Abstract
Huntington’s disease (HD) is an inherited neurodegenerative disorder that causes neurological pathology in the basal ganglia and related circuitry. A key site of HD pathology is striatum, the principal basal ganglia input structure; striatal pathology likely changes basal ganglia output but no existing studies address this issue. In this report, we characterize single-neuron activity in the substantia nigra reticulata (SNr) of awake, freely-behaving 140 CAG knock-in (KI) mice at 16 to 40 weeks. KI mice are a well characterized model of adult HD and are mildly symptomatic in this age range. As the primary basal ganglia output nucleus in rodents, the SNr receives direct innervation from striatum, as well as indirect influence via polysynaptic inputs. We analyzed 32 single neurons recorded from KI animals and 44 from wild-type (WT) controls. We found increased burst rates, without a concordant change in spike discharge rate, in KI animals relative to WTs. Furthermore, although metrics of burst structure, such as the inter-spike interval in bursts, do not differ between groups, burst rate increases with age in KI, but not WT, animals. Our findings suggest that altered basal ganglia output is a physiological feature of early HD pathology.
Keywords: Huntington’s disease, knock-in mice, basal ganglia, substantia nigra reticulata, spike burst
Introduction
Huntington’s disease (HD) arises from an autosomal dominant mutation in the huntingtin (htt) gene and causes severe neurological pathology and eventual death [15]. The hallmark pathology of HD is striatal atrophy [35]. As the principal input center for the basal ganglia, striatum receives input from throughout the cortical mantle, which is then routed through a series of parallel circuits for behavioral output [2].
Among the most well-characterized HD models are R6/2 mice, which carry a transgenic copy of the mutant human htt gene [19] and display an aggressive symptom profile that mirrors juvenile HD [5, 19, 31], and 140 CAG knock-in (KI) mice [21], which carry a chimeric mouse/human gene [21] and show a relatively mild behavioral phenotype [9, 20, 21, 29]. Studies of these and other HD models show that behavioral symptoms emerge before the onset of neuronal atrophy [13, 14, 20, 21, 29, 31], suggesting that neuronal dysfunction, but not cell death, underlies early HD. Striatal neurons from HD animals show altered cellular properties in vitro [4, 6, 18] and in vivo recordings from R6/2 mice revealed increased mean action potential discharge rates relative to wild-type (WT) animals [27], as well as increased temporal variability in the spike trains as measured by the coefficient of variation of the ISI [23]. Conversely, striatal neurons in behaving KI animals do not display altered spike rates; however, they show aberrant collective activity as measured by decreased spike synchrony and tandem bursting [23]. Altered functionality of basal ganglia input neurons is thus a key component of HD in multiple genetic constructs.
It is likely that altered striatal activity leads to abnormal activity in the substantia nigra reticulata (SNr) via descending striatofugal pathways. As a major basal ganglia output center, SNr provides tonic inhibition of thalamic output neurons [1, 10]. Disruption of this inhibitory regulation could contribute to the motor and cognitive symptoms observed in HD patients. To test the hypothesis that dysregulated SNr activity plays a role in HD, we analyzed the firing patterns of single neurons isolated in the SNr of KI animals and WT controls. We use KI animals because they carry a non-truncated version of the mutant gene and their slow symptom progressions mimics the development of adult HD in humans.
Materials and Methods
Homozygous WT (129sv) mice and KI animals were produced in-house using lines descended from heterozygous breeding pairs obtained with permission [21]. Homozygous KI animals and WT littermate controls were identified by standard polymerase chain reaction and gel assays. Heterozygotes were not used as experimental animals. We confirmed the presence of an expanded Hdh gene (the murine analog of htt) (~120 CAG repeats) in KI animals using our in-house genotyping procedure [23]. Animals were housed in individual cages under standard conditions (12 hr light/dark cycle, lights on at 7:30 AM) and provided with food and water ad libitum. All procedures were carried out in accordance with animal care and use guidelines of the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee.
