Abstract
The discovery of endothelial progenitor cells in the 1990s challenged the paradigm of angiogenesis by showing that cells derived from hematopoietic stem cells are capable of forming new blood vessels even in the absence of a pre-existing vessel network, a process termed vasculogenesis. Since then, the majority of studies in the field have found a strong association between circulating endothelial progenitor cells and cardiovascular risk. Several studies have also reported that inflammation influences the mobilization and differentiation of endothelial progenitor cells. In this review, we discuss the emerging role of endothelial progenitor cells as biomarkers of cardiovascular disease as well as the interplay between inflammation and endothelial progenitor cell biology. We will also review the challenges in the field of endothelial progenitor cell-based therapy.
Keywords: biomarker, cardiovascular disease, endothelial progenitor cell, inflammation, vasculogenesis
Angiogenesis, the creation of new blood vessels, has attracted tremendous interest in the field of cardiology and cancer biology in the last decades. However, angiogenesis has always been a double-edged sword; in some diseases, such as coronary artery disease, it may benefit patients by increasing oxygen supply, whereas in others, such as cancer and inflammatory diseases, angiogenesis may lead to further disease progression and tumor growth. It was long believed that angiogenesis, the formation of new blood vessels in humans, could only occur by the new blood vessels sprouting out of pre-existing vessels [1]. This paradigm changed in the mid-1990s, when researchers detected endothelial cells of hematopoietic origin on the surface of left ventricular assist devices [2]. Earlier studies had also described endothelial-like cells within the peripheral blood stream [3]. In 1997, Asahara and colleagues described the so-called endothelial progenitor cells (EPCs) isolated from the circulation. EPCs are also capable of forming new blood vessels [4], mainly after their recruitment and migration into the ischemic tissue, a process later termed ‘vasculogenesis’. In contrast to angiogenesis, during vasculogenesis the formation of new blood vessels can occur in the absence of pre-existing blood vessels.
Since EPCs do not need pre-existing blood vessels to work, interest in EPC biology has been growing continuously since their discovery. EPCs are now regarded as biomarkers in cardiovascular disease and also as a potential therapeutic tool or target. In this article, we discuss the emerging role of EPCs as biomarkers of cardiovascular disease as well as the interplay between inflammation and EPC biology. We will also review the challenges in the field of EPC-based therapy.
Methodology
We conducted a search in PubMed® using the following search terms: endothelial progenitor (precursor) cells, coronary artery disease, myocardial infarction, stroke, heart failure, pulmonary hypertension, allograft vasculopathy and chronic inflammatory diseases. We selected articles with more than ten patients that analyzed the association or relationship between outcome and EPCs. Basic science papers on EPC biology were also reviewed. Furthermore, we screened and analyzed different reviews on the topic. However, because studies often referred to different characterization and quantification techniques for EPCs, the observations may not always be directly comparable.
Definition & characterization of EPCs
The exact definition and characterization of EPCs is still an ongoing and unresolved issue, although researchers have been trying to standardize the definitions of EPCs [5–8]. When quantifying and analyzing EPCs by flow cytometry, staining for the expression of CD34 is essential. Nevertheless, there is no clear consensus on what other markers should be mandatory. Other surface molecules, mainly CD45, have been added to the panel by some investigators to characterize EPCs [9] and may increase the specificity for EPCs. Different technical protocols have also been recommended to stain EPCs; some recommend staining for EPCs directly from full blood after erythrocyte lysis, whereas others use peripheral blood mononuclear cells after density centrifugation, but the latter was recently associated with a loss of EPCs [10].
The majority of outcome studies have quantified absolute or relative numbers of circulating EPCs within the peripheral blood. In these studies, the number of EPCs has been either expressed as an absolute number, or as a percentage of positive cells per certain volume or per acquired cells. Assays that assess and quantify the outgrowth of colonies have also emerged. It is essential to differentiate among three of the most commonly used assays – the so called ‘early outgrowth assay’ [11,12], the related ‘Hill assay’ [13], and the ‘late outgrowth assay’ [14] – because it is now evident that the first two and the latter exhibit two different cell types. In the early outgrowth assay and the Hill assay, cells staining positive for acetylated low-density lipoprotein and Ulex europaeus [12], or forming specific colonies [13] are of hematopoietic and monocytic origin and express CD31, different endothelial markers and von Willebrand factor-secreting proangiogenic factors [15–17]. In the late outgrowth assay, cells are cultivated for a longer time period of 14–21 days, do not express CD45, but do exhibit a clonal proliferative potential [14,18,19].
In addition to the EPC quantities and the potential to form colonies, the characterization of their functional capacities also include proliferation, migration, adhesion and in vitro vasculogenic capacity that can be assessed in various ways using distinct readout systems. The definition of EPCs has also undergone constant changes over the last years. Initially, EPCs were usually classified as a subtype of CD34+ hematopoietic stem and progenitor cells and characterized by the coexpression of CD133, also called prominin 1 [20]. In various studies, EPCs were also characterized by the coexpression of KDR-1, also known as VEGF-R2. Later, accumulating evidence showed that a ‘true’ EPC, which by definition represents a cell that can differentiate into an endothelial cell, is not of hematopoietic origin, whereas the other so-called EPCs were linked to the mononuclear lineage, secreting proangiogenic factors and were referred to as EPCs due to their ability to form colonies (see above). Currently, there is evidence to suggest that ‘true’ EPCs are not of hematopoietic origin, but rather of an already differentiated endothelial origin with clonal proliferative potential, and therefore the term ‘endothelial colony forming cell’ (ECFC) has emerged [14,17,21]. Recently, Richardson and Yoder proposed to subdivide EPCs into mainly two groups, the proangiogenic hematopoietic cell and ECFCs [22].
These findings of distinct and different cell types also led to modifications in the flow cytometry staining protocols. Recently, staining for CD45dim (the hematopoietic cell marker), CD34+ and KDR+ cells was recommended as a modified International Society of Hematotherapy and Graft Engineering (ISHAGE) protocol, although there is still no clear consensus about a definite panel [9]. In another recent approach, both CD45 and KDR were excluded due to difficulties with titration [23]. Moreover, the scaling involved in gating for cells was considered to be very important to detect ECFCs. The constantly evolving flow cytometry staining and gating protocols, of course, are additional factors that complicate comparisons with older studies.
Taken together, both historically named EPCs, which were mostly of hematopoietic origin and the ‘new’ EPCs (now increasingly known as ECFCs) exhibit a potential to form, or at least increase and support the formation of, new vessels. A major problem for researchers is the lack of a unique marker for EPCs, leaving the field in limbo and complicating precise comparisons among different studies. This is, therefore, an important caveat to mention when reviewing the literature, because not all the papers refer to the same cell type. In this review, we will use the term EPCs as it was used in the studies that are referenced. Tables 1–4 highlight important studies and also refer to the methods used for EPC quantification.
Table 1.
Selected outcome and characterization studies on endothelial progenitor cells in patients at risk of cardiovascular disease.
| Author | Population | Study design | n | Methods | Main finding | Ref. |
|---|---|---|---|---|---|---|
| Hill et al. (2003) |
General population |
Cross-sectional | 45 | Hill outgrowth assay | Number of EPCs correlated with Framingham risk score and was a better correlate of endothelial function |
[13] |
| Xiao et al. (2007) |
At risk of CVD | Cross-sectional | 571 | Early outgrowth assay, Hill assay |
EPC number declined with age. EPC number was higher with increasing Framingham risk score (contrasting previous studies) |
[46] |
| Tepper et al. (2002) |
Diabetes mellitus (Type 2) |
Cross-sectional | 20 | FACS, proliferation assay, adhesion assay, Matrigel™ tubule assay |
EPCs were decreased in Type 2 diabetes mellitus and inversely related to HbA1c levels |
[25] |
| Loomans et al. (2004) |
Diabetes mellitus (Type 1) |
Cross-sectional | 20 | FACS, early outgrowth assay, in vitro angiogenesis assay |
EPCs were 44% lower in Type 1 diabetic patients and inversely related to HbA1c levels |
[24] |
| Lorenzen et al. (2010) |
Chronic kidney disease (stage V) |
Prospective | 265 | FACS, early outgrowth assay |
Number of functional active EPCs and age were independently associated with cardiovascular events |
[30] |
CVD: Cardiovascular disease; EPC: Endothelial progenitor cell; FACS: Fluorescence-activated cell sorting.
Table 4.
Selected outcome and characterization studies on endothelial progenitor cells in patients with rheumatic diseases.
| Author | Population | Study design | n | Methods | Main finding | Ref. |
|---|---|---|---|---|---|---|
| Grisar et al. (2005) |
RA: active and nonactive |
Cross-sectional | 52 | FACS, Hill assay, adhesion assay, migration assay |
EPCs are reduced in RA, inverse correlation of EPC levels with disease activity, high serum TNF levels are associated with low EPC counts |
[69] |
| Herbrig et al. (2006) |
RA | Cross-sectional | 15 | FACS, early outgrowth assay, Hill assay |
Reduced EPC levels in RA, inverse correlation of EPC levels and IL-6 levels |
[71] |
| Grisar et al. (2007) |
RA with intermediate and high disease activity |
Prospective | 36 | FACS, early outgrowth assay, Hill assay |
Reduced EPC levels in RA were normalized after 1 week of glucocorticoid therapy |
[70] |
| Westerweel et al. (2007) |
SLE | Cross-sectional | 13 | FACS, outgrowth assay, apoptosis assay, functional assay |
Decreased peripheral levels of EPC in SLE and increased apoptosis rate |
[77] |
| Grisar et al. (2008) |
SLE | Cross-sectional | 31 | FACS, early outgrowth assay, functional assay |
Normal peripheral EPC quantities but reduced adhesion and migration capacity |
[82] |
| Kuwana et al. (2004) |
Systemic sclerosis | Cross-sectional | 11 | FACS | Reduced EPC levels compared with healthy controls and RA patients |
[83] |
EPC: Endothelial progenitor cell; FACS: Fluorescence-activated cell sorting; RA: Rheumatoid arthritis; SLE: Systemic lupus erythematosus.
