Q, Yuan Y, Zhu J, Danishefsky SJ. Angew Chem. 2008;120:8649–8652. [Google Scholar]; Angew Chem Int Ed. 2008;47:8521–8524. doi: 10.1002/anie.200803523. [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Yang R, Pasunooti KK, Li F, Liu XW, Liu CF. J Am Chem Soc. 2009;131:13592–13593. doi: 10.1021/ja905491p. [DOI] [PubMed] [Google Scholar]; i) Kumar KSA, Haj-Yahya M, Olschewski D, Lashuel HA, Brik A. Angew Chem. 2009;121:8234–8238. doi: 10.1002/anie.200902936. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2009;48:8090–8094. doi: 10.1002/anie.200902936. [DOI] [PubMed] [Google Scholar]; j) Chen J, Wang P, Zhu J, Wan Q, Danishefsky SJ. Tetrahedron. 2010;66:2277–2283. doi: 10.1016/j.tet.2010.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]; k) Harpaz S, Siman P, Kumar KS, Brik A. ChemBioChem. 2010;11:1232–1235. doi: 10.1002/cbic.201000168. [DOI] [PubMed] [Google Scholar]; l) Tan Z, Shang S, Danishefsky SJ. Angew Chem. 2010;122:9690–9693. doi: 10.1002/anie.201005513. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2010;49:9500–9503. doi: 10.1002/anie.201005513. [DOI] [PMC free article] [PubMed] [Google Scholar]; m) Shang S, Tan Z, Dong S, Danishefsky SJ. J Am Chem Soc. 2011;133:10784–10786. doi: 10.1021/ja204277b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagorny PN, Fasching B, Li X, Chen G, Aussedat B, Danishefsky SJ. J Am Chem Soc. 2009;131:5792–5799. doi: 10.1021/ja809554x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samaddar M, Catterall JF, Dighe RR. Protein Expr Purif. 1997;10:345–355. doi: 10.1006/prep.1997.0745. [DOI] [PubMed] [Google Scholar]
- 21.(a) Gharib SD, Wierman ME, Shupnik MA, Chin WW. Endocr Rev. 1990;11:177–199. doi: 10.1210/edrv-11-1-177. [DOI] [PubMed] [Google Scholar]; b) Sairam MR. FASEB J. 1989;3:1915–1926. doi: 10.1096/fasebj.3.8.2542111. [DOI] [PubMed] [Google Scholar]; c) Mutsaers JHGM, Kamerling JP, Devos R, Guisez Y, Fiers W, Vliegenthart JFG. Eur J Biochem. 1986;156:651–654. doi: 10.1111/j.1432-1033.1986.tb09627.x. [DOI] [PubMed] [Google Scholar]; d) Damm JBL, Kamerling JP, van Dedem GWK, Vliegenthart JFG. Glycoconjugates. 1987;4:129–144. [Google Scholar]; e) Hokke CH, Berwer AA, van Dedem GWK, van Ostrum JV, Kamerling JP, Vliegenthart JFG. FEBS Lett. 1990;275:9–14. doi: 10.1016/0014-5793(90)81427-p. [DOI] [PubMed] [Google Scholar]; f) Green ED, Baenziger JU. J Biol Chem. 1988;263:25–35. [PubMed] [Google Scholar]; g) Yamashita K, Hitoi A, Tsuchida Y, Nishi S, Kobata A. Cancer Res. 1983;43:4691–4695. [PubMed] [Google Scholar]
- 22.(a) Cohen-Anisfeld ST, Lansbury PT., Jr J Am Chem Soc. 1993;115:10531–10537. [Google Scholar]; b) Miller JS, Dudkin VY, Lyon GJ, Muir TW, Danishefsky SJ. Angew Chem. 2003;115:447–450. doi: 10.1002/anie.200390131. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2003;42:431–434. [Google Scholar]
- 23.(a) Danishefsky SJ, Hu S, Cirillo PF, Eckhardt M, Seeberger PH. Chem-Eur J. 1997;3:1617–1628. [Google Scholar]; b) Dudkin VY, Miller JS, Danishefsky SJ. Tetrahedron Lett. 2003;44:1791–1793. [Google Scholar]; c) Wu B, Hua Z, Warren JD, Ranganathan K, Wan Q, Chen G, Tan Z, Chen J, Endo A, Danishefsky SJ. Tetrahedron Lett. 2006;47:5577–5579. doi: 10.1016/j.tetlet.2006.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Likhosherstov LM, Novikova OS, Derevitskaja V, Kochetkov NK. Carbohydr Res. 1986;146:C1–C5. [Google Scholar]
- 25.Isbell HS, Frush HL. J Org Chem. 1958;23:1309–1319. [Google Scholar]
- 26.Wöhr T, Wahl F, Nefzi A, Rohwedder B, Sato T, Sun X, Mutter M. J Am Chem Soc. 1996;118:9218–9227. [Google Scholar]
- 27.Sakakibara S. Biopolymers. 1995;37:17–28. doi: 10.1002/bip.360370105. [DOI] [PubMed] [Google Scholar]
- 28.Cocktail B (2% triisopropylsilane/5% phenol/5% H2O/88% TFA).
- 29.On the chitobiose model system [Ref. 19], we have observed that the Acm groups on the nine protected cysteines can be removed using AgOAc, as analyzed by LC-MS.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.