In preparation for surgery, animals were anesthetized with chloropent (0.04 ml/kg body weight i.p.). Holes were made bilaterally over SNr (Bregma: 3.4 mm posterior, ±1.3 mm medial/lateral). Electrode bundles, consisting of three Formvar® insulated microwires and an uninsulated stainless steel ground wire (California Fine Wire Company, CA) built in-house, were positioned over SNr, lowered −4.5 mm relative to skull surface and secured in place using dental acrylic and two small screws inserted into the skull. Animals were grounded using two uninsulated stainless steel wires implanted along with the microwire bundles. Both wires lead to a common ground via the Faraday cage and also served as the reference electrodes. After at least one week of recovery animals were habituated to an open field (Plexiglas® cage with standard bedding, 48 cm L×26 cm W×20 cm H) for at least 10 min to minimize effects of a novel environment.
Recording sessions occurred between 10:00 AM and 5:00 PM during the light phase. The technical details of our recording procedures are described elsewhere [23]. Spike activity was recorded for 30 min. An observer blind to genotype monitored the animals periodically and noted spontaneous behaviors (resting, rearing, digging and locomotion) during the recording session. In previous studies, our group has analyzed videotaped behavior of WT and KI mice under similar experimental conditions, including a subsample of mice from this study [36], and found no significant effects of genotype on general behavioral activity patterns [23, 36].
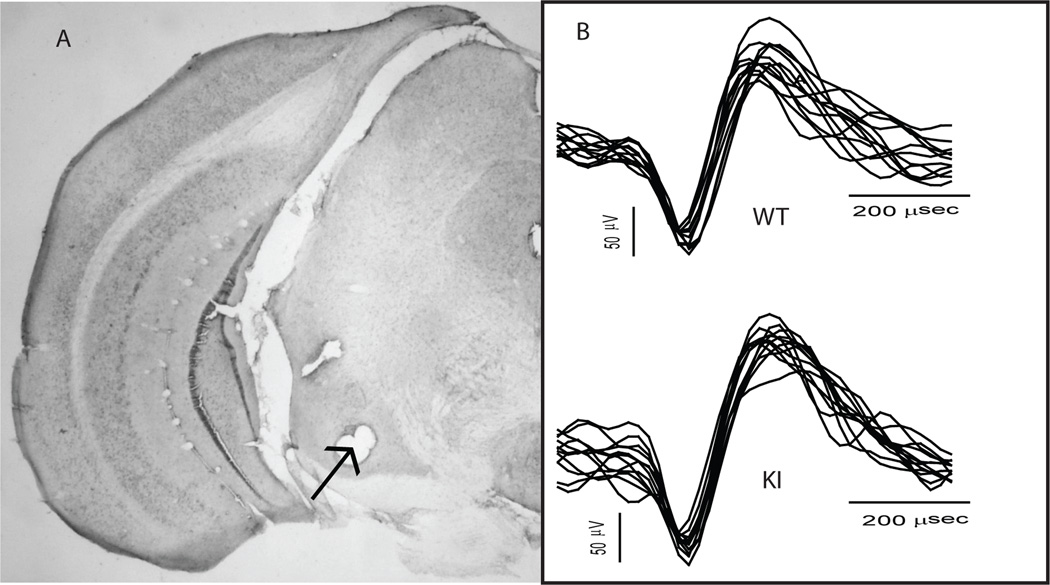
Following data collection, animals were placed under deep anesthesia and transcardially perfused with 10% neutral buffered formalin. Brains were extracted, cryoprotected for at least 24 h and then sectioned on a microtome. Coronal sections were inspected to confirm electrode implantation in SNr. Data recorded from electrodes placed outside SNr were discarded. See Fig. 1A for a representative histological slice showing successful electrode implantation in SNr.
Figure 1.
A representative coronal section from the brain of a KI mouse (A) and representative spike waveforms (B). (A) A coronal section obtained from the brain of a KI mouse. The arrow indicates an electrode-marking lesion centered in the SNr. (B) Multiple (~10) traces of spike waveforms recorded in a WT (top) and KI (bottom) animal.