EPCs as potential biomarkers of cardiovascular & inflammatory diseases
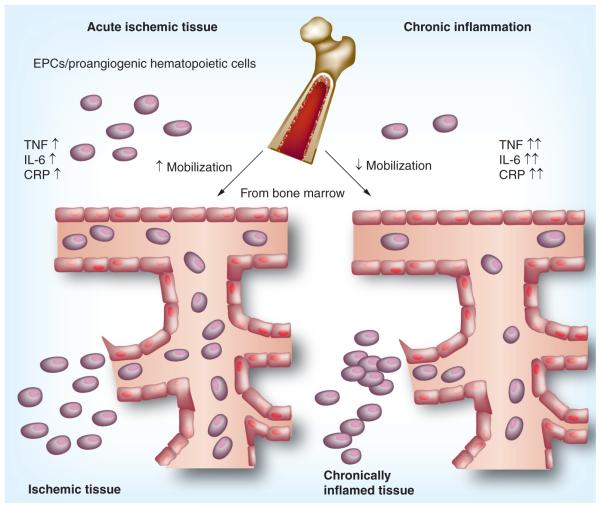
The value of EPCs as biomarkers for disease severity, prognosis and response to therapy has been the focus of several investigations. In patients at an increased risk of cardiovascular disease, including those with comorbid conditions such as diabetes mellitus [24,25], systemic hypertension [26,27] and chronic kidney disease [28–30], the peripheral EPC number is reduced while EPC function is often impaired. The reduction in EPC quantities is also observed in patients with established coronary artery disease [31] and stroke [32]. By contrast, an increased number of EPCs is often observed in patients who present with an acute coronary syndrome such as acute myocardial infarction [33] or unstable angina [34,35], suggesting the mobilization of EPCs during acute ischemic events (Figure 1).
Figure 1. Endothelial progenitor cells in acute ischemia and chronic inflammation.
Acute vascular events lead to a mobilization of EPCs from the bone marrow and an enhancement in the peripheral blood by a variety of factors, including TNF, IL-6 and CRP for contribution in vascular repair. Persistent systemic inflammation, however, is believed to reduce mobilization of EPCs from the bone marrow, reducing their quantities within the circulation. In addition, EPCs have been found to accumulate in chronically inflamed tissue, perpetuating vasculogenesis and further decreasing the peripheral EPC levels. Therefore, the quantities of EPCs taking part in vascular repair might be limited via this mechanism in chronic inflammatory diseases. CRP: C-reactive protein; EPC: Endothelial progenitor cell.
Heart failure has been associated with increased EPC counts in the earlier, less severe stages of the disease, but EPC numbers tend to decrease with further disease progression [36]. In pulmonary hypertension, some studies have shown a decrease in EPCs [37–39], while others report normal levels or an increase [40,41]. The studies on EPCs and transplant allograft vasculopathy thus far have small sample sizes that limit the generalizability of their results [42–44]. Tables 1–4 summarize some of the important studies relating to EPCs and outcomes in distinct diseases. In this section, we expand on each of the disease entities.
EPCs & atherosclerosis
In their landmark study, Hill et al. reported that a lower number of circulating EPCs was associated with a higher Framingham risk score and endothelial dysfunction as measured by flow-mediated brachial artery reactivity [13]. The relationship between EPCs and cardiovascular outcome was later confirmed by Werner and colleagues, who demonstrated that patients with coronary artery disease and a low baseline number of EPCs experience a higher likelihood of death from cardiovascular causes (Table 1 & 2) [31]. Moreover, EPC levels independently predict atherosclerotic disease progression even after adjustment for traditional cardiovascular risk factors [45]. Aging was also associated with a decline in the number of circulating EPCs [46].
Table 2.
Selected outcome and characterization studies on endothelial progenitor cells in patients with coronary artery disease, acute coronary syndrome and stroke.
| Author | Population | Study design | n | Methods | Main finding | Ref. |
|---|---|---|---|---|---|---|
| Stable CAD | ||||||
| Werner et al. (2005) |
CAD | Prospective | 519 | FACS, Hill assay | Lower EPC levels were associated with a higher risk of 1-year mortality of cardiovascular cause or major cardiovascular event |
[31] |
| Schmidt- Lucke et al. (2005) |
CAD ACS |
Prospective | 44 33 |
FACS | Reduced levels of circulating EPCs independently predicted atherosclerotic disease progression even after adjustment for traditional cardiovascular risk factors |
[45] |
| ACS | ||||||
| George et al. (2004) |
Unstable angina |
Prospective | 29 | FACS, Hill assay, adhesion assay |
Increase in circulating EPCs in patients with unstable angina without necrosis |
[34] |
| Guven et al. (2006) |
Patients referred for angiography |
Cross-sectional | 48 | FACS, early outgrowth assay |
In patients referred for angiography, EPCs were found to be higher in patients with significant CAD and correlated with maximum stenosis |
[35] |
| In-stent restenosis | ||||||
| Pelliccia et al. (2010) |
CAD post-bare metal stent |
Prospective | 155 | FACS | Higher levels of subpopulations of EPCs in patients with restenosis compared with those without |
[141] |
| den Dekker et al. (2011) (HEALING 2B study) |
Bioengineered CD34 antibody coated stents |
Prospective | 100 | FACS | Study suggests that statin therapy combined with the EPC capture stent is not contributing to a reduction of in-stent restenosis formation for the treatment of de novo CAD |
[142] |
| Stroke | ||||||
| Ghani et al. (2005) |
Cerebrovascular disease |
Prospective | 88 | Hill assay | Number of EPCs is lower in patients with acute and subacute cerebrovascular disease than in healthy controls |
[32] |
| Sobrino et al. (2007) |
Acute ischemic stroke |
Prospective | 48 | Hill assay | EPC levels increased during the first week after stroke and were associated with good functional outcome at 3 months |
[143] |
| CAV | ||||||
| Simper et al. (2003) |
Heart transplantation |
Prospective | 15 | Early outgrowth assay, long-term outgrowth assay |
Suggests an association between a reduction of EPCs and CAV |
[144] |
| Thomas et al. (2009) |
Heart transplantation |
Cross-sectional | 64 | FACS | Compared with matched patients without CAV, no difference was noted in EPC quantities in patients with CAV |
[42] |
| Osto et al. (2011) |
Heart transplantation |
Cross-sectional | 29 | FACS, immunohistochemistry |
Lower levels of EPCs correlated with microvascular dysfunction |
[44] |
ACS: Acute coronary syndrome; CAD: Coronary artery disease; CAV: Cardiac allograft vasculopathy; EPC: Endothelial progenitor cell; FACS: Fluorescence-activated cell sorting.
In animal models, EPCs were observed to restore carotid injury [47,48] by promoting differentiation into endothelial cells. In another study, however, ApoE-/- mice infused with EPCs were found to have increased plaque size and decreased plaque stability, hence contributing to neointimal hyperplasia [49]. In another observation, hematopoietic stem and progenitor cells, which are considered to be the ancestors of EPCs, were observed to participate in the development of atherosclerosis [50] and have been detected in the atherosclerotic plaque [51]. However, recently, a Danish group analyzed transplanted EPCs within the endothelium of atherosclerotic plaques in a complex mouse model and determined that EPCs do not take part in this process [52].
Since these data provide conflicting evidence regarding the exact role of EPCs, especially in the early stages of atherosclerosis, therapeutic protocols that involve EPC mobilization with the goal of increasing vasculogenesis in ischemic tissues have to be carefully evaluated.
EPCs as a biomarker in diabetes mellitus
Peripheral levels of EPCs have been found to be significantly reduced and associated with complications of diabetes mellitus [53]. The macrovasculopathy as assessed by ankle brachial index or carotid narrowing was reportedly associated with reduced peripheral levels of EPCs [54,55], and EPC levels were also found to be reduced in Type 2 diabetes retinopathy patients [56]. Moreover, EPC counts were negatively correlated with the albumin excretion rates in Type 2 diabetes patients [57]. A recent study performed in 425 patients revealed that the reduction of EPCs can already be observed at the onset of Type 2 diabetes, but a further reduction is seen with longer lasting disease [58]. Taken together, there is tremendous evidence that circulating EPCs are impaired in diabetes and that the reduction of EPCs is also negatively associated with various complications in diabetes [53]. The correlations of peripheral EPC levels with clinical characteristics in both inflammatory diseases and Type 2 diabetes would make serial measurements of EPCs an ideal candidate, possibly for both monitoring the treatment of the disease and measuring the cardiovascular risk.
EPCs as a biomarker in heart failure & pulmonary arterial hypertension
In patients with heart failure, several studies have shown that in mildly symptomatic heart failure (New York Heart Association [NYHA] functional class I), the levels of EPCs are increased compared with matched controls and then decline progressively with increasing severity of heart failure [36]. In a small outcome study, the peripheral quantities of EPCs were an independent predictor of survival along with age and diabetes mellitus in heart failure [59]. In this study, however, diastolic parameters of heart function and pulmonary hypertension were not considered as covariates in the multivariate models. Interestingly, exercise was observed to be associated with an increase of peripheral EPCs in patients with NYHA class III heart failure [60].
EPCs were also studied in patients with pulmonary arterial hypertension, with conflicting results. Whereas some studies reported depleted peripheral EPC levels [37–39], other studies noted increased EPC levels [40,41]. EPCs have further been shown to take part in the vascular remodeling in pulmonary arterial hypertension [61]. These different findings may be related to discrepancies regarding the methods of characterization and quantification of EPCs as well as assessment of patients with different etiologies or stages of pulmonary arterial hypertension [62].
EPCs in rheumatic & chronic inflammatory conditions
In many inflammatory rheumatic diseases, decreased peripheral levels and functionally altered EPCs have been described. Rheumatoid arthritis (RA) is the most common inflammatory joint disease that can lead to joint destruction and disability if insufficiently treated, and it is characterized by an increased cardiovascular mortality and morbidity [63] even after adjustment for traditional risk factors [64]. The increased cardiovascular risk is also true for other chronic inflammatory conditions [65,66], such as systemic lupus erythematosus (SLE), which exhibits up to a 50-fold increase in cardiovascular mortality [67,68].
In RA, the levels of peripheral EPCs are decreased compared with matched healthy controls [69–71], and an inverse relationship has been reported between peripheral EPC levels and disease activity as measured by the disease activity score 28 (DAS-28) [69,72]. EPCs were also observed to accumulate in the inflamed joints [73,74], where an increased blood vessel supply is needed, giving rise to the hypothesis that peripheral EPCs are trapped within the highly vascularized inflamed joints [75]. The trapped EPCs are suspected to contribute to disease progression by helping to maintain the inflammatory process via vasculogenesis, further facilitating the influx of immune cells [75].
SLE, a potentially life-threatening disease that can affect multiple organs, was found to be associated with depleted [76–80] or normal [81,82] peripheral EPC levels. Furthermore, SLE is also associated with impaired EPC differentiation into endothelial cells, depleted adhesion and migration capacity, as well as an increased susceptibility to apoptosis and a reduction of angiogenic growth factors [80,82].
Systemic sclerosis (or scleroderma) is a disease characterized by a suspected endothelial damage, causing fibrosis of the skin and other organs such as the lung and the kidney. Owing to the heterogeneity of the disease and the different surface markers used, the findings on EPCs have been conflicting. One study showed decreased peripheral EPC levels [83], but others found increased EPCs within the circulation when compared with controls [84,85] that correlated with disease activity [86]. These discrepant results may be caused by different markers, as well as the fact that the disease duration and the disease activity might affect the EPC quantities [87]. At present, there is no clear consensus regarding the exact role EPCs play in scleroderma. However, EPCs might develop into a therapeutic target in systemic sclerosis. There is evidence that the healing of digital ulcers, which are common in systemic sclerosis, can be improved by enhancing peripheral EPC levels with erythropoietin [88]. Moreover, treatment with intravenous cyclophosphamide for interstitial lung disease associated with systemic sclerosis was linked to an increase in peripheral EPC levels [89].
Kawasaki’s disease is a rare vasculitis that mainly affects children in the Asian Pacific region [90]. Its hallmark is the occlusion of medium-sized vessels that can lead to life threatening events. Two publications indicated that the disease is characterized by lowered EPC levels [91] that were inversely correlated with serum TNF and C-reactive protein (CRP) levels [92]. In a follow-up study, successful treatment with intravenous immunoglobulins was associated with a reduction of TNF and high-sensitivity CRP levels, and restored the functional deficiencies of the EPCs in the Kawasaki patients [93].
Interplay between EPC biology, inflammation & immunity
Immune activation plays an important role in atherosclerosis and heart failure. Understanding the interplay between EPC biology and inflammation is important from both a biomarker and therapeutic perspective. Moreover, immune modulation of stem cell biology might be essential in the future to improve stem cell survival in the clinical setting. Both markers of inflammation and low numbers of EPCs have been associated with an increased risk of cardiovascular events [31,94–96]. Data published in recent years have convincingly shown that EPCs play a role and participate in inflammation, but are also themselves significantly affected by chronic inflammation and chronic inflammatory diseases [97]. Inflammation is associated with an increased secretion of cytokines by the various immune cell types. Amongst the relevant cytokines, TNF and IL-6 are considered to be key players [98] and have been shown to significantly affect EPC biology. The same is the case for CRP, one of the best surrogate parameters of systemic inflammation [99].
To understand the effects of inflammation on EPC biology, it is important to differentiate between the acute effects of a cytokine on stem cell mobilization and the effect of chronic cytokine activation and inflammatory milieu on EPC biology (Figure 1). Furthermore, recruitment of EPCs in an inflammatory milieu can lead to a reduction of the circulating EPCs.
Inflammation & EPC mobilization & function
CRP is one of the most common acute phase reactants. In 2004, Verma and colleagues showed that CRP inhibits EPC differentiation, survival and function in vitro [100], a finding that was further corroborated by an observation showing that even in healthy subjects, the colony-forming capacity is negatively correlated with the CRP serum levels [101]. CRP itself may impair EPC antioxidant defenses, and may promote EPC sensitivity toward oxidant-mediated apoptosis and telomerase inactivation [102]. This effect might be due to the CRP-induced upregulation of the receptor for advanced glycation end products, leading to an increased EPC sensitivity and oxidative stress-mediated apoptosis [103].
By contrast, CRP release as a consequence of an ischemic event or endothelial injury was shown to lead to rapid EPC mobilization [34,35,104,105]. Together, these findings suggest a dual role of CRP in EPC biology (Figure 1), depending on the cause and duration of CRP secretion. Another possible explanation for this phenomenon was recently published by Ahrens and coworkers, who found somewhat controversial results when studying the effects of native, monomeric, and pentameric CRP on EPCs in a tube formation assay [106]. It turned out that the two types of CRP induced an opposing gene expression profile. Interestingly, the gene expression pattern of monomeric CRP-treated EPCs was related to the one found in patients suffering from SLE. Moreover, pentameric CRP was associated with increased apoptosis and lowered tube formation of EPCs in vitro [106].
The production of CRP in the liver is mainly induced and perpetuated by the proinflammatory cytokines IL-6, -1 and -17 [107]. In recent years, it has become evident that IL-6 plays a key role in inflammation of chronic inflammatory disorders. This cytokine is produced by various cells contributing to inflammatory reactions, with the vast majority believed to be secreted from macrophages and other lymphocytes [108]. In RA, IL-6 is systemically elevated, and recently its blockade with an IL-6 receptor antagonist was shown to be a successful treatment option for this crippling disease and is now a routinely administered drug [109]. Herbrig and coworkers first described that higher levels of serum IL-6 levels are associated with lower EPC numbers while studying these cells in RA patients and healthy controls [71], suggesting a potential role for this cytokine in EPC biology.
Experimental studies also suggest that an acute increase in IL-6 can lead to EPC mobilization, whereas chronic IL-6 secretion is associated with a reduction in peripheral EPCs [110] (Figure 1). In the study by Fan et al., IL-6 improved EPC proliferation, migration and tube formation [110]. This observation is further corroborated by the fact that exercise-induced EPC mobilization was linked to IL-6 secretion in healthy individuals [104,111]. Thus, it is likely that IL-6 plays a promoting role that could be regarded as physiological, whereas chronically increased systemic IL-6 levels might directly and/or indirectly impair EPC function and number.
TNF is another pivotal proinflammatory cytokine that is highly upregulated in many inflammatory diseases. TNF and its relation to EPCs have been investigated in various diseases, and this proinflammatory cytokine indeed has significant effects on EPC biology, mobilization and differentiation. In RA, increased TNF serum levels were shown to be associated with reduced peripheral EPC numbers, and patients treated with antibodies blocking TNF showed either normal EPC levels [69] or an increase of EPCs after drug administration [112]. However, the decreased peripheral levels of EPCs in active RA were reversible after treating the patients with medium-dose glucocorticoids for one week [70], an effect that was partly dependent on TNF and glucocorticoid, as shown by in vitro experiments.
Moreover, in Type 1 diabetes, in line with the results described in RA, an inverse relationship between peripheral EPC levels and TNF serum levels has been demonstrated [113]. Investigating the effects of TNF on EPCs in vitro, Chen and coworkers showed that addition of TNF to EPCs isolated from healthy controls led to a dose-dependent reduction of proliferation, migration, adhesion and tube formation capacity [114]. In addition, the presence of TNF increased the EPC apoptosis rate, and augmented the expression of proinflammatory adhesion molecules and paracrine factors in EPCs. The TNF-induced reduction of EPCs, however, could be reduced or reverted in the presence of statins [115] or reservatrol found in red wine [116]. TNF was also further shown to induce apoptosis of EPCs, an effect that could also be inhibited by the addition of simvastatin [115].
Recruitment of EPCs in the inflammatory milieu
Inflamed tissue is usually characterized by an increased demand for new vessels to sufficiently supply the tissue with oxygen and immune-competent cells. Moreover, inflammation is characterized by hypoxia, which has been shown to be a very strong stimulus for EPC recruitment in order to enhance vasculogenesis [117], which is at least partly driven by hypoxia-inducible factor-1α. In RA, there is strong evidence that EPCs are selectively recruited into the inflamed joint by vascular adhesion molecule-1 [74]. Accumulation of EPCs in inflamed tissues might trigger local synovial inflammation by promoting the process of inflammation via vasculogenesis [75]. As discussed above, inflammatory diseases are mainly associated with increased levels of CRP and reduced number of peripheral EPCs; both of these factors can contribute to the progression of cardiovascular risk. Therefore, the increase of EPCs after successful treatment, might also be a consequence of modulation of both systemic and local inflammation.