Spike data were stored, and burst analysis performed, using Neuroexplorer (Plexon Inc., Dallas, TX). Burst patterns were assessed using the Poisson surprise method [17]. Bursts were defined as clusters of spikes in which the mean inter-spike interval (ISI) exceeds a “surprise” value that quantifies the degree to which the ISI deviates from the expected ISI for an ideal Poisson process. The burst surprise value was set at 5, meaning that all spike clusters counted as bursts would be observed less than 1 percent of the time if the ISI followed a Poisson distribution. Here we present only burst metrics for which there was a significant difference between WT and KI neurons. Differences between the KI and WT groups were assessed using an independent samples t-test, or the nonparametric equivalent Mann-Whitney rank-sum test, where appropriate. Effects of age on burst rate were assessed using linear regression analysis with age as the independent variable and the natural logarithm of burst rate as the dependent variable. We applied a logarithmic transformation to remove the floor effect at zero bursts/min.
Results
We recorded a total of 76 neurons (44 WT, 32 KI) from 19 KI and 22 WT animals. When possible, we recorded from the same mouse during multiple sessions. Although the same neuron may appear on a given electrode over multiple recording sessions, we do not make such an assumption because in most cases waveform templates change over sessions and it is impossible to know how factors such as disease progress or aging affect extracellular signals. This procedure is in keeping with prior reports [23, 27, 36]. Age ranged from 16 to 40 weeks in the KI group and 16 to 41 weeks in the WT group. The mean (+/− SEM) age at recording was 24.8 +/−1.264 weeks in the KI group and 27.5 +/− 1.064 weeks in the WT group. An independent samples t-test confirmed that the difference in age between the groups is non-significant (t39 = −1.676, p = 0.102). Recorded waveforms displayed the characteristic amplitude and biphasic signature of SNr output neurons [12, 37]. Representative waveforms recorded from a WT and KI animal are shown in Fig. 1B.