EPCs as a therapeutic agent or target
Given the hard evidence that an enhancement of the EPC pool and an increase of peripheral EPCs might be beneficial for and/or preventive of cardiovascular disease modalities, increasing the number or improving the function of EPCs may be promising in the treatment of atherosclerotic disease or heart failure. The goal of increasing circulating EPCs to facilitate vasculogenesis in ischemic tissues can be reached by different methods, either by enhanced mobilization from the bone marrow or autologous application of EPCs.
Effects of exercise & drug therapy on EPC mobilization
Exercise has been shown to enhance mobilization of EPCs in both healthy controls and patients with heart failure [118–121]. This provides another mechanistic explanation for why exercise is regarded as beneficial in patients with cardiovascular disease. Dietary and lifestyle factors have also been shown to increase EPC mobilization, including green tea consumption [122], Mediterranean diet [123] and moderate red wine consumption [116]. By contrast, obesity [124] and smoking are related to lower EPC counts [125]. Moreover, several drugs have been shown to be associated with either enhanced peripheral EPC levels or with an improvement of EPC function. Amongst them are various statins [12,126], erythropoietin [127,128], glitazones [129,130] and antihypertensive drugs such as angiotensin-converting enzyme inhibitors [131] and angiotensin II receptor blockers [132,133].
EPC-based therapies
Given the strong association between EPCs and cardiovascular outcomes, there has been a growing interest in investigating the therapeutic role of EPCs. However, several obstacles exist before large scale use of EPCs can commence. For instance, the relatively rare cells must be expanded in sufficient numbers from peripheral blood, and possible changes in phenotype may create a risk of cell senescence after in vitro enumeration of progenitor cells. The clinical application of EPC-based therapy is, therefore, still in very early stages, as critical questions regarding EPC survival, timing of administration, and phase- or activity-dependent efficacy of the disease still need to be resolved.
The available clinical studies on stem cell administration and outcome in cardiovascular disease are mainly based on the administration of CD34+ cells, with only a few studies investigating the role of CD34+/CD133+ cells. In the study by Stamm et al., intracoronary infusion of CD133+ cells after acute myocardial infarction resulted in an early improvement of left ventricular ejection fraction [134], but later was associated with luminal narrowing and failure in remodeling [135]. Another study reported an improvement in left ventricular function following the transepicardial injection of CD133+ cells into the myocardial border zone [136,137]. One multicenter double-blind and randomized placebo-controlled trial with 167 patients who underwent transendocardial application (NOGA® mapping) of mobilized, autologously transplanted CD34+ cells reported a lower weekly rate of angina in the low-cell-dose-treated patients and a significantly greater improvement in exercise response [138]. In patients with dilated cardiomyopathy, Vrotvec et al. have shown that administration of autologously transplanted CD34+ cells led to an improvement in left ventricular ejection fraction and was associated with more clinical stability [139].
Another therapeutic approach, besides increasing mobilization and transplantation of EPCs, is to aim for a better homing of these cells into a ‘target organ’. Data from animal and in vitro experiments showed that blockade of C-X-C chemokine receptor type 4 was sufficient to mobilize EPCs, increase recruitment to the neovasculature and reduce mortality [140]. However, these options of therapeutic interventions in humans remain to be further validated.
Future perspective
EPCs (both ECFCs and hematopoietic cells with angiogenic properties) are emerging as useful biomarkers in cardiovascular disease. They may improve risk stratification, and offer novel tools for monitoring disease progression and response to therapy. However, the search for a unique marker allowing a generalized protocol for quantification is still ongoing. Validation of the findings on the predictive role of EPCs on outcome requires larger sample sizes and appropriate adjustment for covariates of disease such as age, diastolic left heart function and pulmonary hypertension. Future studies will also help define the role of EPC-based therapy and answer challenging questions on patient selection, EPC expansion, EPC survival and EPC administration. Modulation of EPC recruitment in inflamed tissues could also be a promising area of research, because blocking their influx into the inflamed area might reduce local inflammation and progression.
Executive summary.
Definition of endothelial progenitor cells
▪ Endothelial progenitor cells (EPCs) were first described in 1997 as bone marrow-derived cells capable of neovascularization. EPCs were previously thought to differentiate out of the hematopoietic lineage, but there is evidence that the ‘earlier’ termed EPCs can be mainly subdivided into endothelial colony-forming cells and hematopoietic cells with angiogenic properties.
▪ Owing to the lack of a unique marker, the exact definition of EPCs is still being discussed. For flow cytometry, the hematopoietic marker CD45 has been described to be critical in recent recommendations. However, the variations in the methods used in different studies make direct comparisons difficult.
▪ EPCs are decreased in the majority of cardiovascular diseases.
▪ The majority of studies investigating EPCs in diverse disease states report decreased levels of EPCs in chronic cardiovascular disorders, whereas acute ischemic events are associated with an increased mobilization of EPCs. These results reveal that EPCs are potential biomarkers for monitoring the ‘cardiovascular health status’.
Interplay between the immune system & EPCs
▪ Proinflammatory mediators such as cytokines and growth factors are significantly involved in EPC biology and underlie the interplay between the immune system and the cardiovascular system.
EPCs as therapeutics
▪ Increasing the pool of peripheral (or, in ischemic tissue, local) EPCs may improve vascular repair. Several drugs, exercise and dietary factors have been found to enhance EPC levels and improve function. Autologous transplantation of EPCs is another potentially promising avenue.
Table 3.
Selected outcome and characterization studies on endothelial progenitor cells in patients with heart failure and pulmonary hypertension.
| Author | Population | Study design | n | Methods | Main finding | Ref. |
|---|---|---|---|---|---|---|
| Heart failure | ||||||
| Valgimigli et al. (2004) |
Heart failure | Cross-sectional | 91 | FACS, Hill assay | Compared with controls, EPCs were increased in class I heart failure with numbers progressively decreasing with increased severity of disease |
[36] |
| Michowitz et al. (2007) |
Heart failure NYHA class II–IV |
Prospective | 107 | Hill assay | EPC levels, age and diabetes mellitus were independent predictors of all-cause mortality |
[59] |
| Erbs et al. (2010) |
Heart Failure, NYHA class III |
Exercise-based trial |
18 (exercise) 19 (controls) |
FACS | Exercise increased circulating EPCs, endothelial function, left ventricular ejection fraction and maximal oxygen consumption |
[60] |
| PAH | ||||||
| Diller et al. (2008) |
Idiopathic PAH Eisenmenger |
Cross-sectional | 55 41 |
FACS | Circulating EPC numbers were found to be reduced in idiopathic PAH and Eisenmenger, which also showed increased levels of inflammatory mediators |
[37] |
| Junhui et al. (2008) |
Idiopathic PAH | Cross-sectional | 20 | FACS, early outgrowth assay, migration assay, adhesion assay |
EPC number and function impaired compared with healthy controls |
[39] |
| Asosingh et al. (2008) |
Idiopathic PAH | Cross-sectional | 16 | FACS, late outgrowth assay, Hill assay |
Patients with idiopathic PAH have higher numbers of circulating proangiogenic precursors |
[41] |
EPC: Endothelial progenitor cell; FACS: Fluorescence-activated cell sorting; NHYA: New York Heart Association; PAH: Pulmonary arterial hypertension.
Acknowledgements
Due to space limitations, we are unable to include all of the important papers relevant to biomarkers, endothelial cells and cardiovascular disease; we apologize to those investigators whose work we omitted here.
This work was supported by Burroughs Wellcome Foundation, NIH L30 HL085899, NIH EB009689 (JC Wu) and the Austrian Science Fund J2884-B11 (JC Grisar).
Footnotes
Financial & competing interests disclosure The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Folkman J. Angiogenesis. Annu. Rev. Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 2.Rafii S, Oz MC, Seldomridge JA, et al. Characterization of hematopoietic cells arising on the textured surface of left ventricular assist devices. Ann. Thorac. Surg. 1995;60(6):1627–1632. doi: 10.1016/0003-4975(95)00807-1. [DOI] [PubMed] [Google Scholar]
- 3.Sbarbati R, de Boer M, Marzilli M, Scarlattini M, Rossi G, van Mourik JA. Immunologic detection of endothelial cells in human whole blood. Blood. 1991;77(4):764–769. [PubMed] [Google Scholar]
- 4.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–967. doi: 10.1126/science.275.5302.964. ▪▪ First study showing that bone marrow-derived cells are capable of neovascularization.