During recording, the animals spent a considerable amount of time resting, and also displayed bouts of motor activity that included locomotion and grooming. KI animals did not display pronounced HD neurological signs such as tremor or gait abnormalities.
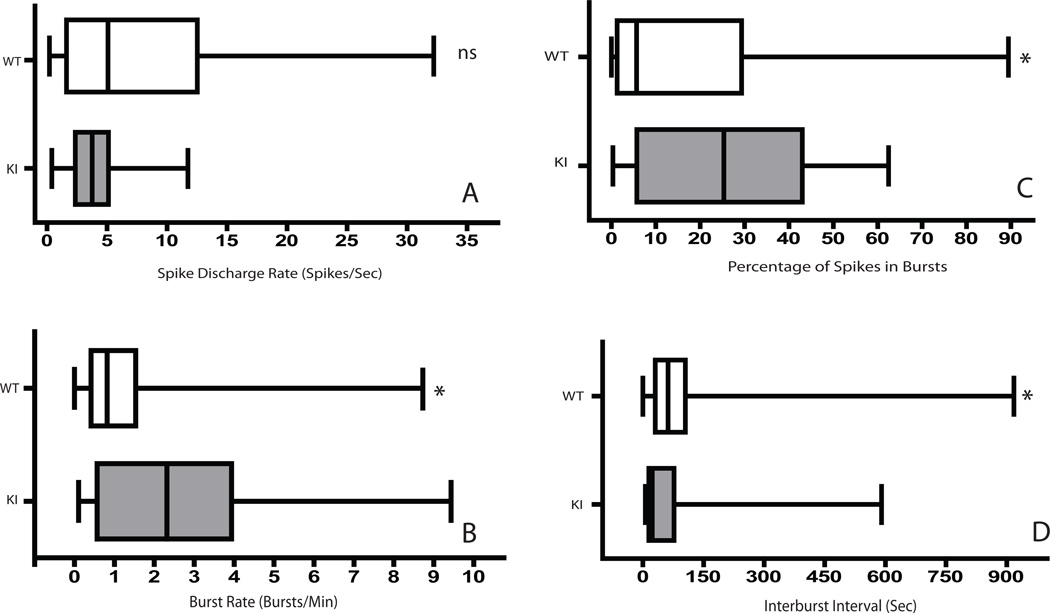
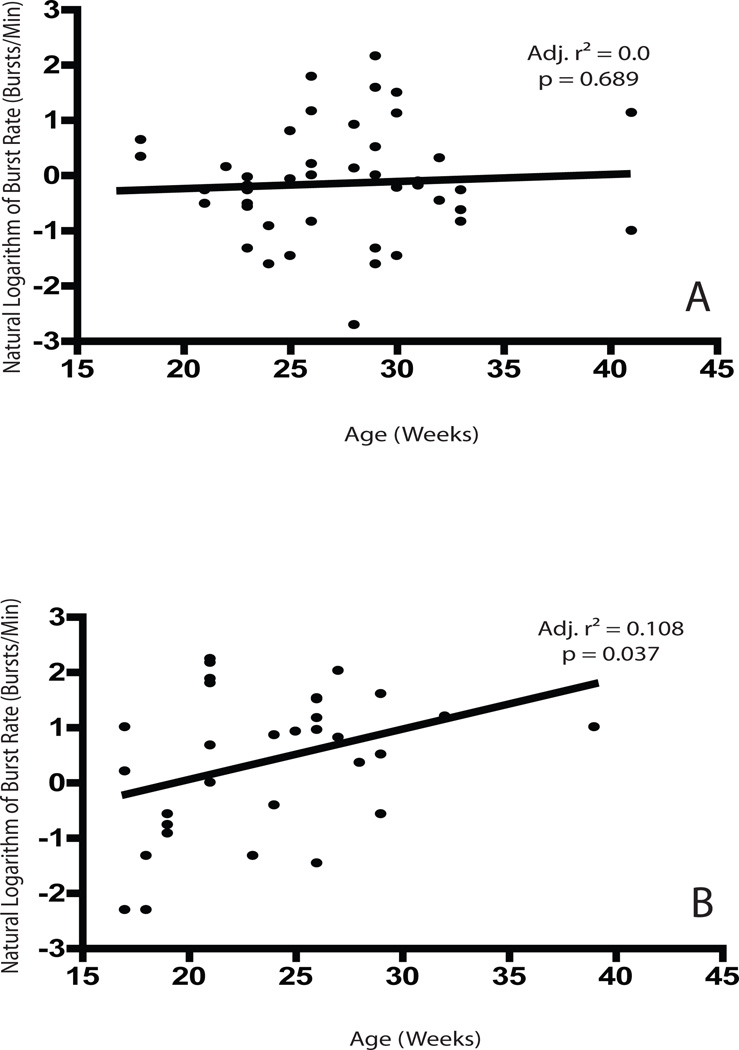
Median spike discharge rates were lower in KI than WT neurons, but this difference was not significant (Fig. 2A). We did, however, observe a significantly greater occurrence of spike-bursts as reflected by a higher burst rate (U = 472.5, p = 0.015), a higher percentage of spikes participating in bursts (U = 501.5, p = 0.034) and lower inter-burst interval (U = 919.5, p = 0.024) in KI neurons (Fig. 2B-D). We also found that the burst rate increases as a function of age in KI but not WT neurons (Fig. 3), suggesting that the abnormalities underlying the observed effects become more pronounced as the mice age. The median spike rate between bursts was higher in WT animals; however, this difference is non-significant (U = 550.5, p = 0.107). Metrics reflecting burst structure, such as the surprise value and the mean ISI within a burst, did not differ significantly between WT and KI neurons (data not shown), suggesting that the occurrence of bursting increases in HD animals without a corresponding change in the clustering of spikes within individual bursts.
Figure 2.
Box plots of the median values of spike discharge rates (A), the mean burst rate (B), the percentage of spikes participating in bursts (C) and the interburst interval (D) for WT and KI animals. Vertical bars within boxes denote the sample median. Box borders correspond to the cutoffs for the 25th and 75th percentiles, and the outermost bars correspond to the highest and lowest values in each sample. The asterisk denotes a difference in the sum of ranks with a two-tailed cutoff at p = 0.05. “ns” indicates a non-significant difference.
Figure 3.
Scatter plots showing the age at recording and the corresponding natural logarithm of the observed burst rate for single neurons for all WT (A) and KI (B) neurons. Black lines are the best-fit line generated by the regression model. Adjusted r-squared values and corresponding p values are shown on the plots.