- 5.Distler JH, Allanore Y, Avouac J, et al. EULAR scleroderma trials and research group statement and recommendations on endothelial precursor cells. Ann. Rheum. Dis. 2009;68(2):163–168. doi: 10.1136/ard.2008.091918. [DOI] [PubMed] [Google Scholar]
- 6.George J, Shmilovich H, Deutsch V, Miller H, Keren G, Roth A. Comparative analysis of methods for assessment of circulating endothelial progenitor cells. Tissue Eng. 2006;12(2):331–335. doi: 10.1089/ten.2006.12.331. [DOI] [PubMed] [Google Scholar]
- 7.Khan SS, Solomon MA, McCoy JP., Jr Detection of circulating endothelial cells and endothelial progenitor cells by flow cytometry. Cytometry B Clin. Cytom. 2005;64(1):1–8. doi: 10.1002/cyto.b.20040. [DOI] [PubMed] [Google Scholar]
- 8.Ingram DA, Caplice NM, Yoder MC. Unresolved questions, changing definitions, and novel paradigms for defining endothelial progenitor cells. Blood. 2005;106(5):1525–1531. doi: 10.1182/blood-2005-04-1509. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt-Lucke C, Fichtlscherer S, Aicher A, et al. Quantification of circulating endothelial progenitor cells using the modified ISHAGE protocol. PLoS One. 2010;5(11):e13790. doi: 10.1371/journal.pone.0013790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Craenenbroeck EM, Conraads VM, Van Bockstaele DR, et al. Quantification of circulating endothelial progenitor cells: a methodological comparison of six flow cytometric approaches. J. Immunol. Methods. 2008;332(1–2):31–40. doi: 10.1016/j.jim.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Kalka C, Masuda H, Takahashi T, et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc. Natl Acad. Sci. USA. 2000;97(7):3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vasa M, Fichtlscherer S, Adler K, et al. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation. 2001;103(24):2885–2890. doi: 10.1161/hc2401.092816. [DOI] [PubMed] [Google Scholar]
- 13.Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N. Engl. J. Med. 2003;348(7):593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 14.Ingram DA, Mead LE, Tanaka H, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104(9):2752–2760. doi: 10.1182/blood-2004-04-1396. ▪▪ Provides evidence that cells capable of vasculogenesis are not of hematopoietic origin. Subsequently, evidence emerged that there exists both hematopoietic cells with proangiogenic properties and the even rarer endothelial cells with colony-forming abilities.
- 15.Rehman J, Li J, Orschell CM, March KL. Peripheral blood ‘endothelial progenitor cells’ are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107(8):1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 16.Gulati R, Jevremovic D, Peterson TE, et al. Diverse origin and function of cells with endothelial phenotype obtained from adult human blood. Circ. Res. 2003;93(11):1023–1025. doi: 10.1161/01.RES.0000105569.77539.21. [DOI] [PubMed] [Google Scholar]
- 17.Yoder MC. Defining human endothelial progenitor cells. J. Thromb. Haemost. 2009;7(Suppl. 1):49–52. doi: 10.1111/j.1538-7836.2009.03407.x. [DOI] [PubMed] [Google Scholar]
- 18.Yoder MC, Mead LE, Prater D, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/ progenitor cell principals. Blood. 2007;109(5):1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ. Res. 2004;95(4):343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 20.Gehling UM, Ergun S, Schumacher U, et al. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood. 2000;95(10):3106–3112. [PubMed] [Google Scholar]
- 21.Case J, Mead LE, Bessler WK, et al. Human CD34+AC133+VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp. Hematol. 2007;35(7):1109–1118. doi: 10.1016/j.exphem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Richardson MR, Yoder MC. Endothelial progenitor cells: quo vadis? J. Mol. Cell. Cardiol. 2011;50(2):266–272. doi: 10.1016/j.yjmcc.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Estes ML, Mund JA, Ingram DA, Case J. Identification of endothelial cells and progenitor cell subsets in human peripheral blood. Curr. Protoc. Cytom. 2010;33:1–11. doi: 10.1002/0471142956.cy0933s52. Chapter 9, Unit 9. [DOI] [PubMed] [Google Scholar]
- 24.Loomans CJ, de Koning EJ, Staal FJ, et al. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of Type 1 diabetes. Diabetes. 2004;53(1):195–199. doi: 10.2337/diabetes.53.1.195. [DOI] [PubMed] [Google Scholar]
- 25.Tepper OM, Galiano RD, Capla JM, et al. Human endothelial progenitor cells from Type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106(22):2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 26.Pirro M, Schillaci G, Menecali C, et al. Reduced number of circulating endothelial progenitors and HOXA9 expression in CD34+ cells of hypertensive patients. J. Hypertens. 2007;25(10):2093–2099. doi: 10.1097/HJH.0b013e32828e506d. [DOI] [PubMed] [Google Scholar]
- 27.Umemura T, Soga J, Hidaka T, et al. Aging and hypertension are independent risk factors for reduced number of circulating endothelial progenitor cells. Am. J. Hypertens. 2008;21(11):1203–1209. doi: 10.1038/ajh.2008.278. [DOI] [PubMed] [Google Scholar]
- 28.Choi JH, Kim KL, Huh W, et al. Decreased number and impaired angiogenic function of endothelial progenitor cells in patients with chronic renal failure. Arterioscler. Thromb. Vasc. Biol. 2004;24(7):1246–1252. doi: 10.1161/01.ATV.0000133488.56221.4a. [DOI] [PubMed] [Google Scholar]
- 29.de Groot K, Bahlmann FH, Sowa J, et al. Uremia causes endothelial progenitor cell deficiency. Kidney Int. 2004;66(2):641–646. doi: 10.1111/j.1523-1755.2004.00784.x. [DOI] [PubMed] [Google Scholar]
- 30.Lorenzen J, David S, Bahlmann FH, et al. Endothelial progenitor cells and cardiovascular events in patients with chronic kidney disease: a prospective follow-up study. PLoS One. 2010;5(7):e11477. doi: 10.1371/journal.pone.0011477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Werner N, Kosiol S, Schiegl T, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N. Engl. J. Med. 2005;353(10):999–1007. doi: 10.1056/NEJMoa043814. ▪▪ Landmark study, showing that reduced endothelial progenitor cell levels are associated with cardiovascular outcomes in patients with cardiovascular disease. Demonstrates that circulating endothelial progenitor cells might be useful as a biomarker.
- 32.Ghani U, Shuaib A, Salam A, et al. Endothelial progenitor cells during cerebrovascular disease. Stroke. 2005;36(1):151–153. doi: 10.1161/01.STR.0000149944.15406.16. [DOI] [PubMed] [Google Scholar]
- 33.Shintani S, Murohara T, Ikeda H, et al. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001;103(23):2776–2779. doi: 10.1161/hc2301.092122. [DOI] [PubMed] [Google Scholar]
- 34.George J, Goldstein E, Abashidze S, et al. Circulating endothelial progenitor cells in patients with unstable angina: association with systemic inflammation. Eur. Heart J. 2004;25(12):1003–1008. doi: 10.1016/j.ehj.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 35.Guven H, Shepherd RM, Bach RG, Capoccia BJ, Link DC. The number of endothelial progenitor cell colonies in the blood is increased in patients with angiographically significant coronary artery disease. J. Am. Coll. Cardiol. 2006;48(8):1579–1587. doi: 10.1016/j.jacc.2006.04.101. [DOI] [PubMed] [Google Scholar]
- 36.Valgimigli M, Rigolin GM, Fucili A, et al. CD34+ and endothelial progenitor cells in patients with various degrees of congestive heart failure. Circulation. 2004;110(10):1209–1212. doi: 10.1161/01.CIR.0000136813.89036.21. [DOI] [PubMed] [Google Scholar]
- 37.Diller GP, van Eijl S, Okonko DO, et al. Circulating endothelial progenitor cells in patients with Eisenmenger syndrome and idiopathic pulmonary arterial hypertension. Circulation. 2008;117(23):3020–3030. doi: 10.1161/CIRCULATIONAHA.108.769646. [DOI] [PubMed] [Google Scholar]
- 38.Fadini GP, Schiavon M, Rea F, Avogaro A, Agostini C. Depletion of endothelial progenitor cells may link pulmonary fibrosis and pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2007;176(7):724–725. doi: 10.1164/ajrccm.176.7.724a. author reply 725. [DOI] [PubMed] [Google Scholar]
- 39.Junhui Z, Xingxiang W, Guosheng F, Yunpeng S, Furong Z, Junzhu C. Reduced number and activity of circulating endothelial progenitor cells in patients with idiopathic pulmonary arterial hypertension. Respir. Med. 2008;102(7):1073–1079. doi: 10.1016/j.rmed.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 40.Toshner M, Voswinckel R, Southwood M, et al. Evidence of dysfunction of endothelial progenitors in pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2009;180(8):780–787. doi: 10.1164/rccm.200810-1662OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Asosingh K, Aldred MA, Vasanji A, et al. Circulating angiogenic precursors in idiopathic pulmonary arterial hypertension. Am. J. Pathol. 2008;172(3):615–627. doi: 10.2353/ajpath.2008.070705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas HE, Parry G, Dark JH, Arthur HM, Keavney BD. Circulating endothelial progenitor cell numbers are not associated with donor organ age or allograft vasculopathy in cardiac transplant recipients. Atherosclerosis. 2009;202(2):612–616. doi: 10.1016/j.atherosclerosis.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 43.Caplice NM, Bunch TJ, Stalboerger PG, et al. Smooth muscle cells in human coronary atherosclerosis can originate from cells administered at marrow transplantation. Proc. Natl Acad. Sci. USA. 2003;100(8):4754–4759. doi: 10.1073/pnas.0730743100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Osto E, Castellani C, Fadini GP, et al. Impaired endothelial progenitor cell recruitment may contribute to heart transplant microvasculopathy. J. Heart Lung Transplant. 2011;30(1):70–76. doi: 10.1016/j.healun.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt-Lucke C, Rossig L, Fichtlscherer S, et al. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111(22):2981–2987. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- 46.Xiao Q, Kiechl S, Patel S, et al. Endothelial progenitor cells, cardiovascular risk factors, cytokine levels and atherosclerosis – results from a large population-based study. PLoS One. 2007;2(10):e975. doi: 10.1371/journal.pone.0000975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hristov M, Zernecke A, Liehn EA, Weber C. Regulation of endothelial progenitor cell homing after arterial injury. Thromb. Haemost. 2007;98(2):274–277. [PubMed] [Google Scholar]
- 48.Hristov M, Zernecke A, Bidzhekov K, et al. Importance of CXC chemokine receptor 2 in the homing of human peripheral blood endothelial progenitor cells to sites of arterial injury. Circ. Res. 2007;100(4):590–597. doi: 10.1161/01.RES.0000259043.42571.68. [DOI] [PubMed] [Google Scholar]
- 49.George J, Afek A, Abashidze A, et al. Transfer of endothelial progenitor and bone marrow cells influences atherosclerotic plaque size and composition in apolipoprotein E knockout mice. Arterioscler. Thromb. Vasc. Biol. 2005;25(12):2636–2641. doi: 10.1161/01.ATV.0000188554.49745.9e. [DOI] [PubMed] [Google Scholar]
- 50.Sata M, Saiura A, Kunisato A, et al. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat. Med. 2002;8(4):403–409. doi: 10.1038/nm0402-403. [DOI] [PubMed] [Google Scholar]
- 51.Torsney E, Mandal K, Halliday A, Jahangiri M, Xu Q. Characterisation of progenitor cells in human atherosclerotic vessels. Atherosclerosis. 2007;191(2):259–264. doi: 10.1016/j.atherosclerosis.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 52.Hagensen MK, Shim J, Thim T, Falk E, Bentzon JF. Circulating endothelial progenitor cells do not contribute to plaque endothelium in murine atherosclerosis. Circulation. 2010;121(7):898–905. doi: 10.1161/CIRCULATIONAHA.109.885459. [DOI] [PubMed] [Google Scholar]
- 53.Fadini GP, Avogaro A. Potential manipulation of endothelial progenitor cells in diabetes and its complications. Diabetes Obes. Metab. 2010;12(7):570–583. doi: 10.1111/j.1463-1326.2010.01210.x. [DOI] [PubMed] [Google Scholar]
- 54.Fadini GP, Miorin M, Facco M, et al. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of Type 2 diabetes mellitus. J. Am. Coll. Cardiol. 2005;45(9):1449–1457. doi: 10.1016/j.jacc.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 55.Fadini GP, Sartore S, Albiero M, et al. Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy. Arterioscler. Thromb. Vasc. Biol. 2006;26(9):2140–2146. doi: 10.1161/01.ATV.0000237750.44469.88. [DOI] [PubMed] [Google Scholar]
- 56.Fadini GP, Sartore S, Baesso I, et al. Endothelial progenitor cells and the diabetic paradox. Diabetes Care. 2006;29(3):714–716. doi: 10.2337/diacare.29.03.06.dc05-1834. [DOI] [PubMed] [Google Scholar]
- 57.Makino H, Okada S, Nagumo A, et al. Decreased circulating CD34+ cells are associated with progression of diabetic nephropathy. Diabet. Med. 2009;26(2):171–173. doi: 10.1111/j.1464-5491.2008.02638.x. [DOI] [PubMed] [Google Scholar]
- 58.Fadini GP, Maruyama S, Ozaki T, et al. Circulating progenitor cell count for cardiovascular risk stratification: a pooled analysis. PLoS One. 2010;5(7):e11488. doi: 10.1371/journal.pone.0011488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Michowitz Y, Goldstein E, Wexler D, Sheps D, Keren G, George J. Circulating endothelial progenitor cells and clinical outcome in patients with congestive heart failure. Heart. 2007;93(9):1046–1050. doi: 10.1136/hrt.2006.102657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Erbs S, Hollriegel R, Linke A, et al. Exercise training in patients with advanced chronic heart failure (NYHA IIIb) promotes restoration of peripheral vasomotor function, induction of endogenous regeneration, and improvement of left ventricular function. Circ. Heart Fail. 2010;3(4):486–494. doi: 10.1161/CIRCHEARTFAILURE.109.868992. [DOI] [PubMed] [Google Scholar]
- 61.Yao W, Firth AL, Sacks RS, et al. Identification of putative endothelial progenitor cells (CD34+CD133+Flk-1+) in endarterectomized tissue of patients with chronic thromboembolic pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009;296(6):L870–L878. doi: 10.1152/ajplung.90413.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Diller GP, Thum T, Wilkins MR, Wharton J. Endothelial progenitor cells in pulmonary arterial hypertension. Trends Cardiovasc. Med. 2010;20(1):22–29. doi: 10.1016/j.tcm.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 63.Wolfe F, Mitchell DM, Sibley JT, et al. The mortality of rheumatoid arthritis. Arthritis Rheum. 1994;37(4):481–494. doi: 10.1002/art.1780370408. [DOI] [PubMed] [Google Scholar]
- 64.del Rincon ID, Williams K, Stern MP, Freeman GL, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001;44(12):2737–2745. doi: 10.1002/1529-0131(200112)44:12<2737::AID-ART460>3.0.CO;2-%23. [DOI] [PubMed] [Google Scholar]
- 65.Sarzi-Puttini P, Atzeni F, Gerli R, et al. Cardiac involvement in systemic rheumatic diseases: an update. Autoimmun. Rev. 2010;9(12):849–852. doi: 10.1016/j.autrev.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 66.Szekanecz Z, Koch AE. Vascular involvement in rheumatic diseases: ‘vascular rheumatology’. Arthritis Res. Ther. 2008;10(5):224. doi: 10.1186/ar2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Urowitz MB, Bookman AA, Koehler BE, Gordon DA, Smythe HA, Ogryzlo MA. The bimodal mortality pattern of systemic lupus erythematosus. Am. J. Med. 1976;60(2):221–225. doi: 10.1016/0002-9343(76)90431-9. [DOI] [PubMed] [Google Scholar]
- 68.Rubin LA, Urowitz MB, Gladman DD. Mortality in systemic lupus erythematosus: the bimodal pattern revisited. Q. J. Med. 1985;55(216):87–98. [PubMed] [Google Scholar]
- 69.Grisar J, Aletaha D, Steiner CW, et al. Depletion of endothelial progenitor cells in the peripheral blood of patients with rheumatoid arthritis. Circulation. 2005;111(2):204–211. doi: 10.1161/01.CIR.0000151875.21836.AE. [DOI] [PubMed] [Google Scholar]
- 70.Grisar J, Aletaha D, Steiner CW, et al. Endothelial progenitor cells in active rheumatoid arthritis: effects of tumour necrosis factor and glucocorticoid therapy. Ann. Rheum. Dis. 2007;66(10):1284–1288. doi: 10.1136/ard.2006.066605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Herbrig K, Haensel S, Oelschlaegel U, Pistrosch F, Foerster S, Passauer J. Endothelial dysfunction in patients with rheumatoid arthritis is associated with a reduced number and impaired function of endothelial progenitor cells. Ann. Rheum. Dis. 2006;65(2):157–163. doi: 10.1136/ard.2005.035378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Prevoo ML, van ‘t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38(1):44–48. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 73.Ruger B, Giurea A, Wanivenhaus AH, et al. Endothelial precursor cells in the synovial tissue of patients with rheumatoid arthritis and osteoarthritis. Arthritis Rheum. 2004;50(7):2157–2166. doi: 10.1002/art.20506. [DOI] [PubMed] [Google Scholar]
- 74.Silverman MD, Haas CS, Rad AM, Arbab AS, Koch AE. The role of vascular cell adhesion molecule-1/very late activation antigen 4 in endothelial progenitor cell recruitment to rheumatoid arthritis synovium. Arthritis Rheum. 2007;56(6):1817–1826. doi: 10.1002/art.22706. [DOI] [PubMed] [Google Scholar]
- 75.Paleolog E. It’s all in the blood: circulating endothelial progenitor cells link synovial vascularity with cardiovascular mortality in rheumatoid arthritis? Arthritis Res. Ther. 2005;7(6):270–272. doi: 10.1186/ar1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moonen JR, de Leeuw K, van Seijen XJ, et al. Reduced number and impaired function of circulating progenitor cells in patients with systemic lupus erythematosus. Arthritis Res. Ther. 2007;9(4):R84. doi: 10.1186/ar2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Westerweel PE, Luijten RK, Hoefer IE, Koomans HA, Derksen RH, Verhaar MC. Haematopoietic and endothelial progenitor cells are deficient in quiescent systemic lupus erythematosus. Ann. Rheum. Dis. 2007;66(7):865–870. doi: 10.1136/ard.2006.065631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Denny MF, Thacker S, Mehta H, et al. Interferon-α promotes abnormal vasculogenesis in lupus: a potential pathway for premature atherosclerosis. Blood. 2007;110(8):2907–2915. doi: 10.1182/blood-2007-05-089086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee PY, Li Y, Richards HB, et al. Type I interferon as a novel risk factor for endothelial progenitor cell depletion and endothelial dysfunction in systemic lupus erythematosus. Arthritis Rheum. 2007;56(11):3759–3769. doi: 10.1002/art.23035. [DOI] [PubMed] [Google Scholar]