Discussion
Our findings indicate that abnormal firing patterns previously reported for HD striatum also occur in SNr, but with an interesting difference. Whereas striatal neurons in behaving KI mice tested at roughly the same ages as reported here show a decreased number of spikes participating in a burst and an increase in burst strength as reflected in a higher burst surprise score relative to WT [23], SNr neurons trend in the opposite direction with higher burst rate and a higher percentage of spikes participating in bursts. Furthermore, metrics such as burst surprise and ISI within a burst do not change, suggesting that burst occurrence but not burst structure changes in KI animals. The increase in SNr bursting, moreover, increases with age in KI mice. Thus, although neuronal processing in the basal ganglia output system is altered in the KI model, the alterations contrast with those reported for the striatum and appear to intensify with age. Although the mechanism underlying this striato-nigral difference is unclear, it is interesting that striatal degeneration in HD occurs sooner among neurons contributing to the indirect projection to SNr than those that comprise the direct pathway [28]. Exactly how an HD-related change in the balance or interaction between these pathways could contribute to the change in SNr activity remains to be determined. Increased bursting also may result from HD-induced changes in individual SNr neurons, or from system-level interactions brought about by the effects of HD on multiple nuclei.
In striatum, there are sharp differences in firing rate between behaving R6/2 mice, which present a robust behavioral phenotype that includes a progressive decline in motor activation, and the KI model, which shows relatively mild neurological signs [23]. R6/2s increase striatal activity, whereas KIs do not – presumably a reflection of the clear behavioral difference. Changes in neuronal activity absent behavioral symptoms have also been observed in presymptomatic human carriers of the mutant huntingtin gene. Both cortical and striatal activation levels, as measured by fMRI, are reduced in HD patients relative to controls before the emergence of a behavioral phenotype [39]. In keeping with the lack of change in KI striatal activity, we found no change in SNr firing rate. Not only does this result highlight rate as a relatively insensitive measure of an underlying neurological dysfunction, but also rules out a change in rate as an explanation for the increase in SNr burst activity. The temporal structure of spike trains as measured by burst metrics appears to be a reliable indicator of basal ganglia dysfunction in HD. To our knowledge, this is the first report describing such pathology in a HD model.
Bursting is uncommon in SNr neurons in WT rodents, and is also associated with a number of pathological conditions [8, 25, 30]. Neurons of the human internal pallidum, the primate equivalent of the rodent SNr, show increased burst rates in HD relative to Parkinson’s disease patients[32]. Interestingly, Tang and colleagues report that the burst rates of pallidal neurons are equal between HD and Parkinsonian patients[33]. This discrepancy highlights the heterogeneity of the HD phenotype and the dependence of neuronal and behavioral symptoms on age and genetic factors such as CAG repeat length.
Increased bursting in SNr neurons could lead to increased thalamic inhibition by elevating nigro-thalamic GABA release. Increased bursting, however, does not necessarily correspond to increased GABAergic output of SNr since pathologies such as impaired GABA receptor trafficking [34] may reduce this effect. It is dangerous, moreover, to speculate on the behavioral implications of a change in SNr firing patterns in light of growing evidence that SNr neurons may maintain simultaneous enhancing and suppressing effects on downstream structures to facilitate appropriate motor responding [12, 16, 24].
Neuronal dysfunction of the striatum is a likely source of altered SNr activity given the extensive network of mono and polysynaptic striatonigral connections; however, other sources, such as the corticosubthalaic hyperdirect pathway, may contribute to changes in SNr activity. Neuronal pathology in HD extends beyond the basal ganglia as multiple studies demonstrate cortical dysfunction in HD [3, 7, 11, 22, 36]. Aberrant cortical output could propagate directly to the STN, bypassing the striatum and influencing SNr via increased subthalamonigral glutamate release. While our data do not preclude the possibility that pathology of the hyperdirect pathway influence SNr activity in HD, our data neither support nor refute this hypothesis. Moreover, it is prudent not to speculate strongly on this issue, as the role of the STN remains unclear. The STN not only projects to the SNr it loops back onto the GPe, possibly forming a rhythm-generating feedback system [26]. Furthermore, SNr neurons are reactive to GABA but relatively insensitive to glutamate [38]. The role of the STN and its cortical inputs is thus more nuanced then the simple “push/pull” dynamics proposed in early work by Albin and colleagues [1].