- 80.Ebner P, Picard F, Richter J, et al. Accumulation of VEGFR-2+/CD133+ cells and decreased number and impaired functionality of CD34+/ VEGFR-2+ cells in patients with SLE. Rheumatology (Oxford) 2010;49(1):63–72. doi: 10.1093/rheumatology/kep335. [DOI] [PubMed] [Google Scholar]
- 81.Deng XL, Li XX, Liu XY, Sun L, Liu R. Comparative study on circulating endothelial progenitor cells in systemic lupus erythematosus patients at active stage. Rheumatol. Int. 2009;30(11):1429–1436. doi: 10.1007/s00296-009-1156-4. [DOI] [PubMed] [Google Scholar]
- 82.Grisar J, Steiner CW, Bonelli M, et al. Systemic lupus erythematosus patients exhibit functional deficiencies of endothelial progenitor cells. Rheumatology (Oxford) 2008;47(10):1476–1483. doi: 10.1093/rheumatology/ken286. [DOI] [PubMed] [Google Scholar]
- 83.Kuwana M, Okazaki Y, Yasuoka H, Kawakami Y, Ikeda Y. Defective vasculogenesis in systemic sclerosis. Lancet. 2004;364(9434):603–610. doi: 10.1016/S0140-6736(04)16853-0. [DOI] [PubMed] [Google Scholar]
- 84.Del Papa N, Colombo G, Fracchiolla N, et al. Circulating endothelial cells as a marker of ongoing vascular disease in systemic sclerosis. Arthritis Rheum. 2004;50(4):1296–1304. doi: 10.1002/art.20116. [DOI] [PubMed] [Google Scholar]
- 85.Allanore Y, Batteux F, Avouac J, Assous N, Weill B, Kahan A. Levels of circulating endothelial progenitor cells in systemic sclerosis. Clin. Exp. Rheumatol. 2007;25(1):60–66. [PubMed] [Google Scholar]
- 86.Avouac J, Juin F, Wipff J, et al. Circulating endothelial progenitor cells in systemic sclerosis: association with disease severity. Ann. Rheum. Dis. 2008;67(10):1455–1460. doi: 10.1136/ard.2007.082131. [DOI] [PubMed] [Google Scholar]
- 87.Nevskaya T, Bykovskaia S, Lyssuk E, et al. Circulating endothelial progenitor cells in systemic sclerosis: relation to impaired angiogenesis and cardiovascular manifestations. Clin. Exp. Rheumatol. 2008;26(3):421–429. [PubMed] [Google Scholar]
- 88.Ferri C, Giuggioli D, Manfredi A, et al. Recombinant human erythropoietin stimulates vasculogenesis and wound healing in a patient with systemic sclerosis complicated by severe skin ulcers. Clin. Exp. Dermatol. 2010;35(8):885–887. doi: 10.1111/j.1365-2230.2010.03847.x. [DOI] [PubMed] [Google Scholar]
- 89.Furuya Y, Okazaki Y, Kaji K, Sato S, Takehara K, Kuwana M. Mobilization of endothelial progenitor cells by intravenous cyclophosphamide in patients with systemic sclerosis. Rheumatology (Oxford) 2010;49(12):2375–2380. doi: 10.1093/rheumatology/keq259. [DOI] [PubMed] [Google Scholar]
- 90.Takahashi K, Oharaseki T, Yokouchi Y. Pathogenesis of Kawasaki disease. Clin. Exp. Immunol. 2011;164(Suppl. 1):20–22. doi: 10.1111/j.1365-2249.2011.04361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kuroi A, Imanishi T, Suzuki H, et al. Clinical characteristics of patients with kawasaki disease and levels of peripheral endothelial progenitor cells and blood monocyte subpopulations. Circ. J. 2010;74(12):2720–2725. doi: 10.1253/circj.cj-10-0317. [DOI] [PubMed] [Google Scholar]
- 92.Xu MG, Men LN, Zhao CY, et al. The number and function of circulating endothelial progenitor cells in patients with Kawasaki disease. Eur. J. Pediatr. 2010;169(3):289–296. doi: 10.1007/s00431-009-1014-0. [DOI] [PubMed] [Google Scholar]
- 93.Xu MG, Men LN, Zu Y, Zhao CY, Meng XC, Wang T. The functions of endothelial progenitor cells were significantly improved after treatment with intravenous immunoglobulin and aspirin in children with Kawasaki disease. Pediatr. Cardiol. 2011;32(4):455–460. doi: 10.1007/s00246-011-9900-4. [DOI] [PubMed] [Google Scholar]
- 94.Ridker PM, Cook NR. Biomarkers for prediction of cardiovascular events. N. Engl. J. Med. 2007;356(14):1472–1473. author reply 1474–1475. [PubMed] [Google Scholar]
- 95.Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. J. Am. Coll. Cardiol. 2009;54(23):2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bartoloni E, Shoenfeld Y, Gerli R. Inflammatory and autoimmune mechanisms in the induction of atherosclerotic damage in systemic rheumatic diseases: two faces of the same coin. Arthritis Care Res. (Hoboken) 2011;63(2):178–183. doi: 10.1002/acr.20322. [DOI] [PubMed] [Google Scholar]
- 97.Westerweel PE, Verhaar MC. Endothelial progenitor cell dysfunction in rheumatic disease. Nat. Rev. Rheumatol. 2009;5(6):332–340. doi: 10.1038/nrrheum.2009.81. [DOI] [PubMed] [Google Scholar]
- 98.Smolen JS, Redlich K, Zwerina J, Aletaha D, Steiner G, Schett G. Pro-inflammatory cytokines in rheumatoid arthritis: pathogenetic and therapeutic aspects. Clin. Rev. Allergy Immunol. 2005;28(3):239–248. doi: 10.1385/CRIAI:28:3:239. [DOI] [PubMed] [Google Scholar]
- 99.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl. J. Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 100.Verma S, Kuliszewski MA, Li SH, et al. C-reactive protein attenuates endothelial progenitor cell survival, differentiation, and function: further evidence of a mechanistic link between C-reactive protein and cardiovascular disease. Circulation. 2004;109(17):2058–2067. doi: 10.1161/01.CIR.0000127577.63323.24. ▪ Underlines that endothelial progenitor cells and inflammation are crosslinked.
- 101.Ciulla MM, Giorgetti A, Silvestris I, et al. Endothelial colony forming capacity is related to C-reactive protein levels in healthy subjects. Curr. Neurovasc. Res. 2006;3(2):99–106. doi: 10.2174/156720206776875876. [DOI] [PubMed] [Google Scholar]
- 102.Fujii H, Li SH, Szmitko PE, Fedak PW, Verma S. C-reactive protein alters antioxidant defenses and promotes apoptosis in endothelial progenitor cells. Arterioscler. Thromb. Vasc. Biol. 2006;26(11):2476–2482. doi: 10.1161/01.ATV.0000242794.65541.02. [DOI] [PubMed] [Google Scholar]
- 103.Chen J, Huang L, Song M, Yu S, Gao P, Jing J. C-reactive protein upregulates receptor for advanced glycation end products expression and alters antioxidant defenses in rat endothelial progenitor cells. J. Cardiovasc. Pharmacol. 2009;53(5):359–367. doi: 10.1097/FJC.0b013e31819b5438. [DOI] [PubMed] [Google Scholar]
- 104.Goussetis E, Spiropoulos A, Tsironi M, et al. Spartathlon, a 246 kilometer foot race: effects of acute inflammation induced by prolonged exercise on circulating progenitor reparative cells. Blood Cells Mol. Dis. 2009;42(3):294–299. doi: 10.1016/j.bcmd.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 105.Adams V, Linke A, Breuckmann F, et al. Circulating progenitor cells decrease immediately after marathon race in advanced-age marathon runners. Eur. J. Cardiovasc. Prev. Rehabil. 2008;15(5):602–607. doi: 10.1097/HJR.0b013e328309c756. [DOI] [PubMed] [Google Scholar]
- 106.Ahrens I, Domeij H, Eisenhardt SU, et al. Opposing effects of monomeric and pentameric C-reactive protein on endothelial progenitor cells. Basic Res. Cardiol. 2011;106(5):879–895. doi: 10.1007/s00395-011-0191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Eklund CM. Proinflammatory cytokines in CRP baseline regulation. Adv. Clin. Chem. 2009;48:111–136. doi: 10.1016/s0065-2423(09)48005-3. [DOI] [PubMed] [Google Scholar]
- 108.Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res. Ther. 2006;8(Suppl. 2):S3. doi: 10.1186/ar1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Patel AM, Moreland LW. Interleukin-6 inhibition for treatment of rheumatoid arthritis: a review of tocilizumab therapy. Drug Des. Devel. Ther. 2010;4:263–278. doi: 10.2147/DDDT.S14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fan Y, Ye J, Shen F, et al. Interleukin-6 stimulates circulating blood-derived endothelial progenitor cell angiogenesis in vitro. J. Cereb. Blood Flow Metab. 2008;28(1):90–98. doi: 10.1038/sj.jcbfm.9600509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bonsignore MR, Morici G, Riccioni R, et al. Hemopoietic and angiogenetic progenitors in healthy athletes: different responses to endurance and maximal exercise. J. Appl. Physiol. 2010;109(1):60–67. doi: 10.1152/japplphysiol.01344.2009. [DOI] [PubMed] [Google Scholar]
- 112.Ablin JN, Boguslavski V, Aloush V, et al. Effect of anti-TNFα treatment on circulating endothelial progenitor cells (EPCs) in rheumatoid arthritis. Life Sci. 2006;79(25):2364–2369. doi: 10.1016/j.lfs.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 113.Sibal L, Aldibbiat A, Agarwal SC, et al. Circulating endothelial progenitor cells, endothelial function, carotid intima-media thickness and circulating markers of endothelial dysfunction in people with Type 1 diabetes without macrovascular disease or microalbuminuria. Diabetologia. 2009;52(8):1464–1473. doi: 10.1007/s00125-009-1401-0. [DOI] [PubMed] [Google Scholar]