Although further research is required to identify the mechanisms underlying increased SNr burst activity in KI mice, our results suggest a role for altered basal ganglia output patterns in HD. It also is noteworthy that these patterns increase with age even in the absence of a strong behavioral phenotype. Research informed by these results may reveal new clinical avenues in treating HD patients, as well as insight into the function of the basal ganglia in health and pathology.
HIGHLIGHTS.
We analyze single neuron data gathered from knock-in Huntington’s disease mice
Knock-in neurons exhibit altered bursting patterns relative to controls
Knock-in mice show substantial neuronal pathology over a broad age range
Altered basal ganglia output characterizes early Huntington’s disease
Our findings offer new insight into the etiology of Huntington’s disease
Acknowledgements
This research was supported by NINDS grant #R01 NS35663 (GVR), the NSF GRFP (JLD, AMN), the NIH (JLD) and the Beckman Foundation (AMN). Marie Francois Chesselet and the University of California at Los Angeles provided mouse breeding pairs. Faye Caylor, Paul Langley, and Scott Barton provided administrative and technical support.
Abbreviations
- HD
Huntington’s disease
- WT
wild-type
- KI
knock-in
- SNr
substantia nigra reticulata
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 2.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 3.Andre VM, Cepeda C, Venegas A, Gomez Y, Levine MS. Altered cortical glutamate receptor function in the R6/2 model of Huntington's disease. Journal of neurophysiology. 2006;95:2108–2119. doi: 10.1152/jn.01118.2005. [DOI] [PubMed] [Google Scholar]
- 4.Ariano MA, Cepeda C, Calvert CR, Flores-Hernandez J, Hernandez-Echeagaray E, Klapstein GJ, Chandler SH, Aronin N, DiFiglia M, Levine MS. Striatal potassium channel dysfunction in Huntington's disease transgenic mice. J Neurophysiol. 2005;93:2565–2574. doi: 10.1152/jn.00791.2004. [DOI] [PubMed] [Google Scholar]
- 5.Carter RJ, Lione LA, Humby T, Mangiarini L, Mahal A, Bates GP, Dunnett SB, Morton AJ. Characterization of progressive motor deficits in mice transgenic for the human Huntington's disease mutation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:3248–3257. doi: 10.1523/JNEUROSCI.19-08-03248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cepeda C, Ariano MA, Calvert CR, Flores-Hernandez J, Chandler SH, Leavitt BR, Hayden MR, Levine MS. NMDA receptor function in mouse models of Huntington disease. J Neurosci Res. 2001;66:525–539. doi: 10.1002/jnr.1244. [DOI] [PubMed] [Google Scholar]
- 7.Cummings DM, Milnerwood AJ, Dallerac GM, Waights V, Brown JY, Vatsavayai SC, Hirst MC, Murphy KP. Aberrant cortical synaptic plasticity and dopaminergic dysfunction in a mouse model of Huntington's disease. Human molecular genetics. 2006;15:2856–2868. doi: 10.1093/hmg/ddl224. [DOI] [PubMed] [Google Scholar]
- 8.Deransart C, Hellwig B, Heupel-Reuter M, Leger JF, Heck D, Lucking CH. Single-unit analysis of substantia nigra pars reticulata neurons in freely behaving rats with genetic absence epilepsy. Epilepsia. 2003;44:1513–1520. doi: 10.1111/j.0013-9580.2003.26603.x. [DOI] [PubMed] [Google Scholar]
- 9.Dorner JL, Miller BR, Barton SJ, Brock TJ, Rebec GV. Sex differences in behavior and striatal ascorbate release in the 140 CAG knock-in mouse model of Huntington's disease. Behav Brain Res. 2007;178:90–97. doi: 10.1016/j.bbr.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graybiel AM, Ragsdale CW., Jr Fiber connections of the basal ganglia. Progress in brain research. 1979;51:237–283. [PubMed] [Google Scholar]