- 114.Chen TG, Zhong ZY, Sun GF, Zhou YX, Zhao Y. Effects of tumour necrosis factor-α on activity and nitric oxide synthase of endothelial progenitor cells from peripheral blood. Cell Prolif. 2011;44(4):352–359. doi: 10.1111/j.1365-2184.2011.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Henrich D, Seebach C, Wilhelm K, Marzi I. High dosage of simvastatin reduces TNF-α-induced apoptosis of endothelial progenitor cells but fails to prevent apoptosis induced by IL-1β in vitro. J. Surg. Res. 2007;142(1):13–19. doi: 10.1016/j.jss.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 116.Balestrieri ML, Schiano C, Felice F, et al. Effect of low doses of red wine and pure resveratrol on circulating endothelial progenitor cells. J. Biochem. 2008;143(2):179–186. doi: 10.1093/jb/mvm209. [DOI] [PubMed] [Google Scholar]
- 117.Ribatti D, Nico B, Crivellato E, Vacca A. Endothelial progenitor cells in health and disease. Histol. Histopathol. 2005;20(4):1351–1358. doi: 10.14670/HH-20.1351. [DOI] [PubMed] [Google Scholar]
- 118.Rehman J, Li J, Parvathaneni L, et al. Exercise acutely increases circulating endothelial progenitor cells and monocyte-/macrophage-derived angiogenic cells. J. Am. Coll. Cardiol. 2004;43(12):2314–2318. doi: 10.1016/j.jacc.2004.02.049. [DOI] [PubMed] [Google Scholar]
- 119.Steiner S, Niessner A, Ziegler S, et al. Endurance training increases the number of endothelial progenitor cells in patients with cardiovascular risk and coronary artery disease. Atherosclerosis. 2005;181(2):305–310. doi: 10.1016/j.atherosclerosis.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 120.Laufs U, Urhausen A, Werner N, et al. Running exercise of different duration and intensity: effect on endothelial progenitor cells in healthy subjects. Eur. J. Cardiovasc. Prev. Rehabil. 2005;12(4):407–414. doi: 10.1097/01.hjr.0000174823.87269.2e. [DOI] [PubMed] [Google Scholar]
- 121.Schlager O, Giurgea A, Schuhfried O, et al. Exercise training increases endothelial progenitor cells and decreases asymmetric dimethylarginine in peripheral arterial disease: a randomized controlled trial. Atherosclerosis. 2011;217(1):240–248. doi: 10.1016/j.atherosclerosis.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 122.Kim W, Jeong MH, Cho SH, et al. Effect of green tea consumption on endothelial function and circulating endothelial progenitor cells in chronic smokers. Circ. J. 2006;70(8):1052–1057. doi: 10.1253/circj.70.1052. [DOI] [PubMed] [Google Scholar]
- 123.Marin C, Ramirez R, Delgado-Lista J, et al. Mediterranean diet reduces endothelial damage and improves the regenerative capacity of endothelium. Am. J. Clin. Nutr. 2010;93(2):267–274. doi: 10.3945/ajcn.110.006866. [DOI] [PubMed] [Google Scholar]
- 124.Westerweel PE, Visseren FL, Hajer GR, et al. Endothelial progenitor cell levels in obese men with the metabolic syndrome and the effect of simvastatin monotherapy vs. simvastatin/ ezetimibe combination therapy. Eur. Heart J. 2008;29(22):2808–2817. doi: 10.1093/eurheartj/ehn431. [DOI] [PubMed] [Google Scholar]
- 125.Ludwig A, Jochmann N, Kertesz A, et al. Smoking decreases the level of circulating CD34+ progenitor cells in young healthy women – a pilot study. BMC Womens Health. 2010;10:20. doi: 10.1186/1472-6874-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dimmeler S, Aicher A, Vasa M, et al. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI3-kinase/Akt pathway. J. Clin. Invest. 2001;108(3):391–397. doi: 10.1172/JCI13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bahlmann FH, DeGroot K, Duckert T, et al. Endothelial progenitor cell proliferation and differentiation is regulated by erythropoietin. Kidney Int. 2003;64(5):1648–1652. doi: 10.1046/j.1523-1755.2003.00279.x. [DOI] [PubMed] [Google Scholar]
- 128.Mueller C, Wodack K, Twelker K, Werner N, Custodis F, Nickenig G. Darbepoetin improves endothelial function and increases circulating endothelial progenitor cell number in patients with coronary artery disease. Heart. 2011;97(18):1474–1478. doi: 10.1136/hrt.2010.220798. [DOI] [PubMed] [Google Scholar]
- 129.Pistrosch F, Herbrig K, Oelschlaegel U, et al. PPARγ-agonist rosiglitazone increases number and migratory activity of cultured endothelial progenitor cells. Atherosclerosis. 2005;183(1):163–167. doi: 10.1016/j.atherosclerosis.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 130.Wang CH, Ciliberti N, Li SH, et al. Rosiglitazone facilitates angiogenic progenitor cell differentiation toward endothelial lineage: a new paradigm in glitazone pleiotropy. Circulation. 2004;109(11):1392–1400. doi: 10.1161/01.CIR.0000123231.49594.21. [DOI] [PubMed] [Google Scholar]
- 131.Cianfrocca C, Loricchio ML, Pelliccia F, et al. C-reactive protein and left atrial appendage velocity are independent determinants of the risk of thrombogenesis in patients with atrial fibrillation. Int. J. Cardiol. 2010;142(1):22–28. doi: 10.1016/j.ijcard.2008.12.052. [DOI] [PubMed] [Google Scholar]
- 132.Bahlmann FH, de Groot K, Mueller O, Hertel B, Haller H, Fliser D. Stimulation of endothelial progenitor cells: a new putative therapeutic effect of angiotensin II receptor antagonists. Hypertension. 2005;45(4):526–529. doi: 10.1161/01.HYP.0000159191.98140.89. [DOI] [PubMed] [Google Scholar]
- 133.Min TQ, Zhu CJ, Xiang WX, Hui ZJ, Peng SY. Improvement in endothelial progenitor cells from peripheral blood by ramipril therapy in patients with stable coronary artery disease. Cardiovasc. Drugs Ther. 2004;18:203–209. doi: 10.1023/B:CARD.0000033641.33503.bd. [DOI] [PubMed] [Google Scholar]
- 134.Stamm C, Kleine HD, Westphal B, et al. CABG and bone marrow stem cell transplantation after myocardial infarction. Thorac. Cardiovasc. Surg. 2004;52(3):152–158. doi: 10.1055/s-2004-817981. [DOI] [PubMed] [Google Scholar]
- 135.Stamm C, Westphal B, Kleine HD, et al. Autologous bone-marrow stem-cell transplantation for myocardial regeneration. Lancet. 2003;361(9351):45–46. doi: 10.1016/S0140-6736(03)12110-1. [DOI] [PubMed] [Google Scholar]
- 136.Bartunek J, Vanderheyden M, Vandekerckhove B, et al. Intracoronary injection of CD133-positive enriched bone marrow progenitor cells promotes cardiac recovery after recent myocardial infarction: feasibility and safety. Circulation. 2005;112(Suppl. 9):I178–I183. doi: 10.1161/CIRCULATIONAHA.104.522292. [DOI] [PubMed] [Google Scholar]
- 137.Vanderheyden M, Vercauteren S, Mansour S, et al. Time-dependent effects on coronary remodeling and epicardial conductance after intracoronary injection of enriched hematopoietic bone marrow stem cells in patients with previous myocardial infarction. Cell Transplant. 2007;16(9):919–925. doi: 10.3727/096368907783338244. [DOI] [PubMed] [Google Scholar]
- 138.Losordo DW, Henry TD, Davidson C, et al. Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circ. Res. 2011;109(4):428–436. doi: 10.1161/CIRCRESAHA.111.245993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vrtovec B, Poglajen G, Sever M, et al. Effects of intracoronary stem cell transplantation in patients with dilated cardiomyopathy. J. Card. Fail. 2010;17(4):272–281. doi: 10.1016/j.cardfail.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 140.Jujo K, Hamada H, Iwakura A, et al. CXCR4 blockade augments bone marrow progenitor cell recruitment to the neovasculature and reduces mortality after myocardial infarction. Proc. Natl Acad. Sci. USA. 2010;107(24):11008–11013. doi: 10.1073/pnas.0914248107. ▪ Reduction of mortality after myocardial infarction in a murine model by small molecule inhibitor attracting cells that support neovascularization.
- 141.Pelliccia F, Cianfrocca C, Rosano G, Mercuro G, Speciale G, Pasceri V. Role of endothelial progenitor cells in restenosis and progression of coronary atherosclerosis after percutaneous coronary intervention: a prospective study. JACC Cardiovasc. Interv. 2010;3(1):78–86. doi: 10.1016/j.jcin.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 142.den Dekker WK, Houtgraaf JH, Onuma Y, et al. Final results of the HEALING IIB trial to evaluate a bioengineered CD34 antibody coated stent (GenousStent) designed to promote vascular healing by capture of circulating endothelial progenitor cells in CAD patients. Atherosclerosis. 2011 doi: 10.1016/j.atherosclerosis.2011.06.032. doi:10.1016/j.atherosclerosis.2011.06.032. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 143.Sobrino T, Hurtado O, Moro MA, et al. The increase of circulating endothelial progenitor cells after acute ischemic stroke is associated with good outcome. Stroke. 2007;38(10):2759–2764. doi: 10.1161/STROKEAHA.107.484386. [DOI] [PubMed] [Google Scholar]
- 144.Simper D, Wang S, Deb A, et al. Endothelial progenitor cells are decreased in blood of cardiac allograft patients with vasculopathy and endothelial cells of noncardiac origin are enriched in transplant atherosclerosis. Circulation. 2003;108(2):143–149. doi: 10.1161/01.CIR.0000081703.34526.5D. [DOI] [PubMed] [Google Scholar]