- 11.Gu X, Li C, Wei W, Lo V, Gong S, Li S, Iwasato T, Itohara S, Li XJ, Mody L, Heintz N, Yang XW. Pathological cell-cell interactions elicited by a neuropathogenic form of mutant Huntingtin contribute to cortical pathogenesis in HD mice. Neuron. 2005;46 doi: 10.1016/j.neuron.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 12.Gulley JM, Kosobud AE, Rebec GV. Behavior-related modulation of substantia nigra pars reticulata neurons in rats performing a conditioned reinforcement task. Neuroscience. 2002;111:337–349. doi: 10.1016/s0306-4522(02)00018-0. [DOI] [PubMed] [Google Scholar]
- 13.Hickey MA, Chesselet MF. The use of transgenic and knock-in mice to study Huntington's disease. Cytogenet Genome Res. 2003;100:276–286. doi: 10.1159/000072863. [DOI] [PubMed] [Google Scholar]
- 14.Hickey MA, Kosmalska A, Enayati J, Cohen R, Zeitlin S, Levine MS, Chesselet MF. Extensive early motor and non-motor behavioral deficits are followed by striatal neuronal loss in knock-in Huntington's disease mice. Neuroscience. 2008;157:280–295. doi: 10.1016/j.neuroscience.2008.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huntington's Disease Collaborative Research Group, A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 16.Jiang H, Stein BE, McHaffie JG. Opposing basal ganglia processes shape midbrain visuomotor activity bilaterally. Nature. 2003;423:982–986. doi: 10.1038/nature01698. [DOI] [PubMed] [Google Scholar]
- 17.Legendy CR, Salcman M. Bursts and recurrences of bursts in the spike trains of spontaneously active striate cortex neurons. J Neurophysiol. 1985;53:926–939. doi: 10.1152/jn.1985.53.4.926. [DOI] [PubMed] [Google Scholar]
- 18.Levine MS, Klapstein GJ, Koppel A, Gruen E, Cepeda C, Vargas ME, Jokel ES, Carpenter EM, Zanjani H, Hurst RS, Efstratiadis A, Zeitlin S, Chesselet MF. Enhanced sensitivity to N-methyl-D-aspartate receptor activation in transgenic and knockin mouse models of Huntington's disease. J Neurosci Res. 1999;58:515–532. [PubMed] [Google Scholar]
- 19.Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, Lawton M, Trottier Y, Lehrach H, Davies SW, Bates GP. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 1996;87:493–506. doi: 10.1016/s0092-8674(00)81369-0. [DOI] [PubMed] [Google Scholar]
- 20.Menalled LB. Knock-in mouse models of Huntington's disease. NeuroRx. 2005;2:465–470. doi: 10.1602/neurorx.2.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menalled LB, Sison JD, Dragatsis I, Zeitlin S, Chesselet MF. Time course of early motor and neuropathological anomalies in a knock-in mouse model of Huntington's disease with 140 CAG repeats. J Comp Neurol. 2003;465:11–26. doi: 10.1002/cne.10776. [DOI] [PubMed] [Google Scholar]
- 22.Miller BR, Walker AG, Barton SJ, Rebec GV. Dysregulated Neuronal Activity Patterns Implicate Corticostriatal Circuit Dysfunction in Multiple Rodent Models of Huntington's Disease. Frontiers in systems neuroscience. 2011;5:26. doi: 10.3389/fnsys.2011.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller BR, Walker AG, Shah AS, Barton SJ, Rebec GV. Dysregulated information processing by medium spiny neurons in striatum of freely behaving mouse models of Huntington's disease. J Neurophysiol. 2008;100:2205–2216. doi: 10.1152/jn.90606.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- 25.Murer MG, Riquelme LA, Tseng KY, Pazo JH. Substantia nigra pars reticulata single unit activity in normal and 60HDA-lesioned rats: effects of intrastriatal apomorphine and subthalamic lesions. Synapse. 1997;27:278–293. doi: 10.1002/(SICI)1098-2396(199712)27:4<278::AID-SYN2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 26.Plenz D, Kital ST. A basal ganglia pacemaker formed by the subthalamic nucleus and external globus pallidus. Nature. 1999;400:677–682. doi: 10.1038/23281. [DOI] [PubMed] [Google Scholar]
- 27.Rebec GV, Conroy SK, Barton SJ. Hyperactive striatal neurons in symptomatic Huntington R6/2 mice: variations with behavioral state and repeated ascorbate treatment. Neuroscience. 2006;137:327–336. doi: 10.1016/j.neuroscience.2005.08.062. [DOI] [PubMed] [Google Scholar]
- 28.Richfield EK, Maguire-Zeiss KA, Vonkeman HE, Voorn P. Preferential loss of preproenkephalin versus preprotachykinin neurons from the striatum of Huntington's disease patients. Annals of neurology. 1995;38:852–861. doi: 10.1002/ana.410380605. [DOI] [PubMed] [Google Scholar]
- 29.Rising AC, Xu J, Carlson A, Napoli VV, Denovan-Wright EM, Mandel RJ. Longitudinal behavioral, cross-sectional transcriptional and histopathological characterization of a knock-in mouse model of Huntington's disease with 140 CAG repeats. Exp Neurol. 2011;228:173–182. doi: 10.1016/j.expneurol.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rohlfs A, Nikkhah G, Rosenthal C, Rundfeldt C, Brandis A, Samii M, Loscher W. Hemispheric asymmetries in spontaneous firing characteristics of substantia nigra pars reticulata neurons following a unilateral 6-hydroxydopamine lesion of the rat nigrostriatal pathway. Brain Res. 1997;761:352–356. doi: 10.1016/s0006-8993(97)00475-7. [DOI] [PubMed] [Google Scholar]
- 31.Stack EC, Kubilus JK, Smith K, Cormier K, Del Signore SJ, Guelin E, Ryu H, Hersch SM, Ferrante RJ. Chronology of behavioral symptoms and neuropathological sequela in R6/2 Huntington's disease transgenic mice. The Journal of comparative neurology. 2005;490:354–370. doi: 10.1002/cne.20680. [DOI] [PubMed] [Google Scholar]
- 32.Starr PA, Kang GA, Heath S, Shimamoto S, Turner RS. Pallidal neuronal discharge in Huntington's disease: support for selective loss of striatal cells originating the indirect pathway. Experimental neurology. 2008;211:227–233. doi: 10.1016/j.expneurol.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang JK, Moro E, Lozano AM, Lang AE, Hutchison WD, Mahant N, Dostrovsky JO. Firing rates of pallidal neurons are similar in Huntington's and Parkinson's disease patients, Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 2005;166:230–236. doi: 10.1007/s00221-005-2359-x. [DOI] [PubMed] [Google Scholar]
- 34.Twelvetrees AE, Yuen EY, Arancibia-Carcamo IL, MacAskill AF, Rostaing P, Lumb MJ, Humbert S, Triller A, Saudou F, Yan Z, Kittler JT. Delivery of GABAARs to synapses is mediated by HAP1-KIF5 and disrupted by mutant huntingtin. Neuron. 2010;65:53–65. doi: 10.1016/j.neuron.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP., Jr. Neuropathological classification of Huntington's disease. J Neuropathol Exp Neurol. 1985;44:559–577. doi: 10.1097/00005072-198511000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Walker AG, Miller BR, Fritsch JN, Barton SJ, Rebec GV. Altered information processing in the prefrontal cortex of Huntington's disease mouse models. J Neurosci. 2008;28:8973–8982. doi: 10.1523/JNEUROSCI.2804-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waszczak BL, Eng N, Walters JR. Effects of muscimol and picrotoxin on single unit activity of substantia nigra neurons. Brain Res. 1980;188:185–197. doi: 10.1016/0006-8993(80)90567-3. [DOI] [PubMed] [Google Scholar]
- 38.Windels F, Kiyatkin EA. GABA, not glutamate, controls the activity of substantia nigra reticulata neurons in awake, unrestrained rats. J Neurosci. 2004;24:6751–6754. doi: 10.1523/JNEUROSCI.1528-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolf RC, Grön G, Sambataro F, Vasic N, Wolf ND, Thomann PA, Saft C, Landwehrmeyer B, Orth M. Brain activation and functional connectivity in premanifest Huntington's disease during states of intrinsic and phasic alertness. Human Brain Mapping. 2011 doi: 10.1002/hbm.21348. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]